Abstract
Breast cancer is the second leading cause of cancer mortality among women. Given its important role in DNA methylation and synthesis, one-carbon metabolism may affect breast cancer mortality. We utilized a population-based cohort of 1,508 women with breast cancer to investigate possible associations of dietary intake of B vitamins prior to diagnosis as well as 9 polymorphisms of one-carbon metabolizing genes and subsequent survival. Women newly diagnosed with a first primary breast cancer in 1996-1997 were followed for vital status for an average of 5.6 years. Kaplan-Meier survival and Cox proportional hazard regression analyses were used to evaluate the association between dietary intakes of B vitamins (1479 cases), genotypes (∼1065 cases) and all-cause as well as breast cancer-specific mortality. We found that higher dietary intake of vitamin B1 and B3 was associated with improved survival during the follow-up period (p for trend = 0.01 and 0.04, respectively). Compared to the major genotype, the MTHFR 677 T allele carriers have reduced all-cause mortality and breast cancer-specific mortality in a dominant model [HR and 95% CI: 0.69(0.49-0.98) and 0.58 (0.38-0.89), respectively). The BHMT 742 A allele was also associated with reduced all-cause mortality [HR 0.70(0.50-1.00)]. ER/PR status modified the association between the MTHFR C677T polymorphism and survival (p=0.05). The survival associations with one-carbon polymorphisms did not differ with the use of chemotherapy, although study power was limited for examining such effect modification. Our results indicate that one-carbon metabolism may be an important pathway that could be targeted to improve breast cancer survival.
Keywords: one-carbon, diet, B vitamin, gene polymorphism, breast cancer, survival
INTRODUCTION
The 5-year survival rate for breast cancer among US women has increased from 75% during 1974-76 to 85% during 1989-95 (1, 2). Despite such marked improvement, breast cancer remains the leading cause of cancer mortality among women 20 – 59 years of age and the second leading cause of cancer mortality among all women (1). Disease-free survival after breast cancer treatment may be partially predicted by tumor size, hormone receptor status and other clinical and pathological factors (3-7). Although a number of lifestyle and host factors have been inconsistently or infrequently reported to impact disease-free or overall survival, only a few have been firmly established to adversely affect survival, including age, race and obesity (8-10); few of these are factors that a patient can actively modify or that can help clinicians to tailor an effective treatment.
One-carbon metabolism may influence breast cancer mortality because of the critical role it plays in both DNA methylation and DNA synthesis (Figure 1). An abnormal methylation profile such as promoter-CpG island hypermethylation is a common molecular defect in cancer cells (11, 12). Estrogen receptor (ER) and progesterone receptor (PR) status have been used widely as a prognostics markers as well as indicators for tailoring breast cancer treatment (endocrine- vs. chemotherapy)(13). Loss of function of these genes has been shown to predict poor prognosis of breast cancer (14). Promoter hypermethylation has been implicated as the underlying mechanism for silencing of these receptors (12, 15-17). Both dietary factors, such as folate and related B vitamins, and genetic variations of one-carbon metabolizing genes may modify the methylation profile of these prognostic genes, thus influencing breast cancer survival.
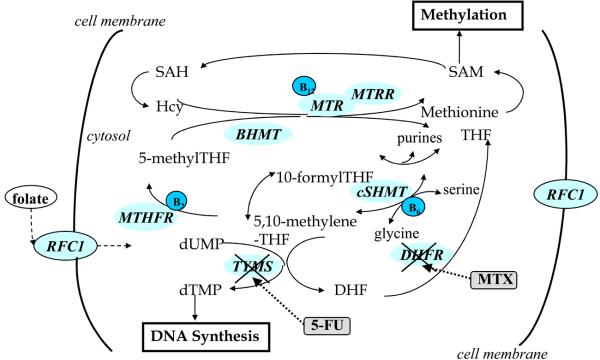
Figure 1.
Schematic illustration of one-carbon metabolism pathway. Key genes involved in one-carbon metabolism include methylenetetrahydrofolate reductase (MTHFR), thymidylate synthase (TYMS), methionine synthase (MTR), methionine synthase reductase (MTRR), cytosol serine hydroxymethyltransferase (cSHMT), dihydrofolate reductase (DHFR), and betaine-homocysteine methyltransferase (BHMT). Reduced folate carrier (RFC1) and human folate receptor (hFR) transport the dietary polyglutamyl folate (the predominant form of folate in diet) in intestinal absorption. B vitamins are co-factors of one-carbon enzymes as shown. Chemotherapy drugs 5-fluorouracil (5-FU) targets TYMS and methotrexate targets DHFR to block DNA synthesis. Hcy, homocysteine; SAM, S-adenosylmethionine; SAH, adenosylhomocysteine; THF, tetrahydrofolate; DHF, dihydrofolate; dUMP, deoxyuridine monophosphate; dUTP, deoxythymidine monophosphate.
Another reason one-carbon metabolism may influence breast cancer survival is that it is the direct target for several widely used anti-cancer drugs. Combination chemotherapy with cyclophosphamide, methotrexate, and 5-Fluorouracil (CMF) has been the treatment of choice for the majority of patients with stage II, node-negative breast cancer, as clinical trials have demonstrated its efficacy and long-term safety (18). 5-fluorouracil targets the enzyme thymidylate synthase (TYMS) while methotrexate targets dihydrofolate reductase (DHFR), both involved in one-carbon metabolism (Figure 1). These drugs block DNA synthesis and ultimately cell replication. Genetic variations of these enzymes may have functional consequences and ultimately impact the efficacy of the CMF treatment modality.
