Abstract
Despite having been identified first, their greater degree of complexity has resulted in our understanding of eukaryotic ribosomes lagging behind that of their bacterial and archaeal counterparts. A much more complicated biogenesis program results in ribosomes that are structurally, biochemically, and functionally more complex. However, recent advances in molecular genetics and structural biology are helping to reveal the intricacies of the eukaryotic ribosome and to address many longstanding questions regarding its many roles in the regulation of gene expression.
Since its initial discovery using differential ultracentrifugation of rat liver homogenates (reviewed in Ref. 1), the ribosome has remained a foundational platform upon which our understanding of the relationship between structure and function at the molecular level has been built. There is a rich history of biochemistry and genetics of eukaryotic ribosomes, including the discovery in the 1950s that they 32 are the site of protein synthesis, the elucidation of the function of the nucleolus, and even the discovery of the first eukaryotic RNA polymerase (reviewed in Ref. 2). Whereas early studies using mammalian ribosomes defined the “integral requirements” for protein synthesis, a switch to bacterial ribosomes in the 1960s facilitated the identification of the “minimal requirements” for the translational machinery, giving rise to a “golden age” of translation. In particular, the greater degree of structural and functional complexity makes eukaryotic ribosomes more challenging to work with than their bacterial and archaeal counterparts. For example, whereas bacterial translation initiation requires only a small set of trans-acting factors and is facilitated by the Shine-Dalgarno sequence, this process in eukaryotes requires a multifactorial complex of trans-acting factors that is almost as massive as the ribosome itself (reviewed in Ref. 3). Here, some of the current topics and challenges in the study of the eukaryotic ribosome are reviewed.
Biochemistry: Different from and Less Advanced than Bacterial Systems
The availability of an in vitro reconstitution system has facilitated highly detailed biochemical analyses of bacterial ribosomes (4). For example, in vitro reconstitution enables construction and assays of otherwise “dead” ribosomes (5), and it has enabled the use of fluorescence resonance energy transfer to examine intra-ribosomal movement at the single molecule level (6). In contrast, despite numerous attempts over the past 40 years, no analogous system has been successfully established for eukaryotic ribosomes, thus presenting significant technical challenges to biochemical studies. These failed efforts suggest that the biochemistry and physical complexity of eukaryotic ribosomes are significantly different from those of their bacterial counterparts. Indeed, recent biochemical analyses showing that salt rather than divalent ion concentrations are more important for subunit joining suggest that protein/protein and protein/RNA interactions are more widespread in eukaryotic as opposed to bacterial ribosomes (7). The strongest biochemistry has been developed in the field of translation initiation, where in vitro systems have existed for some time (reviewed in Ref. 8). More recently, a robust yeast-based in vitro translation initiation system has been developed, allowing yeast molecular genetics methods to complement biochemical approaches (9). However, the current state of the art is limited to steady-state biochemical analyses, and the contemporary challenge is to develop sturdy platforms for true kinetic studies.
Structural Biology
The elucidation of x-ray crystal structures of bacterial and archaeal ribosomes at the turn of the century engendered a “ribosomal renaissance,” enabling relationships between structure and function to be discerned at the atomic level (reviewed in Refs. 10 and 11). Efforts to crystallize eukaryotic ribosomes have lagged, likely due to their more complex biochemistry. Current state of the art in this area is based on medium resolution cryo-EM2 single particle reconstructions fitted to atomic resolution x-ray crystal structures of archaeal and bacterial ribosomes (reviewed in Refs. 12 and 13). Fig. 1 compares yeast and Thermus thermophilus ribosomes. This technological platform is beginning to enable investigators to fit genetic and biochemical knowledge into a structural context. For example, whereas there is a wealth of genetic and biochemical information pertaining to translation initiation in yeast, cryo-EM studies are revealing specific structural rearrangements in the 40 S subunit consequent to binding and release of specific initiation factors (14, 15). Similarly, cryo-EM methods are illuminating the details of the interactions between the ribosome and the signal recognition particle (reviewed in Ref. 16) and are even beginning to enable comparative structural analyses between ribosomes derived from different species of eukaryotes (17). The current limit of resolution for cryo-EM is ∼7 Å, but the newest generation of probes coming on-line is anticipated to reduce this to 5 Å. At this level, individual rRNA helices are clearly discernible, and proteins and rRNAs can be distinguished by their differences in density. This information is currently being used as the foundation for molecular replacement modeling based on bacterial/archaeal atomic resolution ribosome structures to obtain pseudo-high resolution structures (e.g. see Ref. 18). In addition, chemical protection methods are being used in combination with the structural models and yeast molecular genetics approaches to identify and map allosteric information exchange pathways throughout the eukaryotic ribosome (e.g. see Refs. 19–21). In the near future, we anticipate that the application of newer chemical protection and high throughput sequencing methods (e.g. see Ref. 22) will enable us to gain a more complete picture of the allosteric changes that occur in all four rRNAs through each step of the elongation cycle. In addition, the race is currently on to obtain x-ray crystal structures of eukaryotic ribosomes; personal communications suggest strong levels of enthusiasm and keen competition among the competing groups.
