Abstract
The endoplasmic reticulum (ER) has a strict protein quality control system. Misfolded proteins generated in the ER are degraded by the ER-associated degradation (ERAD). Yeast Mnl1p consists of an N-terminal mannosidase homology domain and a less conserved C-terminal domain and facilitates the ERAD of glycoproteins. We found that Mnl1p is an ER luminal protein with a cleavable signal sequence and stably interacts with a protein-disulfide isomerase (PDI). Analyses of a series of Mnl1p mutants revealed that interactions between the C-terminal domain of Mnl1p and PDI, which include an intermolecular disulfide bond, are essential for subsequent introduction of a disulfide bond into the mannosidase homology domain of Mnl1p by PDI. This disulfide bond is essential for the ERAD activity of Mnl1p and in turn stabilizes the prolonged association of PDI with Mnl1p. Close interdependence between Mnl1p and PDI suggests that these two proteins form a functional unit in the ERAD pathway.
The endoplasmic reticulum (ER)2 is the first organelle in the secretory pathway of eukaryotic cells and provides an optimum environment for maturation of newly synthesized secretory and membrane proteins. Protein folding/assembly in the ER is aided by molecular chaperones and folding enzymes. Molecular chaperones in the ER assist folding of newly synthesized proteins and prevent them from premature misfolding and/or aggregate formation (1, 2). Protein folding in the ER is often associated with formation of disulfide bonds, which contribute to stabilization of native, functional states of proteins. Disulfide bond formation could be a rate-limiting step of protein folding both in vitro and in vivo (3, 4), and the ER has a set of folding enzymes including protein-disulfide isomerase (PDI) and its homologs that catalyze disulfide bond formation (5, 6).
In parallel, protein folding/assembly in the ER relies on the inherent failsafe mechanism, i.e. the ER quality control system, to ensure that only correctly folded and/or assembled proteins can exit the ER. Misfolded or aberrant proteins are retained in the ER for refolding by ER-resident chaperones, whereas terminally misfolded proteins are degraded by the mechanism known as ER-associated degradation (ERAD). The ERAD consists of recognition and processing of aberrant substrate proteins, retrotranslocation across the ER membrane, and subsequent proteasome-dependent degradation in the cytosol. More than 20 different components have been identified to be involved in this process in yeast and mammals (7).
The majority of proteins synthesized in the ER are glycoproteins, in which N-linked glycans are not only important for folding but also crucial for their ERAD if they fail in folding. Specifically, trimming of one or more mannose residues of Man9GlcNAc2 oligosaccharide and recognition of the modified mannose moiety represent a key step for selection of terminally misfolded proteins for disposal (8). A mannosidase I-like protein, Mnl1p/Htm1p (yeast), and EDEM (mammals, ER degradation enhancing α-mannosidase-like protein) were identified as candidates for lectins that recognize ERAD substrates with modified mannose moieties (9–11). Both Mnl1p and EDEM contain an N-terminal mannosidase homology domain (MHD), which lacks cysteine residues conserved among α1,2-mannosidase family members and is proposed to function in recognition of mannose-trimmed carbohydrate chains (supplemental Fig. S1). However, whether Mnl1p or EDEM indeed functions as an ERAD-substrate-binding lectin or has a mannosidase activity is still in debate (11–15), and Yos9p was suggested to take the role of ERAD-substrate binding lectin (14, 16–18). Mnl1p, but not EDEM, has a large C-terminal extension, which does not show any homology to known functional domains and is conserved only among fungal Mnl1p homologs (supplemental Fig. S1).
After recognition of the modified mannose signal for degradation, aberrant proteins are maintained or converted to be retrotranslocation competent by ER chaperones including BiP (19). PDI was also indicated to be involved in these steps in the ERAD by, for example, its possible chaperone-like functions (20–23). The yeast PDI, Pdi1p, contains four thioredoxin-like domains, two of which have a CGHC motif as active sites, followed by a C-terminal extension containing the ER retention signal. During its catalytic cycle, PDI transiently forms a mixed disulfide intermediate with its substrate through an intermolecular disulfide bond between the cysteine residues of the active site of PDI and the substrate molecule.
Here we report identification of PDI as an Mnl1p-interacting protein. Stable interactions between the C-terminal domain of Mnl1p and PDI involve intermolecular disulfide bonds. Stably interacting PDI is required for formation of the functionally essential intramolecular disulfide bond in the MHD of Mnl1p, which in turn stabilizes and prolongs the Mnl1p-PDI interactions. Possible roles for those stable interactions between Mnl1p and PDI in the ERAD will be discussed.
EXPERIMENTAL PROCEDURES
Strains, Plasmids, and Culturing Conditions—Yeast strains used in this study are W303-1A (MATa ade2 ura3 leu2 trp1 his3 ade2 can1), SEY6210 (MATa ura3 leu2 trp1 his3 lys2 suc2) (24), SNY1079 (MATa mnl1::HIS3 ura3 leu2 trp1 his3 lys2 suc2) (24), SNY1080 (MATa prc1-1 mnl1::HIS3 ura3 leu2 trp1 his3 lys2 suc2) (25), and PBY3–9B (MATa sec11-7 ura3 leu2 his4) (26). KRY94 (MATa pdi1::HIS3 ade2 ura3 leu2 trp1 his3 ade2 can1 pPDI-URA3) (27) is a gift from K. Römisch (Universität des Saarlandes). Yeast cells were grown in YPD (1% yeast extract, 2% polypeptone, and 2% glucose) or SCD (0.67% yeast nitrogen base without amino acids, 2% glucose, 0.5% casamino acids) with appropriate supplements.
The mnl1::CgTRP1 allele was constructed as follows. A DNA fragment containing the Candida glabrata TRP1 gene (CgTRP1) was amplified by PCR using pCgTRP1 (28) as a template with primers 5′-GAAGACGATGCGTACTCATTCACTTCTAAAGAACTTAAGGGTTGTAAAACGACGGCCAGT-3′ and 5′-GTGGGGGAAACTCCGGAGGACTAAAGTTCCACCTTTCAGGCACAGGAAACAGCTATGACC-3′. The amplified DNA fragment, flanked by 40 base pairs each of the upstream and downstream sequences of the MNL1 gene, was introduced into KRY94, and Trp+ transformants were selected. Disruption of the MNL1 gene was confirmed by PCR, and the resulting strain was named W303-1A mnl1ΔpdiΔ/pPDI-URA3.
Plasmids expressing C-terminally FLAG-tagged Mnl1p (Mnl1p-FLAG) was generated as follows. The MNL1 gene was amplified by PCR using yeast genomic DNA as a template with primers 5′-GCGCTCGAGTGACCGATCCACCCTTTAAG-3′ and 5′-GCGGAGCTCCTTTCCTCAATAGTGGTGTA-3′. The amplified 3.3-kilobase pair DNA fragment was digested with SacI and XhoI and inserted into the SacI-XhoI sites of pRS316 (29) to give pSNA27. A BglII site was inserted between the 796th codon and the stop codon of the MNL1 gene by oligonucleotide-directed mutagenesis to give pKHY1. A DNA fragment for the 3×FLAG tag sequence was amplified by PCR with primers 5′-GGCGAATTGGGATCCGGGCCCGAC-3′ and 5′-CGCGGATCCGTCGACGGGGGGCCTCTT-3′ using pTYE247 (30) as a template. The amplified DNA fragment was digested with BglII and inserted into the BglII site of pKHY1 to give pKHY3. The 3.4-kb SacI-XhoI fragment of pKHY3 was introduced into the SacI-XhoI sites of pYO326 (31) to give pMAY5. A series of the Cys → Ser Mnl1p mutants, the Ala substitution mutants for the conserved residues in the C-terminal domain of Mnl1p, and the ΔC Mnl1p mutant were constructed by oligonucleotide-directed mutagenesis using pMAY5 as a template. pPDI-TRP1 and pPDI-S1S2 plasmids are provided from W. J. Lennarz (Stony Brook University). pPDI-S5S6 and a series of S3S4 mutants of PDI were constructed by oligonucleotide-directed mutagenesis using pPDI-TRP1 as a template. pPDI-TRP1, pPDI-S1S2, or pPDI-S5S6 was introduced into W303-1A mnl1ΔpdiΔ/pPDI-URA3, and then pPDI-URA was removed by growing the transformants on medium containing 1 mg/ml 5-fluoroorotic acid.
