Abstract
Glutathione peroxidase-1 (GPx-1) is a selenocysteine-containing enzyme that plays a major role in the reductive detoxification of peroxides in cells. In permanently transfected cells with approximate 2-fold overexpression of GPx-1, we found that intracellular accumulation of oxidants in response to exogenous hydrogen peroxide was diminished, as was epidermal growth factor receptor (EGFR)-mediated Akt activation in response to hydrogen peroxide or EGF stimulation. Knockdown of GPx-1 augmented EGFR-mediated Akt activation, whereas overexpression of catalase decreased Akt activation, suggesting that EGFR signaling is regulated by redox mechanisms. To determine whether mitochondrial oxidants played a role in these processes, cells were pretreated with a mitochondrial uncoupler prior to EGF stimulation. Inhibition of mitochondrial function attenuated EGF-mediated activation of Akt in control cells but had no additional effect in GPx-1-overexpressing cells, suggesting that GPx-1 overexpression decreased EGFR signaling by decreasing mitochondrial oxidants. Consistent with this finding, GPx-1 overexpression decreased global protein disulfide bond formation, which is dependent on mitochondrially produced oxidants. GPx-1 overexpression, in permanently transfected or adenovirus-treated cells, also caused overall mitochondrial dysfunction with a decrease in mitochondrial potential and a decrease in ATP production. GPx-1 overexpression also decreased EGF- and serum-mediated [3H]thymidine incorporation, indicating that alterations in GPx-1 can attenuate cell proliferation. Taken together, these data suggest that GPx-1 can modulate redox-dependent cellular responses by regulating mitochondrial function.
Accumulation of reactive oxygen species (ROS),2 such as superoxide anion and hydrogen peroxide, is thought to contribute to cellular damage, apoptosis, and cell death (1–3); however, ROS production is part of normal cellular metabolism, and evidence is accumulating that hydrogen peroxide, in particular, may function as a signaling molecule necessary for cell growth and survival (4–8). Superoxide is generated as a byproduct of mitochondrial respiration and by cellular redox enzymes, such as NADPH oxidase, that are stimulated through receptor-mediated mechanisms (9). Hydrogen peroxide is formed from the dismutation of superoxide, which occurs spontaneously or can be catalyzed by superoxide dismutase (10) or, alternatively, is produced by the two-electron enzymatic reduction of molecular oxygen by various oxidases, such as xanthine oxidase (11). Recent studies also suggest that hydrogen peroxide may be directly generated by receptor-ligand interactions (12). One mechanism by which hydrogen peroxide may modulate signal transduction is through the reversible oxidation of proteins at redox-active cysteines, including, for example, thiols in tyrosine kinase phosphatases. Oxidation and inactivation of phosphatases, such as PTEN, have been shown to promote the activity of the pro-growth and -survival kinase, Akt (13).
Antioxidant enzymes, such as glutathione peroxidase, catalase, and peroxiredoxins, serve to eliminate hydrogen peroxide, thereby regulating cellular responses to this endogenous oxidant. GPx-1 is a selenoprotein and one of a family of peroxidases that reductively inactivate peroxides using glutathione as a source of reducing equivalents (14, 15). GPx-1, in particular, is a major intracellular antioxidant enzyme that is found in the cytoplasm and mitochondria of all cell types. In cell culture models as well as in genetic mouse models, GPx-1 overexpression is associated with enhanced protection against oxidative stress (16–19); however, GPx-1-overexpressing mice can become obese and insulin-resistant, and have attenuated insulin-mediated activation of Akt (20). Thus, to study how GPx-1 modulates the effects of cellular oxidants on cell signaling and cell growth, we analyzed cellular responses to hydrogen peroxide and EGF in permanently transfected cells overexpressing GPx-1.
EXPERIMENTAL PROCEDURES
Chemicals and Antibodies—All of the chemicals used were purchased from Sigma, and cell culture reagents were from Invitrogen unless otherwise indicated. The GPx-1 antibody was obtained from MBL, and anti-actin antibody was from Sigma. All other primary antibodies, the secondary anti-rabbit antibody conjugated to horseradish peroxidase, and U0126, a mitogen-activated protein kinase/ERK kinase (MEK) 1/2 inhibitor, were purchased from Cell Signaling Technology (Danvers, MA). Anti-mouse horseradish peroxidase was acquired from Sigma. AG1478, an EGFR inhibitor, was obtained from BioSource International, Inc. (Camarillo, CA).
Cell Culture and Permanent Transfections—Chang liver cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 4.5 g/liter glucose supplemented with 10 ng/ml sodium selenite and 10% fetal calf serum (FCS). Permanently transfected cells were passaged in the presence of G418 at 260 μg/ml. The human GPx-1 cDNA (accession number M83094) was subcloned from the pHIHG GPx-1 construct (16) and inserted at NotI and HindIII sites in the pcDNA3.1(–) vector (Invitrogen), which contains a cytomegalovirus promoter for target gene expression and a neomycin resistance gene for selection. The cells were transfected with the resulting GPx-1 cDNA construct or the control vector, pcDNA3.1 CAT, selected with G418, and grown as clonal cell lines. G418 was excluded from the medium when the cells were seeded on plates for all experiments, for a period of 2–5 days. The cells were cultured for 2 h in medium without FCS prior to hydrogen peroxide or EGF treatment.
GPx-1 Enzyme Assay—The lysates were prepared by sonication of cells in 50 mm Tris, pH 7.5, 5 mm EDTA, and 1 mm dithiothreitol. Enzyme activity was measured in a coupled reaction with glutathione reductase, based on published methods (21). The assay was performed in microtiter plates in 50 mm Tris, pH 7.5, 5 mm EDTA, 1 mm GSH, 0.2 mm NADPH, 0.5 unit/ml glutathione reductase, and 0.002% tert-butyl hydroperoxide. In the assay, GSH is oxidized to GSSG by the GPx-1-mediated reduction of tert-butyl peroxide; NADPH is oxidized, in turn, by the glutathione reductase-mediated reduction of GSSG, resulting in measurable rates of decrease in absorbance of the adenine nucleotide at 340 nm.
Accumulation of Intracellular ROS—Confluent cells in 96-well plates were washed with Dulbecco's phosphate-buffered saline and then loaded with 5-(and 6-)-carboxy 2′-7′dichlorohydrofluorescein diacetate (DCF) at 0.07 mg/ml for 1 h at 37 °C in the dark. Excess DCF was removed by additional phosphate-buffered saline washes. The cells were then treated with 500 μm hydrogen peroxide, and oxidation of DCF to its fluorescent derivative was monitored in a SpectraMax fluorescent plate reader at an excitation wavelength of 485 nm and an emission wavelength of 518 nm. Accumulation of fluorescence (as a percentage of the initial reading) was determined by subtracting the initial fluorescence measurements for each well from each timed measurement, followed by dividing the difference by the initial fluorescence reading.