FIGURE 1.
Comparison of yeast and T. thermophilus ribosomes. Yeast ribosome structures (Protein Data Bank codes 1s1h and 1s1i) were obtained by docking atomic models for RNA and protein components into a 11.7-Å cryo-EM map and subsequent threading onto atomic resolution structure of archaeal ribosomes. T. thermophilus ribosome structures (Protein Data Bank codes 2b64 and 2b66) were obtained by x-ray diffraction at a resolution of 5.90 Å. Note the overall larger size and greater density of the yeast ribosomes. SSU, small subunit; LSU, large subunit.
Genetics and Human Disease
Some of the earliest yeast mutants were selected based on their resistance to translational inhibitors, establishing a connection between yeast molecular genetics and protein synthesis. For example, CYH2/RPL28 (encoding ribosomal protein L28) and TCM1/MAK8/RPL3 (encoding ribosomal protein L3) were identified based on their resistance to cycloheximide and trichodermin, respectively (23). In addition, mutants of many yeast large subunit ribosomal protein genes were found to affect the propagation of the yeast “killer” virus, thus establishing a functional link to translation of viral mRNAs (reviewed in Ref. 24). We have exploited this linkage to identify mutants of yeast ribosomal proteins L2, L3, L5, L10, and L11 with specific defects in translational fidelity, including reading frame maintenance and recognition of termination codons (e.g. see Refs. 19, 20, 25, and 26). Mutants can also be identified and characterized based on phenotypic changes associated with growth at high or low temperatures and by resistance or hypersensitivity to translational inhibitors. Yeast-based systems have also been used to create and identify mutants of 5 S, 25 S, and 18 S rRNAs and in rRNA-modifying enzymes using both classical forward and reverse genetics approaches (e.g. see Refs. 27–30). Although yeast-based ribosome molecular genetics are far more advanced than archaeal systems and are on par with if not more advanced than bacterial systems, one complication is that, unlike Escherichia coli, RNA aptamer-tagged rRNAs appear to be targeted for degradation (31). This forestalls, for example, the ability to coexpress “lethal” rRNA mutants in wild-type cells, followed by affinity purification of the mutants and subsequent biochemical characterization. Thus, researchers are limited to working with mutants that are not lethal to cells.
Ribosome mutants have also been characterized in metazoans. It has long been known that the Drosophila Minute phenotype can be caused by ribosomal protein insufficiency (32), and a similar effect has been observed in Arabidopsis (33). In humans, ribosomal protein defects have been associated with a variety of blood and connective tissue disorders, including Diamond-Blackfan anemia, and rRNA pseudouridylation defects have been shown to be associated with dyskeratosis congenita (reviewed in Refs. 34 and 35). Mitochondrial ribosome defects have also been linked to inherited human diseases (reviewed in Ref. 36).