In humans, folate plays the fundamental role of providing methyl groups for intracellular methylation reactions and de novo deoxynucleoside synthesis. Folate deficiency is associated with genomic instability and could lead to abnormal DNA methylation status (19). Recently, studies on colorectal cancer suggested that folate may play a dual role in tumor progression (20, 21). While folate is in general anti-neoplastic before the tumor foci are established, it may enhance tumor proliferation after the tumor is established. Thus the effect of one-carbon metabolism on breast cancer survival may be complicated. Utilizing the population-based data from the Long Island Breast Cancer Study Project (LIBCSP), we investigated the associations of dietary intakes of B vitamins prior to diagnosis as well as functional polymorphisms in one-carbon metabolism and breast cancer survival.
METHODS
Study population
We utilized data from the follow-up study of the LIBCSP, a population-based study that includes women newly diagnosed with a first primary breast cancer who participated in the original case-control study (22) and were subsequently re-interviewed about five years later and followed for vital status (23). The study protocol was approved by the Institutional Review Boards of the collaborating institutions.
Women considered eligible for the follow-up study included all case participants of the parent case-control study, which includes 1,508 women with a first primary in situ or invasive breast cancer who were newly diagnosed between August 1, 1996 and July 31, 1997, and were residents of Nassau or Suffolk counties, Long Island, NY, at the time of diagnosis. Cases were identified through review of pathology/cytology records of 33 collaborating institutions and were contacted by letter and telephone after obtaining physician permission.
As previously reported (22), at the time of the first primary breast cancer diagnosis, the mean age was 58.8 years (range: 25.1-98.1); 94% were white, 4% were African American, and 2% were other; 235 (15.6%) had carcinoma in situ and 1273 (84.4%) had invasive tumor; 472(31.3%) women were pre-menopausal and 1006 (66.7%) were post-menopausal; and 583 (58.9%) were ER+/PR+; 143 (14.4%) were ER+/PR−; 52 (5.3%) were ER−/PR+; and 212 (21.4%) were ER−/PR−.
Exposure assessment
Exposure data was obtained as part of the 1) case-control (baseline) interview; 2) follow-up interview; and 3) medical record abstraction, as described below. For our analyses, dietary intake data and most of the potential confounders and effect modifiers were derived from the baseline interview. Complete course of treatment for the primary breast cancer was obtained during the follow-up interview and through medical record abstraction.
Case-control data
The baseline questionnaire was administered by a trained interviewer approximately 3 months after diagnosis (average time was 96 days) and elicited information on reproductive/menstrual history, cigarette smoking, alcohol, body size, physical activity, and medical history (http://www.epi.grants.cancer.gov/LIBCSP/projects/Questionnaire.html). Dietary intake in the year prior to the interview (which primarily reflects pre-diagnostic intake) was assessed using a self-administered modified Block Food Frequency Questionnaire (FFQ) that was completed at the time of the baseline interview (24). The frequency and portion sizes data were translated to daily intakes of nutrients from both dietary and supplement sources using the National Cancer Institute's DietSys version 3. Habitual use of multivitamin supplements was also obtained from the FFQ. The questions included multivitamin use over the past 10–15 years before the interview, type of multivitamin, and dosage. Details have been described previously (24-26). Total folate was calculated by using the estimated dietary folate equivalent (DFE) conversion factor of 1.7 for folic acid from supplement (27, 28): total folate = dietary folate + 1.7 × supplement folate. In this study, we examined the B vitamins (folate, B1, B2, B3, B6, and B12) and two cofactors involved in methyl transfer in the one-carbon metabolism pathway, namely, methionine and betaine. Spearman coefficients ranged from a low of 0.41 between total folate and vitamin B3 to a high of 0.90 between dietary folate and vitamin B1 (29). Energy intake was also estimated using data collected from the FFQ. The dietary intake information was available for 1479 cases in our analysis.
Follow-up data
In 2002-2004, case participants, or their proxy (close relative or friend) were re-contacted first by mail, and then by telephone, to invite them to participate in the follow-up study (23, 30). Follow-up data used in this study were the information for tumor characteristics (tumor size and nodal status) and treatment information. Of the 1098 case women for whom we have data from the follow-up questionnaire: 784 (71.4%) completed the full interview themselves; 165 (15.0%) respondents completed the critical interview which was an abbreviated questionnaire that focused on collecting treatment data only; and 84 (7.7%) proxy interviews were conducted. Among the 1022 case women who provided information on chemotherapy at the time of the follow-up interview, 423 (41.4%) reported receiving chemotherapy as part of their first course of treatment for their primary breast cancer diagnosis.
Medical record data
For cases who signed medical record release forms, medical records were abstracted twice: at baseline (as part of the case-control study) and again during the follow-up study. At baseline, information was obtained on disease stage (in situ/invasive), tumor size, ER/PR status (assessed by immunohistochemistry, n = 990), and initial course of treatment for their breast cancer. Over three quarters of the baseline case interviews occurred prior to the initiation of chemotherapy (22). Additional treatment information was obtained by re-abstracting medical records as part of the follow-up study. Among the subset of women for whom we had complete course of treatment data from the medical record (n = 499), we compared their self-reported responses at the follow-up interview with the medical record data using the kappa coefficient; concordance between the two sources was high (kappa = 0.97 for radiation therapy, 0.96 for chemotherapy, and 0.92 for hormone therapy)(23).
