Abstract
Insulin signaling is essential for normal glucose homeostasis. Rho-kinase (ROCK) isoforms have been shown to participate in insulin signaling and glucose metabolism in cultured cell lines. To investigate the physiological role of ROCK1 in the regulation of whole body glucose homeostasis and insulin sensitivity in vivo, we studied mice with global disruption of ROCK1. Here we show that, at 16–18 weeks of age, ROCK1-deficient mice exhibited insulin resistance, as revealed by the failure of blood glucose levels to decrease after insulin injection. However, glucose tolerance was normal in the absence of ROCK1. These effects were independent of changes in adiposity. Interestingly, ROCK1 gene ablation caused a significant increase in glucose-induced insulin secretion, leading to hyperinsulinemia. To determine the mechanism(s) by which deletion of ROCK1 causes insulin resistance, we measured the ability of insulin to activate phosphatidylinositol 3-kinase and multiple distal pathways in skeletal muscle. Insulin-stimulated phosphatidylinositol 3-kinase activity associated with IRS-1 or phospho-tyrosine was also reduced ∼40% without any alteration in tyrosine phosphorylation of insulin receptor in skeletal muscle. Concurrently, serine phosphorylation of IRS-1 at serine 632/635, which is phosphorylated by ROCK in vitro, was also impaired in these mice. Insulin-induced phosphorylation of Akt, AS160, S6K, and S6 was also decreased in skeletal muscle. These data suggest that ROCK1 deficiency causes systemic insulin resistance by impairing insulin signaling in skeletal muscle. Thus, our results identify ROCK1 as a novel regulator of glucose homeostasis and insulin sensitivity in vivo, which could lead to new treatment approaches for obesity and type 2 diabetes.
The ability of insulin to acutely stimulate glucose uptake and metabolism in peripheral tissues such as skeletal muscle and adipose tissue is critical for the regulation of normal glucose homeostasis (1). Impairments in insulin secretion and in the response of peripheral tissues to insulin (i.e. insulin resistance) are major pathogenic features of type 2 diabetes and contribute to the morbidity of obesity (1, 2). Insulin action involves a series of signaling cascades initiated by insulin binding to its receptor, eliciting receptor autophosphorylation and activation of the receptor tyrosine kinase, resulting in tyrosine phosphorylation of insulin receptor substrates (IRSs)4 (3). Phosphorylation of IRSs leads to activation of phosphatidylinositol 3-kinase (PI3K) and subsequently to activation of Akt and its downstream mediator AS160, all of which are important steps for the stimulation of glucose transport induced by insulin (4–6). Although the mechanism(s) underlying insulin resistance are not completely understood in peripheral tissues such as skeletal muscle, they are thought to result, at least in part, from impaired insulin-stimulated signal transduction (7).
Rho-kinase (ROCK) is a Ser/Thr protein kinase identified as a GTP-Rho-binding protein (8). There are two isoforms of Rhokinase, ROCK1 (also known as ROCKβ) (9, 10) and ROCK2 (also known as ROCKα) (9, 11). ROCK activity is enhanced by binding with RhoA GTP through a Rho-binding domain (8). Insulin activates geranylgeranyltranferase and increases the cellular amounts of geranlygeranylated RhoA, leading to increased RhoA activity (12). ROCK plays important roles in many cellular processes, including signal transduction, vesicle trafficking, and cytoskeletal organization (13, 14), key processes involved in insulin-stimulated glucose transport in myocytes and adipocytes (15–17). Previous studies have indicated that ROCK chemical inhibition is beneficial for reversing certain disease abnormalities in hypertension and diabetic nephropathy (18–20). Studies of the effects of ROCK inhibitors on glucose homeostasis in animals have yielded conflicting results, however. In obese Zucker rats, chronic treatment with the ROCK inhibitor fasudil decreases blood pressure and improves glucose tolerance (21). However, very recently, chronic treatment of obese db/db mice with the inhibitor fasudil was reported to have no effect on blood glucose levels (20). In contrast, in normal mice, we found that acute treatment with ROCK inhibitor Y-27632 causes insulin resistance in vivo by reducing insulin-mediated glucose uptake in skeletal muscle (22). In support of this, our previous work demonstrated that overexpression of dominant negative ROCK decreases insulin-stimulated glucose transport in L6 muscle cells, isolated soleus muscle ex vivo, and 3T3-L1 adipocytes via impairing PI3K activity (22). However, the use of different inhibitors, doses, treatment times, and animal models in these in vivo animal studies limits understanding of the roles of ROCK in regulating glucose homeostasis and insulin sensitivity in vivo. The fact that ROCK inhibitors target both ROCK isoforms and that their specificities may not be absolute further complicates interpretation of these studies (23).