Ribosome Biogenesis
The failure to develop an in vitro reconstitution system suggested that ribosome biogenesis is significantly more complex in eukaryotes compared with their bacterial counterparts. Both systems have been the subject of intense investigation. Interestingly, the structures and polarities of the rDNA operons are conserved among all three kingdoms: both the large and small subunit rRNAs are cotranscribed, and the gene encoding the small subunit rRNA is 5′ of and synthesized prior to that encoding the large subunit rRNA (reviewed in Ref. 37). The basic program of pre-rRNA processing is also conserved: the first step involves separating the pre-small and large rRNAs from one another through an endonucleolytic cleavage event, followed by exonucleolytic trimming of the resulting 5′- and 3′-ends. From this juncture, however, the similarities end. For example, whereas a single RNA polymerase synthesizes all rRNAs and mRNAs in bacteria, the 35 S pre-rRNA is transcribed by RNA polymerase I, 5 S rRNA is transcribed by RNA polymerase III, and protein-encoding genes are transcribed by RNA polymerase II in eukaryotes. Another difference is that deletion of the relatively small set of non-ribosomal factors that participate in ribosome assembly in E. coli is not lethal, suggesting that they merely facilitate a self-assembly process endogenous to bacterial ribosomes. The current theory is that bacterial ribosome biogenesis proceeds through an “assembly gradient,” where ribosomal protein binding is coupled to pre-rRNA folding and maturation. In contrast, in addition to ∼70 ribosomal proteins, >170 non-ribosomal proteins and ∼70 small nucleolar rRNAs, most of which are essential, are involved in yeast ribosome biogenesis. Among these are numerous helicases, GTPases, AAA-ATPases, and chaperones, suggesting a significant degree of remodeling during the biogenesis program. Another major difference relates to the compartmentalization of eukaryotic cells. Pre-rRNAs are transcribed, and the first steps of assembly and base modification (predominantly 2′ O-methylation and pseudouridylation) occur in the nucleolus. A pre-ribosome is then exported to the nucleoplasm, where it undergoes further rounds of maturation. The resulting pre-ribosomal subunits must then be transported through the nuclear pores, and the final steps of maturation occur in the cytoplasm.
The basic composition of each of the pre-ribosomal precursors has been the subject of intense study in the past few years. Early EM studies of actively transcribing Xenopus rDNA loci revealed that the termini of pre-rRNA transcripts condensed into knoblike structures. The assembly program has been best defined in yeast. Here, different steps of rRNA maturation and ribosomal subunit maturation are highlighted. More detailed descriptions and figures are available elsewhere (e.g. see Ref. 37). Fully transcribed 35 S pre-rRNAs are first assembled into 90 S pre-ribosomes in the nucleolus. The first rRNA-processing step involves an endonucleolytic cleavage (the A0 step) near the 5′-end to produce the 33 S pre-RNA. This is followed by a second endonucleolytic cleavage (A1) to produce the 32 S pre-rRNA, the 5′-end of which corresponds to that of the mature 18 S rRNA. The next step involves an endonucleolytic cleavage (A2) that separates the 90 S pre-ribosome particle into 43 S and 66 S particles containing 20 S and 27 SA2 pre-rRNAs, respectively. The 43 S precursor particle is exported to the cytoplasm, where the rRNA undergoes a final cleavage, resulting in the mature small subunit. Maturation of the large subunit is much more complex. From the A2 cleavage step, the process bifurcates to produce two species that differ by the presence or absence of sequence at the 5′-end; the two species so produced are named 27 SB(S) and 27 SB(L), i.e. large and small subspecies. Endonucleolytic cleavages next liberate the two pre-5.8 S precursors, 7 S(S) and 7 S(L), from mature 25 S rRNA, and the mature 5.8 S(S) and 5.8 S(L) species are created by 3′-exonuclease trimming. In addition, pre-5 S rRNA is transcribed by RNA polymerase III, processed, and joins the 66 S pre-large subunit accompanied by ribosomal proteins L5 and L11 in the nucleolus.