Blood sample collection and genotyping
Blood samples were collected from 73% of the 1508 cases at the time of the baseline interview by trained field staff (22). DNA was isolated from blood specimens using the methods previously described (25); DNA was available from 1065 individuals for genotyping. Using methods described elsewhere (29, 31), genotyping was conducted on 9 polymorphisms in the one-carbon metabolism pathway, namely: MTHFR C677T (rs1801133) and A1298C (rs1801131); TYMS 5′-UTR tandem repeat; DHFR 19bp deletion; MTR A2756 (rs1805087); MTRR A66G (rs1801394); BHMT G742A (rs3733890); RFC1 A80G (rs1051266); and cSHMT C1420T (rs1979277). The mean call rate was 96%; the main reason for missing genotypes was insufficient DNA. About 10% of the study population samples were randomly duplicated as quality control samples; the concordance rate was >98% for all polymorphisms in this study. All laboratory personnel were blinded to the outcome status of the breast cancer cases as well as quality control status of the specimens. Characteristics were comparable between cases with blood and those with genotype information available.
Study outcome
The National Death Index was used to ascertain all-cause and breast cancer-specific mortality. Among the 1508 women diagnosed with breast cancer in 1996–1997, 198 (13.1%) deaths occurred by December 31, 2002. The mean follow up time was 5.6 years (range: 0.2-7.4). Based on International Classification of Diseases (ICD) codes 174.9 and C-50.9 listed as a primary or secondary code on the death certificate, 124 (62.6%) of these 198 deaths were due to breast cancer. When restricted to the cohort from which DNA was available for genotype analysis (n=1065), a total of 131 (12.3%) deaths were observed; 84 (64.1%) of these were due to breast cancer.
Statistical Analysis
The Kaplan-Meier and the log-rank test were used to examine the crude association between dietary intake or genotypes and survival (32). The Cox proportional hazard regression (32) was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for all-cause and breast cancer-specific mortality, with adjustments made for age at diagnosis (continuous) and energy intake (quintiles). Nutrient intakes in the year prior to the interview were categorized based on the distributions observed among all cases regardless of outcome status; results based on tertiles are shown. Tests of trend were conducted by coding the variables ordinarily in the model. To increase statistical power, heterozygous and variant homozygous genotypes were combined as a single risk group.
Confounding was evaluated using the methods described by Rothman and Greenland (33) starting with a full multivariate model and using backward elimination. Factors considered as potential confounders included: menopausal status (pre-/post-menopausal), family history of breast cancer in a first-degree relative, cancer type (in situ/invasive), active/passive cigarette smoking, body mass index (BMI) at diagnosis, average lifetime alcohol intake (g/day), education, income, tumor size, and radiation treatment and chemotherapy undergone for the original breast cancer diagnosis. If eliminating a covariate from the full Cox regression model changed the effect estimate by 10% or more, the covariate was considered a confounder and kept in the model (33). Otherwise that covariate was dropped from the multivariate model. None of the covariates tested met such criterion, thus, only results adjusted for age and energy intake are presented.
Effect modification on the multiplicative scale was evaluated using the log likelihood ratio test to compare Cox models with and without the interaction term as a cross-product term of genotype and effect modifier. Factors considered as potential effect modifiers of the genotype-mortality association include: menopausal status (pre-/post-menopausal), cancer type (in situ/invasive), ER/PR status [cases were categorized to two groups: ER and PR both positive (ER+/PR+) vs. all others (ER+/PR−, ER−/PR+, ER−/PR−) and when the latter receptor types were analyzed individually, their respective HRs were similar] and chemotherapy. P values for the interaction were evaluated for the potential modification on gene effect by factors examined.
Unconditional logistic regression was used to explore the relationship of one-carbon genotype and hormone receptor (ER/PR) status. Genotypes were used as exploratory variables in the model. ER/PR status (either both positive vs. all others) was treated as outcome in the logistic regression model and the odds ratio (OR) and 95% CI were estimated by modeling the probability of the case tumor being ER/PR positive.
All statistical analyses were performed using SAS statistical software version 9.1(SAS Institute, Cary, NC).
RESULTS
The associations between dietary intake of B vitamins in the year prior to the baseline interview and subsequent all-cause as well as breast cancer specific mortality are summarized in Table 1. Intake of vitamin B1 and B3 were inversely associated with all-cause mortality in this population-based cohort of women with breast cancer. Compared to the low intake group, cases in the high intake group had a 46% (HR and 95% CI: 0.54; 0.34-0.88) and 39% (HR and 95% CI: 0.61; 0.38-0.98) lower risk of death for B1 and B3, respectively. Trend tests for the associations were significant (p for trend =0.01 for B1 and 0.04 for B3). Although beneficial effects were observed for other one-carbon related B vitamins (except for B12), where all the HRs were less than one, the reductions were not statistically significant. No association was observed for intakes of methionine or betaine and all-cause mortality. When we examined the association with breast cancer-specific mortality, similar HRs were observed (Table 1); however, the association for vitamin B3 did not reach significance (p for trend =0.09).
Table 1.