Preparation of Microsomes and Crude Membrane Fractions—The microsomes were prepared as described by McCracken and Brodsky (32). Crude membrane fractions were prepared as described below. Yeast cells (1.5 × 107 cells) collected from exponentially growing cell cultures were suspended in 1 ml of 0.1 m Tris-SO4, pH 9.4, and 10 mm DTT and immediately collected by centrifugation at 9,500 × g for 30 s at 4 °C. The cells were converted to spheroplasts by incubating in 1 ml of 20 mm Tris-HCl, pH 7.4, 1.2 m sorbitol, and 0.02 mg/ml Zymolyase 20T (Seikagaku Corporation) for 15 min at 30 °C. The spheroplasts were suspended in 100 μl of 100 mm sorbitol, 50 mm potassium acetate, 2 mm EDTA, 1 mm PMSF, 10 mm Hepes-KOH, pH 7.4, and 10 mm DTT and disrupted by vortexing for 1 min with glass beads (∼100 mg) for two cycles with an 1.5-min interval on ice. In Fig. 1D, DTT was omitted from this solution. Cell homogenates were diluted 5-fold with the same solution as used in homogenization and centrifuged at 550 × g for 5 min at 4 °C to remove cell debris. The supernatant was centrifuged at 15,000 × g for 15 min at 4 °C, and the resulting pellet was used as the crude membrane fraction.
FIGURE 1.
Mnl1p is an ER luminal protein with a cleavable signal sequence. A, microsomes prepared from cells expressing Mn1p-FLAG from a multicopy plasmid (SNY1080/pMAY5) were solubilized with 1% SDS and 1% 2-mercaptoethanol and incubated with endoglycosidase H (Endo H). The proteins were analyzed by SDS-PAGE and immunoblotting with the anti-FLAG antibody or anti-CPY antibodies. B, microsomes were proteinase K-treated with or without 1% Triton X-100, and the proteins were analyzed by SDS-PAGE and immunoblotting using the anti-FLAG antibody or anti-BiP/Kar2p antibodies. The asterisk indicates a degradation product of BiP. C, microsomes were treated with 0.1 m Na2CO3 (pH 11.5) or 1% Triton X-100 (T, total) and centrifuged to separate the pellet (P) and supernatant (S) fractions. Each fraction was analyzed by SDS-PAGE and immunoblotting with the anti-FLAG antibody or antibodies against indicated proteins. D, wild type (SEY6210; SEC11) and sec11-7 mutant (PBY3–9B) strains harboring pMAY5 were grown to early log phase and incubated at 23 °C or 37 °C for 3 h. The membrane fractions prepared from these cells were solubilized with 1% SDS and 1% 2-mercaptoethanol and treated with (+) or without (–) endoglycosidase H. The proteins were analyzed by SDS-PAGE and immunoblotting using the anti-FLAG antibody or anti-Kar2p antibodies. Filled and open arrowheads indicate the precursor and mature forms, respectively, after endoglycosidase H treatment.
Immunoprecipitation—Microsomes or crude membranes were suspended in 20 mm Hepes-KOH, pH 7.4, 50 mm NaCl, 1% Nonidet P-40, and protease inhibitor mixture for use with mammalian cell and tissue extracts (PiC; Sigma-Aldrich), incubated on ice for 1 h and centrifuged at 15,000 × g for 5 min at 4 °C to remove insoluble materials. The supernatant was diluted 10-fold with 20 mm Hepes-KOH, pH 7.4, 50 mm NaCl, and PiC and incubated with anti-FLAG M2-agarose (Sigma-Aldrich) or anti-PDI antibody-bound protein G-Sepharose at 4 °C for more than 3 h. The immunoprecipitated materials were washed twice with 20 mm Hepes-KOH, pH 7.4, 50 mm NaCl, and 0.1% Nonidet P-40 and eluted with sample buffer for SDS-PAGE without 2-mercaptoethanol.
In Fig. 2E, 3 and 5B, spheroplasts were incubated in medium containing 1.2 m sorbitol for 30 min at 30 °C, and the proteins were precipitated by incubation with 10% trichloroacetic acid on ice for 10 min followed by centrifugation at 15,000 × g for 5 min at 4 °C. The precipitated materials were washed twice with cold acetone, and the proteins were suspended in 2% SDS, 20 mm Hepes-KOH, pH 7.4, 50 mm NaCl, 35 mm iodoacetamide, and PiC and incubated at 94 °C for 5 min. Insoluble materials were removed by centrifugation at 15,000 × g for 5 min at 4 °C. The supernatant was diluted 10-fold with 20 mm Hepes-KOH, pH 7.4, 50 mm NaCl, and PiC and incubated with anti-FLAG M2-agarose at room temperature for 1 h. The immunoprecipitates were washed twice with 20 mm Hepes-KOH, pH 7.4, 50 mm NaCl and eluted with sample buffer for SDS-PAGE without 2-mercaptoethenol. For SDS-PAGE under reducing conditions, 2-mercaptoethanol was added to the sample at 3% prior to the analyses.
FIGURE 2.
