Abstract
Saccharomyces cerevisiae cells lacking the amphiphysin-like orthologs, Rvs161 or Rvs167, are unable to thrive under many stress conditions. Here we show cells lacking Rvs161 require Cdc55, the B subunit of the yeast ceramide-activated protein phosphatase, for viability under heat stress. By using specific rvs mutant alleles, we linked this lethal genetic interaction to loss of Rvs161 endocytic domain function. Recessive mutations in the sphingolipid pathway, such as deletion of the very long-chain fatty acid elongase, Sur4, suppress the osmotic growth defect of rvs161 cells. We demonstrate that Cdc55 is required for sur4-dependent suppressor activity and that protein phosphatase activation, through overexpression of CDC55 alone, can also remediate this defect. Loss of SUR4 in rvs161 cells reinitiates Ste3 a-factor receptor endocytosis and requires Cdc55 function to do so. Moreover, overexpression of CDC55 reinitiates Ste3 endocytic-dependent degradation and restores fluid phase endocytosis in rvs161 cells. In contrast, loss of SUR4 or CDC55 overexpression does not remediate the actin polarization defects of osmotic stressed rvs161 cells. Importantly, remediation of rvs161 defects by protein phosphatase activation requires the ceramide-activated protein phosphatase catalytic subunit, Sit4, and the protein phosphatase 2A catalytic subunits, Pph21/Pph22. Finally, genetic analyses reveal a synthetic lethal interaction between loss of CDC55 and gene deletions lethal with rvs161, all of which function in endocytosis.
The Saccharomyces cerevisiae amphiphysin orthologs, Rvs161 and Rvs167, are members of the BAR (BIN/Amphiphysin/RVS domain)2 family of proteins (1–3, 4, 5). BAR proteins function to regulate early endocytosis and the actin cytoskeleton, likely through facilitating the development of a tubulovesicular membrane system required for clathrin-mediated endocytosis and regulation of membrane dynamics (3, 4, 6–11). Failure to form these tubular structures leads to defects in intracellular trafficking (11).
In S. cerevisiae, the RVS161/END6 gene (Table 1) was identified by isolating recessive mutations causing reduced viability upon starvation (12) but was subsequently shown to be involved in actin cytoskeleton polarization (13), cell polarity (14), endocytosis (15), and secretory vesicle trafficking (16). Mutations in RVS161 are highly pleiotropic, resulting in delocalization of the actin cytoskeleton (13), high salt sensitivity (12), mating/cell fusion defects (17), and a random budding pattern in diploid cells (13); however, the RVS161 gene is not essential for cell viability (12).
TABLE 1.
Description of yeast gene acronyms referred to in the text
| Acronym | Acronym translationa | Biological processa | Functiona |
|---|---|---|---|
| ABP1 | Actin Binding Protein | Actin cortical patch assembly; establishment of cell polarity | Actin-binding protein; activation of the Arp2/3 complex |
| CDC55 | Cell Division Cycle | Protein amino acid dephosphorylation; actin filament organization; translation; cell bud growth; pseudohyphal growth; mitotic cell cycle spindle assembly checkpoint; negative regulation of exit from mitosis | B regulatory subunit of protein phosphatase 2A and ceramide-activated protein phosphatase; protein serine/threonine phosphatase activity |
| EXO70 | EXOcyst | Bipolar bud site selection; cytokinesis; exocytosis; Golgi to plasma membrane transport vesicle docking during exocytosis; vesicle fusion | Phosphatidylinositol 4,5-bisphosphate binding; protein binding |
| PEP4 | carboxyPEPtidase Y-deficient | Cellular response to starvation; microautophagy; formation of a cellular spore during sporulation; vacuolar protein catabolic process | Vacuolar aspartyl protease (proteinase A) |
| PPH21 and PPH22 | Protein PHosphatase | Actin filament organization; cell bud growth; G1/S transition of mitotic cell cycle; mitotic cell cycle spindle assembly checkpoint; protein amino acid dephosphorylation; translation | Catalytic subunit C of protein phosphatase 2A; protein serine/threonine phosphatase activity; functionally redundant |
| RTS1 | Rox Three Suppressor | Meiotic chromosome cohesion translation protein amino acid dephosphorylation | B regulatory subunit of PP2A |
| RVS161 | Reduced Viability upon nutrient Starvation | Endocytosis; polar budding; response to osmotic stress; mating | Cytoskeletal protein binding |
| RVS167 | Reduced Viability upon nutrient Starvation | Endocytosis; polar budding; response to osmotic stress | Cytoskeletal protein binding |
| SAC6 | Suppressor of ACtin mutations | Actin filament organization endocytosis; polar budding; response to osmotic stress | Actin filament binding; protein binding, bridging |
| SEC8 | SECretory | Bipolar bud site selection; cytokinesis; exocytosis; Golgi to plasma membrane transport vesicle docking during exocytosis; vesicle fusion | Protein binding |
| SEC20 | SECretory | SNAP receptor activity | Vesicle fusion; retrograde vesicle-mediated transport, Golgi to endoplasmic reticulum |
| SIT4 | Sporulation-Induced Transcript | G1/S transition of the mitotic cycle; actin cytoskeleton and cell wall organization; dephosphorylation; response to oxidative stress; protein kinase cascade; TOR signaling pathway; DNA repair; replicative cell aging | Type 2A-related serine-threonine phosphatase |
| SLA1 | Synthetically Lethal with ABP1 | Actin cortical patch assembly; actin filament organization cell wall; organization and biogenesis; endocytosis | Cytoskeletal protein-binding protein; interacts with proteins regulating actin dynamics and endocytosis |
| SLA2 | Synthetically Lethal with ABP1 | Actin filament organization; cell polarization cell wall organization and biogenesis; endocytosis; exocytosis | Transmembrane actin-binding protein; links actin to clathrin |
| STE3 | STErile | Pheromone-dependent signal transduction during conjugation with cellular fusion; transcribed in alpha cells | Receptor for a factor; mediates pheromone response through MAP kinase cascade |
| SUR4 | SUppressor of Rvs | Fatty acid elongation; sphingolipid biosynthesis | Fatty acid elongase activity |
| TPD3 | tRNA Processing Deficient | Actin filament organization; cell bud growth; mitotic cell cycle spindle assembly checkpoint protein amino acid dephosphorylation; translation | Regulatory subunit A of protein phosphatase 2A and ceramide-activated protein phosphatase protein serine/threonine phosphatase activity |
| VPS20 | Vacuolar Protein Sorting | Late endosome to vacuole transport; ubiquitin-dependent protein catabolic process via the multivesicular body sorting pathway | Myristoylated subunit of ESCRTIII, the endosomal sorting complex |
| VPS21 | Vacuolar Protein Sorting | Transport during endocytosis; protein-vacuolar targeting | GTPase activity |
Obtained from the S. cerevisiae genome database.
Almost all rvs161-related phenotypes are suppressed by mutations in the sphingolipid biosynthetic pathway (18). For instance, loss of the SUR4/ELO3/VBM1 gene, which encodes a very long-chain fatty acid elongase required for the production of the C26 fatty acids found only in yeast complex sphingolipids, alters sphingolipid biosynthesis (sur4 cells accumulate the long-chain base, phytosphingosine (19)) and suppresses rvs defects (18, 20). Sphingolipids are essential lipids and are conserved structurally throughout evolution (21). In addition to roles as structural components of membranes (22), sphingolipids act as signaling molecules regulating biological processes, such as cell growth, endocytosis, differentiation, stress, and apoptosis; the sphingolipid, ceramide, and multiple long-chain sphingoid bases are key signaling intermediates regulating these events in mammalian cells and in S. cerevisiae (23–28).
In S. cerevisiae, ceramide serves as the backbone for all complex sphingolipids, and its transient accumulation may be a signal to adapt to cellular stresses such as heat (29–32), amino acid deprivation (33), and cell cycle arrest (34). Several ceramide-regulated enzymes have been identified (35, 36), including the conserved ceramide-activated protein phosphatase (CAPP) (37–41), which was identified as a member of the mammalian 2A class of serine/threonine protein phosphatases (PP2A) (42). Ubiquitous among all eukaryotes, PP2A is a heterotrimer composed of two regulatory subunits (A and B), and a catalytic subunit (C) (43, 44). In yeast, the B regulatory subunit of PP2A is encoded by two genes, CDC55 and RTS1 (45–47), the A scaffolding subunit is encoded by TPD3 (48), and the C catalytic subunit is redundantly encoded by PPH21 and PPH22 (49).