Age- and energy-adjusted HRs and 95% CIs for the association between B vitamin intakes and all-cause as well as breast cancer-specific mortality
| Nutrient‡ Dietary folate |
Low | Medium | High | P, trend | |
|---|---|---|---|---|---|
| Range(μg/d) | <194.1 | 194.1-300.8 | >300.8 | ||
| HR(95% CI)* | 1.00(ref) | 0.82(0.57-1.17) | 0.79(0.52-1.12) | 0.28 | |
| HR(95% CI) † | 1.00(ref) | 0.88(0.55-1.39) | 0.81(0.47-1.39) | 0.44 | |
| Total folate (diet + supplements) | |||||
| Range(μg/d) | <291.0 | 291.0-869.0 | >869.0 | ||
| HR(95% CI)* | 1.00(ref) | 0.82(0.57-1.18) | 0.97(0.69-1.36) | 0.85 | |
| HR(95% CI) † | 1.00(ref) | 0.80(0.49-1.31) | 1.24(0.81-1.90) | 0.27 | |
| Vitamin B1 (thiamin) | |||||
| Range(mg/d) | <0.87 | 0.87-1.25 | >1.25 | ||
| HR(95% CI)* | 1.00(ref) | 0.72(0.49-1.04) | 0.54(0.38-0.88) | 0.01 | |
| HR(95% CI) † | 1.00(ref) | 0.71(0.44-1.12) | 0.44(0.24-0.81) | 0.01 | |
| Vitamin B2 (riboflavin) | |||||
| Range(mg/d) | <1.17 | 1.17-1.75 | >1.75 | ||
| HR(95% CI)* | 1.00(ref) | 0.75(0.51-1.09) | 0.92(0.58-1.44) | 0.67 | |
| HR(95% CI) † | 1.00(ref) | 0.86(0.54-1.38) | 0.72(0.41-1.29) | 0.27 | |
| Vitamin B3 (niacin) | |||||
| Range(mg/d) | <11.9 | 11.9-16.7 | >16.7 | ||
| HR(95% CI)* | 1.00(ref) | 0.68(0.47-0.99) | 0.61(0.38-0.98) | 0.04 | |
| HR(95% CI) † | 1.00(ref) | 0.76(0.47-1.21) | 0.61(0.34-1.09) | 0.09 | |
| Vitamin B6 (pyridoxine) | |||||
| Range(mg/d) | <1.05 | 1.05-1.54 | >1.54 | ||
| HR(95% CI)* | 1.00(ref) | 0.99(0.68-1.43) | 0.95(0.61-1.48) | 0.82 | |
| HR(95% CI) † | 1.00(ref) | 0.95(0.60-1.51) | 0.77(0.44-1.36) | 0.37 | |
| Vitamin B12 (cobalamin) | |||||
| Range(mcg/d) | <3.05 | 3.05-5.12 | >5.12 | ||
| HR(95% CI)* | 1.00(ref) | 0.96(0.67-1.40) | 1.20(0.80-1.81) | 0.38 | |
| HR(95% CI) † | 1.00(ref) | 0.93(0.58-1.49) | 1.10(0.65-1.85) | 0.71 | |
| Methionine | |||||
| Range(g/d) | <0.79 | 0.79-1.14 | >1.14 | ||
| HR(95% CI)* | 1.00(ref) | 0.84(0.59-1.21) | 0.70(0.44-1.13) | 0.14 | |
| HR(95% CI) † | 1.00(ref) | 0.93(0.59-1.49) | 0.70(0.39-1.28) | 0.25 | |
| Betaine | |||||
| Range(mg/d) | <86.5 | 86.5-149.6 | >149.6 | ||
| HR(95% CI)* | 1.00(ref) | 0.87(0.61-1.25) | 0.81(0.54-1.20) | 0.28 | |
| HR(95% CI) † | 1.00(ref) | 0.63(0.40-1.02) | 0.72(0.44-1.17) | 0.19 | |
All-cause mortality
Breast cancer-specific mortality
Intake of B vitamins was tertiled for analysis
Table 2 summarizes the association between one-carbon polymorphisms and all-cause as well as breast cancer-specific mortality. The variant alleles of two polymorphisms, MTHFR C677T and BHMT G742A, were significantly associated with better survival. The MTHFR677 T allele carriers had 31% lower risk of death than patients with the MTHFR677 CC genotype (HR and 95% CI: 0.69; 0.49-0.98). BHMT 742 A allele carriers had 30% lower risk of death than those with the BHMT GG genotype (HR and 95% CI: 0.70; 0.50-1.00). Two other single nucleotide polymorphisms (SNPs), MTR2756 G and cSHMT1420 T alleles were associated with better survival with borderline significance. When we examined breast cancer-specific mortality, similar results were observed (Table 2); however, the association with BHMT did not reach statistical significance (p=0.11).
Table 2.
Age-adjusted HRs and 95% CIs for the associations of polymorphisms of the one-carbon metabolizing genes and all-cause as well as breast cancer-specific mortality
| All-cause mortality | Breast cancer-specific mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Genotype | No. death | No. censored (%) | HR* | 95% CI | No. death | No. censored (%) | HR* | 95% CI |
| MTHFR | CC | 60 | 338(84.9) | 1.00 | Ref. | 42 | 356(89.5) | 1.00 | Ref. |
| (C677T) | CT/TT | 71 | 594(89.3) | 0.69 | 0.49-0.98 | 42 | 623(93.7) | 0.58 | 0.38-0.89 |
| MTHFR | CC | 69 | 489(87.6) | 1.00 | Ref. | 41 | 517(92.7) | 1.00 | Ref. |
| (A1298C) | AC/AA | 61 | 443(87.9) | 1.01 | 0.71-1.42 | 43 | 461(91.5) | 1.16 | 0.76-1.78 |
| TSTR | 3R/3R | 43 | 268(86.2) | 1.00 | Ref. | 26 | 285(91.6) | 1.00 | Ref. |
| (5′-UTR) | 3R/2R/2R/2R | 85 | 657(88.5) | 0.80 | 0.56-1.16 | 57 | 685(92.5) | 0.91 | 0.57-1.45 |
| DHFR | +/+ | 46 | 288(86.2) | 1.00 | Ref. | 31 | 303(90.7) | 1.00 | Ref. |
| (19bp del) | +/− / −/− | 84 | 645(88.5) | 0.85 | 0.60-1.22 | 52 | 677(92.9) | 0.76 | 0.49-1.18 |
| MTR | GG | 96 | 609(86.4) | 1.00 | Ref. | 58 | 647(91.8) | 1.00 | Ref. |
| (A2756G) | AG/AA | 34 | 315(90.3) | 0.70 | 0.47-1.03 | 25 | 324(92.8) | 0.85 | 0.53-1.35 |
| MTRR | GG | 42 | 237(85.0) | 1.00 | Ref. | 26 | 253(90.7) | 1.00 | Ref. |
| (A66G) | AG/AA | 88 | 691(88.7) | 0.75 | 0.52-1.08 | 57 | 722(92.7) | 0.77 | 0.49-1.23 |
| BHMT | GG | 74 | 436(85.5) | 1.00 | Ref. | 47 | 463(90.8) | 1.00 | Ref. |
| (G742A) | AG/AA | 56 | 495(89.8) | 0.70 | 0.50-1.00 | 36 | 515(93.5) | 0.70 | 0.45-1.08 |
| RFC1 | GG | 34 | 253(88.2) | 1.00 | Ref. | 19 | 268(93.4) | 1.00 | Ref. |
| (A80G) | AG/AA | 97 | 682(87.5) | 1.02 | 0.69-1.51 | 65 | 714(91.7 | 1.25 | 0.75-2.08 |
| cSHMT | CC | 70 | 438(86.2) | 1.00 | Ref. | 47 | 461(90.8) | 1.00 | Ref. |
| (C1420T) | CT/TT | 60 | 493(89.2) | 0.73 | 0.52-1.03 | 36 | 517(93.5) | 0.69 | 0.44-1.06 |
Note: HR: hazards ratio, adjusted for age (continuous); CI: confidence interval.