In this study, we examined the physiological role of ROCK1 in the regulation of glucose homeostasis, whole body insulin sensitivity, and insulin action in mice with particular emphasis on the molecular basis of insulin resistance. Here we provide the evidence that global ROCK1 deficiency in mice causes insulin resistance in vivo in part via serine 632/635 phosphorylation of IRS-1. These data identify ROCK1 as a novel regulator of whole body glucose homeostasis and insulin signaling in vivo.
MATERIALS AND METHODS
Animals—ROCK1 knock-out (ROCK1–/–) mice used for experiments were generated on a C57BL/6–129/SvJ mixed background and then backcrossed at least 10 generations onto an FVB background (24). Heterozygous ROCK1-deficient (ROCK1+/–) and ROCK1–/– mice were viable with no detectable anatomic abnormalities (24). ROCK1+/– mice were intercrossed to generate male and female wild type (WT), ROCK1+/–, and ROCK1–/– littermates, which were studied at ∼16–18 weeks of age. The mice were fed standard chow (PMI Feeds, Inc., St. Louis, MO) ad libitum and housed under controlled temperature at 24 °C and a 12-h light-dark cycle with light from 6:30 a.m. to 6:30 p.m. All aspects of animal care and experimentation were conducted in accordance with the National Institutes Health Guide for the Care and Use of Laboratory Animals (34) and approved by the Institutional Animal Care and Use Committees of Indiana University and Beth Israel Deaconess Medical Center.
Metabolic Measurements—Blood was collected from random fed or overnight fasted mice via tail bleed between 8 and 10 a.m. Blood glucose was measured using an OneTouch Ultra glucose meter (LifeScan, Inc., Milpitas, CA). Serum insulin was measured by enzyme-linked immunosorbent assay (Crystal Chem Inc., Chicago, IL). For glucose tolerance tests, male mice were fasted overnight, and blood glucose was measured immediately before and 15, 30, 45, 60, 90, and 120 min after intraperitoneal injection of glucose (1.0 g/kg of body weight). For insulin tolerance tests, food was removed for 5 h in the morning, and blood glucose was measured immediately before and 15, 30, 45, 60, and 75 min after intraperitoneal injection of human insulin (0.75 unit/kg of body weight; HumulinR, Lilly). The area under the curve or above the curve for glucose or insulin was calculated (see Fig. 1) using the trapezoidal rule for glucose or insulin data.
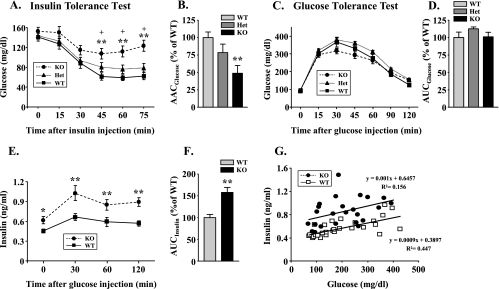
FIGURE 1.