In addition to rRNA processing and modification, eukaryotic ribosome biogenesis involves a number of remodeling steps and the formation of a set of discrete assembly intermediates, during which different ribosomal proteins are added to the nascent subunits, and non-ribosomal proteins associate and dissociate as needed. As noted above, the first intermediate is the 90 S pre-ribosome, consisting of the 35 S rRNA, the U3 small nuclear ribonucleoprotein complex, and a large number of small subunit ribosomal proteins and non-ribosomal proteins. The different rRNA cleavage steps result in pre-ribosome complexes called 90 SA0 and 90 SA1 (large and small subunit precursors) and 90 SA3 (small subunit processosome). The biogenesis process for the small subunit progresses to the 43 S particle in the nucleus, followed by export to the cytoplasm, where the final rRNA cleavage event activates the mature 40 S subunit. Large subunit biogenesis involves formation of a series of at least four 66 S intermediates traveling from the nucleolus to the nucleus, followed by export to the cytoplasm. The large subunit is also finally “activated” in the cytoplasm by exchanging ribosomal protein L10 for Crm1p. We believe that this “concerted assembly” process (to borrow a term from virology) explains the biochemical fragility of eukaryotic ribosomes. The past few years have seen the identification and preliminary characterization of each of the pre-ribosome complexes. In particular, tandem affinity purification tagging and mass spectrophotometric methods have enabled the identification of all of the ∼170 proteins involved in each of the precursors. The challenge is now focused on the order of assembly, nuclear export, and structural biology. Affinity pulldown and two-hybrid methods are being used to construct interaction maps for each stage of the biogenesis program. In parallel, cryo-EM methods are being employed to structurally characterize the small nuclear ribonucleoproteins and the pre-ribosome intermediates.
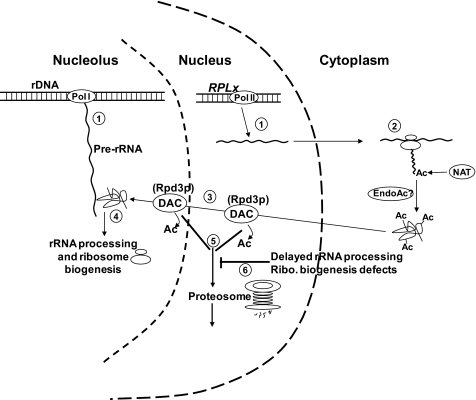
Recent proteomic studies of yeast ribosomes (e.g. see Ref. 38) reveal a puzzling paradox. Specifically, although the primary amino acid sequences of many integral ribosomal proteins conform to the “N-end rules” and their N-terminal methionine residues have been removed (reviewed in Ref. 39), mature ribosomal proteins isolated from intact ribosomes are not N-terminally acetylated. This suggests that these proteins were initially N-terminally acetylated but deacetylated at some later point. Are these ribosomal proteins initially cotranslationally acetylated but deacetylated later during the ribosome biogenesis program, or do they bypass the stability requirements for cotranslational N-terminal processing and acetylation? The former hypothesis is supported by the notion that ribosome assembly may be facilitated by interactions between highly basic, unstructured extensions on ribosomal proteins and negatively charged rRNA phosphate groups. Acetylation of N termini might interfere with assembly into rRNA by reducing positive charges, potentially inhibiting RP-rRNA interactions and promoting structural motifs in regions of RPs that need to be unstructured to assemble with rRNA. Thus, we suggest that although acetylation may be required to stabilize RPs in the cytoplasm, acetyl groups are later removed to ensure proper ribosome biogenesis in the nucleolar compartment. If so, what is the deacetylase? We showed previously that mutation or deletion of RPD3, best known as a histone deacetylase, and of proteins that target Rpd3p to heterochromatin, but not to euchromatin, resulted in phenotypic defects similar to those observed with many L3 mutants (40). A potential model for this process is shown in Fig. 2. The hypothesis that Rpd3p may also play a critical role in deacetylating ribosomal proteins prior to their incorporation into nascent ribosomes is currently being tested using proteomic methods.
FIGURE 2.
Model of post-translational acetylation and deacetylation of eukaryotic ribosomes. Step 1, pre-rRNA is transcribed in the nucleolus, whereas rRNAs encoding RPs are transcribed in the nucleus. Step 2, cotranslational N-terminal acetylation (NAT) of RPs occurs in the cytoplasm. endo-Acetylases (EndoAc) may also be active. Step 3, mature RPs are re-imported into the nucleolus via the nucleus. We propose that they are deacetylated along this pathway (exactly where is unknown, as shown by presence of deacetylases in both compartments). Deacetylation (DAC) eliminates negative charges on RPs, which could promote charge repulsion with phosphate groups of pre-rRNAs and structure in regions of RPs that need to be unstructured for assembly with rRNA. Step 4, deacetylated, highly basic RPs can properly associate with pre-rRNAs to begin nucleation of pre-ribosomes, rRNA processing, and ribosome biogenesis. Step 5, regulation. Excess free RPs are deacetylated in the nucleus or nucleolus, where they are targeted to the proteasome for degradation. Step 6, dysregulation. Loss of deacetylase activity, e.g. in mof6-1 or rpd3Δ cells (40), interferes with Step 5, resulting in delayed rRNA processing and ribosome (Ribo.) biogenesis defects.