Yeast CAPP is thought to be comprised of the subunits, Tpd3 (A), Cdc55 (B), and Sit4 (C), and its activation by addition of exogenous short-chain ceramides results in G1 arrest (50). Putative CAPP activation through the addition of C2-ceramide, SUR4/ELO3/VBM1 gene inactivation, or overexpression of SIT4 can rescue the endocytic defects of cells lacking the Tlg1 or Tlg2 t-SNAREs involved in endocytosis (51) and confers normal growth and secretion to cells lacking the exocytic v-SNAREs, Snc1 and Snc2 (52). On the other hand, deletion of the CAPP B subunit, CDC55, or the catalytic subunits of PP2A abolishes the sphingoid base requirement in endocytosis (53). So the exact role of CAPP in regulating endocytosis is unclear. PP2A seems to regulate endocytosis in mammalian cells, and activation of PP2A is necessary for dynamin 2 dephosphorylation, resulting in the clathrin-dependent endocytosis of Na+-ATPase and K+-ATPase molecules (54).
How defects in sphingolipid biosynthesis suppress rvs phenotypes is unclear and likely very complex. Deletion of sphingolipid genes suppresses the actin depolarization and growth defects of osmotic stressed rvs161 cells (55). However, steady-state actin cytoskeletal defects persist in rvs161 sur4 cells under conditions of glucose starvation, yet these cells are viable (20). Thus, understanding the molecular basis for how this suppression mechanism works should give valuable insight into how sphingolipid metabolites function as signaling lipids regulating many diverse cell events (23–28).
To further understand how mutating sphingolipid biosynthesis suppresses rvs defects, we determined the roles of PP2A/CAPP in this process. Here we show that PP2A/CAPP activation, through overexpression of CDC55 or loss of SUR4, is an essential prerequisite for suppressing rvs growth defects. Suppression requires the PP2A catalytic subunits, Pph21/Pph22, and/or the CAPP catalytic subunit, Sit4. Importantly, CAPP/PP2A activation reinitiates endocytosis but does not remediate the actin polarization defects of rvs161 cells. We propose that sphingolipid-dependent PP2A/CAPP activation results in the reinitiation of endocytosis, and this is essential for cells to thrive under stress in the absence of amphiphysin function.
EXPERIMENTAL PROCEDURES
Strains and Plasmids—The S. cerevisiae strains used in this study were derived from W303 (MATa ura3-1 leu2–3, 112 his3–11,15 trp1-1 ade2-1 can1–100) or MY2792 (MATa ura3–52 leu2-1 his3–200) backgrounds (17). Yeast strains were transformed with the Yep24-CDC55-URA3 or Yep24-URA3 vector using the procedure described by Ito et al. (56). For routine propagation of plasmids, Escherichia coli XL1-Blue cells were used and grown in LB medium supplemented with ampicillin (200 μg/ml). Yeast haploid rvs161, sur4, cdc55, sit4, pph21, pph22, tpd3, and pep4 null mutants were generated by the one-step disruption method of Rothstein (57). GAL-STE3-HA strains were constructed using the PCR cassette pFA6a-TRP1-GAL1 (58). Integrating YIp-GPD-CDC55-URA3 and YIp-GPD-URA3 into the ura3–52 locus, respectively, generated strains containing GPD-CDC55 and GPD. The sla1, sla2, vps20, vps21, rvs167, abp1, and sac6 null strains were generated by several backcrosses of W303 to haploid null strains obtained from the Research Genetics Yeast Strain Collection.
Media and Growth Conditions—Yeast strains were grown in YP media (1% yeast extract, 2% bactopeptone) supplemented with either 2% glucose (YEPD), 2% glycerol, 2% glucose plus 3.4% NaCl, or 2% glucose plus 6% NaCl as indicated. Yeast strains were also grown in synthetic minimal media containing 0.67% yeast nitrogen base supplemented with the appropriate amino acids and carbon source. To assay for the ability to grow under different stress conditions, yeast strains were grown to exponential phase in YEPD or selective minimal media plus 2% glucose. 2 × 105 cells were spotted as 10-fold serial dilutions onto various media plates or streaked for single colonies and incubated at 30 °C or 37 °C as indicated. Cell growth was examined after 48 or 72 h as indicated.
Fluorescence Microscopy of Polymerized Actin and Lucifer Yellow Internalization—For all microscopic observations, yeast strains were grown to exponential phase in YEPD or ura– plus 2% glucose. For NaCl stress-induced experiments, cells were pelleted by centrifugation and resuspended in YEPD plus 3.4% NaCl, YEPD plus 6% NaCl, or ura– media containing 2% glucose plus 3.4% NaCl. Actin was stained with rhodamine phalloidin as described by Adams and Pringle (59) with some modifications.
Briefly, cells grown to exponential phase were fixed by addition of formaldehyde to a final concentration of 3.7%. After 30 min, cells were pelleted by centrifugation, washed three times in 1× phosphate-buffered saline, pH 7.3, resuspended in 45 μl of 1× phosphate-buffered saline containing 3 units of Rhodamine-phalloidin (Molecular Probes), and incubated at room temperature for 2 h. Cells were then washed eight times with 1× phosphate-buffered saline and resuspended in 20 μl of 1× phosphate-buffered saline. To visualize the internalization of lucifer yellow (LY), 107 cells grown to exponential phase were resuspended in 90 μl of YEPD and stained with 10 μl of 40 mg/ml lucifer yellow solution (Sigma). The stained cells were then incubated at 30 °C for 1 h. Cells were then washed four times with ice-cold LY buffer (50 mm sodium succinate, pH 5), resuspended in 10 μl of cold LY buffer, and stored on ice before visualization. The actin cytoskeleton and lucifer yellow internalization was visualized using a Leica DMRBE fluorescence microscope and rhodamine isothiocyanate optics and a PlanAPO 100× objective. Data were collected using a Hamamatsu DIG-15 charge-coupled digital camera and Open Labs software (version 2.1). For lucifer yellow internalization studies, a total of 350 cells were counted during each experiment and the values represented are the average values of three independent experiments.
Classification of the Actin Polarization State of Cells—Only cells with small buds were counted. Cells with actin patches concentrated in the small bud, with five or fewer patches in the mother cell and visible actin cables, were classified as polarized cells. Cells with most actin patches in the mother cell rather than in the small bud were classified as depolarized cells. A total of 350 cells was counted during each experiment, and the values are the average values of six independent experiments.
Determination of Ste3 Stability—The yeast strains used carry a functional STE3::GAL1-STE3-HA::TRP1 chromosomal allele. Cultures grown to exponential phase in YEPD or ura– plus 2% glucose at 30 °C were pelleted, washed, and shifted to YP or ura– media containing 2% raffinose for 1.5 h. To induce expression of Ste3-HA, cultures were washed and shifted to YP or ura– media containing 2% galactose for 3 h. Cultures were then washed and shifted to YEPD, ura– plus 2% glucose, YEPD plus 3.4% NaCl, or ura– plus 2% glucose plus 3.4% NaCl for 2 h to shut off the expression of Ste3-HA. Samples were taken before induction and at the indicated times post initiation of shut-off with glucose. Ste3-HA level was assayed by Western analysis and immunoblotting using 16B12 mouse anti-HA antibody (Covance).
Western Analysis—Total cell extracts were obtained using a modified procedure from Hsiung et al. (60). Exponentially growing yeast cells were pelleted and resuspended in yeast lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.1% Nonidet P-40, 10% glycerol) containing 50 mm sodium fluoride, protease inhibitors (0.4 mm phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin, 1 μg/ml leupeptin, and 1 μg/ml aprotinin), and phosphatase inhibitors (0.1 mm sodium orthovanadate, 5 mm EDTA, 5 mm EGTA, and 10 mm sodium pyrophosphate). Cells were then lysed with glass beads by using seven cycles of vortexing for 1 min followed by 1-min incubation on ice. Extracts were collected after centrifugation for 5 min at 3,000 rpm. The protein concentrations of the cell lysates were determined by the BCA protein assay (Pierce). Proteins were resolved by 10% SDS-PAGE and subsequently transferred to a nitrocellulose membrane. Membranes were blocked overnight at 4 °C with 5% nonfat dry milk in Buffer A (10 mm Tris-HCl, pH 7.4, 150 mm NaCl) plus 0.05% Tween 20. Incubations with primary and secondary antibodies were performed at room temperature for 1 h in buffer B (Buffer A containing 1% milk and 10% goat serum). Membranes were washed four times after antibody incubations with Buffer A containing 0.05% Tween 20. The primary antibody was monoclonal mouse 16B12 anti-HA (Covance, 1:500 dilution) and the secondary antibody was polyclonal goat anti-mouse horseradish peroxidase (Amersham Biosciences, 1:1000 dilution). Proteins were detected using ECL chemiluminescence (Amersham Biosciences). For a loading control, actin protein was detected using monoclonal rabbit anti-β-actin primary antibody (Rockland, 1:1000 dilution) and polyclonal goat anti-rabbit horseradish peroxidase secondary antibody (Amersham Biosciences, 1:2000 dilution).