To explore whether two SNPs, MTHFR C677T and BHMT G742A, influence breast cancer survival via the hormone receptor (ER/PR) status of the tumor, we examined the associations between genotypes and ER/PR status (Table 3). Tumors of cases with the MTHFR677 TT genotype had an ∼60% higher chance to be ER/PR positive compared to tumors of individuals with the CC genotype. Tumors of the T allele carriers had an ∼28% higher chance to be ER/PR positive. There was no relationship between the BHMT polymorphism and ER/PR status in this population. Although no main effects on mortality were observed for other 7 polymorphisms investigated in the study, we also examined the associations between genotypes and ER/PR status but no association was found (data not shown).
Table 3.
Relation of one-carbon genotype to ER/PR status
| ER/PR status* | |||||
|---|---|---|---|---|---|
| Gene | Genotype | positive(%) | negative(%) | OR (95%CI) † | P(trend) |
| MTHFR | CC | 145(34.6) | 107(40.2) | 1.00 (referent) | 0.05 |
| (C677T) | CT | 195(46.5) | 123(46.2) | 1.17(0.84-1.64) | |
| TT | 79(18.9) | 36(13.5) | 1.63(1.02-2.60) | ||
| CT/TT | 274(75.4) | 159(59.8) | 1.28(0.93-1.75) | ||
| BHMT | GG | 192(45.9) | 128(47.9) | 1.00 (referent) | 0.89 |
| (G742A) | GA | 183(43.8) | 108(40.5) | 1.13(0.82-1.57) | |
| AA | 43(10.3) | 31(11.6) | 0.92(0.55-1.54) | ||
| GA/AA | 226(54.1) | 139(52.1) | 1.09(0.80-1.48) | ||
ER/PR status: positive represents for ER+PR+ cases and negative represents for ER+/PR−, ER−/PR+ and ER−/PR− cases.
OR: odds ratio; CI: confidence interval
We also examined whether the polymorphism-survival relationship differed by hormone receptor (ER/PR) status. Effect modification by ER/PR status was observed with respect to the MTHFR C677T polymorphism and all-cause mortality (p for interaction = 0.05). The T allele was associated with better survival with borderline significant in all-cause mortality only among the grouped cases with ER+/PR−, ER-/PR+ and ER−/PR− status (HR: 0.61; 0.36-1.01). The associations between other 8 polymorphisms and survival did not differ with respect to hormone receptor status (data not shown).
To explore the potential modifying effect of one-carbon gene polymorphisms on chemotherapy response in relation to breast cancer survival, we stratified the cases by whether they received chemotherapy or not. About 800 cases with both genotype and chemotherapy treatment information were include in this analysis. Associations of one-carbon metabolism polymorphisms and overall survival did not differ by chemotherapy status (data not shown).
DISCUSSION
Utilizing data from a population-based cohort of breast cancer cases, we found inverse associations between several micronutrients and genotypes in the one-carbon metabolism and all-cause mortality. To the best of our knowledge, our study is the first to systematically evaluate pre-diagnostic intake of B vitamins involved in one-carbon metabolism pathway in relation to breast cancer survival. This study is based on a strong biological rationale because one-carbon metabolism not only involves in regulation of prognosis-predictive genes in breast cancer but also is the major target for treatment of the disease (Figure 1). Considering the high prevalence of these polymorphisms in the general population, results from the study can help us to identify factors that may influence disease outcomes.
B vitamins (B1, thiamin; B2, riboflavin; B3, niacin; B6, pyridoxine; B9, folate; B12, cobalamin) play important roles in cell metabolism, and some of them are cofactors involved in the one-carbon pathway. In a previous report (29), we found that increased dietary intakes of B vitamins were associated with reduced risk of developing breast cancer. Herein, we reported a beneficial effect of B vitamins, B1 and B3 in particular, on survival in the same population of breast cancer cases. These findings imply that a healthy diet reduces a woman's risk of developing breast cancer, but should a breast cancer occur, the tumor would also display characteristics associated with a more favorable prognosis.
In this study, we found two genetic polymorphisms, MTHFR C677T and BHMT G742A, were inversely associated with all-cause mortality. MTHFR catalyzes an irreversible reaction and it is the rate-limiting step in folate metabolism. Changes in MTHFR activity may tilt the balance of one-carbon metabolism in favor of DNA synthesis at the expense of methyl supply (i.e. S-Adenosylmethionine) for methylation reactions. The C677T polymorphism of MTHFR results in an alanine to valine substitution and has been correlated with enzyme thermolability and reduced enzyme activity (34). Lower MTHFR activity has been shown to decrease DNA methylation in animal experiments (35). DNA methylation has been implicated in the silencing of ER, a prognosis-predictive gene (12, 15-17). Consistent with this reasoning, the TT genotype was associated with ER+/PR+ tumors in our study population (Table 3) leading to improved survival. This finding is based on a small sample size, and replication is warranted.