Regulation
Ribosomal proteins that are not deeply intertwined with rRNA (i.e. non-integral ribosomal proteins) can potentially dissociate from mature ribosomes. Recent reports have demonstrated that this class of proteins can have ribosome-independent regulatory functions. For example, phosphorylation of human ribosomal protein L13a in response to interferon-γ promotes its release from the large subunit, whereupon it silences translation of ceruloplasmin by binding to the 3′-untranslated region of its mRNA (41). Other ribosomal proteins have been linked to signal transduction pathways relevant to cell growth/apoptosis, e.g. L11 and c-Myc (reviewed in Ref. 42), S6 phosphorylation and mTOR (reviewed in Ref. 43), S19 and the leukocyte C5a receptor (reviewed in Ref. 44), and S3a as a nuclear phosphatidylinositol 3-kinase target (45). A more broad idea that has been considered for at least 20 years is that there may be special ribosomes for specialized circumstances. Why are most but not all yeast ribosomal protein genes duplicated, and why does deletion of one isogene, but not of its paralog, result in specific phenotypes? For example, many mutants of one but not of the other ribosomal protein isogene tend to produce the Mak– (maintenance of killer) phenotype, wherein the affected cells cannot maintain the double-stranded RNA killer virus (reviewed in Ref. 24). More recently, transcriptional and phenotypic profiling of cells lacking specific ribosomal protein genes revealed differences between the functional roles of ribosomal protein paralogs and that ribosomal protein paralogs have disparate requirements for localization and assembly, suggesting that ribosomal protein isoforms have become specialized for specific cellular processes (46). In addition, knockdown of specific nonessential ribosomal proteins has been shown to cause a wide variety of developmental defects in Danio rerio, demonstrating a deep linkage between the ribosome and developmental biology (47). The existence of ribosomes specialized in cap-independent translation under conditions of nutritional stress has also been postulated (48). We are currently using proteomic methods to identify differences in post-translational modifications of specific yeast ribosomal proteins that correspond to changes in nutritional status and also the possibility that ribosomal proteins are also differentially modified at different stages in the cell cycle. In vertebrates, it has been suggested that specialized “immunoribosomes” exist for the express purpose of synthesizing proteins to be degraded into major histocompatibility complex class I peptide ligands for immune surveillance purposes (reviewed in Ref. 49). Finally, the discovery that specific mRNAs are targeted for translation in specific areas of cells, e.g. localized translation of the GluR2 mRNA in axons, supports the notion of specialized ribosomes, and recent studies suggest that defects associated with these processes may account for some autistic phenotypes (reviewed in Ref. 50).
Conclusions and Outlook
The eukaryotic ribosome can be viewed as a paradigm for understanding complex molecular machines. The emerging understanding of ribosome assembly and structure/function relationships is helping to establish the foundational underpinnings for the development of nanoscale biomaterials. It is also anticipated that atomic resolution structures will be obtained and that these will provide platforms for the development and design of novel therapeutics. The longstanding issue of “specialized ribosomes” appears to be nearing resolution as well. The characterization of these will have far reaching impacts on our understanding of cell and developmental biology and on human disease.
Supplementary Material
Acknowledgments
I thank all of the members of my laboratory, both past and present, whose hard work and determination have made the entire enterprise exciting, enjoyable, and prosperous. Thanks also to my many colleagues in the ribosome field, some of the smartest and nicest people that I have ever had the pleasure to work with.
Author's Choice—Final version full access.
This work was supported, in whole or in part, by National Institutes of Health Grant GM058859. This minireview will be reprinted in the 2009 Minireview Compendium, which will be available in January, 2010.
Footnotes
The abbreviations used are: EM, electron microscopic/microscopy; RP, ribosomal protein.