Protein Phosphatase Assay—Phosphatase activity was measured for 15 min at 30 °C by following the dephosphorylation of phosphohistone in the absence and presence of C2-ceramide as previously described (50).
Synthetic Lethal Interaction Studies—Haploid matings between cdc55 cells and strains harboring null alleles of genes having a lethal genetic interaction with loss of RVS161 were carried out using standard procedures (61). Heterozygous diploids were sporulated, and double null haploid progeny were obtained by tetrad dissection. For each mating cross, the haploid segregants were allowed to grow on YEPD medium at 30 °C, and at least 96 asci were tested.
RESULTS
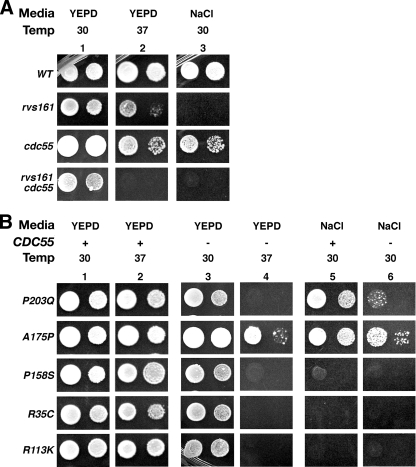
The B Regulatory Subunit of Yeast CAPP/PP2A, Cdc55, Is Required for the Viability of rvs161 Cells under Stress—While we were exploring whether CAPP had a role in sur4-dependent suppression of rvs defects, we discovered rvs161 cdc55 cells were inviable at 37 °C, while both rvs161 and cdc55 cells grew (Fig. 1A). In addition, we noted that loss of CDC55 did not remediate high salt rvs growth defects (6% NaCl).
FIGURE 1.
CDC55 is required for the viability of rvs161 cells during conditions of stress. A and B, 10-fold serial dilutions of cells were spotted on YEPD or YEPD plus 6% salt (NaCl) solid media. Cells were grown for 3 days at 30 °C or 37 °C.
To understand why Cdc55 was required for growth of rvs161 cells during stress, we examined how its loss affected the growth of cells harboring rvs161 mutant alleles isolated by Brizzio et al. (17). These alleles cause either endocytosis (End– Fus+) or cell fusion/mating (End+ Fus–) defects, thus separating specific Rvs161 functions. R35C, R59K, R113K, P158S, and P166S alleles cause endocytosis defects, whereas A175P and P203Q are cell fusion/mating-defective, based on several criteria (17). CDC55 was deleted in the two cell fusion (A175P and P203Q) and three endocytosis mutants (R35C, R113K, and P158S), and we determined whether these strains could grow at 37 °C or when under osmotic stress.
rvs161-R35C cdc55, rvs161-R113K cdc55, and rvs161-P158S cdc55 endo– cells were inviable at 37 °C (Fig. 1B, lanes 3 versus 4). Loss of CDC55 was also synthetic lethal in rvs161-P203Q fus– endo+ cells at this temperature, suggesting this allele may give rise to subtle stress-induced defects, which become more severe in the absence of Cdc55 function. rvs161-A175P cdc55 cells grew. Moreover, loss of CDC55 did not suppress the osmotic growth defect of rvs161-P158S, rvs161-R35C, and rvs161-R113K cells, and actually compromised the growth of rvs161-P203Q cells (Fig. 1B, lanes 5 versus 6). Thus CAPP and/or Cdc55-dependent PP2A are essential for cell growth under stress in the absence of Rvs161 endocytic function.
Suppression of rvs161 Defects by Loss of SUR4 Requires CDC55—Because rvs161 endo– cells required Cdc55 during heat stress, we tested whether this B regulatory subunit had a role in sur4-dependent suppression of rvs defects. We focused on the rvs osmotic growth defect as a representative mutant phenotype (18). As previously published, loss of SUR4 suppressed the high salt osmotic growth defect of rvs161 cells (18, 20) (Fig. 2A). Importantly, Cdc55 function was absolutely essential for rvs161 sur4 cells to grow under this condition (Fig. 2A, rvs161 sur4 versus rvs161 sur4 cdc55). Moreover, loss of Sur4 function remediated the high salt osmotic defect of rvs161 R35C, rvs161 R113K, and rvs161 P158S cells (Fig. 2B, lanes 1 versus 2), and in all cases Cdc55 was required for this suppression (Fig. 2B, lanes 2 versus 3).
FIGURE 2.
sur4-dependent suppression of rvs161 growth defects requires CDC55. A and B, Cells were grown and assayed as described for Fig. 1.
The Putative CAPP Catalytic Subunit, Sit4, Is Not Required for Sur4-dependent Suppressor Activity—It was important to determine what molecular species of PP2A/CAPP was required for sur4-dependent suppression. To delineate molecular structure, we first examined what was the consequence of deleting the PP2A catalytic subunits, Pph21 and Pph22 (49), or the putative CAPP catalytic subunit, Sit4 (50, 62), on osmotic stress-induced growth of rvs161 sur4 cells. Deletion of both Pph21 and Pph22, and not Sit4, attenuated sur4-dependent suppressor activity (Fig. 3). Loss of function of the PP2A/CAPP A regulatory subunit, Tpd3, also resulted in loss of viability of rvs161 sur4 cells (not shown). Thus, a heterotrimeric PP2A/CAPP species comprised of Tpd3/Cdc55/Pph21–22 is required for sphingolipid-dependent suppression of rvs161 growth defects under osmotic stress.
FIGURE 3.
CAPP and PP2A catalytic subunits are required for sur4-dependent suppression of rvs161 growth defects. Each strain was streaked for single colonies on YEPD or YEPD plus 6% salt (NaCl) solid media. Cells were grown for 2–3 days at 30 °C.
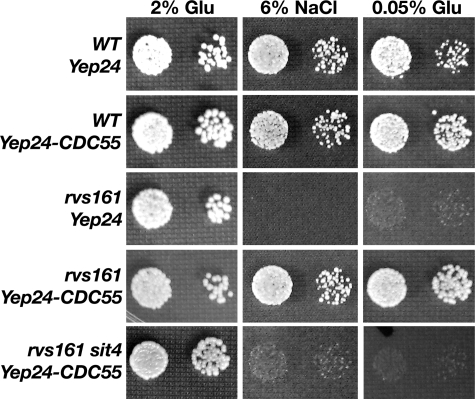
Activation of PP2A/CAPP through Overexpression of CDC55 Suppresses Multiple Growth Defects of rvs161 Cells—Our hypothesis is that during stress PP2A/CAPP is activated in a sur4-dependent manner and is essential in the absence of Rvs161 endocytic function. If this is the case, activation of PP2A/CAPP alone, through overexpression of Cdc55 should suppress rvs defects in the absence of any sphingolipid defects. Thus, we asked if increased expression of CDC55 could compensate for loss of Rvs161. The rvs161 mutant phenotypes examined were lack of growth under osmotic stress, under nutrient (glucose) starvation, and on a nonfermentable carbon source (not shown), as well as, the inability to mate (18).
Wild-type and rvs161 cells expressing either control Yep24-URA3 or Yep24-CDC55-URA3 were grown on selective medium containing 2% glucose, 2% glucose plus 6% NaCl, 2% glycerol, or 0.05% glucose, and viability was determined. CDC55 overexpression alone suppressed all rvs161 growth defects observed (Fig. 4, rvs161 Yep24 versus rvs161 Yep24-CDC55). Overexpression or deletion of CDC55 did not remediate the mating defects of rvs161 cells when quantitative limited mating assays were used (not shown). We next asked what catalytic subunits were necessary for Cdc55-dependent suppressor activity. Suppression required the presence of the putative CAPP catalytic subunit, Sit4 (Fig. 4, rvs161 Yep-CDC55 versus rvs161 sit4 Yep-CDC55) or the PP2A catalytic subunits, Pph21 and Pph22 (not shown). Thus, Cdc55 can form two CAPP species to suppress rvs defects that use the PP2A catalytic subunits, Pph21–22, or the putative CAPP subunit, Sit4.