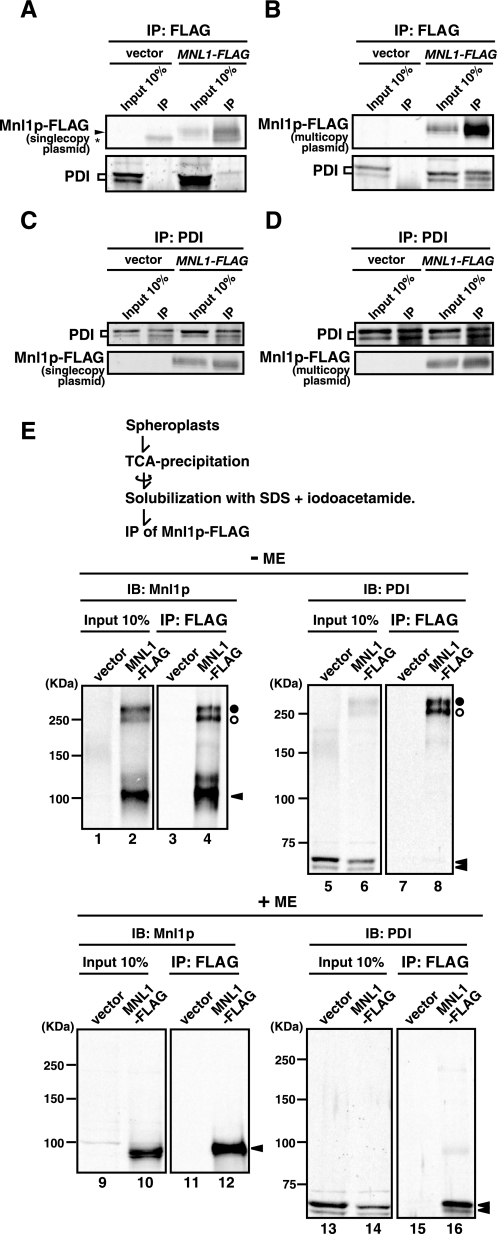
Mnl1p interacts with PDI. A and C, microsomes prepared from cells expressing Mnl1p-FLAG from a single copy plasmid (SNY1079/pKHY3; MNL1-FLAG) or from those with a vector alone (SNY1079/pRS316; vector) were solubilized with 1% Nonidet P-40 and subjected to immunoprecipitation with the anti-FLAG antibody (A) or anti-PDI antibodies (C). Immunoprecipitated materials (IP) and 10% of the solubilized membranes prior to immunoprecipitation (Input 10%) were analyzed by SDS-PAGE and immunoblotting with the anti-FLAG antibody (Mnl1p-FLAG) or anti-PDI antibodies (PDI). The asterisk indicates IgG bands. B and D, microsomes prepared from cells expressing Mnl1p-FLAG from a multicopy plasmid (SNY1079/pMAY5; MNL1-FLAG) or from those with a vector alone (SNY1079/pYO326; vector) were treated as in A and C. E, spheroplasts prepared from cells expressing Mnl1p-FLAG from a multicopy plasmid (SNY1079/pMAY5; MNL1-FLAG) or from those with a vector alone (SNY1079/pYO326; vector) were subjected to trichloroacetic acid precipitation. The precipitated proteins were solubilized with 2% SDS in the presence of 35 mm iodoacetamide and subjected to immunoprecipitation with the anti-FLAG antibody. Immunoprecipitated materials (IP) and 10% of the solubilized membranes prior to immunoprecipitation (Input 10%) were analyzed by nonreducing (–ME) or reducing (+ME) SDS-PAGE and immunoblotting with antibodies against Mnl1p (IB: Mnl1p) or PDI (IB: PDI). The open and filled circles indicate the PDI-Mnl1p-FLAG complexes formed by the intermolecular disulfide bonds, and arrowheads indicate the Mnl1p and PDI monomers.
FIGURE 3.
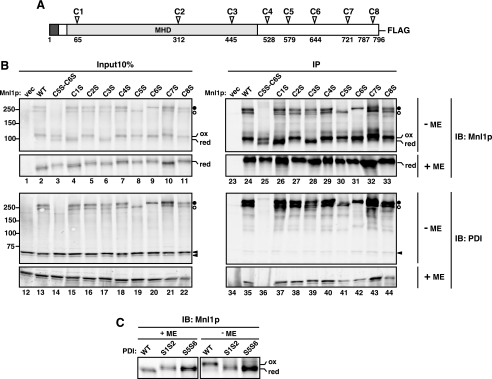
Cys → Ser mutants of Mnl1p. A, schematic representation of Mnl1p with eight Cys residues. The signal sequence and the MHD are shown in black and gray boxes, respectively. B, spheroplasts prepared from cells expressing WT Mnl1p-FLAG and a series of Cys → Ser Mnl1p-FLAG mutants and from those with a vector alone (vec) were analyzed as in Fig. 2E. The open and filled circles indicate the PDI-Mnl1p-FLAG complexes formed by the intermolecular disulfide bonds involving C6 or C5, respectively. Reduced and oxidized forms of the Mnl1p-FLAG monomer are indicated as red and ox, respectively. C, proteins in the cell lysate prepared in the presence of iodoacetamide from WT (W303-1A mnl1ΔpdiΔ/pPDI1, pMAY5), pdi1-S1S2 (S1S2; W303-1A mnl1ΔpdiΔ/pPDI1-S1S2, pMAY5) or pdi1-S5S6 (S5S6; W303-1A mnl1ΔpdiΔ/pPDI1-S5S6, pMAY5) cells expressing Mnl1p-FLAG from a multicopy plasmid were analyzed by nonreducing SDS-PAGE and immunoblotting (IB) with anti-Mnl1p antibodies.
FIGURE 5.
Interactions between the C-terminal domain of Mnl1p and PDI without intermolecular disulfide bonds. A, sequence alignment of Mnl1p (residues 600–667) with its fungal orthologs. The accession numbers are: Vanderwaltozyma polyspora, XP_001643561; C. glabrata, XP_446361; Ashbya gossyppi, NP_983706; Kluyveromyces lactis, XP_451695; Pichia guilliermondii, XP_001484730; and Pichia stipitis, XP_001383807. Identical and similar residues are denoted with double (**) and single asterisks (*), respectively. Amino acid residues denoted with filled circles were replaced with Ala. B, spheroplasts prepared from cells expressing wild type Mnl1p-FLAG and a series of Ala substitution mutants from a multicopy plasmid and from those with a vector alone (vec) were analyzed as in Fig. 2E. The open and filled circles indicate the PDI-Mnl1p-FLAG complexes formed by the intermolecular disulfide bonds. Reduced and oxidized forms of the Mnl1p-FLAG monomer are indicated as red and ox, respectively. Mutants that do not form a disulfide-linked Mnl1p-PDI complex are highlighted by black boxes. C, membrane fractions were prepared from cells expressing wild type Mnl1p-FLAG and a series of Ala substitution mutants from a multicopy plasmid and from those with a vector alone in the presence of 10 mm DTT and were subjected to analyses as in Fig. 4. IP, immunoprecipitation; IB, immunoblotting.
Protease Protection Assay—The microsomes were incubated with or without 0.2 mg/ml proteinase K in 20 mm Hepes-KOH, pH 7.4, 100 mm KCl, 300 mm mannitol in the presence or absence of 1% Triton X-100 on ice for 30 min. The reaction was terminated by addition of 2 mm PMSF. The samples were incubated with 10% trichloroacetic acid for 10 min on ice, and the proteins were recovered by centrifugation at 15,000 × g for 5 min at 4 °C. The precipitates were washed twice with cold acetone and were solubilized by sample buffer for SDS-PAGE.
Endoglycosidase H Treatment—Microsomes were suspended in 1% SDS and 1% 2-mercaptoethanol and incubated at 94 °C for 5 min. The samples were diluted 5-fold with 50 mm sodium citrate, pH 4.5, 1% Triton X-100, 1 mm PMSF, and 10 mm pepstatin A. Endoglycosidase H (Seikagaku Corporation) was added to 10 units/ml, and the samples were incubated for 16 h at 37 °C.
Extraction of Mnl1p—The microsomes were incubated in 20 mm Hepes-KOH pH 7.4, 100 mm KCl, 300 mm mannitol, 1 mm EGTA, and 1 mm PMSF containing 0.1 m Na2CO3, pH 11.5, or 1% Triton X-100 on ice for 30 min. The samples were centrifuged at 80,000 × g for 30 min at 4 °C to separate soluble and insoluble fractions.
Cycloheximide Chase Experiments—The cells were grown to OD600 = 1.5–2, and cycloheximide was added directly to the cell culture at 0.2 mg/ml. 0, 30, 60, or 90 min after the addition of cycloheximide, an equal volume of cell culture was removed and was incubated further in the presence of 10 mm NaN3 for 10 min on ice. Cell extracts were prepared as described by Yaffe and Schatz (33).