Characterization of glucose metabolism in ROCK1–/– mice. A, ITT in WT, ROCK1+/– (HET), and ROCK1–/– (KO) mice. An ITT was performed 5 h after food removal. Mice were injected intraperitoneally with insulin at 0.75 units/kg of body weight. Blood glucose was measured from tail bleeds at the indicated times. B, the area above the glucose curve was calculated during the ITT. C, GTT in WT, ROCK1+/–, and ROCK1–/– mice. A GTT was performed in overnight fasted mice with intraperitoneal injection of glucose at 1.0 g/kg of body weight. Blood glucose was determined from tail bleeds at the indicated times. D, the area under the glucose curve was calculated during the GTT. E, serum insulin levels during the GTT from WT and ROCK1–/– mice. Insulin levels were measured from tail bleeds during GTT. F, the area under the insulin curve was calculated as described under “Materials and Methods.” G, correlation between glucose and insulin levels during the GTT in WT and ROCK1–/– mice. All data are from male mice. Data are means ± S.E. for 6–8 mice. *, p < 0.05, **, p < 0.01 versus WT mice, #, p < 0.05 versus ROCK1+/– mice.
Insulin Signaling Studies—Female WT, ROCK1+/–, and ROCK–/– mice were fasted overnight. Mice were injected intraperitoneally with human insulin (10 unit/kg of body weight; HumulinR) or saline and scarified 10 min later. Gastrocnemius muscle was rapidly removed and snap-frozen in liquid nitrogen and stored at –80 °C until analysis. Muscle was homogenized in lysis buffer as described (22). PI3K activities were measured as described (25). Muscle lysates (20–50 μg of protein) were resolved by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were incubated with: polyclonal antibodies against phospho-Tyr972 IR (Invitrogen); phospho-Tyr612 IRS-1 (Invitrogen); phospho-Ser632/635IRS-1 (Cell Signaling); phospho-Ser307IRS-1 (Millipore, Billerica, MA); phospho-Ser473Akt (Cell Signaling); phospho-Thr308Akt (Cell Signaling); phospho-Thr642AS160 (Cell Signaling); phospho-The389S6 kinase (Cell Signaling); phospho-Ser240/244 S6 ribosomal protein (Cell Signaling); phosphor-Thr/Glu/Tyr MAPK antibody (Promega; Madison, WI); ROCK1 (Santa Cruz Biotechnology) or ROCK2 (Santa Cruz Biotechnology); IR (Santa Cruz Biotechnology); Akt (Santa Cruz Biotechnology); S6 kinase (Cell Signaling); S6 ribosomal protein (Cell Signaling); Glut4 (Millipore); glyceraldehyde-3-phosphate dehydrogenase (Santa Cruz Biotechnology); or monoclonal antibodies specific for RhoA (Santa Cruz Biotechnology) or for RhoE (Millipore). The bands were visualized with enhanced chemiluminescence and quantified by densitometry (25). All phosphorylation data were normalized by the total protein levels.
Statistical Analysis—Data are expressed as mean ± S.E. Differences between two groups were assessed using unpaired two-tailed t tests and among more than two groups by ANOVA. Data involving more than two repeated measures were assessed by repeated measures ANOVA. When a significant difference was found with ANOVA, post hoc analyses were performed with Fisher's protected least significant difference test. Differences were considered significant at p < 0.05. Analyses were performed using StatView software (BrainPower Inc.).
RESULTS
ROCK1 Deficiency Impairs Insulin Sensitivity in Mice—Body weight tended to be slightly decreased in both female and male ROCK1–/– mice as compared with WT littermates, but differences were not statistically significant (supplemental Fig. 1, A and E). Adipose tissue mass in female ROCK1–/– mice tended to be lower than that in WT littermates (0.72 ± 0.10 g in WT mice versus 0.65 ± 0.12 g in ROCK1–/– mice). However, the effect was no longer present when normalized for body weight (2.52 ± 0.33% in WT mice versus 2.49 ± 0.37% in ROCK1–/– mice). There were no differences in liver or heart weight normalized to body weight between genotypes (liver, 4.20 ± 0.19% in WT mice versus 4.08 ± 0.14% in ROCK1–/– mice; heart, 0.47 ± 0.02% in WT mice versus 0.46 ± 0.02% in ROCK1–/– mice).