Thymidine Incorporation—Incorporation of thymidine ([3H]methyl) was accomplished using previously published methods (22) with some modifications in the incubation conditions, as described below. Briefly, the cells were seeded at various densities (10,000–40,000/well) in 24-well plates. After culturing cells for 24 h with DMEM plus 0.1% bovine serum albumin (BSA), the cells were additionally grown for 16 h in the presence of DMEM plus 0.1% BSA, with no additives or with the addition of 10% FCS or 20 ng/ml EGF. Thymidine ([3H]methyl) at 1 μCi/ml (70–90Ci/mmol specific activity) was added to cultures for the final 6 h of incubation. In a separate set of experiments, the cells were maintained in DMEM plus 0.1% BSA for up to 96 h with an addition of 1 μCi/ml [3H]thymidine 1.5 h before each harvest at 48, 72, and 96 h. The cells were harvested as described (22), and thymidine incorporation was determined by scintillation counting.
Western Blotting—After treatments, the cells were washed twice in ice-cold phosphate-buffered saline and incubated on ice for 5 min in lysis buffer containing 20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 1 mm sodium vanadate, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate plus protease inhibitors (100 nm 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride, 80 nm aprotinin, 5 μm bestatin, 1.5 μm E-64, 3.0 μm leupeptin, 1.0 μm pepstatin), and 50 mm N-ethylmaleimide to block free thiols. The cell extracts were collected, briefly sonicated, and cleared by centrifugation at 13,000 × g at 4 °C for 10 min. The proteins were separated by polyacrylamide gel electrophoresis on reducing or nonreducing SDS-PAGE gels and transferred to nitrocellulose membranes (Hybond; Amersham Biosciences). Immunodetection was completed using horseradish peroxidase-conjugated secondary antibodies and ECL (Amersham Biosciences).
Detection of Cellular Disulfide-containing Proteins—Disulfide-containing proteins were imaged according to published methods (23). Briefly, free cysteines were blocked in methanolfixed cells with 200 mm iodoacetamide for 1 h at 37 °C, followed by reduction of disulfide bonds with 1 mm tris(2-carboxyethyl) phosphine, pH 8.3, and subsequent incubation in 1 mm iodoacetamidofluorescein to label previously oxidized thiols. The cells were visualized with a Nikon fluorescence TE 2000 microscope.
Mitochondrial Potential and ATP Measurements—JC-1 dye (Molecular Probes™, Invitrogen) was used to measure mitochondrial inner membrane potential (ΔΨm) in cells grown in 96-well plates. JC-1 accumulates in the mitochondria in proportion to ΔΨm, forming aggregates that fluoresce red. In the cytoplasm, JC-1 exists as monomers that fluoresce green. The ratio of red-to-green fluorescence is proportional to ΔΨm. Red fluorescence (excitation, 550 nm; emission, 600 nm) and green fluorescence (excitation, 485 nm; emission, 535 nm) were measured using a SpectraMax Gemini microplate fluorimeter following a 2-h incubation with 2 μm JC-1. ATP was measured using the luminescence setting of the SpectraMax Gemini microplate reader to quantitate light emitted from luciferase in the presence of cellular lysates or standard ATP samples. The ATP levels were normalized to protein concentrations.
siRNA Transfection and Adenovirus Infection—siGPx-1 and control scrambled siRNA sequences detailed in Ref. 19 were transfected into Chang liver cells using Lipofectamine 2000 (Invitrogen) as in Ref. 19. MitoCatalase (MitoCAT) and catalase adenoviruses were obtained from David Pimental (Boston University School of Medicine) and used as described (23). GPx-1 adenovirus containing a native GPx-1 cDNA was prepared in our laboratory using standard procedures described in Ref. 24 except that the PacI/SphI restriction fragment overlapping the GPx-1 cDNA and adenovirus homology regions of pHIH-GPx-1 was used for recombination.
RESULTS
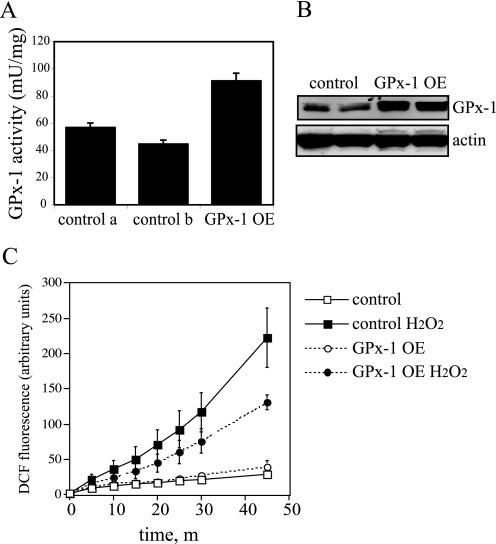
Overexpression of GPx-1 Decreases ROS Accumulation—GPx-1 overexpression in permanently transfected Chang liver cells was maximal in the presence of selenium, resulting in a 1.6–2.0-fold increase in enzyme activity (p < 0.0005) and a 2.4-fold increase in immunodetectable protein (p < 0.001) with cells grown in medium supplemented with 10 ng/ml sodium selenite (Fig. 1, A and B). Because GPx-1 is a vital intracellular antioxidant protein, we determined whether this level of overexpression could lower intracellular ROS levels as measured by DCF accumulation. GPx-1 overexpression was effective in decreasing DCF fluorescence in response to exogenous hydrogen peroxide with a 42% decrease in the maximal accumulated ROS (p < 0.01); however, basal levels of DCF fluorescence were unchanged (over time) between control and GPx-1-overexpressing cells (Fig. 1C).
FIGURE 1.
Overexpression of GPx-1 and ROS accumulation. Transfected cells were maintained in medium with 10 ng/ml sodium selenite. A, GPx-1 enzyme activity was determined following growth in 100-mm dishes. The data show the mean activities plus standard error from three independent assays. GPx-1 activity is 1.6-fold higher in GPx-1 transfectants than control a and 2.0-fold higher than control b (p < 0.0005, by analysis of variance). control a and control b represent two vector transfected control lines; activity in these lines is not significantly different between these two lines (or with each control line compared with nontransfected cells; data not shown). B, Western blot. Proteins (50 μg) were separated on 12% SDS-PAGE gels and transferred to HyBond membrane. A monoclonal antibody to human GPx-1 (MBL) was used at 1:100 dilution to detect GPx-1 protein, and an anti-actin polyclonal antibody was used at 1:4000 dilution to detect actin. C, DCF fluorescence. The cells were grown in 96-well plates to confluence and loaded with DCF prior to treatment with 500 μm hydrogen peroxide. Fluorescent DCF accumulation was monitored using an excitation wavelength of 485 nm and recording emission at 518 nm. Shown is an average of four experiments ± S.E. At 45 min, GPx-1 overexpressing cells showed a 42% reduction in intracellular ROS accumulation (p < 0.01).
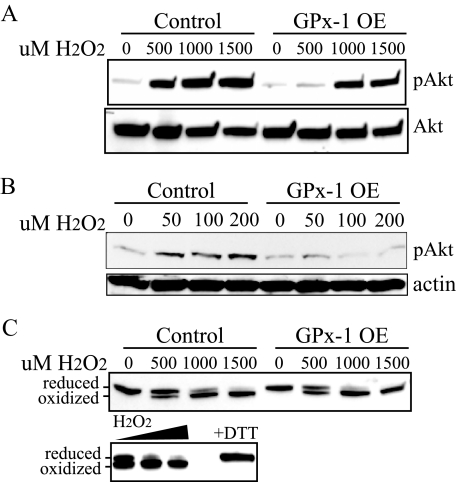
Akt Activation Is Suppressed by GPx-1 Overexpression via an EGFR-mediated Mechanism—To determine whether the enhanced detoxification of hydrogen peroxide altered the cellular signaling response to oxidants, Akt activation was monitored in cells treated with hydrogen peroxide. Akt phosphorylation has been associated with pro-survival and pro-growth signaling. Akt activation (phosphorylation at Ser473; pAkt) in response to 500 μm hydrogen peroxide treatment was attenuated by GPx-1 overexpression, with twice as much hydrogen peroxide necessary to detect Akt phosphorylation in GPx-1-overexpressing cells as in control cells (Fig. 2A). Lower doses of hydrogen peroxide (50–200 μm) also caused detectable Akt activation in control cells but had little effect on Akt phosphorylation in GPx-1-overexpressing cells (Fig. 2B).