References
- 1.Palade, G. E., and Siekevitz, P. (1956) J. Biophys. Biochem. Cytol. 2 171–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rheinberger, H. J. (2004) in Protein Synthesis and Ribosome Structure (Nierhaus, K. H., and Wilson, D. N., eds) pp. 1–51, Wiley-VCN Verlag GmbH & Co., Weinheim, Germany
- 3.Hinnebusch, A. G., Asano, K., Olsen, D. S., Phan, L., Nielsen, K. H., and Valasek, L. (2004) Ann. N. Y. Acad. Sci. 1038 60–74 [DOI] [PubMed] [Google Scholar]
- 4.Hosokawa, K., Fujimura, R. K., and Nomura, M. (1966) Proc. Natl. Acad. Sci. U. S. A 55 198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polacek, N., and Mankin, A. S. (2005) Crit. Rev. Biochem. Mol. Biol. 40 285–311 [DOI] [PubMed] [Google Scholar]
- 6.Munro, J. B., Vaiana, A., Sanbonmatsu, K. Y., and Blanchard, S. C. (2008) Biopolymers 89 565–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budkevich, T. V., El'skaya, A. V., and Nierhaus, K. H. (2008) Nucleic Acids Res. 36 4736–4744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merrick, W. C. (1979) Methods Enzymol. 60 108–123 [DOI] [PubMed] [Google Scholar]
- 9.Acker, M. G., Kolitz, S. E., Mitchell, S. F., Nanda, J. S., and Lorsch, J. R. (2007) Methods Enzymol. 430 111–145 [DOI] [PubMed] [Google Scholar]
- 10.Steitz, T. A. (2008) Nat. Rev. Mol. Cell Biol. 9 242–253 [DOI] [PubMed] [Google Scholar]
- 11.Korostelev, A., Ermolenko, D. N., and Noller, H. F. (2008) Curr. Opin. Chem. Biol. 12 674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank, J. (2003) Biopolymers 68 223–233 [DOI] [PubMed] [Google Scholar]
- 13.Stark, H. (2002) Curr. Protein Pept. Sci. 3 79–91 [DOI] [PubMed] [Google Scholar]
- 14.Passmore, L. A., Schmeing, T. M., Maag, D., Applefield, D. J., Acker, M. G., Algire, M. A., Lorsch, J. R., and Ramakrishnan, V. (2007) Mol. Cell 26 41–50 [DOI] [PubMed] [Google Scholar]
- 15.Gilbert, R. J., Gordiyenko, Y., von der Haar, T., Sonnen, A. F., Hofmann, G., Nardelli, M., Stuart, D. I., and McCarthy, J. E. (2007) Proc. Natl. Acad. Sci. U. S. A 104 5788–5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wild, K., Halic, M., Sinning, I., and Beckmann, R. (2004) Nat. Struct. Mol. Biol. 11 1049–1053 [DOI] [PubMed] [Google Scholar]
- 17.Nilsson, J., Sengupta, J., Gursky, R., Nissen, P., and Frank, J. (2007) J. Mol. Biol. 369 429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen, C. B., Becker, T., Blau, M., Anand, M., Halic, M., Balar, B., Mielke, T., Boesen, T., Pedersen, J. S., Spahn, C. M., Kinzy, T. G., Andersen, G. R., and Beckmann, R. (2006) Nature 443 663–668 [DOI] [PubMed] [Google Scholar]
- 19.Meskauskas, A., and Dinman, J. D. (2008) Nucleic Acids Res. 36 6175–6186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrov, A. N., Meskauskas, A., Roshwalb, S. C., and Dinman, J. D. (2008) Nucleic Acids Res. 36 6187–6198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakauskaite, R., and Dinman, J. D. (2008) Nucleic Acids Res. 36 1497–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkinson, K. A., Gorelick, R. J., Vasa, S. M., Guex, N., Rein, A., Mathews, D. H., Giddings, M. C., and Weeks, K. M. (2008) PLoS Biol. 6 e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fried, H. M., and Warner, J. R. (1982) Nucleic Acids Res. 10 3133–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickner, R. B. (1996) Annu. Rev. Genet. 30 109–139 [DOI] [PubMed] [Google Scholar]