At 16–18 weeks of age, female and male ROCK1–/– mice had similar fed and fasted blood glucose levels as compared with WT mice (supplemental Fig. 1, B and F). Fasted serum insulin levels tended to be higher in female ROCK1–/– mice (p < 0.10) but were significantly increased in male ROCK1–/– mice (p < 0.05) as compared with WT mice (supplemental Fig. 1, C and G). Female and male ROCK1–/– mice had a higher risk for insulin resistance, as revealed by higher insulin levels in the fed state. Fed serum insulin levels were increased ∼1.7-fold in female ROCK1–/– mice and ∼1.5-fold in male ROCK1–/– mice as compared with WT littermates (supplemental Fig. 1, C and G). When insulin sensitivity was expressed as the glucose × insulin product, both female and male ROCK1–/– mice displayed higher values than WT (1.8- and 2.0-fold, respectively), indicating that insulin sensitivity is decreased in these mice (supplemental Fig. 1, D and H).
To determine the physiological consequence of the absence of ROCK1 in glucose metabolism, we evaluated glucose homeostasis in ROCK1–/– mice by insulin tolerance tests (ITTs) and glucose tolerance tests (GTTs). ROCK1–/– mice were insulin-resistant as evidenced by a lack of decrease in blood glucose levels after insulin injection (0.75 units/kg of body weight), whereas in WT mice, glucose levels decreased about 56% after insulin injection (Fig. 1A). The area above the glucose curve was decreased ∼25% in ROCK1+/– mice, although this did not reach significance (p < 0.10), and ∼50% in ROCK1–/– mice (p < 0.001) as compared with WT littermates (Fig. 1B).
In contrast, glucose tolerance was normal in ROCK1–/– mice, as indicated by similar areas under the glucose curve during the GTT (Fig. 1, C and D). After an overnight fast, ROCK1–/– mice had elevated insulin levels at the start of the GTT. Moreover, glucose-induced insulin secretion during the GTT was significantly increased in ROCK1–/– mice as compared with WT littermates (Fig. 1E). The area under the insulin curve was increased by ∼50% in ROCK1–/– mice as compared with WT mice (p < 0.01) (Fig. 2F). When plotting glucose versus insulin during the GTT, ROCK1–/– and WT mice had similar slopes, indicating similar incremental pancreatic β-cell responsiveness to a glucose load (Fig. 1G). However, the regression intercept was significantly shifted upward for ROCK1–/– mice as compared with WT, such that for a given glucose level, the pancreas secreted 65% more insulin (Fig. 1G).
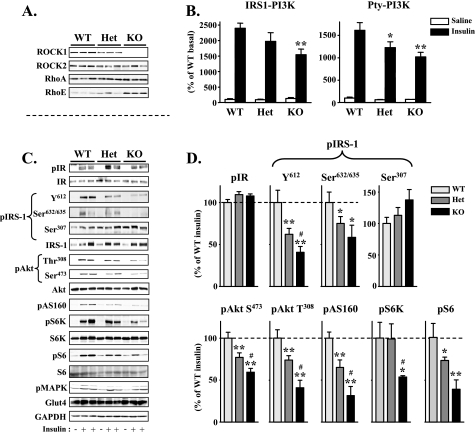
FIGURE 2.
Molecular analysis of insulin signaling in ROCK1–/– mice. A, immunoblot analysis of ROCK1 in muscle of WT, ROCK1+/– (HET), and ROCK1–/– (KO) mice. Muscle lysates were subjected to SDS-PAGE and immunoblot with antibodies for ROCK1, ROCK2, RhoA, and RhoE.B, PI3K activity in muscle of WT, ROCK1+/–, and ROCK1–/– mice. After an overnight fast, 4-month-old female mice were injected intraperitoneally with saline (white bars) or 10 units/kg of insulin (black bars). Ten min later, muscle was removed. Muscle lysates were subjected to immunoprecipitation with an IRS-1 antibody or a phospho-tyrosine antibody. PI3K activity was measured and quantitated using a PhosphorImager. p indicates phosphorylation; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. C, insulin signaling in muscle of WT, ROCK1+/–, and ROCK1–/– mice. Muscle lysates were subjected to SDS-PAGE and immunoblot with antibodies as indicated. The bands were quantitated using densitometry and normalized by the total protein levels. Note: The IRS-1 antibody reacts more strongly with phosphorylated IRS-1 protein. D, quantitation of insulin signaling molecules in muscle of WT, ROCK1+/–, and ROCK1–/– mice. The immunoblots shown are representative of three blots for each antibody. For each genotype, n = 4 for saline and n = 6–7 for insulin. Data are means ± S.E. *, p < 0.05, **, p < 0.01 versus WT mice. #, p < 0.05 versus ROCK1+/– mice.