Modification of Cys with AMS and Maleimde-PEG5000—The cells were suspended in 10% trichloroacetic acid and disrupted by agitation with glass beads. Trichloroacetic acid-precipitated proteins were collected by centrifugation at 15,000 × g for 5 min at 4 °C followed by washing twice with cold acetone. Protein pellets were suspended in 80 mm Tris-HCl, pH 6.8, 2% SDS, 6 m urea, 1 mm PMSF in the presence of 25 mm AMS (Invitrogen) or 5 mm maleimide-PEG5000 (Laysan Bio, Inc). After incubation on ice for 15 min at 37 °C for 10 min, the samples were boiled for 2 min and analyzed by SDS-PAGE under reducing conditions. To generate a fully reduced form of PDI, the trichloroacetic acid-precipitated pellets were boiled in 80 mm Tris-HCl, pH 6.8, 2% SDS, 6 m urea, 1 mm PMSF for 5 min, and the soluble fraction was incubated with 100 mm DTT at 30 °C for 30 min. Then proteins were precipitated with 10% trichloroacetic acid again and subsequently solubilized in 80 mm Tris-HCl, pH 6.8, 2% SDS, 6 m urea, 1 mm PMSF in the presence of 25 mm AMS or 5 mm maleimide-PEG5000. After incubation on ice for 15 min, at 37 °C for 10 min, the samples were boiled for 2 min and analyzed by SDS-PAGE under reducing conditions.
RESULTS
Mnl1p Is an ER Luminal Protein with a Cleavable Signal Sequence—Mnl1p was previously shown to reside in the yeast ER (9). We first asked whether the hydrophobic segment at the N terminus of Mnl1p functions as a cleavable signal sequence or a transmembrane segment to anchor the protein to the ER membrane. Immunoblotting of microsomes prepared from cells expressing FLAG-tagged Mnl1p (Mnl1p-FLAG) with the anti-FLAG antibody showed a 102-kDa band, which shifted to 91 kDa after treatment with endoglycosidase H, indicating that Mnl1p contains N-linked carbohydrate chains (Fig. 1A). Carboxypeptidase Y (CPY), which has four N-glycan sites, was used as a positive control. Mnl1p was, like an ER luminal protein BiP, resistant against externally added proteinase K but became protease-susceptible after disruption of the membrane with Triton X-100 (Fig. 1B). When treated with alkaline sodium carbonate, Mnl1p and BiP were mainly recovered in the supernatant after centrifugation, whereas Sec63p, an integral ER membrane protein, was recovered in the pellet (Fig. 1C, lanes 2 and 3). Treatment of the microsomes with Triton X-100 solubilized all of these proteins (Fig. 1C, lanes 4 and 5). These results collectively indicate that Mnl1p is an ER luminal protein, but not anchored to the ER membrane. Indeed, Mnl1p-FLAG and BiP in the yeast sec11 mutant, a temperature-sensitive mutant of the subunit of the ER signal peptidase (26), exhibited higher molecular weight bands (the bands from Mnl1p-FLAG with different extents of N-glycosylation became clearer after endonuclease H treatment) at restrictive temperature (37 °C) than at permissive temperature (23 °C) (Fig. 1D). Therefore the N-terminal hydrophobic segment of Mnl1p most likely functions as a cleavable signal sequence that guides the mature protein to the ER lumen.
Mnl1p Interacts with PDI—Because Mnl1p facilitates ERAD of glycoproteins in the ER (9, 10), we searched for its possible partner proteins cooperating with Mnl1p in the ERAD. When microsomes with Mnl1p-FLAG were solubilized with 1% Nonidet P-40 and subjected to immunoprecipitation with the immobilized anti-FLAG antibody, PDI was found to be retained on the beads in particular when Mnl1p-FLAG was expressed from a multicopy plasmid (Fig. 2, A and B). When solubilized microsomes were subjected to immunoprecipitation with the anti-PDI antibodies, Mnl1p-FLAG was in turn detected in the co-immunoprecipitated fractions (Fig. 2, C and D). Hemagglutinin-tagged Yos9p, hemagglutinin-tagged Mns1p (α1,2-mannosidase in yeast), or BiP was not detected by the anti-hemagglutinin antibody (for Yos9p and Mns1p) or anti-BiP antibodies (for BiP) in the co-immunoprecipitated fractions (data not shown).
We next asked whether the interactions between Mnl1p and PDI involve intermolecular disulfide bonds. To minimize oxidation of Mnl1p after cell lysis, the proteins were precipitated by trichloroacetic acid from the spheroplasts expressing Mnl1p-FLAG and solubilized with SDS in the presence of iodoacetamide, which can alkylate thiol groups to prevent artificial disulfide shuffling. Then Mnl1p-FLAG was immunoprecipitated with the anti-FLAG antibody and analyzed by SDS-PAGE under reducing or nonreducing conditions followed by immunoblotting with anti-Mnl1p antibodies and anti-PDI antibodies (Fig. 2E). Mnl1p exhibited two high molecular weight bands of ∼250 kDa (denoted by circles) in addition to the band of 102 kDa corresponding to the Mnl1p monomer (denoted by a triangle) under nonreducing conditions (Fig. 2E, lanes 2 and 4) but not under reducing conditions (Fig. 2E, lanes 10 and 12). The 250–300-kDa bands were also detected by anti-PDI antibodies under nonreducing conditions (Fig. 2E, lanes 6 and 8), suggesting that the 250–300-kDa bands correspond to complexes formed by intermolecular disulfide bond(s) between Mnl1p and PDI. Quantification of the pulldown results under the conditions of Mnl1p being expressed from a multicopy plasmid suggests that about half the Mnl1p molecules interact with PDI and that ∼30% of PDI with Mnl1p through disulfide bonds (data not shown).
Inter- and Intramolecular Disulfide Bond Formation of Mnl1p by PDI—Mnl1p contains eight cysteine residues in its mature domain, which are named C1–C8 (Fig. 3A); C1–C3 are in the MHD and C4–C8 in the C-terminal domain of Mnl1p. To identify the cysteine residues involved in the intermolecular disulfide bond with PDI, we made a series of Cys → Ser mutants of Mnl1p (C1S to C8S), in which each of the eight Cys was replaced by Ser, and analyzed their disulfide bond formation by nonreducing SDS-PAGE followed by immunoblotting with anti-Mnl1p antibodies and anti-PDI antibodies (Fig. 3B). The upper (filled circles) and lower (open circles) bands of the 250–300-kDa Mnl1p-PDI complexes disappeared in C5S and C6S mutant cells, respectively (Fig. 3B, lanes 30, 31, 41, and 42). Combination of the C5S and C6S mutations (C5S,C6S) resulted in the complete disappearance of the two bands (Fig. 3B, lanes 25 and 36). Therefore C5 and/or C6 of Mnl1p form intermolecular disulfide bonds with PDI, and the upper and lower bands of 250–300 kDa correspond to Mnl1p-PDI complexes with disulfide bonds involving C5 and C6, respectively.