FIGURE 2.
Akt activation by hydrogen peroxide and PTEN oxidation. A, cells were grown in 100-mm dishes to near confluence and treated with various concentrations (from 500–1500 μm) of hydrogen peroxide in DMEM with no FCS for 15 min. B, cells were treated with 50–200 μm hydrogen peroxide. The detection of phospho-Akt (pAkt), Akt, or actin was determined by Western blotting. C, cells were treated as in A, extracts were isolated in the presence of N-ethylmaleimide to block free cysteines, and samples were separated on 10% SDS-PAGE gels under nonreducing conditions. The blots were probed for PTEN. Lower panel, increasing amounts of hydrogen peroxide (500–1500 μm) lead to increasing amounts of oxidized PTEN, and GPx-1 overexpression (GPx-1 OE) did not affect this oxidation. The addition of dithiothreitol reduces the oxidized form to the reduced form. GPx-1 OE.
Akt activation in response to hydrogen peroxide has been shown to be partially regulated by oxidative inactivation of phosphatases, such as the PTEN phosphatase, which normally attenuates phosphatidylinositol 3-kinase-mediated activation of Akt (25–27). To determine whether the ability of GPx-1 to decrease intracellular oxidant accumulation maintains PTEN in the active (reduced) state, the cells were treated with hydrogen peroxide, extracts were isolated in the presence of N-ethylmaleimide to block free cysteines and prevent oxidation during sample processing, and the redox state of PTEN was assessed by electrophoresis on nonreducing gels. Under these conditions, the reduced form of PTEN can be separated from the more quickly migrating oxidized form (25). Under basal conditions, PTEN primarily existed in the reduced form, whereas hydrogen peroxide, in a dose-dependent manner, converted PTEN to the oxidized form (Fig. 2C). PTEN oxidation is reversible because the addition of dithiothreitol to samples prior to electrophoresis converts PTEN to the reduced form. There was no detectable difference in the sensitivity of PTEN to hydrogen peroxide-mediated oxidation in cells overexpressing GPx-1, even when lower doses of hydrogen peroxide were used (data not shown). Thus, the antioxidant effects of GPx-1 do not alter sensitivity of PTEN to oxidation in these cells, indicating that alterations in PTEN redox state do not contribute to the lack of Akt activation in GPx-1-overexpressing cells.
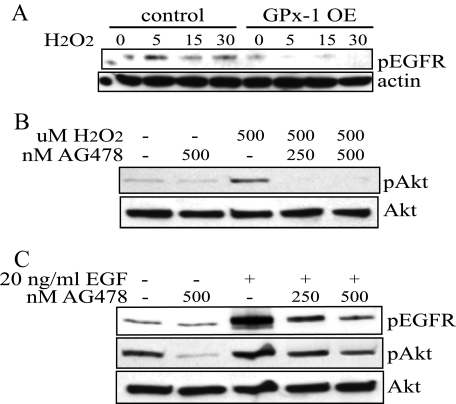
Transactivation of EGFR has also been shown to play a role in the hydrogen peroxide activation of Akt (6). To determine a possible role of EGFR in GPx-1-overexpressing cells, the cells were stimulated with 500 μm hydrogen peroxide, and EGFR phosphorylation (pEGFR) was monitored at 5, 15, and 30 min. Stimulation occurred rapidly in the control cells but was nearly undetectable in the GPx-1-overexpressing cells (Fig. 3A). We found that transactivation of EGFR is difficult to detect at lower doses of hydrogen peroxide, possibly because of the endogenous antioxidant activity in cells that modulates this response (data not shown). To confirm that EGFR was necessary for downstream activation of Akt, control cells were preincubated with AG478, an EGFR inhibitor, for 30 min prior to hydrogen peroxide stimulation (Fig. 3B). AG478 pretreatment blocked hydrogen peroxide-mediated activation of Akt in control cells, supporting the role of EGFR in this pathway. To confirm that AG478 would block EGFR signaling, control cells were stimulated with 20 ng/ml EGF in the presence and absence of AG478. The EGFR inhibitor showed a dose-dependent attenuation of EGF-stimulated pEGFR and downstream activation of Akt (Fig. 3C).
FIGURE 3.
Akt activation and EGFR transactivation. A, cells were grown in 100 mm dishes to near confluence and treated with 500 μm hydrogen peroxide in DMEM with no FCS for 5, 15, or 30 min. Extracts were separated on SDS-PAGE and transferred to Hybond membranes. The membranes were probed with an anti-phospho-EGFR antibody specific for phosphorylation at tyrosine 992. The blots were stripped and reprobed with an anti-actin antibody. B and C, to determine the role of EGFR activation in hydrogen peroxide and EGF-mediated signaling, control cells were pretreated for 30 min with 250 or 500 nm AG478, an EGFR-specific inhibitor. B, following preincubation, the cells were treated with 500 μm hydrogen peroxide for 15 min prior to isolation. The membranes were probed with the anti-pAkt antibody, stripped, and reprobed with an anti-Akt antibody. C, following preincubation, control cells were treated with 20 ng/ml EGF for 10 min. The blots were screened sequentially for pEGFR, pAkt, and Akt.
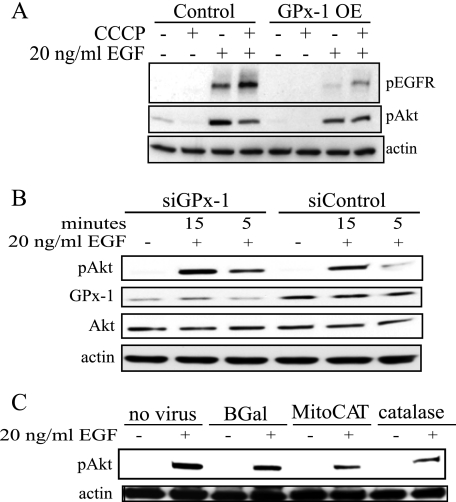
GPx-1 Overexpression Decreases EGF Activation of EGFR—Because growth factor receptor-mediated events are also thought to stimulate oxidant generation, the effect of GPx-1 overexpression on EGF-mediated activation of EGFR was examined. EGF, at concentrations between 0.8 and 100 ng/ml, stimulated pEGFR in a dose-dependent manner in both GPx-1-overexpressing and control cell lines, although the overall levels of phosphorylation were lower in GPx-1-overexpressing cells (data not shown). Based on these findings, we chose to examine further the effects of EGF at 20 ng/ml in these cells (Fig. 4A). Both pEGFR and the resulting Akt activation were decreased in GPx-1-overexpressing cells (Fig. 4A), whereas EGF-mediated pAkt is enhanced by GPx-1 knockdown with siRNA (Fig. 4B). As further support for the role of oxidants in these mechanisms, we treated cells with adenoviruses overexpressing catalase and a form of catalase that is targeted to mitochondria (MitoCAT). Using equivalent multiplicity of infection of these adenoviruses, MitoCAT increased catalase levels 3.2 ± 0.8-fold (p < 0.05) over uninfected or control infected cells, whereas the catalase construct increased catalase activity 9.5 ± 0.9-fold (p < 0.05). Both constructs attenuated Akt phosphorylation (Fig. 4C).