ROCK1 Deficiency Impairs Skeletal Muscle Insulin Signaling in Mice—To explore the mechanism by which deletion of ROCK1 causes systemic insulin resistance, we measured the ability of insulin to activate PI3K and multiple distal pathways in skeletal muscle of ROCK1–/– mice. As expected, ROCK1 protein was not detected in muscle of ROCK1–/– mice, whereas ROCK2 protein expression was preserved (Fig. 2A). The levels of ROCK1 protein in ROCK1+/– mice were reduced by ∼35% in muscle as compared with WT mice (Fig. 2A). There is no significant difference in RhoA protein amounts among WT, ROCK1+/–, and ROCK1–/– mice (Fig. 2A). Interestingly, the protein levels of RhoE were increased in ROCK1–/– mice as compared with WT littermates (Fig. 2A).
Insulin-stimulated PI3K activity associated with IRS-1 or phospho-tyrosine was significantly decreased in skeletal muscle of ROCK1–/– mice as compared with WT (Fig. 2B). This decrease in PI3K activity corresponded closely with a reduction in IRS-1 tyrosine 612 phosphorylation, the binding site for the p85 subunit of PI3K, in ROCK1–/– mice (Fig. 2, C and D). In parallel, phosphorylation of IRS-1 at serine 632/635, which is phosphorylated by ROCK in vitro (22), was also impaired in ROCK1–/– mice (Fig. 2, C and D). Concurrently, insulin stimulation of Akt and AS160 phosphorylation in skeletal muscle was decreased in ROCK1–/– mice as compared with WT (Fig. 2, C and D). Furthermore, ROCK1 deficiency decreased phosphorylation of S6K, and its downstream S6 ribosomal protein was induced by insulin. Interestingly, S6 phosphorylation was markedly reduced in ROCK1+/– mice despite normal S6K phosphorylation (Fig. 2, C and D). Except for S6K phosphorylation, the activation of skeletal muscle insulin signaling in ROCK1+/– mice in response to insulin stimulation was intermediate between ROCK1–/– and WT mice. Importantly, ROCK1 deficiency did not affect insulin-stimulated IR tyrosine phosphorylation, IRS-1 serine 307 phosphorylation, and MAPK phosphorylation in skeletal muscle (Fig. 2, C and D). Protein expression of insulin signaling molecules was not altered by ROCK1 deficiency. Loss of ROCK1 also had no effect on the expression of Glut4, the insulin-responsive glucose transporter in skeletal muscle (Fig. 2, C and D).
Our previous work demonstrated that overexpression of dominant negative ROCK inhibits insulin-stimulated IRS-1-associated PI3K but not the phosphorylation of Akt at both Ser473 and Thr308 and p70 S6 kinase in 3T3-L1 adipocytes (22). However, in the present study, insulin-stimulated phosphorylation of Akt and p70 S6kinase was significantly reduced in ROCK1-deficient mice. This discrepancy is likely due to the fact that previous studies were done in vitro in adipocytes, whereas the current studies were performed in vivo in skeletal muscle. Furthermore, we used a dominant negative form of ROCK2, which inhibits the activity of both ROCK1 and ROCK2 (26, 27). Thus, another explanation for this might be differences in ROCK isoform specificity.
