Abstract
Photoaffinity labeling of γ-aminobutyric acid type A (GABAA)-receptors (GABAAR) with an etomidate analog and mutational analyses of direct activation of GABAAR by neurosteroids have each led to the proposal that these structurally distinct general anesthetics bind to sites in GABAARs in the transmembrane domain at the interface between the β and α subunits. We tested whether the two ligand binding sites might overlap by examining whether neuroactive steroids inhibited etomidate analog photolabeling. We previously identified (Li, G. D., Chiara, D. C., Sawyer, G. W., Husain, S. S., Olsen, R. W., and Cohen, J. B. (2006) J. Neurosci. 26, 11599–11605) azietomidate photolabeling of GABAAR α1Met-236 and βMet-286 (in αM1 and βM3). Positioning these two photolabeled amino acids in a single type of binding site at the interface of β and α subunits (two copies per pentamer) is consistent with a GABAAR homology model based upon the structure of the nicotinic acetylcholine receptor and with recent αM1 to βM3 cross-linking data. Biologically active neurosteroids enhance rather than inhibit azietomidate photolabeling, as assayed at the level of GABAAR subunits on analytical SDS-PAGE, and protein microsequencing establishes that the GABAAR-modulating neurosteroids do not inhibit photolabeling of GABAAR α1Met-236 or βMet-286 but enhance labeling of α1Met-236. Thus modulatory steroids do not bind at the same site as etomidate, and neither of the amino acids identified as neurosteroid activation determinants (Hosie, A. M., Wilkins, M. E., da Silva, H. M., and Smart, T. G. (2006) Nature 444, 486–489) are located at the subunit interface defined by our etomidate site model.
GABAA3 receptors (GABAAR) are major mediators of brain inhibitory neurotransmission and participate in most circuits and behavioral pathways relevant to normal and pathological function (1). GABAAR are subject to modulation by endogenous neurosteroids, as well as myriad clinically important central nervous system drugs including general anesthetics, benzodiazepines, and possibly ethanol (1, 2). The mechanism of GABAAR modulation by these different classes of drugs is of major interest, including identification of the receptor amino acid residues involved in binding and action of the drugs.
In the absence of high resolution crystal structures of drug-receptor complexes, the locations of anesthetic binding sites in GABAARs have been predicted based upon analyses of functional properties of point mutant receptors, which identified residues in the α and β subunit M1–M4 transmembrane helices important for modulation by volatile anesthetics (primarily α subunit) and by intravenous agents, including etomidate and propofol (β subunit) (3–5). Position βM2–15, numbered relative to the N terminus of the helix, functions as a major determinant of etomidate and propofol potency as GABA modulators in vitro and in vivo (6–8). By contrast, this residue is not implicated for modulation by the neurosteroids, potent endogenous modulators of GABAAR (9).
Photoaffinity labeling, which allows the identification of residues in proximity to drug binding sites (10, 11), has been used to identify two GABAAR amino acids covalently modified by the etomidate analog [3H]azietomidate (12): α1Met-236 within αM1 and βMet-286 within βM3. Photolabeling of these residues was inhibited equally by nonradioactive etomidate and enhanced proportionately by GABA present in the assay, consistent with the presence of these two residues in a common drug binding pocket that would be located at the interface between the β and α subunits in the transmembrane domain (12). Mutational analyses identify these positions as etomidate and propofol sensitivity determinants (13–15).
A recent mutagenesis study (16) identified two other residues in GABAAR αM1 and βM3 as critical for direct activation by neurosteroids, αThr-236 (rat numbering, corresponding to α1Thr-237, bovine numbering used here and by Li et al. (12))4 and βTyr-284. These residues were also proposed to contribute to a neurosteroid binding pocket in the transmembrane domain at the interface between β and α subunits, based upon their location in an alternative GABAAR structural model that positioned those amino acids, and not α1Met-236 or βMet-286, at the subunit interface. For GABAARs and other members of the Cys-loop superfamily of neurotransmitter-gated ion channels, the transmembrane domain of each subunit is made up of a loose bundle of four α helices (M1–M4), with M2 from each subunit contributing to the lumen of the ion channel and M4 positioned peripherally in greatest contact with lipid, as seen in the structures of the Torpedo nicotinic acetylcholine receptor (nAChR) (17) and in distantly related prokaryote homologs (18). However, uncertainties in the alignment of GABAAR subunit sequences relative to those of the nAChR result in alternative GABAAR homology models (12, 19, 20) that differ in the location of amino acids in the M3 and M4 membrane-spanning helices and in the M1 helix in some models (16, 21).