FIGURE 4.
EGFR stimulation and expression in GPx-1-overexpressing cells. A, cells were serum-starved for 1 h followed by treatments with 20 ng/ml EGF. The extracts were separated on reducing SDS-PAGE gradient gels (4–15%), transferred to Hybond membranes, and probed with the indicated antibodies. A, control or GPx-1 OE were treated with EGF for 10 min in the presence or absence of CCCP, a mitochondrial uncoupler. B, cells were treated with siGPx-1 or control siRNA for 48 h followed by stimulation with EGF for 15 and 5 min. C, cells were pretreated with β-galactosidase (BGal), MitoCAT, or catalase containing adenoviruses for 48 h followed by 10 min EGF.
GPx-1 Overexpression Attenuates Akt Phosphorylation via a Mitochondrial Mechanism—Previous studies have shown that EGFR signal transduction requires mitochondrial oxidants (6). To determine whether GPx-1 modifies mitochondrial-mediated oxidative events, GPx-1-overexpressing cells and control cells were treated with carbonyl cyanide 3-chlorophenyl-hydrazone (CCCP), a mitochondrial uncoupler, prior to EGF stimulation. Under these conditions, control cells showed diminished Akt activation (Fig. 4A). In contrast, EGF signaling in GPx-1-overexpressing cells was not affected by CCCP pretreatment, suggesting GPx-1 may be working through mitochondrial mechanisms.
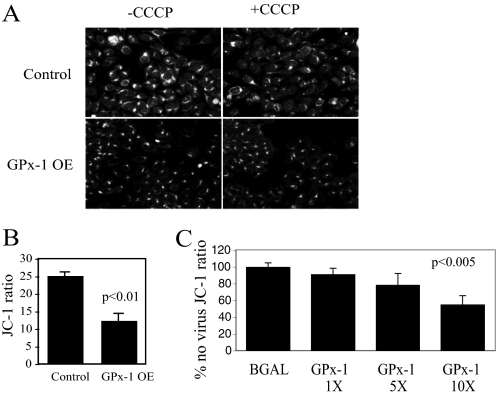
Recent studies from our group have correlated mitochondrial production of ROS with cellular protein disulfide content, indicating that mitochondrial ROS are major determinants of intracellular disulfide formation (23). To determine whether overexpression of GPx-1 disrupted this effect of normal mitochondrial function, the cells were stained for global disulfide bond formation. The GPx-1-overexpressing cells showed significantly less staining than control cells (Fig. 5A); CCCP pretreatment decreased labeling in control cells but had little additional effect in GPx-1-overexpressing cells, suggesting that GPx-1-overexpressing cells have decreased mitochondrial electron flux-mediated ROS production.
FIGURE 5.
Mitochondrial function and GPx-1 overexpression. A, to determine global protein disulfide bond formation, GPx-1-overexpressing and control cells were cultured on chamber slides, fixed with methanol, processed to block free thiols with iodoacetamide, and incubated in tris(2-carboxyethyl) phosphine to reduce disulfides. The resulting free thiols were tagged with 5-iodoacetamidofluorescein according to the protocol of Yang et al. (23). The cells were imaged on a fluorescence microscope. CCCP, a mitochondrial uncoupler, was added to some wells to demonstrate the role of mitochondrial activity in the formation of disulfides. B, mitochondrial potential was determined in cells cultured in 96-well plates in the presence of 2 μm JC-1, a dye that accumulates in the mitochondria proportionate to mitochondrial membrane potential. The ratio of red fluorescence (mitochondrial JC-1) to green fluorescence (cytoplasmic JC-1) was used as a surrogate for mitochondrial potential. GPx-1 OE have a significantly lower JC-1 ratio (p < 0.01, by t test). C, adenovirus treatment of cells to enhance GPx-1 expression resulted in a dose-dependent decrease in JC-1 ratio (p < 0.005) compared with control. The values are plotted compared with no adenovirus treatments, which was set to 100%. JC-1 ratio for BGal adenovirus was not significantly different from no virus control.
To determine whether excess GPx-1 could alter mitochondrial electron flux, we measured mitochondrial membrane potential with JC-1, a dye that accumulates in the mitochondria in proportion to inner membrane potential. Accumulation of JC-1 in mitochondria causes aggregates that fluoresce red; in the cytoplasm, JC-1 exists as monomers that fluoresce green. The ratio of red-to-green fluorescence provides a measure of mitochondrial membrane potential, ΔΨm. In GPx-1-overexpressing cells, ΔΨm is significantly lower (p < 0.01) than in control cells (Fig. 5B). Overexpression of native GPx-1 with adenovirus infection under conditions that significantly increase GPx-1 activity 12–20-fold over control (p < 0.001 by analysis of variance) caused a dose-dependent decrease in JC-1 ratio (p < 0.005) (Fig. 5C). Taken together, these data suggest that GPx-1 overexpression significantly alters ROS output from mitochondria.
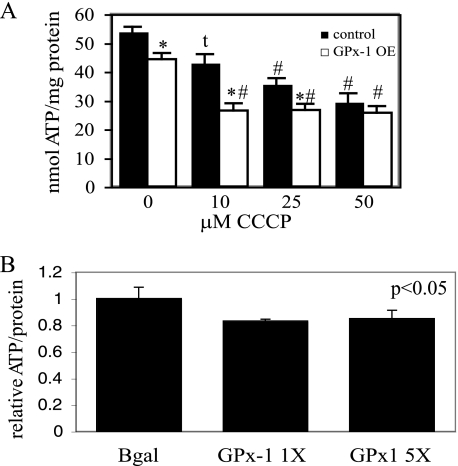
ATP/mg protein was also significantly decreased in GPx-1-overexpressing cells, and adenovirus-mediated overexpression of GPx-1 also caused a decrease in ATP levels, confirming that excess GPx-1 causes a reduction in mitochondrial electron flux and proton-electromotive force (Fig. 6). To illustrate the mitochondrial-sensitive production of ATP, we used CCCP to block mitochondrial function. Overnight treatment with 10 μm CCCP decreased the concentration of ATP in control cells to the level found in GPx-1 OE without CCCP, and control cells showed a dose-dependent decrease of ATP with increasing concentrations of CCCP, up to 50 μm. In contrast, 10 μm CCCP was sufficient to cause the maximal loss of ATP in GPx-1-overexpressing cells (Fig. 6A). These findings suggest a decrease in mitochondrial function caused by mitochondrial uncoupling in GPx-1-overexpressing cells.
FIGURE 6.