DISCUSSION
The current study provides in vivo evidence of a physiological role of ROCK1 in the regulation of glucose homeostasis and insulin signaling and identifies a potentially novel therapeutic target for the prevention and treatment of type 2 diabetes. Our previous work demonstrated that dominant negative ROCK overexpression or chemical ROCK inhibition decreases insulin-stimulated glucose transport in L6 muscle cells and isolated skeletal muscle ex vivo via impairing IRS-1-associated PI3K activity (22). Consistent with these in vitro data, we report here that ROCK1 deficiency in mice results in whole body insulin resistance and impaired skeletal muscle insulin signaling. These effects are independent of changes in body adiposity. Akt and AS160 phosphorylation, both of which are required for insulin-dependent Glut4 translocation (6, 28), were markedly decreased in skeletal muscle of ROCK1–/– mice in response to insulin, suggesting that glucose transport into muscle may be impaired in these mice. Despite this, glucose tolerance of ROCK1–/– mice was normal, which may result from increased glucose-induced insulin secretion. Indeed, our results show that ROCK1–/– mice have elevated serum insulin during the glucose excursion of a glucose tolerance test as well as in the fasted and fed states. The increased serum insulin in ROCK1–/– mice may be due to β-cell compensation for insulin resistance. Alternatively, ROCK1 may directly regulate insulin secretion, as evidenced by the fact that ROCK inhibition in primary pancreatic β-cells prolongs glucose-stimulated insulin secretion (29). Moreover, ROCK inhibition in pancreatic β-cell-derived HIT-T15 cells markedly increased insulin gene expression (30). Importantly, however, ROCK1 deficiency had no effect on insulin-stimulated tyrosine phosphorylation of IR in skeletal muscle, indicating that the effects of ROCK1 on whole body glucose homeostasis are downstream of IR. Thus, our data suggest that ROCK1 functions in skeletal muscle may be critical in regulating whole body glucose homeostasis.
Data emerging from several laboratories over the past several years indicate that serine phosphorylation of IRS-1 is a key regulator of insulin signaling (31). However, the effects of phosphorylation of individual IRS-1 serine residues on insulin signaling appear to be complex, maybe context-dependent, and are still being defined. The mechanism(s) by which dominant negative ROCK overexpression or chemical ROCK inhibition decreases insulin-stimulated glucose transport in cultured cells in vitro is due in part to the absence of ROCK-dependent IRS-1 serine 632/635 phosphorylation, which impairs interaction of IRS-1 with the p85 regulatory subunit of PI3K (22). Consistent with these data, insulin-stimulated IRS-1 serine 632/635 phosphorylation and PI3K activity associated with IRS-1 or phospho-tyrosine are impaired in skeletal muscle of ROCK1–/– mice. Impaired insulin-induced IRS-1 serine 632/635 phosphorylation also accompanies decreased PI3K and ROCK activation in skeletal muscle of obese, insulin-resistant, or diabetic mice and rats,5 suggesting that impaired IRS-1 serine 632/635 phosphorylation may be an important mechanism contributing to the pathogenesis of insulin resistance in obesity. Reducing phosphorylation of IRS-1 serine 632/635 may impair PI3K activity by altering the affinity of the adjacent tyrosine 612 YXXM motif for PI3K (3, 22). Interestingly, mammalian target of rapamycin (mTOR)-mediated IRS-1 serine 632/635 phosphorylation has been shown to inhibit insulin-stimulated IRS-1 tyrosine phosphorylation in 293 HEK cells (32, 33). Further studies will be needed to determine whether the disparate effects of IRS-1 serine 632/635 phosphorylation in these models reflects other concurrent modifications of IRS-1. Regardless, our data provide key in vivo experimental evidence that ROCK1 regulates insulin action in controlling whole body glucose homeostasis and insulin sensitivity.
In conclusion, results reported here show that deficiency of ROCK1 causes whole body insulin resistance by impairing insulin signaling in skeletal muscle, suggesting that activation of ROCK1 is essential for the normal actions of insulin on glucose transport in skeletal muscle in vivo. Thus, our studies identify ROCK1 as a novel stimulator of insulin action in vivo. Further studies of ROCK1 and ROCK2 functions in different metabolic tissues will be needed to precisely delineate ROCK isoform functions to regulate tissue and whole body insulin sensitivity and glucose homeostasis. The emergence of ROCK1 as an important regulator of insulin action could further lead to new treatment approaches for obesity and type 2 diabetes.
Supplementary Material
Acknowledgments
We thank Karyn Catalano and YunShin Jung for important contributions to this work.