If etomidate and neurosteroids both bind at the same β/α interface in the GABAAR transmembrane domain, the limited space available for ligand binding suggests that their binding pockets might overlap and that ligand binding would be mutually exclusive. To address this question, we photolabeled purified bovine brain GABAAR with [3H]azietomidate in the presence of different neuroactive steroids and determined by protein microsequencing whether active neurosteroids inhibited labeling of α1Met-236 and βMet-286, as expected for mutually exclusive binding, or resulted in [3H]azietomidate photolabeling of other amino acids, a possible consequence of allosteric interactions. Active steroids failed to inhibit labeling and enhanced labeling of α1Met-236, clearly indicating that the neurosteroid and the etomidate sites are distinct. Our GABAAR homology model that positions α1Met-236 and βMet-286 at the β/α interface, but not that of Hosie et al. (16), is also consistent with cysteine substitution cross-linking studies (20, 22), which define the proximity relations between amino acids in the αM1, αM2, αM3, and βM3 helices, and these results support the interpretation that the two residues photolabeled by [3H]azietomidate are part of a single subunit interface binding pocket, whereas the steroid sensitivity determinants identified by mutagenesis neither are at the β/α subunit interface nor are contributors to a common binding pocket.
EXPERIMENTAL PROCEDURES
Solubilization and Purification of Bovine Brain GABAARs—GABAAR was purified as reported previously (12) on a benzodiazepine Ro7/1986-1 affinity column. Detailed conditions for solubilization and purification are described there.
Photoaffinity Labeling of Purified GABAAR—The peak [3H]muscimol binding fraction (5 ml) from each affinity column elution was used for photolabeling without further dialysis or concentration. KCl was added to a final concentration of 0.1 m. An aliquot of GABAAR (∼40 nm [3H]muscimol binding sites, 2.5 ml for preparative labeling or 0.14 ml for analytical) was equilibrated with [3H]azietomidate (final concentration, 2 μm) ± additional drugs, incubated for 1 h in the dark (4 °C), and irradiated (30 min, 365 nm). After photolabeling, the total protein was precipitated with methanol/chloroform, solubilized in sample loading buffer, and fractionated by SDS-PAGE. The resulting gel lanes were cut into 3-mm slices, and 3H incorporation was determined either directly by liquid scintillation counting (analytical labeling) or after elution from each slice into 1 ml of elution buffer (12). Aliquots of the eluted bands were assayed for 3H and pooled for proteolytic digestion.
Enzymatic Digestion, Reversed-phase HPLC, and Protein Microsequencing—Digestion with EndoLys-C (Princeton Separations) was carried out using 2.5 μg of protease for aliquots of GABAAR subunit in 50 μl of digestion buffer for 3 days at 25 °C. Reversed-phase HPLC and peptide microsequencing were performed as described (12). 3H release in each cycle of Edman degradation was determined by liquid scintillation counting of fractions for 6 × 10 min. Standard error bars are included in the plots (see Figs. 1 and 2). To determine the amount of peptide sequenced, the individual residues were fit to the equation Ix = I0 × Rx, where Ix is the pmol detected in cycle x, I0 is the starting amount of the peptide, and R is the average repetitive yield. The range of specific residue photolabeling ((2×(cpmn – cpmn–1)/I0 × Rx) was calculated using the I0 × Rx values calculated with 95% confidence. Treatment with o-phthalaldehyde (OPA) during sequencing was as described (12, 23). OPA reacts preferentially with primary rather than secondary amines (i.e. proline) and can be used at any cycle of Edman degradation to block sequencing of peptides not containing an N-terminal proline.