During the analyses of the Mnl1p-PDI complex, we noted that the migration rates of the Mnl1p monomer varied for different Cys mutants under nonreducing conditions, but not under reducing conditions (Fig. 3B). The C1S and C3S mutants showed faster migration rates than the wild type (WT) Mnl1p, suggesting that the upper band corresponds to the Mnl1p monomer with fully oxidized C1 and C3 (denoted as ox) and the lower band to the one without a disulfide bond between C1 and C3 (denoted as red). The S1S2 (C61S,C64S) and S5S6 (C406S,C409S) mutants of PDI have Cys → Ser mutations in the CGHC motif in one of the two active site thioredoxin domains (see Fig. 7A) and still have disulfide forming activity (34, 35). Mnl1p in cells expressing the S1S2 or S5S6 PDI mutants showed only the lower bands for the reduced Mnl1p monomer under nonreducing conditions (Fig. 3C), suggesting that oxidation of C1 and C3 requires both of the two active thioredoxin domains in PDI. Interestingly, the C5S,C6S mutant exhibited double bands for the Mnl1p monomer (Fig. 3B, lane 25), indicating that C1 and C3 were only partially oxidized. In other words, interactions of Mnl1p with PDI through C5 and/or C6 in the C-terminal domain of Mnl1p promote efficient disulfide formation between C1 and C3 in the N-terminal MHD by PDI.
FIGURE 7.
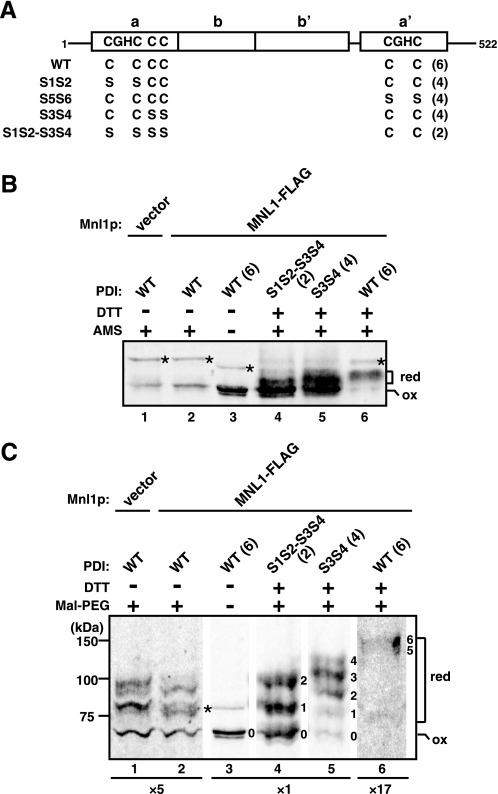
Oxidation states of PDI with and without Mnl1p. A, schematic representation of PDI and its Cys → Ser mutants. The total numbers of Cys are shown in parentheses. PDI has a tandemly arranged thioredoxin-like domain structure of a-b-b′-a′, and have four cysteines in the CGHC motifs in the two active site thioredoxin domains (Cys61, Cys64, Cys406, and Cys409) and two additional cysteines (Cys89 and Cys97). S1S2, S5S6, S3S4, and S1S2-S5S6 have Cys → Ser mutations at positions 61 and 64; positions 406 and 409; positions 89 and 97; and positions 61, 64, 89, and 97, respectively. B and C, proteins were precipitated with trichloroacetic acid from mnl1ΔpdiΔ cells expressing WT or indicated mutant of PDI (S1S2, S5S6, S3S4, and S1S2-S3S4) without (vector) or with Mnl1p-FLAG (MNL1-FLAG) and modified with AMS (B) or maleimide-PEG5000 (C) with or without pretreatment with DTT. The samples were analyzed by SDS-PAGE and immunoblotting with anti-PDI antibodies. Parts of the same gel were shown with different intensity enhancements; for lanes 7 and 8 the gels were enhanced 5-fold, and that for lane 12 was enhanced 17-fold. Asterisks indicate unrelated bands. The number on the right side of each band indicates an estimated number of modified cysteines. red, reduced form; ox, fully oxidized form. The total numbers of Cys are shown in parentheses.
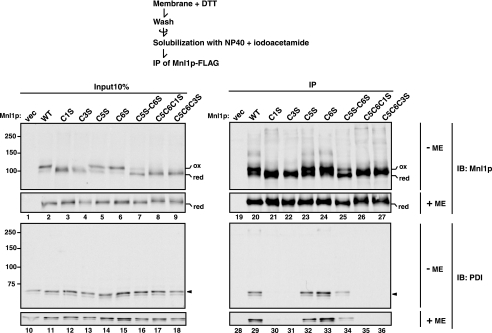
PDI Associated with Mnl1p through Noncovalent Interactions as Well as Intermolecular Disulfide Bonds—Even after formation of the disulfide bond between C1 and C3, Mnl1p appears to interact with PDI via the intermolecular disulfide bond involving C5 and/or C6. We thus asked whether the intermolecular disulfide bond is prerequisite for the prolonged interactions between Mnl1p and PDI. To address this question, we prepared membrane fractions from cells expressing Mnl1p-FLAG in the presence of 10 mm DTT because Mnl1p is enriched in the microsomal fraction. Subsequent nonreducing SDS-PAGE analyses showed that the prepared membranes did not contain the Mnl1p-PDI complex linked by a disulfide bond (Fig. 4, lane 2, –ME). Nevertheless, the observed monomeric form of Mnl1p-FLAG is still predominantly in the oxidized form, suggesting that the intramolecular disulfide bond between C1 and C3 was more resistant against reduction by DTT than the intermolecular disulfide bond between Mnl1p-FLAG and PDI. Then the DTT-treated membranes were washed with DTT-free buffer, solubilized with Nonidet P-40 in the presence of iodoacetamide, and subjected to immunoprecipitation with the anti-FLAG antibody. PDI was still co-immunoprecipitated with Mnl1p-FLAG, indicating the presence of interactions between Mnl1p and PDI independent of the intermolecular disulfide bond (Fig. 4, lane 29).
FIGURE 4.
PDI associates with Mnl1p through noncovalent interactions as well as disulfide bonds. Membrane fractions were prepared from cells expressing WT Mnl1p-FLAG and a series of Cys → Ser Mnl1p-FLAG mutants and from those with a vector alone (vec) in the presence of 10 mm DTT. The membranes were washed, solubilized with Nonidet P-40 in the absence of DTT, and subjected to immunoprecipitation with the anti-FLAG antibody. Immunoprecipitated materials (IP) and 10% of the solubilized membranes prior to immunoprecipitation (Input 10%) were analyzed by nonreducing (–ME) or reducing (+ME) SDS-PAGE and immunoblotting with antibodies against Mnl1p (IB: Mnl1p) or PDI (IB: PDI). Reduced and oxidized forms of the Mnl1p-FLAG monomer are indicated as red and ox, respectively.
Although PDI was co-precipitated with C5S and C6S Mnl1p-FLAG mutants as efficiently as the wild type protein after DTT treatment (Fig. 4, lanes 32 and 33), efficiency of PDI co-precipitation decreased for the C5S,C6S mutant, which is less efficient in formation of the intramolecular disulfide bond between C1 and C3 (Fig. 4, lane 34). Interestingly, PDI was not co-precipitated with the C1S or C3S mutants (Fig. 4, lanes 30 and 31), suggesting that the intact C1–C3 disulfide bond is essential for prolonged association of PDI with Mnl1p in the absence of C5 and C6.
Which residues in Mnl1p are involved in interactions with PDI without via the intermolecular disulfide bond? C5 and C6 of Mnl1p are present in the C-terminal domain, which is not conserved in mammalian EDEM proteins; the C-terminal domain is only found in fungal orthologs of Mnl1p and does not show any homology to known protein domains. Because several residues in the proximity of C5 and C6 are conserved among the fungal Mnl1 proteins (Fig. 5A), we made mutant Mnl1p (D607A, F610A, L614A, P617A, E627A, W636A, T650A, and E654A) with replacement of each of these residues with alanine to analyze their interactions with PDI.