This work was supported, in whole or in part, by National Institutes of Health Grants DK43051 (to Y. B. K.), DK38712 (to M. F. W.), CA127247 (to S. W. L.), and HL072897 and HL085098 (to L. W.). This work was also supported by American Diabetes Association Grants 7-05-PPG-02 and 1-09-RA-87 (to Y. B. K.), Showalter Trust Grant 89829 (to J. S.), Korean Ministry of Health and Welfare Grant A06-2363 (to M. S. K.), and Korea Science and Engineering Foundation Grant R-01-2006-000-108060-0 (to M. S. K.).
The on-line version of this article (available at http://www.jbc.org) contains one supplemental figure.
Footnotes
The abbreviations used are: IR, insulin receptor; IRS, insulin receptor substrate; ROCK, Rho-kinase; PI3K, phosphatidylinositol 3-kinase; WT, wild type; MAPK, mitogen-activated protein kinase; ANOVA, analysis of variance; ITT, insulin tolerance tests; GTT, glucose tolerance tests.
Y. B. Kim, unpublished data.
References
- 1.DeFronzo, R. A. (1997) Diabetes Rev. 5 177–269 [Google Scholar]
- 2.Kahn, B. B., and Flier, J. S. (2000) J. Clin. Investig. 106 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White, M. F. (2002) Am. J. Physiol. 283 E413–E422 [DOI] [PubMed] [Google Scholar]
- 4.Cheatham, B., Vlahos, C. J., Cheatham, L., Wang, L., Blenis, J., and Kahn, C. R. (1994) Mol. Cell. Biol. 14 4902–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hara, K., Yonezawa, K., Sakaue, H., Ando, A., Kotani, K., Kitamura, T., Kitamura, Y., Ueda, H., Stephens, L., Jackson, T. R., Hawkins, P. T., Dhand, R., Clark, A. E., Holman, G. D., Waterfield, M. D., and Kasuga, M. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 7415–7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sano, H., Kane, S., Sano, E., Miinea, C. P., Asara, J. M., Lane, W. S., Garner, C. W., and Lienhard, G. E. (2003) J. Biol. Chem. 278 14599–14602 [DOI] [PubMed] [Google Scholar]
- 7.Kahn, B. B. (1998) Cell 92 593–596 [DOI] [PubMed] [Google Scholar]
- 8.Matsui, T., Amano, M., Yamamoto, T., Chihara, K., Nakafuku, M., Ito, M., Nakano, T., Okawa, K., Iwamatsu, A., and Kaibuchi, K. (1996) EMBO J. 15 2208–2216 [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa, O., Fujisawa, K., Ishizaki, T., Saito, Y., Nakao, K., and Narumiya, S. (1996) FEBS Lett. 392 189–193 [DOI] [PubMed] [Google Scholar]
- 10.Ishizaki, T., Maekawa, M., Fujisawa, K., Okawa, K., Iwamatsu, A., Fujita, A., Watanabe, N., Saito, Y., Kakizuka, A., Morii, N., and Narumiya, S. (1996) EMBO J. 15 1885–1893 [PMC free article] [PubMed] [Google Scholar]
- 11.Leung, T., Manser, E., Tan, L., and Lim, L. (1995) J. Biol. Chem. 270 29051–29054 [DOI] [PubMed] [Google Scholar]
- 12.Chappell, J., Golovchenko, I., Wall, K., Stjernholm, R., Leitner, J. W., Goalstone, M., and Draznin, B. (2000) J. Biol. Chem. 275 31792–31797 [DOI] [PubMed] [Google Scholar]
- 13.Van Aelst, L., and D'Souza-Schorey, C. (1997) Genes Dev. 11 2295–2322 [DOI] [PubMed] [Google Scholar]
- 14.Kaibuchi, K., Kuroda, S., and Amano, M. (1999) Annu. Rev. Biochem. 68 459–486 [DOI] [PubMed] [Google Scholar]
- 15.Omata, W., Shibata, H., Li, L., Takata, K., and Kojima, I. (2000) Biochem. J. 346 321–328 [PMC free article] [PubMed] [Google Scholar]