FIGURE 1.
Neuroactive steroids (A, allopregnanolone; B, THDOC; C, alphaxalone) enhance [3H]azietomidate photolabeling of GABAAR, whereas isopregnanolone, an inactive allopregnanolone isomer, does not. Left panels, GABAAR (6 pmol) was photolabeled with [3H]azietomidate in the presence of 1 mm GABA and concentrations of neurosteroid (for active steroids, 0, □; 100 nm, ▵; 300 nm, ⋄; 1 μm, ▿; 10 μm, ○; and for isopregnanolone, 10 μm, ♦), and covalent 3H incorporation was determined by SDS-PAGE. Right panels, the concentration dependence of neurosteroid modulation, determined by normalizing the 3H in the GABAAR subunit gel band(s) in the presence of neurosteroid (active steroids, ▪; inactive steroid isopregnanolone, ♦) to that in the absence. The data points are the average and range from two independent photolabeling experiments. The solid lines are fits of the data to a logistic function. Error bars indicate S.E.
FIGURE 2.
The neurosteroid THDOC does not inhibit [3H]azietomidate photolabeling of either α1Met-236 (αM1) or βMet-286 (βM3). Solubilized and affinity-purified GABAAR from bovine cortex (∼100 pmol muscimol sites/sample) was equilibrated with [3H]azietomidate and 1 mm GABA in the absence (○,□) or presence (•,▪) of 10 μm THDOC and photolabeled. Subunits were isolated and digested with EndoLys-C, and fragments were isolated for sequence analysis as described in supplemental Figs. 1–4. 3H (○, •) and phenylthiohydantoin-amino acids (□, ▪) released during Edman sequencing. A, sequence analysis of a sample containing αM1 beginning at α1Ile-223 (○, 1,020 cpm sequenced; •, 1,665 cpm sequenced). After treatment with OPA prior to cycle 11, the fragment beginning at α1Ile-223 was present in both samples (–THDOC, I0 = 470 ± 70 fmol, r = 90 ± 1%; +THDOC, I0 = 370 ± 110 fmol, r = 93 ± 2%). Release of 3H in cycle 14 in both samples confirmed labeling at α1Met-236 (–THDOC, 260–380 cpm/pmol; +THDOC, 440–1060 cpm/pmol). Error bars indicate S.E. B, sequence analysis of a sample containing βM3 beginning at Ala-280 (○, 530 cpm sequenced; •, 590 cpm sequenced). The fragment beginning at βAla-280 was present in both samples (–THDOC, I0 = 1.8 ± 0.3 pmol, r = 90 ± 2%; +THDOC, I0 = 2.1 ± 0.5 pmol, r = 89 ± 2%), and release in cycle 7 is consistent with labeling of βMet-286 (–THDOC, 20–43 cpm/pmol; +THDOC, 18–57 cpm/pmol).
RESULTS AND DISCUSSION
Neuroactive Steroids Potentiate GABAAR Photolabeling—When purified bovine brain GABAARs photolabeled with [3H]azietomidate were fractionated by SDS-PAGE, 3H incorporation was detected in GABAAR subunit polypeptides of ∼50–55 kDa that was inhibitable by >90% in the presence of nonradioactive etomidate and was shown to result from labeling of α1Met-236 (or the homologous methionine in α2, -3, -5) and βMet-286 (12). To test for interactions between azietomidate and neurosteroids, we photolabeled GABAAR with [3H]azietomidate in the presence of 1 mm GABA (which enhances binding) and included (individually) in the labeling mixture three different neuroactive steroids: 5α-pregnan-3α-ol-20-one (allopregnanolone), tetrahydro-deoxy-corticosterone (THDOC), or alphaxolone, at various concentrations (0, 0.1, 0.3, 1, 10 μm) in parallel. As shown in Fig. 1, all three steroids enhanced GABAAR subunit labeling in a concentration-dependent manner. The concentration dependence of enhancement could be fit to logistic curves, yielding half-effect concentrations of 0.6, 0.9, and 0.8 μm for allopregnanolone, THDOC, and alphaxalone, respectively, under the experimental conditions employed. An inactive isomer of allopregnanolone, isopregnanolone (5α-pregnan-3β-ol-20-one), produced no modulation of labeling (Fig. 1A), demonstrating the pharmacological specificity of the observed enhancement of labeling.