First, we analyzed the effects of those mutations in the C-terminal domain of Mnl1p on formation of the intramolecular disulfide bond between C1 and C3 in the MHD and intermolecular disulfide bond involving C5 and/or C6 with PDI. Although these amino acid replacements did not abolish interactions of Mnl1p-FLAG with PDI completely (Fig. 5B, lanes 36, 41, and 42, +ME), the D607A, E627A, and W636A mutants are significantly defective in formation of the disulfide bond-mediated interactions with PDI (Fig, 5B, lanes 25, 30, 31, 36, 41, and 42, –ME) despite the presence of C5 and C6. Besides, the D607A, E627A, and W636A mutants lack ability to form the disulfide bond between C1 and C3, as reflected in accumulation of only reduced form of the Mnl1p-FLAG monomer under nonreducing conditions (Fig. 5B, lanes 25, 30, and 31, –ME; supplemental Fig. S2). Notably, the L614A mutant did not appear defective in interaction with PDI but was partially defective in introduction of the disulfide bond between C1 and C3 (Fig. 5B, lane 28, –ME).
When we analyzed the interactions of the C-terminal domain mutants of Mnl1p with PDI after DTT treatment, PDI was not co-immunoprecipitated with D607A, L614A, E627A, or W636A mutant of Mnl1p-FLAG (Fig. 5C, lanes 36, 39, 41, and 42, –ME). In the L614A mutant, which could partially form the C1–C3 disulfide bond, the C1–C3 disulfide bond was evidently more susceptible to reduction by DTT treatment than in wild type Mnl1p-FLAG (Fig. 5C, lane 28, –ME). Although some of those mutants exhibited high molecular weight bands (Fig. 5C, lanes 25, 30, and 31, –ME), they were not detected with anti-PDI antibodies, suggesting that they are aggregated forms of the mutant Mnl1p. These results collectively suggest that the conserved amino acid residues in the C-terminal domain including Asp607, Leu614, Glu627, and Trp636 are critical for stable interactions with PDI, which is essential for correct disulfide-bond formation between C1 and C3 in the MHD of Mnl1p, although it is not clear whether these residues are directly involved in the interactions with PDI or affect folding of the C-terminal domain. The intermolecular disulfide bond involving C5 and/or C6 of Mnl1p with PDI also facilitates the stable association of the C-terminal domain of Mnl1p with PDI to form the C1–C3 disulfide bond in the MHD. Once the disulfide bond between C1 and C3 is formed, it apparently contributes to prolonged association of PDI with Mnl1p as well.
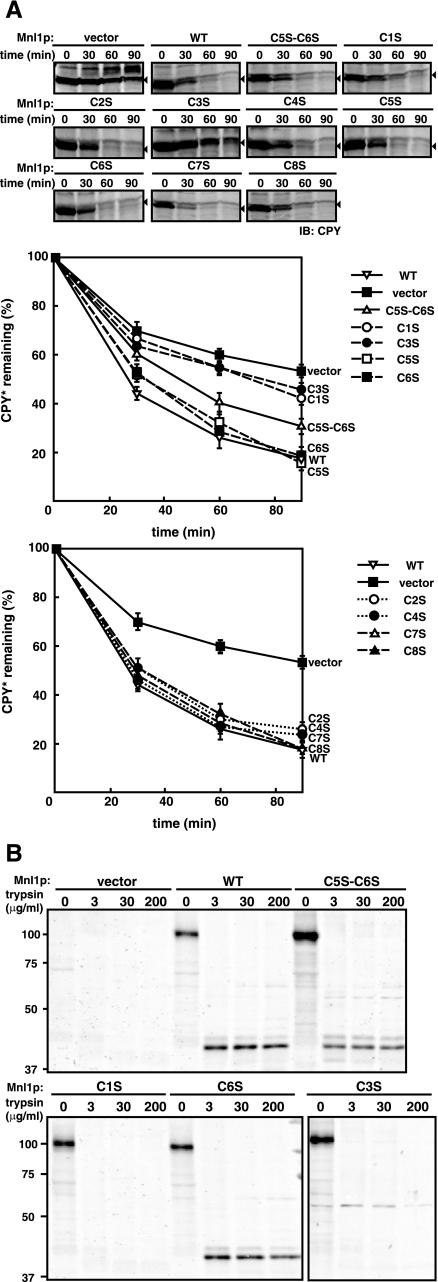
Disulfide Bond between C1 and C3 Is Important for the Mnl1p Function in ERAD—Next, we analyzed the roles of the disulfide bonds in Mnl1p in the ERAD. Mnl1p is required for the ERAD of misfolded glycoproteins including CPY*, a mutant of carboxypeptidase Y (9, 10). When protein synthesis was blocked with cycloheximide, the remaining CPY* was degraded in wild type cells with a half-life of 30 min (Fig. 6A, WT), whereas in the mnl1Δ mutant lacking Mnl1p, CPY* was stabilized and degraded with a half-life of 90 min (Fig. 6A, vector), as observed previously (9). However, expression of the C1S or C3S mutant in the mnl1Δ mutant did not restore the ERAD activity. In contrast, expression of the other single Cys → Ser substitution mutants fully suppressed the ERAD defects of the mnl1Δ mutant. When the C5S,C6S double mutant, in which the C1–C3 disulfide bond was only partially formed, was expressed, the ERAD defects of the mnl1Δ mutant was partially restored. These results indicate that the intramolecular disulfide bond formation between C1 and C3 in the MHD is essential for the ERAD activity of Mnl1p.
FIGURE 6.
Roles of the C1–C3 disulfide bond in the ERAD activity of Mnl1p. A, the prc1-1 mutant cells expressing WT Mnl1p-FLAG and a series of Cys → Ser Mnl1p-FLAG mutants and from those with a vector alone were grown to early log phase. Cycloheximide was added, and the cells were further incubated for indicated times. Then the cell extracts were prepared, and the proteins were analyzed by SDS-PAGE and immunoblotting with anti-CPY antibodies to follow the fate of CPY*. The arrowheads indicate CPY*, and other bands are all nonspecific bands. The amount of CPY* present at 0 min was set to 100%. B, trypsin digestion of WT Mnl1p-FLAG and the Cys → Ser Mnl1p-FLAG mutants. The membrane fractions prepared from cells expressing WT Mnl1p-FLAG and the C5S,C6S, C1S and C6S mutants, and from those with a vector alone were solubilized with 2% Triton X-100 and incubated with indicated concentrations of trypsin on ice for 30 min. The reaction was stopped by addition of 1 mm PMSF. Proteins were trichloroacetic acid-precipitated and analyzed by SDS-PAGE and immunoblotting with anti-Mnl1p antibodies.
What is the role of the disulfide bond between C1 and C3 of Mnl1p in its ERAD activity? We assessed the structure of the MHD in Mnl1p by trypsin digestion. Briefly, membrane fractions prepared from cells expressing wild type or mutant Mnl1p were solubilized with Triton X-100 and subjected to incubation with various concentrations of trypsin (Fig. 6B). Although wild type and C6S Mnl1p generated 70% of trypsin-resistant 41-kDa fragments, after treatment with 3 μg/ml trypsin, C5S,C6S mutant with partially oxidized C1 and C3 formed only 18% of the trypsin-resistant fragment, and the C1S and C3S mutants were completely susceptible to 3 μg/ml trypsin. Therefore, the disulfide bond between C1 and C3 is essential for the stably folded structure of the MHD, which appears important for the ERAD activity of Mnl1p.