- 16.Patki, V., Buxton, J., Chawla, A., Lifshitz, L., Fogarty, K., Carrington, W., Tuft, R., and Corvera, S. (2001) Mol. Biol. Cell 12 129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsakiridis, T., Tong, P., Matthews, B., Tsiani, E., Bilan, P. J., Klip, A., and Downey, G. P. (1999) Microsc. Res. Tech. 47 79–92 [DOI] [PubMed] [Google Scholar]
- 18.Hu, E., and Lee, D. (2003) Curr. Opin. Investig. Drugs 4 1065–1075 [PubMed] [Google Scholar]
- 19.Hu, E., and Lee, D. (2005) Expert Opin. Ther. Targets 9 715–736 [DOI] [PubMed] [Google Scholar]
- 20.Kolavennu, V., Zeng, L., Peng, H., Wang, Y., and Danesh, F. R. (2008) Diabetes 57 714–723 [DOI] [PubMed] [Google Scholar]
- 21.Kanda, T., Wakino, S., Homma, K., Yoshioka, K., Tatematsu, S., Hasegawa, K., Takamatsu, I., Sugano, N., Hayashi, K., and Saruta, T. (2006) FASEB J. 20 169–171 [DOI] [PubMed] [Google Scholar]
- 22.Furukawa, N., Ongusaha, P., Jahng, W. J., Araki, K., Choi, C. S., Kim, H. J., Lee, Y. H., Kaibuchi, K., Kahn, B. B., Masuzaki, H., Kim, J. K., Lee, S. W., and Kim, Y. B. (2005) Cell Metab. 2 119–129 [DOI] [PubMed] [Google Scholar]
- 23.Riento, K., and Ridley, A. J. (2003) Nat. Rev. Mol. Cell Biol. 4 446–456 [DOI] [PubMed] [Google Scholar]
- 24.Zhang, Y. M., Bo, J., Taffet, G. E., Chang, J., Shi, J., Reddy, A. K., Michael, L. H., Schneider, M. D., Entman, M. L., Schwartz, R. J., and Wei, L. (2006) FASEB J. 20 916–925 [DOI] [PubMed] [Google Scholar]
- 25.Kim, Y. B., Shulman, G. I., and Kahn, B. B. (2002) J. Biol. Chem. 277 32915–32922 [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi, K., Takahashi, M., Matsushita, N., Miyazaki, J., Koike, M., Yaginuma, H., Osumi, N., and Kaibuchi, K. (2004) J. Neurosci. 24 3480–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirai, H., Autieri, M., and Eguchi, S. (2007) Curr. Opin. Nephrol. Hypertens. 16 111–115 [DOI] [PubMed] [Google Scholar]
- 28.Jiang, Z. Y., Zhou, Q. L., Coleman, K. A., Chouinard, M., Boese, Q., and Czech, M. P. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 7569–7574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammar, E., Tomas, A., Bosco, D., and Halban, P. A. (2009) Endocrinology, in press [DOI] [PubMed]
- 30.Nakamura, Y., Kaneto, H., Miyatsuka, T., Matsuoka, T. A., Matsuhisa, M., Node, K., Hori, M., and Yamasaki, Y. (2006) Biochem. Biophys. Res. Commun. 350 68–73 [DOI] [PubMed] [Google Scholar]
- 31.Zick, Y. (2001) Trends Cell Biol. 11 437–441 [DOI] [PubMed] [Google Scholar]
- 32.Um, S. H., Frigerio, F., Watanabe, M., Picard, F., Joaquin, M., Sticker, M., Fumagalli, S., Allegrini, P. R., Kozma, S. C., Auwerx, J., and Thomas, G. (2004) Nature 431 200–205 [DOI] [PubMed] [Google Scholar]
- 33.Ozes, O. N., Akca, H., Mayo, L. D., Gustin, J. A., Maehama, T., Dixon, J. E., and Donner, D. B. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 4640–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Institutes Health (1996) Guide for the Care and Use of Laboratory Animals, National Institutes of Health Publication Number 85-23, National Institutes of Health, Bethesda, MD
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.