Neurosteroid Modulation of Photolabeling of α1Met-236/ βMet-286—Protein microsequencing established that the GABAAR subunit photolabeling seen in the absence or presence of GABA resulted from photolabeling of α1Met-236 and βMet-2865 (12). As neurosteroids actually increased [3H]azietomidate photolabeling of GABAAR subunits, we wanted to determine whether the presence of neurosteroids increased the labeling of either (or both) of those residues or potentially reduced labeling at those positions while enhancing labeling of other residues in αM1 or βM3. Purified GABAAR was photolabeled on a preparative scale (110 pmol [3H]muscimol binding sites) with [3H]azietomidate and 1 mm GABA in the absence or presence of 10 μm THDOC. After fractionation of photolabeled GABAAR subunit by SDS-PAGE (supplemental Fig. S1), the gel regions most enriched in labeled α and β subunits were identified by sequencing aliquots after digestion with EndoLys-C, which results in 3H release in cycle 7 of Edman degradation for βMet-286 and in cycle 14 for α1Met-236 (supplemental Figs. S2 and S3). There was no evidence of labeling of other amino acids in αM1 or βM3. Material enriched in labeled α subunits, found in the gel slices containing the peak of 3H, and in labeled β subunits, enriched in the adjacent shoulders, were pooled separately and digested with EndoLys-C, and the digests were fractionated by reversed-phase HPLC (supplemental Fig. S4). For quantification of the labeling of αMet-236, during sequence analysis of the samples enriched in photolabeled α subunits, the sequencing filter was treated with o-phthalaldehyde prior to cycle 11 (α1Pro-233) to block sequencing of all fragments not containing a proline in that cycle (Fig. 2A). The 3H release in cycle 14 (+THDOC, 40 cpm; –THDOC, 16 cpm) indicated labeling of α1Met-236, and the fragment beginning at α1Ile-223 was present at ∼0.4 pmol in both the samples. Taking into account the uncertainties inherent in the mass quantification at subpicomole levels, the observed 3H releases in cycle 14 indicated photolabeling of α1Met-236 at 310 cpm/pmol (260–380 cpm/pmol) in the absence of THDOC that was increased to 620 cpm/pmol (440–1000 cpm/pmol) in the presence of THDOC. Sequence analysis of the HPLC fractions containing the peak of 3H from the digests enriched in GABAAR β subunits (Fig. 2B) revealed the presence of the fragment beginning at βAla-280 at ∼1 pmol, and 3H release in cycle 7 (12 cpm) in both samples corresponded to labeling of βMet-286. Photolabeling of βMet-286 (20–43 cpm/pmol) was not inhibited by THDOC (18–57 cpm/pmol).