Our findings that the intact C1–C3 disulfide bond contributes to prolonged association of PDI with Mnl1p suggest the possibility that associated PDI may also further facilitate the ERAD involving Mnl1p. In mammalian cells, EDEM recruits a reductase ERdj5, which was proposed to unfold misfolded proteins for their efficient retrotranslocation to the cytosol for degradation (36). However, it is not clear whether PDI in yeast can take the role of ERdj5 in the ERAD because whether PDI with six cysteines (Fig. 7A) can function as a reductase as well as an oxidase in the ER is in dispute (5, 6). We thus first tried to assess the oxidation state of PDI in yeast cells by treatment of yeast cells with trichloroacetic acid and subsequently with the thiol-conjugating reagent AMS in the presence of denaturants. We confirmed the previous findings (37) that PDI resisted modification by AMS, suggesting that PDI was mainly in the oxidized state (Fig. 7B, lanes 1 and 2). However, comparison of the AMS-modified forms of WT PDI with six cysteines, S1S2-S3S4 mutant with two cysteines, and S3S4 mutants with four cysteines indicates that the resolution of SDS-PAGE may not be high enough to discriminate partially modified forms (Fig. 7B). We thus used another thiol-conjugating reagent maleimide-PEG5000 instead of AMS (38) to observe partially thiol-modified forms with larger mobility difference. After DTT treatment followed by modification with maleimide-PEG5000, the S1S2-S3S4 and S3S4 mutants, which cannot interact with Mnl1p, exhibited three major bands likely representing the forms with no, one, and two modified cysteines (the S1S2-S3S4 mutant) and two, three, and four modified cysteines (the S3S4 mutant) (Fig. 7C, lanes 4 and 5), although the migration rates for the Cys-modified species were faster for the S3S4 mutant than the S1S2-S3S4 mutant for unknown reasons. Now we could observe substantial amounts of Cys-modified species for WT PDI (Fig. 7C, lanes 1 and 2), probably with two modified Cys, irrespective of the presence of Mnl1p. These results indicate that a part of PDI is in a partially reduced state, which may have escaped detection in the previous analyses using AMS, in cells.
DISCUSSION
In the present study, we found that the disulfide bond between C1 and C3 in the MHD of Mnl1p is essential for the ERAD activity, and its formation requires PDI in the ER lumen, which is stably associated with Mnl1p. The stable association of PDI with Mnl1p requires several conserved residues in the C-terminal domain of Mnl1p and is facilitated further by the intermolecular disulfide bonds involving either C5 or C6 in the C-terminal domain of Mnl1p. Indeed, Mnl1p mutant lacking the entire C-terminal domain failed to form a disulfide bond between C1 and C3 as expected (supplemental Fig. S3). Intact PDI, which has both catalytic and putative peptide-binding domains, is in turn required for its stable association with Mnl1p (supplemental Fig. S4). The C1–C3 disulfide bond in the MHD, once formed, stabilizes the prolonged association of PDI with Mnl1p. During preparation of the present manuscript, Clerc et al. (15) reported that Mnl1p physically interacts with PDI through its C-terminal domain, which agrees well with our findings.
Fig. 8 shows our current model of disulfide bond formation of Mnl1p by PDI; PDI first recognizes the C-terminal domain of Mnl1p containing Asp607, Glu627, and Trp636 (Fig. 8, step 1 to step 2); PDI forms an intermolecular disulfide bond with C5 or C6 of Mnl1p (Fig. 8, step 2 to step 3); PDI introduces a disulfide bond between C1 and C3 in the MHD of Mnl1p (Fig. 8, step 3 to step 4); the disulfide bond between C1 and C3 in turn stabilizes association of PDI with Mnl1p; and the intermolecular disulfide bond between PDI and C5 or C6 of Mnl1p is partially reduced, whereas maintaining association of PDI and Mnl1p (Fig. 8, step 4 to step 5). The reason for the requirement of PDI bound to the C-terminal domain of Mnl1p for introduction of the disulfide bond in the MHD is not clear. The C-terminal domain of Mnl1p may provide a scaffold for PDI to oxidize C1 and C3 efficiently with appropriate geometry of PDI relative to the MHD. Alternatively, correct folding of the MHD, which is a prerequisite for the correct disulfide bond formation between C1 and C3, may require the chaperone activity of PDI in the proximity. Evidently, because formation of the disulfide bond between C1 and C3 in the N-terminal MHD requires the presence of the C-terminal domain (supplemental Fig. S3), the native state of Mnl1p with a correct set of intermolecular disulfide bonds can be achieved post-translocationally.
FIGURE 8.
Model for disulfide-bond formation in Mnl1p by PDI.
What is the role of the C1–C3 disulfide bond in the MHD of Mnl1p in the ERAD? Both C1 and C3 are conserved between Mnl1p and mammalian EDEM, whereas C3, but not C1, of Mnl1p is conserved in Mns1p, a yeast α1,2-mannosidase (supplemental Fig. S1). The C1–C3 disulfide bridge is essential for the functional folded structure of the MHD (Fig. 6B), which is consistent with its resistance against reduction by externally added DTT (Fig. 4A). The folded structure aided by the C1–C3 disulfide bond thus appears essential for such Mnl1p functions as recognition and/or cleavage of the carbohydrate chains of the ERAD substrates. It is to be noted that, although mammalian EDEM proteins contain conserved C1 and C3 in the MHD, they lack the C-terminal domain found in Mnl1p and its fungal homologs (supplemental Fig. S1). Besides, mammalian EDEM proteins possess additional cysteines that are not conserved in Mnl1p. Therefore the mechanisms of formation of the possible C1–C3 disulfide in EDEM proteins may be considerably different from that for Mnl1p revealed here.
What is the role of PDI stably associated with Mnl1p even after formation of the C1–C3 disulfide bond in the ERAD? The intermolecular disulfide bond between Mnl1p and PDI appears stable at least for 60 min in the cycloheximide chase experiments (supplemental Fig. S5), suggesting that the Mnl1p-PDI complex does not merely represent a folding and/or disulfide bond forming intermediate of Mnl1p. Instead, the disulfide bond between C1 and C3 appears to stabilize the noncovalent interactions between PDI and Mnl1p (Fig. 4A, lanes 30 and 31), thereby stabilizing the mixed disulfide between PDI and Mnl1p (supplemental Fig. S5). In mammalian cells, PDI functions as a subunit of triglyceride transfer protein complex and of prolyl 4-hydroxylase complex (39), and a member of the PDI family, ERp57, is stably associated with tapasin via interactions including a intermolecular disulfide bond for assembly of major histocompatibility complex class I molecules (40). Therefore, yeast PDI may perform a specific function when being in a complex with Mnl1p. In this sense, it would be interesting to note that retrotranslocation of disulfide-containing proteins from the ER to the cytosol for degradation appears redox-potential dependent and requires reduction of disulfide bonds before export. Indeed, ERdj5 in mammals was found to cleave disulfide bonds of misfolded proteins, thereby accelerating their ERAD processes (36), and PDI in yeast was suggested to play a similar role (22). PDI stably associated with Mnl1p may thus perhaps facilitate the ERAD by reducing disulfide bonds in misfolded substrate proteins that were recognized or processed by Mnl1p. Our observation that PDI is not only in the oxidized state but also in the reduced form fits well with the role of PDI as a reductase for the ERAD substrate captured by Mnl1p. Alternatively, PDI associated with Mnl1p may function as a chaperone to maintain solubility of the ERAD substrates, which is reminiscent of the role of BiP in yeast (19) and calnexin interacting with EDEM in mammalian cells (41).