ATP production and GPx-1 overexpression. A, ATP-dependent generation of light by luciferase was used to measure ATP content in cells grown on 96-well plates in the presence or absence of various does of CCCP (n = 8). The samples were analyzed by analysis of variance followed by pairwise comparison with the Student-Newman-Keul's test, which assigns significance at p < 0.05. GPx-1 OE, permanently transfected GPx-1-overexpressing cells. *, significantly different between control and GPx-1; #, significantly different between treatment group and either untreated control. t, significantly different between treatment and untreated control. B, adenovirus treatments with GPx-1 significantly reduced ATP levels form β-galactosidase (BGal) controls (p < 0.05). β-Galactosidase values were not significantly different from no virus controls.
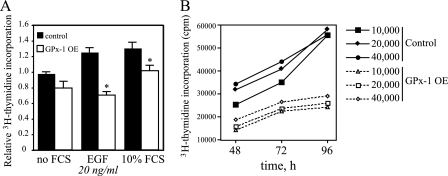
GPx-1 Overexpression Reduces Cellular Proliferation—To determine whether the decrease in growth factor-mediated signaling affects cellular proliferation, the incorporation of [3H]thymidine into DNA was monitored in cells grown in the absence or presence of FCS or EGF (Fig. 7A). FCS stimulation increased [3H]thymidine incorporation into DNA of both control and GPx-1-overexpressing cells; however, the overall increase in [3H]thymidine incorporation was significantly lower in the latter (p < 0.005). In the absence of other growth factors, 20 ng/ml EGF stimulation elicited a 25% increase in proliferation in control cells (p < 0.01) but had no effect on proliferation in GPx-1-overexpressing cells. These data suggest GPx-1-overexpressing cells have blunted proliferative responses to growth factor stimulation. The levels of basal [3H]thymidine incorporation in GPx-1-overexpressing cells cultured in medium with BSA were slightly lower than levels in control cells; however, this difference did not achieve statistical significance. Under the BSA only conditions, the cells continued to divide, and there was a significant incorporation of [3H]thymidine. To compare basal cell growth in these cells, the cells were cultured for up to 96 h in BSA only medium. Under these conditions, the apparent growth rate of the control cells was faster than that of the GPx-1-overexpressing cells (Fig. 7B). These findings confirm that GPx-1 overexpression attenuates cell growth.
FIGURE 7.
GPx-1 overexpression and cellular proliferation. A, cells were seeded at 10,000 cells/well in 24-well dishes, grown for 24 h in medium with no FCS (0.1% BSA), and then treated an additional 16 h with no FCS, EGF 20 ng/ml, or 10% FCS containing media. [3H]Thymidine was added for the last 6 h of the incubation, the cells were harvested, and [3H]thymidine incorporation was determined by scintillation counting. Shown are relative levels from an average of three experiments that measured each condition in six to eight separate wells. The samples were analyzed by analysis of variance followed by pairwise comparison with the Student-Newman-Keul's test, which assigns significance at p < 0.05. Significant differences between control and GPx-1-overexpressing cells are indicated (*). B, to determine the difference in basal cell proliferation, the cells were seeded at various densities in 24-well plates. [3H]Thymidine was added 1.5 h prior to isolation at each time point. A representative experiment is shown in the figure.
DISCUSSION
GPx-1 is one of five selenocysteine-containing glutathione peroxidases capable of eliminating hydroperoxides (28). By regulating peroxide tone, glutathione peroxidases can modulate inflammatory responses and cell death (28–35). However, hydrogen peroxide, in particular, is an essential signaling molecule involved in mediating the mitogenic effect of growth factor receptors (4–8). In our study, we sought to understand how GPx-1 regulates these essential functions of hydrogen peroxide. A previous study (20) found that overexpression of GPx-1 decreased insulin receptor activation of Akt, an important cell growth and survival signal; however, the mechanism for this effect had not been explored. Our study is the first to show that GPx-1 can modulate growth factor receptor signaling by mitochondrial mechanisms. Specifically, we found that GPx-1 expression levels modulate EGFR responses to oxidants or EGF treatment. Functionally, increased GPx-1 expression decreased EGFR activation, leading to decreased cellular proliferation. Mechanistically, we have shown a novel effect for GPx-1 in modulating mitochondrial function. By reducing ROS, GPx-1 overexpression diminished mitochondrial-dependent protein disulfide bond formation and lowered mitochondrial inner membrane potential, leading to decreased production of ATP.
Among the mammalian glutathione peroxidases, only GPx-1 and GPx-4 are found in mitochondria (36, 37). Similar to GPx-1, GPx-4 is also found in other cellular compartments. GPx-4 is also unique in that it preferentially reduces phospholipid hydroperoxides (29). GPx-4 deficiency is embryonically lethal (38, 39), possibly because of its unique role in modulating membrane lipid peroxidation. In fact, the lack of GPx-4 activates unique mitochondrial pathways of cell death involving the translocation of apoptosis-inducing factor from mitochondria to the nucleus (35). GPx-1, in contrast, preferentially acts in the cytosol or mitochondrial matrix environments to remove soluble peroxides. Its deficiency is not lethal, although the lack of GPx-1 potentiates lethality and apoptosis in response to oxidant stress (40, 41). Thus, peroxide tone, modulated by GPx-1 and GPx-4 in mitochondria, may regulate cell signaling and cell death.
Phospholipid hydroperoxides and hydrogen peroxide may regulate different redox-sensitive pathways. Alternatively, there may be cross-talk between membrane-based and soluble pools of peroxides (42) and/or overlap among mechanisms by which membrane bound and soluble peroxides regulate cell function. In support of overlapping pathways, GPx-4, GPx-2, and GPx-1 have all been found to regulate eicosanoid metabolism to some extent (43–45). In addition, by removing cellular oxidants, GPx-4 and GPx-1 can both modulate activation of redox-sensitive transcription factors (28, 34, 46). GPx-4 as well as other cellular GPxs can also suppress the activation of the acetyltransferase essential for the formation of platelet activating factor (47). Interestingly, adenovirus overexpression of phospholipid glutathione peroxidase in mitochondria only diminished pancreatic tumor cell growth in in vitro and in vivo assays, whereas the nonmitochondrial form of GPx-4 had little effect on cell growth (48). However, unlike our studies with GPx-1, there is no evidence that inhibition of cellular proliferation by GPx-4 affects growth factor-mediated signaling; rather, it has been suggested that GPx-4 regulates tumor cell growth, in part by modulating cyclooxygenase-2 and lipoxygenase activities and cyclooxygenase expression (43).
Hydrogen peroxide has been shown to activate growth factor receptors directly, an effect that can be attenuated by catalase overexpression (49). Direct treatment of cells with hydrogen peroxide has also been shown to stimulate EGFR resulting in Akt (6) and ERK1/2 phosphorylation (50, 51), thereby promoting activation pathways associated with cell survival. In our system, we found that 500 μm hydrogen peroxide could transactivate EGFR in control cells, but this activation was nearly undetectable in GPx-1-overexpressing cells. At lower concentrations of hydrogen peroxide (50–200 μm), we were unable to detect EGFR phosphorylation consistently even in control cells (data not shown), possibly owing to the lack of sensitivity of the pEGFR antibody, because we could detect downstream Akt phosphorylation with this lower concentration of hydrogen peroxide, at least in control cells. GPx-1-overexpressing cells had an attenuation of downstream Akt activation that was noticeable at both high (500–1500 μm) and low (50–200 μm) concentrations of hydrogen peroxide. Taken together with our findings that showed decreased ROS accumulation in GPx-1-overexpressing cells, these results suggest that by eliminating ROS, GPx-1 can attenuate oxidant-mediated growth factor receptor transactivation.