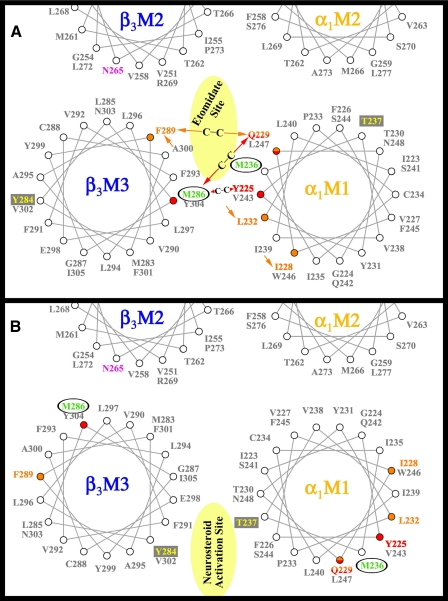
Location of Labeled Residues in GABAAR Homology Models—The proposal that etomidate binds in the GABAAR transmembrane domain at the interface of β and α subunits followed from the locations of α1Met-236 and βM3–286 in a GABAAR homology model (12) derived from the structure of the Torpedo nAChR (Fig. 3A), whereas the proposal that neurosteroids bind at that interface was consistent with the locations of αThr-237 and βTyr-284, positions determining sensitivity to direct activation by neurosteroids, in an alternative structural model (16) (Fig. 3B) that positions neither α1Met-236 nor βMet-286 at the interface between the subunits. The location of α1Met-236 at the β-α interface was consistent with other models based upon the nAChR structure (19, 20), and as shown in Fig. 3, the recent identification (22) of the positions in αM1 that can be cross-linked to βM286C or βF289C is consistent with the homology model of Li et al. (12) but not that of Hosie et al. (16). Further support for this alignment comes from Jansen and Akabas (20), who used Cys mutagenesis and sulfhydryl cross-linking to orient the αM3 segment relative to αM2, which suggests proximity between βM2-Thr-262 and βM3 residues Leu-296, Tyr-299, and Ala-300, as in Fig. 3A. The proposal that each GABAAR contains two equivalent etomidate binding sites, one at each β-α interface (12), is consistent with kinetic data obtained by electrophysiology, suggesting that two equivalent sites can mediate both agonist enhancement and direct channel gating by etomidate (24).
FIGURE 3.
Helical wheel representations of the GABAAR β-α interface in the transmembrane domain illustrating the proposed binding sites for etomidate and neurosteroids. The model in A illustrates the orientation of residues from a homology model built on the nicotinic acetylcholine receptor cryo-EM derived structure (PDB: 2BG9) (12), with the residues in αM1 and βM3 photolabeled by [3H]azietomidate (circled residues in green) contributing to a common binding pocket at the β-α subunit interface. Also included in pink is the position in βM2 that functions as a determinant of etomidate/azietomidate anesthetic potency in vivo (7, 29), the residues in αM1 and βM3 identified as sensitivity determinants for direct activation by neurosteroids (boxed residues in yellow) (16), and the positions in αM1 and βM3 that when mutated to Cys can form intersubunit cross-links (red and orange) (22). B illustrates the locations of those residues in an alternative GABAAR model (16).
Photoaffinity labeling with [3H]azietomidate led to the identification of α1Met-236 and βMet-286 in an unbiased search for photolabeled amino acids in GABAAR subunit digests (12). Although photoaffinity labeling is a powerful tool to identify amino acids contributing directly to drug binding sites in proteins, results can be biased because the photoreactive intermediate may react preferentially with only certain amino acid side chains, and there is also the possibility that highly reactive side chains distant from the binding site may be labeled (10, 11).
The aliphatic diazirine group in [3H]azietomidate results in a reactive aliphatic carbocation intermediate following irradiation, which reacts most efficiently with Asp, Glu, and Tyr and also with Gln, Ser, Cys, and His (23). The labeling of two relatively less reactive methionines with the etomidate analog (12) indicates that they might be the most reactive side chains within the binding site, but they are unlikely to be labeled as distant, highly reactive side chains. In addition, aliphatic carbocation intermediates are known to be highly reactive with water (t½ <1 ns (25)), further limiting the possibility of diffusional encounters with reactive side chains distant from the binding site. That the photolabeling of α1Met-236 and βMet-286 was potentiated in the presence of GABA, fully inhibitable by etomidate (12), and inhibitable by propofol6 provides further evidence that [3H]azietomidate is acting as a specific affinity label and that the photolabeling results provide a direct identification of two amino acids contributing to the etomidate binding site.