FIGURE 4.
Activation of CAPP, through overexpression of CDC55, suppresses multiple growth defects of rvs161 cells. 10-fold serial dilutions of cells expressing either Yep24-URA3 or Yep24-CDC55-URA3 were spotted on synthetic rich (2% Glu), synthetic rich plus 6% salt (NaCl), or synthetic starvation (0.05% Glu) solid media and grown for 3 days at 30 °C.
Cdc55-dependent Suppressor Activity Does Not Function through Regulating Actin Polarization—rvs161 cells harbor defects in actin cytoskeletal structure (13), which contribute to the observed pleiotropic phenotypes. To ascertain how Cdc55 activation of PP2A/CAPP suppresses rvs161 defects, we first asked whether it does so through regulating actin dynamics during stress. Cells were grown to exponential phase and shifted to media containing 3.4% NaCl, and at various times the actin cytoskeleton was visualized using rhodamine phalloidin.
Under normal growth conditions, wild-type cells contained ∼91% polarized cells (Fig. 5A). Upon a shift to 3.4% NaCl, cells became depolarized by 30 min (5% polarized), and after 2 h adapted and repolarized their actin (60% polarized). rvs161 cells were constitutively depolarized (14% polarized) under normal growth conditions (Fig. 5A, WT versus rvs161). Shifting these cells to high salt resulted in the entire population becoming depolarized, and they remained depolarized. Cells lacking CDC55 were polarized (76% polarized), became depolarized after 30 min in high salt, but were defective in repolarizing their actin after 2 h (28% polarized) (Fig. 5A, WT versus cdc55). The actin dynamics of rvs161 cdc55 cells mimicked rvs161 cells (Fig. 5A, rvs161 versus rvs161 cdc55).
FIGURE 5.
Cdc55-dependent sur4 suppressor activity in rvs161 cells does not function through regulating actin polarization. Strains were grown in YEPD or selective minimal media at 30 °C to exponential phase, and at time zero, NaCl was added to a final concentration of 3.4% (A and B) or 6% (C). Actin structure was visualized at the indicated times using Rhodamine-phalloidin and fluorescence microscopy. Histogram showing the average percentage of polarized cells versus total number of cells was determined (n = 350). Error bars represent the standard deviation. D, cells lacking Rvs161 are viable on 3.4% NaCl. 10-fold serial dilutions of cells were spotted on YEPD, 3.4% NaCl, and 6% NaCl solid media. Cells were grown for 3 days at 30 °C.
Interestingly, when we overexpressed CDC55 in wild-type cells, either less cells depolarized their actin, or a greater number of cells repolarized more quickly after 30 min in high salt media (50% polarized) (Fig. 5, A, WT versus B, WT+Yep24-CDC55). Moreover, the percentage of polarized cells observed in high salt after 2 h was almost identical to that seen under normal vegetative growth conditions (92% polarized). CDC55 overexpression in rvs161 cells did remediate rvs-associated actin polarization defects (77% polarized), but only under normal vegetative growth conditions. Under high salt conditions, actin was completely depolarized, and it remained depolarized (Fig. 5, A, rvs161 versus B, rvs161+Yep24-CDC55). Similar results were seen in 6% NaCl. Thus, any regulation of actin dynamics by Cdc55 during stress requires Rvs161 function.
Loss of SUR4 Does Not Remediate the Actin Polarization Defects of rvs161 Cells under Lethal Osmotic Stress Conditions—Loss of SUR4 suppresses the actin polarization defect of rvs161 cells under osmotic stress, and it was suggested this remediation plays a role in sur4-dependent suppressor activity (55). However, studies were performed using 3.4% NaCl, a concentration sublethal for rvs161 cells (Fig. 5D). If sur4 suppressor activity functions through regulating actin depolarization/repolarization, and this is essential for rvs161 sur4 cells to grow under stress conditions, it should regulate actin dynamics at a NaCl concentration lethal to rvs161 cells, but not rvs161 sur4 cells.
We first examined actin dynamics in cells grown under sublethal 3.4% NaCl conditions. As published, the actin polarization defect of rvs161 cells was suppressed (Fig. 5A, rvs161 versus rvs161 sur4). However, Cdc55 function is not required for rvs161 sur4 cells to grow at this salt concentration, because rvs161 sur4 cdc55 cells are viable (not shown). Cdc55 is only required for sur4-dependent suppressor activity under lethal 6% NaCl conditions (Fig. 2A). Thus, we determined if loss of SUR4 suppressed the actin polarization defect of rvs161 cells grown under this lethal growth condition. This was not the case, because rvs161 sur4 cells remained depolarized in 6% NaCl for 2 h (Fig. 5C, rvs161 versus rvs161 sur4) and up to 5 h. Moreover, we observed steady-state actin defects in rvs161 sur4 cells grown exponentially in media containing 6% NaCl (not shown). Based on these results, we conclude that sur4-dependent suppressor activity and subsequent PP2A/CAPP activation does not regulate actin dynamics to ensure rvs161 cells remain viable under stress.
Sur4-dependent Suppressor Activity in rvs161 Cells Reinitiates Endocytosis of the Pheromone Receptor, Ste3, and Requires Cdc55—rvs161 cells harbor defects in endocytosis (15). Neither the overexpression of CDC55 nor loss of SUR4 suppressed the actin polarization defects of rvs161 cells. Thus we asked if the endocytosis defect of rvs161 cells was affected. We did so by determining the stability of the membrane-associated a-factor pheromone receptor, Ste3. Ste3 is subject to two modes of endocytosis, constitutive (or ligand-independent) and ligand-stimulated (63–67), leading to vacuolar-dependent degradation. Ste3 stability assays are frequently used when studying mutants defective in endocytosis (15, 68). We constructed strains carrying an endogenous chromosomal integrated galactose-inducible HA-tagged STE3 allele. Cells were grown to exponential phase, pulse/chase experiments were performed by addition and removal of galactose, and stability of Ste3 was examined by Western analysis.
Ste3 was unstable in wild-type cells, having a half-life of ∼20 min (Fig. 6A). In contrast, Ste3 levels in rvs161 cells remained stable for up to 45 min and gradually declined at 60 min. These results are similar to those previously published (15, 66). Loss of SUR4 in rvs161 cells reinitiated Ste3 degradation (Fig. 6A, rvs161 versus rvs161 sur4), and CDC55 was required for sur4 suppressor activity (Fig. 6A, rvs161 sur4 versus rvs161 sur4 cdc55). Although sur4 and cdc55 cells degraded Ste3 (Fig. 6B), sur4 cdc55 cells harbored a severe defect (Fig. 6B, sur4 versus sur4 cdc55). Thus Cdc55 function is essential for Ste3 degradation in cells lacking SUR4 alone. Loss of cdc55 in rvs161 cells did not restore normal Ste3 degradation (Fig. 6A, rvs161 versus rvs161 cdc55).
FIGURE 6.
CDC55 regulates the endocytosis of the a-factor receptor, Ste3. All strains carry a STE3::GAL-STE3-HA::TRP1 allele. Strains were grown to exponential phase in YEPD or selective minimal media at 30 °C, shifted to YP or selective minimal media containing 2% raffinose for 1.5 h, and then shifted to YP or selective minimal media containing 2% galactose for 3 h to induce expression of Ste3-HA. To shut off expression of Ste3-HA, yeast strains were shifted to YEPD or selective media containing 2% glucose with (E) or without 3.4% NaCl (A–D). Samples were taken at the indicated times following the initial shut-off by glucose. Ste3-HA levels were determined by Western analysis. Actin levels (lower panel) were used as a loading control.
CDC55 Overexpression Restores Ste3 Endocytosis in rvs161 Cells—We next determined whether CDC55 overexpression and subsequent PP2A/CAPP activation leads to reinitiation of endocytosis. This was the case, as CDC55 overexpression alone reduced Ste3 levels in rvs161 cells grown under normal vegetative conditions. In fact, rvs161 cells overexpressing CDC55 had dramatically less steady-state levels of Ste3 compared with wild-type cells (Fig. 6C). The deletion of PEP4, which encodes the vacuolar proteinase A required for endocytosis-dependent degradation of Ste3 (63), restored Ste3 accumulation (Fig. 6, C, rvs161 Yep24-CDC55 versus D, pep4 rvs161 Yep24-CDC55). Therefore, Ste3 is being expressed and produced to wild-type levels, and its disappearance is solely due to vacuolar-dependent proteolysis.