Oxidants are also believed to be necessary for growth factor-mediated signal transduction, and, in fact, recent studies suggest that hydrogen peroxide generated as a consequence of EGF-binding to EGFR is necessary for receptor activation and downstream signal transduction (12). We found that EGF-mediated EGFR signaling and EGF-mediated Akt activation were lower in GPx-1-overexpressing cells. By using catalase to inhibit EGF signaling, we provide additional evidence that this signaling is oxidant-mediated. Knockdown of GPx-1 expression augmented EGF-stimulated Akt activation, confirming a role for GPx-1 in modulating this oxidant-mediated pathway.
Functionally, suppression of EGF-induced signaling had profound effects on cell growth because GPx-1-overexpressing cells showed no response to EGF in a cellular proliferation assay. Other reports have shown that overexpression of antioxidant enzymes, such as catalase, decreased cell growth in response to EGF (52), indicating the importance of oxidants and their regulation by antioxidant enzymes in controlling the outcomes of EGF-mediated pathways.
ROS are thought to modify cellular responses, at least in part, by oxidative modification of redox-sensitive cysteinyl residues. Reversible inactivation of PTEN phosphatase by oxidation is one mechanism that promotes Akt signal transduction by attenuating the inhibitory actions of PTEN on phosphatidylinositol kinase/Akt pathways. Loss of PTEN expression in transformed cells is associated with increased activity of Akt in response to growth factor stimulation, whereas overexpression of PTEN in PTEN null cells leads to apoptosis and inhibition of cell growth (53). In another study of hydrogen peroxide-regulated PTEN inactivation, both catalase and MitoCAT were found to attenuate PTEN oxidation (54), whereas in our cell system GPx-1 overexpression had no effect on oxidation of this phosphatase. In fact, PTEN was reversibly oxidized to a similar extent in GPx-1-overexpressing and control cells, even though GPx-1 overexpression was sufficient to attenuate intracellular accumulation of ROS. These data suggest that PTEN is not the only factor influencing Akt activation in these cells.
In other cell lines, mitochondrial generation of ROS, rather than phosphatase inactivation, has been shown to be necessary for hydrogen peroxide-dependent transactivation of EGFR (6). Thus, our data are consistent with these findings, but additionally show that GPx-1 can modulate these effects by regulating mitochondrial function. Surprisingly, in our studies both catalase and MitoCAT, which is a recombinant catalase that targets to mitochondria, had effects on Akt activation, suggesting that although oxidants generated by mitochondria may be essential regulators of growth factor mediated signaling, some of the effects of these oxidants may be due to their release and accumulation in the cytoplasm. Other studies have found that only mitochondrially targeted antioxidant enzymes can regulate certain oxidant-mediated cell events (23, 55, 56), possibly indicating the importance in regulating local oxidant accumulation for some cellular processes, such as disulfide bond formation or apoptosis. Taken together, these data suggest complex regulation of oxidant fluxes and their cellular consequences by cellular antioxidants.
In a recent report, our group found that ROS produced by mitochondria promote protein disulfide bond formation in cells. Specifically, mitochondrial ROS are necessary for the maintenance of global disulfide formation in cells (23). In addition, cells treated with mitochondrial uncouplers or cells with a deficiency of functional mitochondria (pseudo-Rho0 cells) were found to have less intracellular disulfides than normal cells. MitoCAT, but not catalase, also decreased disulfide bond formation in cells. GPx-1 is an important intracellular antioxidant that localizes both to the cytoplasm and to the mitochondria. The distribution of this antioxidant protein may explain why GPx-1 overexpression can decrease global protein disulfide formation, a process specifically dependent on mitochondrial oxidant generation. We also found that mitochondrial potential was significantly lower in cells with excess GPx-1 and that this effect was observed in permanently transfected cells as well as in cells treated with a GPx-1 adenovirus to increase GPx-1 expression. Mitochondrial potential is known to reflect the ROS output of mitochondria (57, 58). Functionally, the lack of mitochondrial ROS in GPx-1-overexpressing cells coincides with modest uncoupling of mitochondria. This observation would explain why CCCP, a mitochondrial uncoupler, had less effect on GPx-1-overexpressing cells in terms of disulfide bond formation, ATP production, and signal transduction than on control cells. In contrast, EGF-mediated Akt activation was decreased in control cells pretreated with CCCP to eliminate mitochondrial electron flux. Our results suggest that EGFR signaling involves a pathway that can be attenuated by GPx-1 overexpression by diminishing ROS and reducing mitochondrial function.
An unexpected finding of the current study is that a mere 2-fold increase in GPx-1 activity altered cell growth, attenuated transactivation of the EGFR receptor by hydrogen peroxide, and decreased EGF-mediated EGFR stimulation. GPx-1 is a selenoprotein, with a selenocysteine at the active site of the enzyme. The 2-fold level of overexpression of GPx-1 in our cell system is modest but led to an interesting observation regarding the narrow range over which such an antioxidant enzyme can be expressed without altering biological processes, such as signal transduction and cell growth. In fact, GPx-1 null cells isolated from GPx-1 knock-out mice also have decreased proliferative capacity, along with increased susceptibility to oxidant-induced cell death (59), suggesting that both too little and too much ROS accumulation can have deleterious effects. The findings presented here also support the notion of reductive stress, caused by the overabundance of the antioxidant enzyme GPx-1. The contributions of reductive stress to disease have only recently been appreciated, with, for example, overabundance of antioxidant enzymes shown to promote the pathogenesis of cardiomyopathy caused by mutations in αB-crystallin (60). Taken together, these data suggest that a change (in either direction) in cellular redox balance may alter protein folding, signal transduction, and proliferative responses.
Overall, GPx-1 deficiency is correlated with enhanced susceptibility to oxidant stress; GPx-1-deficient mice have endothelial dysfunction and abnormalities in vascular and cardiac structure (61, 62), are more susceptible to injury in cerebral models of ischemia-reperfusion (46, 63), and have defects in endothelial progenitor cell function that impair angiogenesis in a model of hindlimb ischemia (64). Although overexpression of GPx-1 may be protective against oxidative stress generated in a variety of pathological settings, including hyperhomocysteinemia and ischemia-reperfusion injury (16–18), excess GPx-1 may cause other problems because of the elimination of endogenous intracellular hydrogen peroxide necessary for normal oxidative signaling. In GPx-1-overexpressing mice, modest increases of GPx-1 activity, between 21 and 300%, were associated with obesity and insulin resistance and diminished Akt phosphorylation in response to insulin (20). Our findings provide evidence for a unique role of GPx-1 in modulating growth factor-mediated signaling by mitochondrial mechanisms. These data also suggest that GPx-1 regulates mitochondrial function.
Acknowledgments
We thank Dr. David Pimental for the kind gift of MitoCAT and catalase adenoviruses. We also thank Stephanie Tribuna for expert assistance in manuscript preparation.
This work was supported, in whole or in part, by National Institutes of Health Grants HL 58976, HL 61795, NO1 HV 28178, and HL 81587. This work was also supported by Deutsche Forschungsgemeinschaft Grant LU 1452/1-1.