In contrast, Li et al. (12) were not able to determine whether or not the M2 residue (βAsn-265) identified as a determinant of etomidate and propofol sensitivity (6–8, 26) is labeled by [3H]azietomidate, although propofol does not protect βN265C from reaction with sulfhydryl reagents, whereas it protects βM286C (14). In our homology model, this position (Fig. 3A) may be accessible both from the interior of the β subunit helix bundle and from the etomidate binding pocket at the β/α interface, and further studies are needed to determine whether it contributes directly to the etomidate binding site and/or is crucial for the coupling between anesthetic binding and GABAAR gating.
Our results and complementary cross-linking studies (22) indicate that the neurosteroid activation determinants in αM1 and βM3 are not contributing to a common binding pocket at the interface between β and α subunits, although Akk et al. (27) support the data of Hosie et al. (16), implicating residues in αM1 for steroid effects. Further studies with photoreactive neurosteroids (28) may be necessary to identify neurosteroid binding sites.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant GM 58448 (to R. W. O., J. B. C., and Keith W. Miller). This work was also supported by an award to Harvard Medical School from the Howard Hughes Biomedical Research Support Program for Medical School.
The on-line version of this article (available at http://www.jbc.org) contains four supplemental figures.
Footnotes
The abbreviations and trivial names used are: GABA, γ-aminobutyric acid; GABAAR, GABA type A receptor; azietomidate, 2-(3-methyl-3H-diaziren-3-yl)ethyl 1-(phenylethyl)-1H-imidazole-5-carboxylate; nAChR, nicotinic acetylcholine receptor; THDOC, tetrahydro-deoxy-corticosterone; EndoLys-C, endoproteinase Lys-C; HPLC, high pressure liquid chromatography; OPA, o-phthalaldehyde.
The GABAAR α subunit protein sequence numbering is slightly different in those reports (12, 16) because mouse (16) mature sequence lacks one amino acid in the N-terminal found in cow sequence (12) (otherwise, they are exactly the same). The α1M1-Thr-236 in Hosie et al. (16) (refer to its Fig. 1a) is α1M1-Thr-237 here in Fig. 3 and in Li et al. (12) (refer to its Fig. 4c).
As discussed in Li et al. (12), because of the high degree of sequence conservation for α subunit subtypes in the M1 region and β subunit subtypes in the M3 region, including protease cleavage sites, the labeled amino acids cannot be assigned to specific subunit subtypes, and therefore they are referred to as αMet-236 (using the α1 subunit numbering) and βMet-286.
G. D. Li, D. C. Chiara, J. B. Cohen, and R. W. Olsen, unpublished data.
References
- 1.Olsen, R. W., and Sieghart, W. (2008) Pharmacol. Rev. 60 243–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanchar, H. J., Dodson, P. D., Olsen, R. W., Otis, T. S., and Wallner, M. (2005) Nat. Neurosci. 8 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mihic, S. J., Ye, Q., Wick, M. J., Koltchine, V. V., Krasowski, M. D., Finn, S. E., Mascia, M. P., Valenzuela, C. F., Hanson, K. K., Greenblatt, E. P., Harris, R. A., and Harrison, N. L. (1997) Nature 389 385–389 [DOI] [PubMed] [Google Scholar]
- 4.Hemmings, H. C., Akabas, M. H., Goldstein, P. A., Trudell, J. R., Orser, B. A., and Harrison, N. L. (2005) Trends Pharmacol. Sci. 26 503–510 [DOI] [PubMed] [Google Scholar]
- 5.Franks, N. P. (2008) Nat. Rev. Neurosci. 9 370–386 [DOI] [PubMed] [Google Scholar]