If CDC55 overexpression suppresses rvs161 defects through reinitiation of endocytosis, it should do so under stress conditions; thus we tested this hypothesis. Under osmotic stress, Ste3 levels still did not accumulate in rvs161 cells overexpressing CDC55 (Fig. 6E, rvs161 versus rvs161 Yep24-CDC55).
CDC55 Overexpression or Loss of SUR4 Reinitiates Fluid-phase Endocytosis in rvs161 Cells—rvs161 cells harbor defects in receptor-mediated and fluid phase endocytosis (15). Thus, we asked whether CDC55 overexpression could restore fluid-phase endocytosis to mutant cells. We did so by monitoring the time-dependent internalization and vacuolar localization of the fluid-phase endocytic maker, lucifer yellow. CDC55 was overexpressed as described under “Experimental Procedures” using YIp-GPD-CDC55-URA3. Cells were designated as having either rim staining (plasma membrane), or rim and vacuolar staining.
Lucifer yellow staining in wild-type cells was almost completely localized to the rim and vacuole (∼99%), whereas most rvs161 cells showed only rim staining (∼85%) (Fig. 7, WT GPD versus rvs161 GPD). Overexpression of CDC55 in rvs161 cells resulted in a ∼3.5-fold increase in the percentage of cells with rim and vacuolar staining (Fig. 7, rvs161 GPD versus rvs161 GPD-CDC55). We next asked what PP2A/CAPP catalytic subunits were required. The putative CAPP catalytic subunit, Sit4, and the PP2A catalytic subunits, Pph21/22, were required for the observed Cdc55-dependent increase, as lucifer yellow staining returned to the rim of CDC55-overexpressing rvs161 cells lacking these catalytic subunits (92–98%) (Fig. 7, rvs161 GPD-CDC55 versus rvs161 sit4 GPD-CDC55 and rvs161 pph21 pph22 GPD-CDC55). Finally, we found no difference in lucifer yellow internalization between wild-type and rvs161 sur4 cells (not shown). Based on these results, we conclude PP2A/CAPP activation, either by CDC55 overexpression or loss of SUR4, results in reinitiating endocytosis, which is essential for the viability of rvs161 cells under stress.
FIGURE 7.
CDC55 regulates the internalization of lucifer yellow. LY internalization was assayed in cells containing the GPD promoter alone or CDC55 under the regulation of the GPD promoter. Cells were grown to early logarithmic phase in rich medium at 30 °C, and then allowed to internalize LY at 30 °C for 60 min. The average percentage of cells that accumulated LY versus total number of cells was determined (n = 350) and standard deviations are indicated.
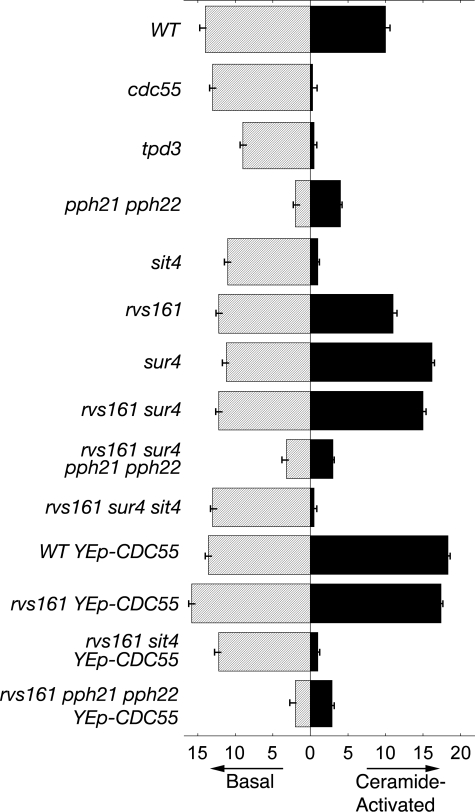
CAPP Activity Is Elevated in Cells Overexpressing CDC55 or Lacking SUR4—Our hypothesis is that PP2A/CAPP activation is a necessary event for bypassing the need for Rvs161 function during stress. Thus, we asked if loss of SUR4 or overexpression of CDC55 causes an increase in in vitro CAPP activity. We measured basal/ceramide-independent (Fig. 8, shaded bars) and ceramide-activated (Fig. 8, solid bars) protein phosphatase activities as described (50).
FIGURE 8.
Loss of SUR4 or the overexpression of CDC55 increases CAPP activity. Cytosolic extracts were assayed for basal PP2A activity (shaded bars) and CAPP activity (solid bars) as described under “Experimental Procedures” using 5 μm [32P]phosphohistone as a substrate. The results are the average values of two separate experiments.
In all strains tested, the majority of the basal/ceramide-independent catalytic phosphatase activity required the presence of the PP2A subunits, Pph21/Pph22, and not the CAPP catalytic subunit, Sit4 (Fig. 8, shaded bars). CAPP activity was absolutely dependent on Cdc55 and Tpd3 functions (Fig. 8, solid bars, WT versus cdc55, tpd3). These results are in good agreement with published work (50). In contrast to what was previously published, our results indicate two CAPP species can be activated and are comprised of Tpd3/Cdc55/Sit4 and Tpd3/Cdc55/Pph21-Pph22. Strains lacking Pph21 and Pph22 together, or Sit4 alone, had severely reduced CAPP activity (Fig. 8, WT versus sit4 or pph21 pph22). Thus Cdc55 can target either Sit4 or Pph21/Pph22 for ceramide activation.
The loss of SUR4 in rvs161 cells caused an increase in a CAPP activity (∼1.7-fold) (Fig. 8, WT versus rvs161 sur4) that was lost when either PPH21 and PPH22, or SIT4, were deleted (Fig. 8, rvs161 sur4 versus rvs161 sur4 pph21 pph22 or rvs161 sur4 sit4). Identical results were obtained when we examined the relationship between CDC55 overexpression and CAPP activity (∼1.9-fold) (Fig. 8, WT Yep-CDC55, rvs161 Yep-CDC55 versus rvs161 sit4 Yep-CDC55, rvs161 pph21 pph22 Yep-CDC55). Overexpression of CDC55 alone increased a CAPP activity that was dependent on Pph21 and Pph22, or Sit4. Thus, mechanisms suppressing rvs161 endocytosis defects, namely loss of SUR4 or CDC55 overexpression, increase CAPP activity in cells (Figs. 2, 6, and 7). When CAPP activity is lost, both through deletion of PPH21 and PPH22, or SIT4, suppressor activity is lost and this correlates with the reappearance of endocytosis defects in mutant cells (Figs. 2, 3, 6, and 7).
Lethal Genetic Interactions Exist between Loss of CDC55 and Loss of Factors Regulating Endocytosis and/or Actin Cytoskeletal Structure—Our hypothesis is PP2A/CAPP functions under stress to regulate endocytosis. To begin to uncover factors regulated by PP2A/CAPP when cells are stressed, we determined whether lethal genetic interactions existed between loss of CDC55 and loss of genes synthetic lethal with loss of RVS161 (20, 69, 70). These genes regulate endocytosis and/or actin cytoskeletal organization (69, 71–74, 75). Double null haploid cells were generated and tested as described under “Experimental Procedures.”
Loss of CDC55 was synthetic lethal with loss of SLA2 (Table 2), which encodes an adaptor protein involved in membrane cytoskeleton assembly (72), and loss of VPS20, which encodes for a subunit of the endosomal sorting complex, ESCRTIII, required for transport of transmembrane proteins from multivesicular bodies to the lysosomal/vacuolar lumen (73). Loss of CDC55 was also synthetic lethal at 37 °C with loss of ABP1. ABP1 encodes for an actin-binding protein, which regulates actin cytoskeleton through binding to the SH3 domain of Rvs167 (69, 71).
TABLE 2.
cdc55 synthetic lethal genetic interactions with genes regulating endocytosis Haploid progeny from crosses were grown on rich media for 72 h at 30 °C or 37 °C.
| Strain | 30 °C | 37 °C |
|---|---|---|
| rvs161 | + | + |
| cdc55 | + | + |
| rvs161 cdc55 | + | - |
| abp1 | + | + |
| abp1 cdc55 | + | - |
| sac6 | + | - |
| sac6 cdc55 | + | - |
| sla1 | + | - |
| sla1 cdc55 | + | - |
| sla2 | + | - |
| sla2 cdc55 | - | - |
| vps20 | + | - |
| vps20 cdc55 | - | - |
| vps21 | + | - |
| vps21 cdc55 | + | - |
In contrast, cdc55 sla1, cdc55 sac6, and cdc55 vps21 mutants were all viable. Sla1 interacts with proteins that regulate actin dynamics and/or are required for endocytosis, such as Rvs167 (76). Sac6, also known as fimbrin, is an actin-bundling protein important in the organization and maintenance of the actin cytoskeleton (74), whereas Vps21, a mammalian Rab5 homolog, is a GTPase required for transport during endocytosis and for correct sorting of vacuolar hydrolases (72, 75). Thus Cdc55, and presumably PP2A/CAPP, genetically interacts with a specific subset of factors involved in regulating endocytosis and the actin cytoskeleton.