Footnotes
The abbreviations used are: ROS, reactive oxygen species; BSA, bovine serum albumin; CCCP, carbonyl cyanide3-chlorophenyl-hydrazone; DCF, 5-(and 6-)-carboxy 2′-7′dichlorohydrofluorescein diacetate; DMEM, Dulbecco's modified Eagle's medium; EGF, epidermal growth factor; EGFR, EGF receptor; FCS, fetal calf serum; GPx-1, glutathione peroxidase-1; GPx-1 OE, GPx-1-overexpressing cells; GPx-4, phospholipid glutathione peroxidase; MitoCAT, adenovirus with mitochondrially targeted catalase; PTEN, phosphatase and tensin homolog deleted on chromosome ten; ERK, extracellular signal-regulated kinase; siRNA, small interfering RNA; pEGFR, phosphorylated EGFR.
References
- 1.Goossens, V., Grooten, J., De Vos, K., and Fiers, W. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 8115–8119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berlett, B. S., and Stadtman, E. R. (1997) J. Biol. Chem. 272 20313–20316 [DOI] [PubMed] [Google Scholar]
- 3.Ryter, S. W., Kim, H. P., Hoetzel, A., Park, J. W., Nakahira, K., Wang, X., and Choi, A. M. (2007) Antioxid. Redox Signal. 9 49–89 [DOI] [PubMed] [Google Scholar]
- 4.Finkel, T. (1998) Curr. Opin. Cell Biol. 10 248–253 [DOI] [PubMed] [Google Scholar]
- 5.Griendling, K. K., Sorescu, D., Lassegue, B., and Ushio-Fukai, M. (2000) Arterioscler. Thromb. Vasc. Biol. 20 2175–2183 [DOI] [PubMed] [Google Scholar]
- 6.Chen, K., and Keaney, J. (2004) Endothelium 11 109–121 [DOI] [PubMed] [Google Scholar]
- 7.Goldstein, B. J., Mahadev, K., Wu, X., Zhu, L., and Motoshima, H. (2005) Antioxid. Redox Signal. 7 1021–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone, J. R., and Yang, S. (2006) Antioxid. Redox Signal. 8 243–270 [DOI] [PubMed] [Google Scholar]
- 9.Lambeth, J. D. (2004) Nat. Rev. Immunol. 4 181–189 [DOI] [PubMed] [Google Scholar]
- 10.Liochev, S. I., and Fridovich, I. (2007) Free Radic. Biol. Med. 42 1465–1469 [DOI] [PubMed] [Google Scholar]
- 11.Berry, C. E., and Hare, J. M. (2004) J. Physiol. 555 589–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeYulia, G. J., Jr., Carcamo, J. M., Borquez-Ojeda, O., Shelton, C. C., and Golde, D. W. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 5044–5049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee, S. G., Kang, S. W., Jeong, W., Chang, T. S., Yang, K. S., and Woo, H. A. (2005) Curr. Opin. Cell Biol. 17 183–189 [DOI] [PubMed] [Google Scholar]
- 14.Raes, M., Michiels, C., and Remacle, J. (1987) Free Radic. Biol. Med. 3 3–7 [DOI] [PubMed] [Google Scholar]
- 15.Ursini, F., Maiorino, M., Brigelius-Flohe, R., Aumann, K. D., Roveri, A., Schomburg, D., and Flohe, L. (1995) Methods Enzymol. 252 38–53 [DOI] [PubMed] [Google Scholar]
- 16.Weiss, N., Zhang, Y. Y., Heydrick, S., Bierl, C., and Loscalzo, J. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 12503–12508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoehn, B., Yenari, M. A., Sapolsky, R. M., and Steinberg, G. K. (2003) Stroke 34 2489–2494 [DOI] [PubMed] [Google Scholar]
- 18.Shiomi, T., Tsutsui, H., Matsusaka, H., Murakami, K., Hayashidani, S., Ikeuchi, M., Wen, J., Kubota, T., Utsumi, H., and Takeshita, A. (2004) Circulation 109 544–549 [DOI] [PubMed] [Google Scholar]
- 19.Zhang, Y., Handy, D. E., and Loscalzo, J. (2005) Circ. Res. 96 831–837 [DOI] [PubMed] [Google Scholar]
- 20.McClung, J. P., Roneker, C. A., Mu, W., Lisk, D. J., Langlais, P., Liu, F., and Lei, X. G. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 8852–8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flohe, L., and Gunzler, W. A. (1984) Methods Enzymol. 105 114–121 [DOI] [PubMed] [Google Scholar]
- 22.Leopold, J. A., Walker, J., Scribner, A. W., Voetsch, B., Zhang, Y. Y., Loscalzo, A. J., Stanton, R. C., and Loscalzo, J. (2003) J. Biol. Chem. 278 32100–32106 [DOI] [PubMed] [Google Scholar]
- 23.Yang, Y., Song, Y., and Loscalzo, J. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 10813–10817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, Y. Y., Walker, J. L., Huang, A., Keaney, J. F., Clish, C. B., Serhan, C. N., and Loscalzo, J. (2002) Biochem. J. 361 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, S. R., Yang, K. S., Kwon, J., Lee, C., Jeong, W., and Rhee, S. G. (2002) J. Biol. Chem. 277 20336–20342 [DOI] [PubMed] [Google Scholar]
- 26.Leslie, N. R., Bennett, D., Lindsay, Y. E., Stewart, H., Gray, A., and Downes, C. P. (2003) EMBO J. 22 5501–5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon, J., Lee, S. R., Yang, K. S., Ahn, Y., Kim, Y. J., Stadtman, E. R., and Rhee, S. G. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 16419–16424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brigelius-Flohe, R. (2006) Biol. Chem. 387 1329–1335 [DOI] [PubMed] [Google Scholar]
- 29.Conrad, M., Schneider, M., Seiler, A., and Bornkamm, G. W. (2007) Biol. Chem. 388 1019–1025 [DOI] [PubMed] [Google Scholar]
- 30.Arthur, J. R. (2000) Cell Mol. Life Sci. 57 1825–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Haan, J. B., Crack, P. J., Flentjar, N., Iannello, R. C., Hertzog, P. J., and Kola, I. (2003) Redox Rep. 8 69–79 [DOI] [PubMed] [Google Scholar]
- 32.Kuhn, H., and Borchert, A. (2002) Free Radic. Biol. Med. 33 154–172 [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa, Y. (2004) Biol. Pharm. Bull. 27 956–960 [DOI] [PubMed] [Google Scholar]