Although not directly involved in folate metabolism, BHMT is involved in the metabolism of homocysteine. BHMT may play a critical role in the remethylation of homocysteine when the folate-dependent pathway is compromised by either genetic or dietary factors (36). The BHMT G742A polymorphism was associated with overall survival in our study population; it is unknown whether homocysteine level correlates with breast cancer prognosis in our study population.
Many studies have been conducted to examine the effects of one-carbon metabolism polymorphisms on breast cancer risk, but reports of effects on subsequent survival are relatively sparse. There are two other studies that examined the effect of MTHFR genotypes on breast cancer survival. Results from the Shanghai Breast Cancer study showed that MTHFR genotypes were not associated with all-cause mortality, but the 677TT genotype was associated with poor survival among those with late stage disease (37). In our study, we observed a beneficial effect of the MTHFR677 T allele on all-cause mortality. Difference among these reports could be due to the difference in study populations, given that our population is overwhelmingly Caucasian, whereas the Shanghai Breast Cancer Study is restricted to Chinese women. Factors that may influence the MTHFR-survival relationship, such as treatment modality, may also be different in these two populations. Results from a small cohort reported by Martin et al. showed that MTHFR1298 C allele was associated with worse survival compared to the AA genotype; furthermore, this effect was stronger in ER- patients (38). This latter study is based on a cohort containing a mixture of Caucasian and African-American women and the sample size was relatively small (∼250).
We did not observe any survival differences by genotype stratified by chemotherapy. Although our sample size is much larger than previous studies, statistical power is still limited in stratified analyses. In addition, because the LIBCSP is a population-based study, the breast cancer patients were treated in multiple institutions with non-standardized protocols. The majority of women in our study received adjuvant chemotherapy as part of their regular treatment for breast cancer. Specific information such as chemotherapy dose and duration were not available for all our study subjects, which limited our ability to examine the potential interaction between one-carbon metabolism and chemotherapy. Investigation of such relationships in the context of a larger population of breast cancer patients with more complete information on treatment modality is warranted, since it is plausible that these factors could potentially be used to tailor treatment and ultimately improve survival.
In our study, we did not find any substantial differences in terms of associations of one-carbon metabolism with all-cause mortality and breast cancer specific mortality. Vitamin B3 intake and BHMT (G742A) polymorphism were associated with all-cause mortality but did not reach statistical significance for breast cancer specific mortality. Given the point estimates are similar, the wide confidence interval could be a result of limited study outcome (∼130 death). Results on breast cancer-specific mortality may help us better understand the role of one-carbon metabolism in breast cancer progression and to develop more efficient treatment for this disease. Accurate assessment on cause of death is crucial in this type of investigation (39-41). The reliability of the cause of death listed on the death certificate, particularly when looking at a specific cancer site may be questionable, e.g. sometimes a metastatic site may be recorded as the cause of death (42, 43). Thus, our findings based on all-cause mortality may be more valid. In addition, estimation of overall survival (with death as the end point regardless of the specific cause) is of public health significance and provides us with better power for detecting associations (44).
We utilized the dietary information collected at the baseline interview, which reflects intake patterns one year prior to the interview including the months just prior to and at diagnosis. Recall of this information was ascertained prior to the study outcome (i.e. death), thus any possible misclassification is likely to be non-differential. However, it is possible that patients change their lifestyle after a diagnosis of cancer (45, 46). Consequently, our results should be interpreted with caution. Studies have indicated that after breast cancer diagnosis, women are motivated to change their diet, but this is observed primarily in younger women (47, 48); our study population, on the other hand, is primarily composed of postmenopausal women. Interestingly, a substantial percentage (50%) of cancer survivors has been reported to continue to engage in health-risk behaviors (45). As discussed in a recent review, supplement use among breast cancer patients is high and frequently increases after diagnosis (49). We were unable to identify studies that specifically address the issue of whether breast cancer survivors change their dietary intake of foods containing B vitamins after diagnosis. If women in our study continued to follow their pre-diagnostic diets after their breast cancer diagnosis, then the implications of our results are that women in the general population should be encouraged to consume more B vitamins.
One potential issue to consider in interpreting our results is whether our analyses should have considered the potential confounding effects of tumor stage (or its surrogates tumor size and first course of treatment). However, because tumor stage is more likely a causal intermediate (e.g. the biological link between the exposure with the outcome), it would be epidemiologically inappropriate to include tumor stage in the model. However, in sub-analyses we did consider the potential confounding effects of tumor size and first course of treatment (as surrogates for tumor stage, which was not reliably recorded on the medical record for all cases), and found that our results remain unchanged.
A potential limitation of our study is that we focused solely on the dominant model in our genetic association analyses. Consideration of other models would be of interest, but because our study power was constrained by the number of deaths (about 130) in our cohort of breast cancer cases, the results would be unstable and perhaps misleading. Another limitation of our study is its limited power for testing potential gene-gene and gene-diet interactions. Thus we did not investigate these interactions in our analysis.
In summary, results from our population-based study suggest that in addition to its role in breast cancer etiology, one-carbon metabolism may be an important pathway that can be targeted to improve breast cancer survival among women with breast cancer.