PP2A/CAPP Can Only Compensate for rvs161 Function under Stress—Our results show that PP2A/CAPP can replace Rvs161 function under stress (Fig. 4). Thus, we asked whether PP2A/CAPP activation through CDC55 overexpression could compensate for loss of SLA1, SLA2, SAC6, or VPS20 and suppress the ts stress phenotype of cells lacking these genes. This was not the case, because sla1, sla2, sac6, and vps20 cells overexpressing CDC55 remained incapable of growing at high temperature. Thus PP2A/CAPP can only compensate for Rvs161 function under stress.
DISCUSSION
We have presented evidence clearly showing sphingolipid-dependent suppression of rvs defects functions through the PP2A/CAPP-dependent reinitiation of endocytosis. Loss of SUR4 suppresses the lethal NaCl osmotic growth defect of rvs161 cells (Fig. 2) (18, 20). Loss of SUR4 does not remediate the actin defects of mutant cells under this condition (Fig. 5), however, it does reinitiate endocytosis (Fig. 6). Suppression and reinitiation of endocytosis by loss of SUR4 requires a PP2A/CAPP composed of Tpd3/Cdc55/Pph21-Pph22 (Figs. 2 and 3). Moreover, direct activation of PP2A/CAPP, through CDC55 overexpression, suppressed the growth (Fig. 4) and endocytosis defects (Figs. 6 and 7) of rvs161 cells. It did not remediate their actin defects (Fig. 5). Interestingly, in this case Cdc55 targeted either Pph21/Pph22 or Sit4 for CAPP activity (Fig. 7).
Thus, a second important result from our study is that two CAPP species can be formed in yeast cells. This is borne out by our in vitro CAPP assays, where decreased CAPP activity was observed in pph21 pph22 and sit4 cells (Fig. 8). Heterotrimeric CAPP activities have been purified from glioma cells and rat brain (39, 42). They are members of the PP2A family, and they contain catalytic subunits orthologous to Pph21/Pph22. Small interference RNA-mediated depletion of this catalytic subunit in rat insulin-secreting cells (INS 832/13) significantly reduces CAPP activity (77), as does combined loss of PPH21 and PPH22 in yeast cells (Fig. 8).
The human ortholog of the putative CAPP catalytic subunit, Sit4, is the phosphatase, Pp6 (78). Sit4 may be the target of viral-induced cell death of yeast, through its association with Cdc55. Yeast cells are killed by overexpression of the adenovirus early region 4 open reading frame 4 (E4orf4), and this E4orf4-induced toxicity can be suppressed by deleting CDC55 (79–81). This suggests E4orf4 uses Cdc55 to target a phosphatase. The catalytic subunit may be Sit4, because sit4 cells are highly sensitive to E4orf4 killing, and this phenotype cannot be suppressed by deletion of CDC55 (79). Human cells lacking Pp6 are also highly sensitive to killing by E4orf4 expression (79). To date, there is no direct evidence that Cdc55 and Sit4, or their metazoan counterparts, directly interact. However, association may only occur under very specific circumstances, like the stress of a viral assault.
Heat stress increases the levels of several ceramide species in S. cerevisiae through de novo biosynthesis (29), whereas the largest heat-induced increase of sphingoid base includes phytosphingosine and dihydrosphingosine (32, 34). sur4 mutants constitutively accumulate the long-chain sphingoid base, phytosphingosine, and they have reduced levels of ceramide and complex sphingolipids (19). Based on these results, phytosphingosine is the only common sphingolipid accumulating that could directly activate CAPP.
In vitro CAPP assays have extensively examined the lipid requirements for CAPP activation (38, 41, 42, 82, 83). CAPP can be activated by C2-, C6-, and C18-ceramides, as well as natural ceramides derived from bovine brain. Sphingosine, sphingolmyelin, and diacylglycerol have also been tested and do not activate CAPP. Structural requirements for CAPP activation by ceramide include the presence of the 4,5-trans double bond in the sphingoid base, and the primary and secondary hydroxyl groups of the sphingoid backbone. Although ceramide has long been recognized as the putative lipid activator of CAPP, there are reports that palmitate (84, 85), linoleic acid (86), and cholesterol (87) can also stimulate activity. Thus, lipids other than ceramide can be activators of CAPP.
lcb1–100ts cells are defective in endocytosis at high temperature; lcb1–100ts cells harbor a weakened serine pamitoyltransferase activity required for the first step in sphingolipid biosynthesis (88). The addition of exogenous phytosphingosine to these cells restores endocytosis (89). Riezman and colleagues (53) showed loss of Cdc55, or overexpression of the yeast casein kinase 2, Yck2, or protein kinase C, Pkc1, restores endocytosis to lcb1–100ts cells. Based on these results, they suggest Cdc55 functions to negatively regulate the activities of Yck2 and/or Pkc1 in response to a sphingolipid generated signal. Our results show Cdc55-dependent PP2A/CAPP acts as a sphingolipid-dependent positive regulator of endocytosis, because its loss causes the reappearance of endocytosis defects. The fact that PP2A/CAPP activity can be differentially modulated by various lipid signals adds an additional tier of sophistication for regulating endocytosis.
The overexpression of SIT4 or loss of SUR4, remediates the endocytic/exocytic defects of cells lacking specific v- or t-SNARES (51, 52). Defects are suppressed through Sit4-dependent regulation of the phosphorylation states of the plasma membrane t-SNARES, Sso1/Sso2, or v-SNARES Tlg1/Tlg2 (51, 52). The roles of Tpd3, Cdc55, Pph21, and Pph22 were not explored as they were in the present study, nor was it tested whether SIT4 overexpression or loss of SUR4 suppress the endocytosis defects of these mutants when they are under osmotic stress. So the exact molecular species of PP2A/CAPP were not resolved.
Our synthetic lethal studies indicate stress-induced PP2A/CAPP regulates some Rvs161-dependent function (Table 2). Rvs161 functions to “sense” and induce membrane curvature through its N-terminal BAR domain (1). rvs161 mutants accumulate vesicles at the plasma membrane (16, 90), and synthetic lethal screening has establishing a putative role for Rvs161 in vesicle trafficking (70). Two-hybrid screens using Rvs161 as bait (20, 91) have not uncovered putative interactions with proteins regulating vesicle secretion. Rvs167, which also contains a BAR domain and binds Rvs161, interacts by two-hybrid with Sec8, Sec20, and Exo70, which are involved in vesicle transport (Table 2 (91).
Based on our results, we have put forth a model to explain how multiple PP2A/CAPP species can be targeted and activated (Fig. 9). rvs161 cells harbor endocytosis defects under stress conditions, which causes cell death (Fig. 9A). During some types of stress, Cdc55 levels increase, modeled by our overexpression studies (Fig. 9B). The increase in Cdc55 levels shifts the cellular balance of PP2A from predominately a single Tpd3/Rts1/Pph21–22 heterotrimer to at least two species comprised of Tpd3/Cdc55/Pph21–22 and Tpd3/Cdc55/Sit4; Rts1 is the other PP2A B regulatory subunit in yeast (Table 1), and it is in 10-fold excess compared with Cdc55 (92). The reshuffling of PP2A activities results in targeting cell factors regulating endocytosis, restoring viability to rvs161 cells.
FIGURE 9.
Model detailing how multiple PP2A/CAPP species can be activated. A, rvs161 cells harbor defects in endocytosis and do not survive under osmotic stress. B, the overexpression of CDC55 or, C, the loss of SUR4 increases PP2A/CAPP activity resulting in the reinitiation of endocytosis and survival under osmotic stress.
The PP2A catalytic subunits, Pph21 and Pph22, and the CAPP catalytic subunit, Sit4, are required. Here, PP2A/CAPP activation occurs in a sphingolipid-independent manner. Microarray analyses have shown CDC55 expression increases in response to heat shock, nitrogen depletion, and during stationary phase (93). rvs61 cells are inviable when starved for nitrogen, which results in their premature entry into stationary phase (12).