- 25.Meskauskas, A., and Dinman, J. D. (2001) RNA 7 1084–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meskauskas, A., Russ, J. R., and Dinman, J. D. (2008) Nucleic Acids Res. 36 1826–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiparisov, S., Petrov, A., Meskauskas, A., Sergiev, P. V., Dontsova, O. A., and Dinman, J. D. (2005) Mol. Genet. Genomics 27 235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rakauskaite, R., and Dinman, J. D. (2006) Mol. Cell. Biol. 26 8992–9002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong, J., Nanda, J. S., Rahman, H., Pruitt, M. R., Shin, B. S., Wong, C. M., Lorsch, J. R., and Hinnebusch, A. G. (2008) Genes Dev. 22 2242–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King, T. H., Liu, B., McCully, R. R., and Fournier, M. J. (2003) Mol. Cell 11 425–435 [DOI] [PubMed] [Google Scholar]
- 31.LaRiviere, F. J., Cole, S. E., Ferullo, D. J., and Moore, M. J. (2006) Mol. Cell 24 619–626 [DOI] [PubMed] [Google Scholar]
- 32.Melnick, M. B., Noll, E., and Perrimon, N. (1993) Genetics 135 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weijers, D., Franke-van Dijk, M., Vencken, R. J., Quint, A., Hooykaas, P., and Offringa, R. (2001) Development (Camb.) 128 4289–4299 [DOI] [PubMed] [Google Scholar]
- 34.Scheper, G. C., van der Knaap, M. S., and Proud, C. G. (2007) Nat. Rev. Genet. 8 711–723 [DOI] [PubMed] [Google Scholar]
- 35.Ellis, S. R., and Massey, A. T. (2006) Med. Hypotheses 66 643–648 [DOI] [PubMed] [Google Scholar]
- 36.Sylvester, J. E., Fischel-Ghodsian, N., Mougey, E. B., and O'Brien, T. W. (2004) Genet. Med. 6 73–80 [DOI] [PubMed] [Google Scholar]
- 37.Hage, A. E., and Tollervey, D. (2004) RNA Biol. 1 45–50 [PubMed] [Google Scholar]
- 38.Lee, S. W., Berger, S. J., Martinovic, S., Pasa-Tolic, L., Anderson, G. A., Shen, Y., Zhao, R., and Smith, R. D. (2002) Proc. Natl. Acad. Sci. U. S. A 99 5942–5947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varshavsky, A. (1992) Cell 69 725–735 [DOI] [PubMed] [Google Scholar]
- 40.Meskauskas, A., Baxter, J. L., Carr, E. A., Yasenchak, J., Gallagher, J. E., Baserga, S. J., and Dinman, J. D. (2003) Mol. Cell. Biol. 23 1602–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazumder, B., Sampath, P., Seshadri, V., Maitra, R. K., DiCorleto, P. E., and Fox, P. L. (2003) Cell 115 187–198 [DOI] [PubMed] [Google Scholar]
- 42.Dai, M. S., Sears, R., and Lu, H. (2007) Cell Cycle 6 2735–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruvinsky, I., and Meyuhas, O. (2006) Trends Biochem. Sci. 31 342–348 [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto, T. (2007) Pathol. Int. 57 1–11 [DOI] [PubMed] [Google Scholar]
- 45.Hamaguchi, N., Ohdaira, T., Shinohara, A., Iwamatsu, A., Ihara, S., and Fukui, Y. (2002) Cytotechnology 40 85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komili, S., Farny, N. G., Roth, F. P., and Silver, P. A. (2007) Cell 131 557–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uechi, T., Nakajima, Y., Nakao, A., Torihara, H., Chakraborty, A., Inoue, K., and Kenmochi, N. (2006) PLoS ONE 1 e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilbert, W. V., Zhou, K., Butler, T. K., and Doudna, J. A. (2007) Science 317 1224–1227 [DOI] [PubMed] [Google Scholar]
- 49.Yewdell, J. W., and Nicchitta, C. V. (2006) Trends Immunol. 27 368–373 [DOI] [PubMed] [Google Scholar]
- 50.Kelleher, R. J., III, and Bear, M. F. (2008) Cell 135 401–406 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.