- 34.Wenk, J., Schuller, J., Hinrichs, C., Syrovets, T., Azoitei, N., Podda, M., Wlaschek, M., Brenneisen, P., Schneider, L. A., Sabiwalsky, A., Peters, T., Sulyok, S., Dissemond, J., Schauen, M., Krieg, T., Wirth, T., Simmet, T., and Scharffetter-Kochanek, K. (2004) J. Biol. Chem. 279 45634–45642 [DOI] [PubMed] [Google Scholar]
- 35.Seiler, A., Schneider, M., Forster, H., Roth, S., Wirth, E. K., Culmsee, C., Plesnila, N., Kremmer, E., Radmark, O., Wurst, W., Bornkamm, G. W., Schweizer, U., and Conrad, M. (2008) Cell Metab. 8 237–248 [DOI] [PubMed] [Google Scholar]
- 36.Esworthy, R. S., Ho, Y. S., and Chu, F. F. (1997) Arch. Biochem. Biophys. 340 59–63 [DOI] [PubMed] [Google Scholar]
- 37.Borchert, A., Wang, C. C., Ufer, C., Schiebel, H., Savaskan, N. E., and Kuhn, H. (2006) J. Biol. Chem. 281 19655–19664 [DOI] [PubMed] [Google Scholar]
- 38.Yant, L. J., Ran, Q., Rao, L., Van Remmen, H., Shibatani, T., Belter, J. G., Motta, L., Richardson, A., and Prolla, T. A. (2003) Free Radic. Biol. Med. 34 496–502 [DOI] [PubMed] [Google Scholar]
- 39.Imai, H., Hirao, F., Sakamoto, T., Sekine, K., Mizukura, Y., Saito, M., Kitamoto, T., Hayasaka, M., Hanaoka, K., and Nakagawa, Y. (2003) Biochem. Biophys. Res. Commun. 305 278–286 [DOI] [PubMed] [Google Scholar]
- 40.de Haan, J. B., Bladier, C., Griffiths, P., Kelner, M., O'Shea, R. D., Cheung, N. S., Bronson, R. T., Silvestro, M. J., Wild, S., Zheng, S. S., Beart, P. M., Hertzog, P. J., and Kola, I. (1998) J. Biol. Chem. 273 22528–22536 [DOI] [PubMed] [Google Scholar]
- 41.Cheng, W. H., Ho, Y. S., Valentine, B. A., Ross, D. A., Combs, G. F., Jr., and Lei, X. G. (1998) J. Nutr. 128 1070–1076 [DOI] [PubMed] [Google Scholar]
- 42.Loscalzo, J. (2008) Cell Metab. 8 182–183 [DOI] [PubMed] [Google Scholar]
- 43.Heirman, I., Ginneberge, D., Brigelius-Flohe, R., Hendrickx, N., Agostinis, P., Brouckaert, P., Rottiers, P., and Grooten, J. (2006) Free Radic. Biol. Med. 40 285–294 [DOI] [PubMed] [Google Scholar]
- 44.Banning, A., Florian, S., Deubel, S., Thalmann, S., Muller-Schmehl, K., Jacobasch, G., and Brigelius-Flohe, R. (2008) Antioxid. Redox Signal. 10 1491–1500 [DOI] [PubMed] [Google Scholar]
- 45.Brigelius-Flohe, R. (1999) Free Radic. Biol. Med. 27 951–965 [DOI] [PubMed] [Google Scholar]
- 46.Crack, P. J., Taylor, J. M., Ali, U., Mansell, A., and Hertzog, P. J. (2006) Stroke 37 1533–1538 [DOI] [PubMed] [Google Scholar]
- 47.Sakamoto, H., Tosaki, T., and Nakagawa, Y. (2002) J. Biol. Chem. 277 50431–50438 [DOI] [PubMed] [Google Scholar]
- 48.Liu, J., Du, J., Zhang, Y., Sun, W., Smith, B. J., Oberley, L. W., and Cullen, J. J. (2006) Hum. Gene Ther. 17 105–116 [DOI] [PubMed] [Google Scholar]
- 49.Finch, J. S., Tome, M. E., Kwei, K. A., and Bowden, G. T. (2006) Free Radic. Biol. Med. 40 863–875 [DOI] [PubMed] [Google Scholar]
- 50.Preston, T. J., Muller, W. J., and Singh, G. (2001) J. Biol. Chem. 276 9558–9564 [DOI] [PubMed] [Google Scholar]
- 51.Madamanchi, N. R., Li, S., Patterson, C., and Runge, M. S. (2001) Arterioscler. Thromb. Vasc. Biol. 21 321–326 [DOI] [PubMed] [Google Scholar]
- 52.Shi, M., Yang, H., Motley, E. D., and Guo, Z. (2004) Am. J. Hypertens. 17 450–456 [DOI] [PubMed] [Google Scholar]
- 53.Choi, Y., Zhang, J., Murga, C., Yu, H., Koller, E., Monia, B. P., Gutkind, J. S., and Li, W. (2002) Oncogene 21 5289–5300 [DOI] [PubMed] [Google Scholar]
- 54.Connor, K. M., Subbaram, S., Regan, K. J., Nelson, K. K., Mazurkiewicz, J. E., Bartholomew, P. J., Aplin, A. E., Tai, Y. T., Aguirre-Ghiso, J., Flores, S. C., and Melendez, J. A. (2005) J. Biol. Chem. 280 16916–16924 [DOI] [PubMed] [Google Scholar]
- 55.Arita, Y., Harkness, S. H., Kazzaz, J. A., Koo, H. C., Joseph, A., Melendez, J. A., Davis, J. M., Chander, A., and Li, Y. (2006) Am. J. Physiol. 290 L978–L986 [DOI] [PubMed] [Google Scholar]
- 56.Gurgul, E., Lortz, S., Tiedge, M., Jorns, A., and Lenzen, S. (2004) Diabetes 53 2271–2280 [DOI] [PubMed] [Google Scholar]
- 57.Boveris, A., and Chance, B. (1973) Biochem. J. 134 707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyer, L. E., Machado, L. B., Santiago, A. P., da-Silva, W. S., De Felice, F. G., Holub, O., Oliveira, M. F., and Galina, A. (2006) J. Biol. Chem. 281 37361–37371 [DOI] [PubMed] [Google Scholar]
- 59.de Haan, J. B., Bladier, C., Lotfi-Miri, M., Taylor, J., Hutchinson, P., Crack, P. J., Hertzog, P., and Kola, I. (2004) Free Radic. Biol. Med. 36 53–64 [DOI] [PubMed] [Google Scholar]
- 60.Rajasekaran, N. S., Connell, P., Christians, E. S., Yan, L. J., Taylor, R. P., Orosz, A., Zhang, X. Q., Stevenson, T. J., Peshock, R. M., Leopold, J. A., Barry, W. H., Loscalzo, J., Odelberg, S. J., and Benjamin, I. J. (2007) Cell 130 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forgione, M. A., Cap, A., Liao, R., Moldovan, N. I., Eberhardt, R. T., Lim, C. C., Jones, J., Goldschmidt-Clermont, P. J., and Loscalzo, J. (2002) Circulation 106 1154–1158 [DOI] [PubMed] [Google Scholar]
- 62.Forgione, M. A., Weiss, N., Heydrick, S., Cap, A., Klings, E. S., Bierl, C., Eberhardt, R. T., Farber, H. W., and Loscalzo, J. (2002) Am. J. Physiol. 282 H1255–H1261 [DOI] [PubMed] [Google Scholar]
- 63.Crack, P. J., Taylor, J. M., Flentjar, N. J., de Haan, J., Hertzog, P., Iannello, R. C., and Kola, I. (2001) J. Neurochem. 78 1389–1399 [DOI] [PubMed] [Google Scholar]
- 64.Galasso, G., Schiekofer, S., Sato, K., Shibata, R., Handy, D. E., Ouchi, N., Leopold, J. A., Loscalzo, J., and Walsh, K. (2006) Circ. Res. 98 254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]