When a specific stress generates a PP2A/CAPP-responsive sphingolipid signal, activation occurs and functions through the PP2A catalytic subunits, Pph21 and Pph22 (Fig. 9C). Cdc55 levels remain constant, and its affinity for Pph21 and Pph22, and not Sit4, is increased due to post-translational modifications and/or changes in Cdc55-protein interactions. Thus, the Cdc55 activity state is regulated by various stresses to ultimately activate and regulate the substrate specificities of multiple PP2A/CAPP species.
Acknowledgments
We thank Drs. Mark Rose and Valeria Brizzio for the rvs point mutant strains. We are grateful for the helpful discussions with the Bergman, Haines, and Edlind laboratories. We appreciate discussions with Drs. Martin Adelson, Eli Mordechai, Jason Trama, Scott Gygax, Kathy Iacono, John Hoey, and John Blaho.
This work was supported, in whole or in part, by National Institutes of Health Grant HL67401. This work was also supported by Medical Diagnostics Laboratories, LLC.
Footnotes
The abbreviations used are: BAR, BIN/amphiphysin/RVS domain; CAPP, ceramide-activated protein phosphatase; PP2A, protein phosphatase 2A; RVS, reduced viability upon starvation; SNARE, soluble NSF attachment protein receptors; LY, luciferase yellow.
References
- 1.Ren, G., Vajjhala, P., Lee, J. S., Winsor, B., and Munn, A. L. (2006) Microbiol. Mol. Biol. Rev. 70 37–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallop, J. L., and McMahon, H. T. (2005) Biochem. Soc. Symp. 72 223–231 [DOI] [PubMed] [Google Scholar]
- 3.Peter, B. J., Kent, H. M., Mills, I. G., Vallis, Y., Butler, P. J., Evans, P. R., and McMahon, H. T. (2004) Science 303 495–499 [DOI] [PubMed] [Google Scholar]
- 4.Zhang, B., and Zelhof, A. C. (2002) Traffic 3 452–460 [DOI] [PubMed] [Google Scholar]
- 5.Sivadon, P., Crouzet, M., and Aigle, M. (1997) FEBS Lett. 417 21–27 [DOI] [PubMed] [Google Scholar]
- 6.Itoh, T., Erdmann, K. S., Roux, A., Habermann, B., Werner, H., and De Camilli, P. (2005) Dev. Cell 9 791–804 [DOI] [PubMed] [Google Scholar]
- 7.Zimmerberg, J., and McLaughlin, S. (2004) Curr. Biol. 14 R250–R252 [DOI] [PubMed] [Google Scholar]
- 8.Takei, K., Slepnev, V. I., Haucke, V., and De Camilli, P. (1999) Nat. Cell Biol. 1 33–39 [DOI] [PubMed] [Google Scholar]
- 9.Lee, E., Marcucci, M., Daniell, L., Pypaert, M., Weisz, O. A., Ochoa, G. C., Farsad, K., Wenk, M. R., and De Camilli, P. (2002) Science 297 1193–1196 [DOI] [PubMed] [Google Scholar]
- 10.Farsad, K., Ringstad, N., Takei, K., Floyd, S. R., Rose, K., and De Camilli, P. (2001) J. Cell Biol. 155 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlton, J., Bujny, M., Peter, B. J., Oorschot, V. M., Rutherford, A., Mellor, H., Klumperman, J., McMahon, H. T., and Cullen, P. J. (2004) Curr. Biol. 14 1791–1800 [DOI] [PubMed] [Google Scholar]
- 12.Crouzet, M., Urdaci, M., Dulau, L., and Aigle, M. (1991) Yeast 7 727–743 [DOI] [PubMed] [Google Scholar]
- 13.Sivadon, P., Bauer, F., Aigle, M., and Crouzet, M. (1995) Mol. Gen. Genet. 246 485–495 [DOI] [PubMed] [Google Scholar]
- 14.Durrens, P., Revardel, E., Bonneu, M., and Aigle, M. (1995) Curr. Genet. 27 213–216 [DOI] [PubMed] [Google Scholar]
- 15.Munn, A. L., Stevenson, B. J., Geli, M. I., and Riezman, H. (1995) Mol. Biol. Cell 6 1721–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breton, A. M., Schaeffer, J., and Aigle, M. (2001) Yeast 18 1053–1068 [DOI] [PubMed] [Google Scholar]
- 17.Brizzio, V., Gammie, A. E., and Rose, M. D. (1998) J. Cell Biol. 141 567–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desfarges, L., Durrens, P., Juguelin, H., Cassagne, C., Bonneu, M., and Aigle, M. (1993) Yeast 9 267–277 [DOI] [PubMed] [Google Scholar]
- 19.Oh, C. S., Toke, D. A., Mandala, S., and Martin, C. E. (1997) J. Biol. Chem. 272 17376–17384 [DOI] [PubMed] [Google Scholar]
- 20.Germann, M., Swain, E., Bergman, L., and Nickels, J. T., Jr. (2005) J. Biol. Chem. 280 4270–4278 [DOI] [PubMed] [Google Scholar]
- 21.Dickson, R. C. (1998) Annu. Rev. Biochem. 67 27–48 [DOI] [PubMed] [Google Scholar]
- 22.Masserini, M., and Ravasi, D. (2001) Biochim. Biophys. Acta 1532 149–161 [DOI] [PubMed] [Google Scholar]
- 23.Hannun, Y. A. (1996) Science 274 1855–1859 [DOI] [PubMed] [Google Scholar]
- 24.Smyth, M. J., Obeid, L. M., and Hannun, Y. A. (1997) Adv. Pharmacol. 41 133–154 [DOI] [PubMed] [Google Scholar]
- 25.Kolesnick, R. N., and Kronke, M. (1998) Annu. Rev. Physiol. 60 643–665 [DOI] [PubMed] [Google Scholar]
- 26.Dickson, R. C., and Lester, R. L. (2002) Biochim. Biophys. Acta 1583 13–25 [DOI] [PubMed] [Google Scholar]
- 27.Ohanian, J., and Ohanian, V. (2001) Cell Mol. Life Sci. 58 2053–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickson, R. C., Sumanasekera, C., and Lester, R. L. (2006) Prog. Lipid Res. 45 447–465 [DOI] [PubMed] [Google Scholar]
- 29.Wells, G. B., Dickson, R. C., and Lester, R. L. (1998) J. Biol. Chem. 273 7235–7243 [DOI] [PubMed] [Google Scholar]
- 30.Dickson, R. C., Nagiec, E. E., Skrzypek, M., Tillman, P., Wells, G. B., and Lester, R. L. (1997) J. Biol. Chem. 272 30196–30200 [DOI] [PubMed] [Google Scholar]
- 31.Jenkins, G. M., Richards, A., Wahl, T., Mao, C., Obeid, L., and Hannun, Y. (1997) J. Biol. Chem. 272 32566–32572 [DOI] [PubMed] [Google Scholar]
- 32.Jenkins, G. M. (2003) Cell Mol. Life Sci. 60 701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skrzypek, M. S., Nagiec, M. M., Lester, R. L., and Dickson, R. C. (1998) J. Biol. Chem. 273 2829–2834 [DOI] [PubMed] [Google Scholar]
- 34.Jenkins, G. M., and Hannun, Y. A. (2001) J. Biol. Chem. 276 8574–8581 [DOI] [PubMed] [Google Scholar]
- 35.Liu, J., Mathias, S., Yang, Z., and Kolesnick, R. N. (1994) J. Biol. Chem. 269 3047–3052 [PubMed] [Google Scholar]
- 36.Muller, G., Ayoub, M., Storz, P., Rennecke, J., Fabbro, D., and Pfizenmaier, K. (1995) EMBO J. 14 1961–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fishbein, J. D., Dobrowsky, R. T., Bielawska, A., Garrett, S., and Hannun, Y. A. (1993) J. Biol. Chem. 268 9255–9261 [PubMed] [Google Scholar]
- 38.Chalfant, C. E., Kishikawa, K., Mumby, M. C., Kamibayashi, C., Bielawska, A., and Hannun, Y. A. (1999) J. Biol. Chem. 274 20313–20317 [DOI] [PubMed] [Google Scholar]
- 39.Galadari, S., Kishikawa, K., Kamibayashi, C., Mumby, M. C., and Hannun, Y. A. (1998) Biochemistry 37 11232–11238 [DOI] [PubMed] [Google Scholar]
- 40.Chalfant, C. E., Kishikawa, K., Bielawska, A., and Hannun, Y. A. (2000) Methods Enzymol. 312 420–428 [DOI] [PubMed] [Google Scholar]
- 41.Chalfant, C. E., Szulc, Z., Roddy, P., Bielawska, A., and Hannun, Y. A. (2004) J. Lipid Res. 45 496–506 [DOI] [PubMed] [Google Scholar]
- 42.Dobrowsky, R. T., Kamibayashi, C., Mumby, M. C., and Hannun, Y. A. (1993) J. Biol. Chem. 268 15523–15530 [PubMed] [Google Scholar]