- 6.Belelli, D., Lambert, J. J., Peters, J. A., Wafford, K., and Whiting, P. J. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 11031–11036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurd, R., Arras, M., Lambert, S., Drexler, B., Siegwart, R., Crestani, F., Zaugg, M., Vogot, K. E., Ledermann, B., Antkowiak, B., and Rudolph, U. (2003) FASEB J. 17 250–252 [DOI] [PubMed] [Google Scholar]
- 8.Reynolds, D. S., Rosahl, T. W., Cirone, J., O'Meara, G. F., Haythornthwaite, A., Newman, R. J., Myers, J., Sur, C., Howell, O., Rutter, A. R., Atack, J., Macaulay, A. J., Hadingham, K. L., Hutson, P. H., Belelli, D., Lambert, J. J., Dawson, G. R., McKernan, R., Whiting, P. J., and Wafford, K. A. (2003) J. Neurosci. 23 8608–8617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belelli, D., and Lambert, J. J. (2005) Nat. Rev. Neurosci. 6 565–575 [DOI] [PubMed] [Google Scholar]
- 10.Kotzyba-Hibert, F., Kapfer, I., and Goeldner, M. (1995) Angew. Chem. Int. Ed. Engl. 34 1296–1312 [Google Scholar]
- 11.Vodovozova, E. L. (2007) Biochemistry (Mosc.) 72 1–20 [DOI] [PubMed] [Google Scholar]
- 12.Li, G. D., Chiara, D. C., Sawyer, G. W., Husain, S. S., Olsen, R. W., and Cohen, J. B. (2006) J. Neurosci. 26 11599–11605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krasowski, M. D., Koltchine, V. V., Rick, C. E., Ye, Q., Finn, S. E., and Harrison, N. L. (1998) Mol. Pharmacol. 53 530–538 [DOI] [PubMed] [Google Scholar]
- 14.Bali, M., and Akabas, M. H. (2004) Mol. Pharmacol. 65 68–76 [DOI] [PubMed] [Google Scholar]
- 15.Stewart, D., Desai, R., Cheng, Q., Liu, A., and Forman, S. A. (2008) Mol. Pharmacol. 74 1687–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosie, A. M., Wilkins, M. E., da Silva, H. M., and Smart, T. G. (2006) Nature 444 486–489 [DOI] [PubMed] [Google Scholar]
- 17.Unwin, N. (2005) J. Mol. Biol. 346 967–989 [DOI] [PubMed] [Google Scholar]
- 18.Hilf, R. J., and Dutzler, R. (2008) Nature 452 375–379 [DOI] [PubMed] [Google Scholar]
- 19.Ernst, M., Bruckner, S., Boresch, S., and Sieghart, W. (2005) Mol. Pharmacol. 68 1291–1300 [DOI] [PubMed] [Google Scholar]
- 20.Jansen, M., and Akabas, M. H. (2006) J. Neurosci. 26 4492–4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamakura, T., Bertaccini, E., Trudell, J. R., and Harris, R. A. (2001) Annu. Rev. Pharmacol. Toxicol. 41 23–51 [DOI] [PubMed] [Google Scholar]
- 22.Bali, M., Jansen, M., and Akabas, M. H. (2009) J. Neurosci. 29 3083–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziebell, M. R., Nirthanan, S., Husain, S. S., Miller, K. W., and Cohen, J. B. (2004) J. Biol. Chem. 279 17640–17649 [DOI] [PubMed] [Google Scholar]
- 24.Ruesch, D., Zhong, H. J., and Forman, S. A. (2004) J. Biol. Chem. 277 20982–20992 [DOI] [PubMed] [Google Scholar]
- 25.McClelland, R. A. (2004) in Reactive Intermediate Chemistry (Moss, R. A., Platz, M. S., and Jones, M., Jr., eds), pp. 3–40, Wiley-Interscience, Hoboken, NJ
- 26.Siegwart, R., Jurd, R., and Rudolph, U. (2002) J. Neurochem. 80 140–148 [DOI] [PubMed] [Google Scholar]
- 27.Akk, G., Li, P., Bracamontes, J., Reichert, D. E., Covey, D. F., and Steinbach, J. H. (2008) Mol. Pharmacol. 74 614–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akk, G., Covey, D. F., Evers, A. S., Steinbach, J. H., Zorumski, C. F., and Mennerick, S. (2007) Pharmacol. Ther. 116 35–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao, M., Sonner, J. M., Husain, S. S., Miller, K. W., Jurd, R., Rudolph, U., and Eger, E. I., II (2005) Anesth. Analg. 101 131–135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.