Acknowledgments
Financial support: This work was supported by National Cancer Institute grant R01CA109753; and in part by grants from the Department of Defense (BC990191 and BC031746), the National Cancer Institute and the National Institutes of Environmental Health and Sciences (UO1CA/ES66572, UO1CA66572, P30ES09089 and P30ES10126); and the University of North Carolina Center for Environmental Health and Susceptibility Nutrient Assessment Facility Core (Core Director: Dr. Steven Zeisel). Xu, X. is the recipient of a Predoctoral Traineeship Award (W81XWH-06-1-0298) from the Department of Defense Breast Cancer Research Program.
REFERENCES
- 1.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999 Jan-Feb;49(1):8,31, 1. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000 Jan-Feb;50(1):7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989 Jan 1;63(1):181–7. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Page DL, Jensen RA, Simpson JF. Routinely available indicators of prognosis in breast cancer. Breast Cancer Res Treat. 1998;51(3):195–208. doi: 10.1023/a:1006122716137. [DOI] [PubMed] [Google Scholar]
- 5.Osborne CK. Steroid hormone receptors in breast cancer management. Breast Cancer Res Treat. 1998;51(3):227–38. doi: 10.1023/a:1006132427948. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 7.Frei E. Section 31: The breast. In: Holland, James F, Kufe, Donald W, editors. Cancer medicine 6. BC Decker, Inc; Hamilton, Ont. ; Lewiston, N.Y.: 2003. p. 1425. [Google Scholar]
- 8.Carmichael AR, Bates T. Obesity and breast cancer: A review of the literature. Breast. 2004 Apr;13(2):85–92. doi: 10.1016/j.breast.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Barnett JB. The relationship between obesity and breast cancer risk and mortality. Nutr Rev. 2003 Feb;61(2):73–6. doi: 10.1301/nr.2003.febr.73-76. [DOI] [PubMed] [Google Scholar]
- 10.Weitzen R, Tichler T, Kaufman B, Catane R, Shpatz Y. Body weight, nutritional factors and physical activity--their influence on prognosis after breast cancer diagnosis. Harefuah. 2006 Nov;145(11):820,5, 861. [PubMed] [Google Scholar]
- 11.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: A fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–96. [PubMed] [Google Scholar]
- 12.Widschwendter M, Jones PA. DNA methylation and breast carcinogenesis. Oncogene. 2002 Aug 12;21(35):5462–82. doi: 10.1038/sj.onc.1205606. [DOI] [PubMed] [Google Scholar]
- 13.early breast cancer trialists' collaborative group Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet. 1998 May 16;351(9114):1451–67. [PubMed] [Google Scholar]
- 14.James CR, Quinn JE, Mullan PB, Johnston PG, Harkin DP. BRCA1, a potential predictive biomarker in the treatment of breast cancer. Oncologist. 2007 Feb;12(2):142–50. doi: 10.1634/theoncologist.12-2-142. [DOI] [PubMed] [Google Scholar]
- 15.Yan PS, Chen CM, Shi H, et al. Dissecting complex epigenetic alterations in breast cancer using CpG island microarrays. Cancer Res. 2001 Dec 1;61(23):8375–80. [PubMed] [Google Scholar]
- 16.Yang X, Yan L, Davidson NE. DNA methylation in breast cancer. Endocr Relat Cancer. 2001 Jun;8(2):115–27. doi: 10.1677/erc.0.0080115. [DOI] [PubMed] [Google Scholar]
- 17.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002 Jun;3(6):415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 18.Goldhirsch A, Colleoni M, Coates AS, Castiglione-Gertsch M, Gelber RD. Adding adjuvant CMF chemotherapy to either radiotherapy or tamoxifen: Are all CMFs alike? the international breast cancer study group (IBCSG) Ann Oncol. 1998 May;9(5):489–93. doi: 10.1023/a:1008236502420. [DOI] [PubMed] [Google Scholar]
- 19.Kim YI. Folate and DNA methylation: A mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol Biomarkers Prev. 2004 Apr;13(4):511–9. [PubMed] [Google Scholar]
- 20.Cole BF. Folic acid for the prevention of colorectal adenomas: A randomized clinical trial. JAMA. 2007 Jun 6;297(21):2351–9. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 21.Ulrich CM, Potter JD. Folate and cancer--timing is everything. JAMA. 2007 Jun 6;297(21):2408–9. doi: 10.1001/jama.297.21.2408. [DOI] [PubMed] [Google Scholar]
- 22.Gammon MD, Neugut AI, Santella RM, et al. The long island breast cancer study project: Description of a multi-institutional collaboration to identify environmental risk factors for breast cancer. Breast Cancer Res Treat. 2002 Jun;74(3):235–54. doi: 10.1023/a:1016387020854. [DOI] [PubMed] [Google Scholar]
- 23.Cleveland RJ, Eng SM, Abrahamson PE, et al. Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiol Biomarkers Prev. 2007 Sep;16(9):1803–11. doi: 10.1158/1055-9965.EPI-06-0889. [DOI] [PubMed] [Google Scholar]
- 24.Gaudet MM, Britton JA, Kabat GC, et al. Fruits, vegetables, and micronutrients in relation to breast cancer modified by menopause and hormone receptor status. Cancer Epidemiol Biomarkers Prev. 2004 Sep;13(9):1485–94. [PubMed] [Google Scholar]
- 25.Gammon MD, Santella RM, Neugut AI, et al. Environmental toxins and breast cancer on long island. I. polycyclic aromatic hydrocarbon DNA adducts. Cancer Epidemiol Biomarkers Prev. 2002 Aug;11(8):677–85. [PubMed] [Google Scholar]