- 43.Wera, S., and Hemmings, B. A. (1995) Biochem. J. 311 17–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janssens, V., and Goris, J. (2001) Biochem. J. 353 417–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Healy, A. M., Zolnierowicz, S., Stapleton, A. E., Goebl, M., DePaoli-Roach, A. A., and Pringle, J. R. (1991) Mol. Cell Biol. 11 5767–5780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shu, Y., Yang, H., Hallberg, E., and Hallberg, R. (1997) Mol. Cell Biol. 17 3242–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao, Y., Boguslawski, G., Zitomer, R. S., and DePaoli-Roach, A. A. (1997) J. Biol. Chem. 272 8256–8262 [DOI] [PubMed] [Google Scholar]
- 48.van Zyl, W., Huang, W., Sneddon, A. A., Stark, M., Camier, S., Werner, M., Marck, C., Sentenac, A., and Broach, J. R. (1992) Mol. Cell Biol. 12 4946–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sneddon, A. A., Cohen, P. T., and Stark, M. J. (1990) EMBO J. 9 4339–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nickels JT, B. J. (1996) Genes Dev. 10 382–394 [DOI] [PubMed] [Google Scholar]
- 51.Gurunathan, S., Marash, M., Weinberger, A., and Gerst, J. E. (2002) Mol. Biol. Cell 13 1594–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marash, M., and Gerst, J. E. (2001) EMBO J. 20 411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friant, S., Zanolari, B., and Riezman, H. (2000) EMBO J. 19 2834–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Efendiev, R., Yudowski, G. A., Zwiller, J., Leibiger, B., Katz, A. I., Berggren, P. O., Pedemonte, C. H., Leibiger, I. B., and Bertorello, A. M. (2002) J. Biol. Chem. 277 44108–44114 [DOI] [PubMed] [Google Scholar]
- 55.Balguerie, A., Bagnat, M., Bonneu, M., Aigle, M., and Breton, A. M. (2002) Eukaryot. Cell 1 1021–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ito, H., Fukuda, Y., Murata, K., and Kimura, A. (1983) J. Bacteriol. 153 163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rothstein, R. J. (1983) Methods Enzymol. 101 202–211 [DOI] [PubMed] [Google Scholar]
- 58.Longtine, M. S., McKenzie, A., 3rd, Demarini, D. J., Shah, N. G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J. R. (1998) Yeast 14 953–961 [DOI] [PubMed] [Google Scholar]
- 59.Adams, A. E., and Pringle, J. R. (1991) Methods Enzymol. 194 729–731 [DOI] [PubMed] [Google Scholar]
- 60.Hsiung, Y. G., Chang, H. C., Pellequer, J. L., La Valle, R., Lanker, S., and Wittenberg, C. (2001) Mol. Cell Biol. 21 2506–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sherman, F. (1991) Methods Enzymol. 194 3–21 [DOI] [PubMed] [Google Scholar]
- 62.Arndt, K. T., Styles, C. A., and Fink, G. R. (1989) Cell 56 527–537 [DOI] [PubMed] [Google Scholar]
- 63.Davis, N. G., Horecka, J. L., and Sprague, G. F., Jr. (1993) J. Cell Biol. 122 53–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roth, A. F., and Davis, N. G. (1996) J. Cell Biol. 134 661–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roth, A. F., Sullivan, D. M., and Davis, N. G. (1998) J. Cell Biol. 142 949–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen, L., and Davis, N. G. (2000) J. Cell Biol. 151 731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roth, A. F., and Davis, N. G. (2000) J. Biol. Chem. 275 8143–8153 [DOI] [PubMed] [Google Scholar]
- 68.Naqvi, S. N., Zahn, R., Mitchell, D. A., Stevenson, B. J., and Munn, A. L. (1998) Curr. Biol. 8 959–962 [DOI] [PubMed] [Google Scholar]
- 69.Lila, T., and Drubin, D. G. (1997) Mol. Biol. Cell 8 367–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Friesen, H., Humphries, C., Ho, Y., Schub, O., Colwill, K., and Andrews, B. (2006) Mol. Biol. Cell 17 1306–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drubin, D. G., Mulholland, J., Zhu, Z. M., and Botstein, D. (1990) Nature 343 288–290 [DOI] [PubMed] [Google Scholar]
- 72.Holtzman, D. A., Yang, S., and Drubin, D. G. (1993) J. Cell Biol. 122 635–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeo, S. C., Xu, L., Ren, J., Boulton, V. J., Wagle, M. D., Liu, C., Ren, G., Wong, P., Zahn, R., Sasajala, P., Yang, H., Piper, R. C., and Munn, A. L. (2003) J. Cell Sci. 116 3957–3970 [DOI] [PubMed] [Google Scholar]
- 74.Adams, A. E., Botstein, D., and Drubin, D. G. (1991) Nature 354 404–408 [DOI] [PubMed] [Google Scholar]
- 75.Horazdovsky, B. F., Busch, G. R., and Emr, S. D. (1994) EMBO J. 13 1297–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stamenova, S. D., Dunn, R., Adler, A. S., and Hicke, L. (2004) J. Biol. Chem. 279 16017–16025 [DOI] [PubMed] [Google Scholar]
- 77.Jangati, G. R., Veluthakal, R., and Kowluru, A. (2006) Biochem. Biophys. Res. Commun. 348 649–652 [DOI] [PubMed] [Google Scholar]
- 78.Bastians, H., and Ponstingl, H. (1996) J. Cell Sci. 109 2865–2874 [DOI] [PubMed] [Google Scholar]
- 79.Li, Y., Wei, H., Hsieh, T. C., and Pallas, D. C. (2008) J. Virol. 82 3612–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roopchand, D. E., Lee, J. M., Shahinian, S., Paquette, D., Bussey, H., and Branton, P. E. (2001) Oncogene 20 5279–5290 [DOI] [PubMed] [Google Scholar]
- 81.Kornitzer, D., Sharf, R., and Kleinberger, T. (2001) J. Cell Biol. 154 331–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dobrowsky, R. T., and Hannun, Y. A. (1993) Adv. Lipid Res. 25 91–104 [PubMed] [Google Scholar]
- 83.Dobrowsky, R. T., and Hannun, Y. A. (1992) J. Biol. Chem. 267 5048–5051 [PubMed] [Google Scholar]
- 84.Mott, D. M., Stone, K., Gessel, M. C., Bunt, J. C., and Bogardus, C. (2008) Am. J. Physiol. 294 E444–E450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu, Y., Song, P., Xu, J., Zhang, M., and Zou, M. H. (2007) J. Biol. Chem. 282 9777–9788 [DOI] [PubMed] [Google Scholar]
- 86.Liu, J., and Sidell, N. (2005) Breast Cancer Res. Treat. 94 161–169 [DOI] [PubMed] [Google Scholar]
- 87.Wang, P. Y., Liu, P., Weng, J., Sontag, E., and Anderson, R. G. (2003) EMBO J. 22 2658–2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nagiec, M. M., Baltisberger, J. A., Wells, G. B., Lester, R. L., and Dickson, R. C. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 7899–7902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chung, N., Jenkins, G., Hannun, Y. A., Heitman, J., and Obeid, L. M. (2000) J. Biol. Chem. 275 17229–17232 [DOI] [PubMed] [Google Scholar]
- 90.Gammie, A. E., Brizzio, V., and Rose, M. D. (1998) Mol. Biol. Cell 9 1395–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bon, E., Recordon-Navarro, P., Durrens, P., Iwase, M., Toh, E. A., and Aigle, M. (2000) Yeast 16 1229–1241 [DOI] [PubMed] [Google Scholar]
- 92.Gentry, M. S., and Hallberg, R. L. (2002) Mol. Biol. Cell 13 3477–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gasch, A. P., Spellman, P. T., Kao, C. M., Carmel-Harel, O., Eisen, M. B., Storz, G., Botstein, D., and Brown, P. O. (2000) Mol. Biol. Cell 11 4241–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]