Supplementary Material
Acknowledgments
We thank Kenji Inaba, Jun Hoseki, Kunio Nakatsukasa, and the members of the Endo lab for helpful discussions; Karin Römisch for pdi1Δ strain; William J. Lennarz for plasmids for the expression of PDI; Satoshi Harashima for the TRP1 gene of C. glabrata; and Hiroyuki Tachikawa and Mami Oi for anti-PDI antibodies.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S5.
Footnotes
The abbreviations used are: ER, endoplasmic reticulum; ERAD, ER-associated degradation; MHD, mannosidase homology domain; EDEM, ER degradation enhancing α-mannosidase-like protein; DTT, dithiothreitol; CPY, caboxypeptidase Y; CPY*, a mutated version of caboxypeptidase Y; PMSF, phenylmethylsulfonyl fluoride; AMS, 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid; PDI, protein disulfide isomerase; WT, wild type.
References
- 1.Buck, T. M., Wright, C. M., and Brodsk, J. L. (2007) Semin. Cell Dev. Biol. 18 751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anelli, T., and Sitia, R. (2008) EMBO J. 27 315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creighton, T. E., Zapun, A., and Darby, N. J. (1995) Trends. Biotech. 13 18–23 [DOI] [PubMed] [Google Scholar]
- 4.Molinari, M., and Helenius, A. (1999) Nature 402 90–93 [DOI] [PubMed] [Google Scholar]
- 5.Sevier, C. S., and Kaiser, C. A. (2006) Antioxid. Redox. Signal 8 797–811 [DOI] [PubMed] [Google Scholar]
- 6.Appenzeller-Herzog, C., and Ellgaard, L. (2008) Biochim. Biophys. Acta 1783 535–548 [DOI] [PubMed] [Google Scholar]
- 7.Nakatsukasa, K., and Brodsky, J. L. (2008) Traffic 9 861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakob, C. A., Burda, P., Roth, J., and Aebi, M. (1998) J. Cell Biol. 142 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakatsukasa, K., Nishikawa, S., Hosokawa, N., Nagata, K., and Endo, T. (2001) J. Biol. Chem. 276 8635–8638 [DOI] [PubMed] [Google Scholar]
- 10.Jakob, C. A., Bodmer, D., Spirig, U., Battig, P., Marcil, A., Dignard, D., Bergeron, J. J., Thomas, D. Y., and Aebi, M. (2001) EMBO Rep. 2 423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosokawa, N., Wada, I., Hasegawa, K., Yorihuzi, T., Tremblay, L. O., Herscovics, A., and Nagata, K. (2001) EMBO Rep. 2 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirao, K., Natsuka, Y., Tamura, T., Wada, I., Morito, D., Natsuka, S., Romero, P., Sleno, B., Tremblay, L. O., Herscovics, A., Nagata, K., and Hosokawa, N. (2006) J. Biol. Chem. 281 9650–9658 [DOI] [PubMed] [Google Scholar]
- 13.Olivari, S., Cali, T., Salo, K. E., Paganetti, P., Ruddock, L. W., and Molinari, M. (2006) Biochem. Biophys. Res. Commun. 349 1278–1284 [DOI] [PubMed] [Google Scholar]
- 14.Quan, E. M., Kamiya, Y., Kamiya, D., Denic, V., Weibezahn, J., Kato, K., and Weissman, J. S. (2008) Mol. Cell 32 870–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clerc, S., Hirsch, C., Oggier, D. M., Deprex, P., Jakob, C., Sommer, T., and Aebi, M. (2009) J. Cell Biol. 184 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhamidipati, A., Denic, V., Quan, E. M., and Weissman, J. S. (2005) Mol. Cell 16 741–751 [DOI] [PubMed] [Google Scholar]
- 17.Kim, W., E. D. Spear, and D. T. Ng. (2005) Mol. Cell 16 753–764 [DOI] [PubMed] [Google Scholar]
- 18.Szathmary, R., Bielmann, R., Nita-Lazar, M., Burda, P., and Jakob, C. A. (2005) Mol. Cell 16 765–775 [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa, S., Fewell, S. W., Kato, Y., Brodsky, J. L., and Endo, T. (2001) J. Cell Biol. 153 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert, H. F. (1997) J. Biol. Chem. 272 29399–29402 [DOI] [PubMed] [Google Scholar]
- 21.Klappa, P., Hawkins, H. C., and Freedman, R. B. (1997) Eur. J. Biochem. 238 38–42 [DOI] [PubMed] [Google Scholar]
- 22.Gillece, P., Luz, J. M., Lennarz, W., de la Cruz, F. J., and Römisch. K. (1999) J. Cell Biol. 147 1443–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nørgaard, R., Westphal, V., Tachibana, C., Alsøe, L., Horst, B., and Winther, J. R. (2001) J. Cell Biol. 152 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson, J. S., Klionsky, D. J., Banta, L. M., and Emr, S. D. (1988) Mol. Cell Biol. 8 4936–4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishikawa, S., and Endo, T. (1997) J. Biol. Chem. 272 12889–12892 [DOI] [PubMed] [Google Scholar]
- 26.Böhni, P. C., Deshaies, R. J., and Schekman, R. W. (1988) J. Cell Biol. 106 1035–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaMantia, M. L., and Lennarz, W. J. (1993) Cell 74 899–908 [DOI] [PubMed] [Google Scholar]
- 28.Kitada, K., Yamaguchi, E., and Arisawa, M. (1995) Gene (Amst.) 165 203–206 [DOI] [PubMed] [Google Scholar]
- 29.Sikorski, R. S., and Hieter, P. (1989) Genetics 122 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshihisa, T., Yunoki-Esaki, K., Ohshima, C., Tanaka, N., and Endo, T. (2003) Mol. Biol. Cell 14 3266–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qadota, H., Ishii, I., Fujiyama, A., Ohya, Y., and Anraku, Y. (1992) Yeast 8 735–741 [DOI] [PubMed] [Google Scholar]
- 32.McCracken, A. A., and Brodsky, J. L. (1996) J. Cell Biol. 132 291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaffe, M. P., and Schatz, G. (198) Proc. Natl. Acad. Sci. U. S. A. 81 4819–4823 [DOI] [PMC free article] [PubMed]
- 34.Holst, B., Tachibana, C., and Winther, J. R. (1997) J. Cell Biol. 138 1229–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulp, M. S., Frickel, E. M., Ellgaard, L., and Weissman, J. S. (2006) J. Biol. Chem. 281 876–884 [DOI] [PubMed] [Google Scholar]
- 36.Ushioda, R., Hoseki, J., Araki, K., Jansen, G., Thomas, D. Y., and Nagata, K. (2008) Science 321 569–572 [DOI] [PubMed] [Google Scholar]
- 37.Frand, A. R., and Kaiser, C. A. (1999) Mol. Cell 4 469–477 [DOI] [PubMed] [Google Scholar]
- 38.Tsai, B., and Rapoport, T. A. (2002) J. Cell Biol. 159 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koivu, J., Myllyla, R., Helaakoski, T., Pihlajaniemi, T., Tasanen, K., and Kivirikko, K. I. (1987) J. Biol. Chem. 262 6447–6449 [PubMed] [Google Scholar]
- 40.Wearsch, P. A., and Cresswell, P. (2008) Curr. Opin. Cell Biol. 20 624–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oda, Y., Hosokawa, N., Wada, I., and Nagata, K. (2003) Science 299 1394–1397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.