- 26.Xu X, Gammon MD, Wetmur JG, et al. A functional 19-base pair deletion polymorphism of dihydrofolate reductase (DHFR) and risk of breast cancer in multivitamin users. Am J Clin Nutr. 2007 Apr;85(4):1098–102. doi: 10.1093/ajcn/85.4.1098. [DOI] [PubMed] [Google Scholar]
- 27.Sauberlich HE, Kretsch MJ, Skala JH, Johnson HL, Taylor PC. Folate requirement and metabolism in nonpregnant women. Am J Clin Nutr. 1987 Dec;46(6):1016–28. doi: 10.1093/ajcn/46.6.1016. [DOI] [PubMed] [Google Scholar]
- 28.Hannon-Fletcher MP, Armstrong NC, Scott JM, et al. Determining bioavailability of food folates in a controlled intervention study. Am J Clin Nutr. 2004 Oct;80(4):911–8. doi: 10.1093/ajcn/80.4.911. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Gammon MD, Chan W, et al. One-carbon metabolism, MTHFR polymorphisms, and risk of breast cancer. Cancer Res. 2005 Feb 15;65(4):1606–14. doi: 10.1158/0008-5472.CAN-04-2630. [DOI] [PubMed] [Google Scholar]
- 30.Fink BN, Gaudet MM, Britton JA, et al. Fruits, vegetables, and micronutrient intake in relation to breast cancer survival. Breast Cancer Res Treat. 2006 Jul;98(2):199–208. doi: 10.1007/s10549-005-9150-3. [DOI] [PubMed] [Google Scholar]
- 31.Xu X, Gammon MD, Zhang H, et al. Polymorphisms of one-carbon metabolizing genes and risk of breast cancer in a population-based study. Carcinogenesis. 2007 Mar 19; doi: 10.1093/carcin/bgm061. [DOI] [PubMed] [Google Scholar]
- 32.Hosmer DW. Applied survival analysis : Regression modeling of time to event data. Wiley; New York: 1999. [Google Scholar]
- 33.Rothman, Kenneth J, Greenland S. Modern epidemiology. 2nd ed. ed. Lippincott-Raven Publishers; Philadelphia, Pa: 1998. [Google Scholar]
- 34.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995 May;10(1):111–3. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Karaplis AC, Ackerman SL, et al. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001 Mar 1;10(5):433–43. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- 36.Schwahn BC, Chen Z, Laryea MD, et al. Homocysteine-betaine interactions in a murine model of 5,10-methylenetetrahydrofolate reductase deficiency. FASEB J. 2003 Mar;17(3):512–4. doi: 10.1096/fj.02-0456fje. [DOI] [PubMed] [Google Scholar]
- 37.Shrubsole MJ, Shu XO, Ruan ZX, et al. MTHFR genotypes and breast cancer survival after surgery and chemotherapy: A report from the shanghai breast cancer study. Breast Cancer Res Treat. 2005 May;91(1):73–9. doi: 10.1007/s10549-004-7265-6. [DOI] [PubMed] [Google Scholar]
- 38.Martin DN, Boersma BJ, Howe TM, et al. Association of MTHFR gene polymorphisms with breast cancer survival. BMC Cancer. 2006 Oct 27;6:257. doi: 10.1186/1471-2407-6-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kesteloot HE, Verbeke G. On the relationship between all-cause, cardiovascular, cancer and residual mortality rates with age. Eur J Cardiovasc Prev Rehabil. 2005 Apr;12(2):175–81. doi: 10.1097/01.hjr.0000164691.57823.eb. [DOI] [PubMed] [Google Scholar]
- 40.D'Amico M, Agozzino E, Biagino A, Simonetti A, Marinelli P. Ill-defined and multiple causes on death certificates--a study of misclassification in mortality statistics. Eur J Epidemiol. 1999 Feb;15(2):141–8. doi: 10.1023/a:1007570405888. [DOI] [PubMed] [Google Scholar]
- 41.Goldacre MJ. Cause-specific mortality: Understanding uncertain tips of the disease iceberg. J Epidemiol Community Health. 1993 Dec;47(6):491–6. doi: 10.1136/jech.47.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Percy C, Dolman A. Comparison of the coding of death certificates related to cancer in seven countries. Public Health Rep. 1978 Jul-Aug;93(4):335–50. [PMC free article] [PubMed] [Google Scholar]
- 43.Percy C, Stanek E, 3rd, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 1981 Mar;71(3):242–50. doi: 10.2105/ajph.71.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaffar S, Leach A, Smith PG, Cutts F, Greenwood B. Effects of misclassification of causes of death on the power of a trial to assess the efficacy of a pneumococcal conjugate vaccine in the gambia. Int J Epidemiol. 2003 Jun;32(3):430–6. doi: 10.1093/ije/dyg082. [DOI] [PubMed] [Google Scholar]
- 45.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000 Feb 1;88(3):674–84. [PubMed] [Google Scholar]
- 46.McBride CM, Clipp E, Peterson BL, Lipkus IM, Demark-Wahnefried W. Psychological impact of diagnosis and risk reduction among cancer survivors. Psychooncology. 2000 Sep-Oct;9(5):418–27. doi: 10.1002/1099-1611(200009/10)9:5<418::aid-pon474>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 47.Maunsell E, Drolet M, Brisson J, Robert J, Deschenes L. Dietary change after breast cancer: Extent, predictors, and relation with psychological distress. J Clin Oncol. 2002 Feb 15;20(4):1017–25. doi: 10.1200/JCO.2002.20.4.1017. [DOI] [PubMed] [Google Scholar]
- 48.Salminen EK. Does breast cancer change patients' dietary habits? Eur J Clin Nutr. 2000;54(11):844–8. doi: 10.1038/sj.ejcn.1601103. [DOI] [PubMed] [Google Scholar]
- 49.Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: A systematic review. J Clin Oncol. 2008 Feb 1;26(4):665–73. doi: 10.1200/JCO.2007.13.5905. [DOI] [PubMed] [Google Scholar]