Abstract
Recent studies in receptor-transfected cell lines have demonstrated that extracellular signal-regulated kinase (ERK) activation by angiotensin type 1A receptor and other G protein-coupled receptors can be mediated by both G protein-dependent and β-arrestin-dependent mechanisms. However, few studies have explored these mechanisms in primary cultured cells expressing endogenous levels of receptors. Accordingly, here we utilized the β-arrestin biased agonist for the angiotensin type 1A receptor, SII-angiotensin (SII), and RNA interference techniques to investigate angiotensin II (ANG)-activated β-arrestin-mediated mitogenic signaling pathways in rat vascular smooth muscle cells. Both ANG and SII induced DNA synthesis via the ERK activation cascade. Even though SII cannot induce calcium influx (G protein activation) after receptor stimulation, it does cause ERK activation, although less robustly than ANG. Activation by both ligands is diminished by depletion of β-arrestin2 by small interfering RNA, although the effect is more complete with SII. ERK activation at early time points but not later time points is strongly inhibited by those protein kinase C inhibitors that can block protein kinase Cζ. Moreover, ANG- and SII-mediated ERK activation require transactivation of the epidermal growth factor receptor via metalloprotease 2/9 and Src kinase. β-Arrestin2 facilitates ANG and SII stimulation of Src-mediated phosphorylation of Tyr-845 on the EGFR, a known site for Src phosphorylation. These studies delineate a convergent mechanism by which G protein-dependent and β-arrestin-dependent pathways can independently mediate ERK-dependent transactivation of the EGFR in vascular smooth muscle cells thus controlling cellular proliferative responses.
G protein-coupled receptors, also known as seven transmembrane (7TM)2 receptors, control virtually all known physiological processes in mammals (1). The various functions of these receptors are mediated and modulated by three families of proteins, which share the property that they interact virtually universally with the receptors in a strictly stimulus-dependent way (1). These three families of proteins are the heterotrimeric G proteins, the G protein-coupled receptor kinases (GRKs), and the β-arrestins. Activation of the receptors stimulates classical G protein-dependent signaling, often involving regulation of levels of second messengers such as cAMP and diacyglycerol. However, as has been known for many years, interaction of activated receptors with GRKs leading to their phosphorylation, and subsequent interaction with β-arrestins leads to desensitization of G protein signaling.
In recent years, however, it has become increasingly clear that the β-arrestin-GRK system is in fact bifunctional (2). Thus, even as it desensitizes G protein signaling by the receptors, it also serves as a signal transduction system in its own right, activating a growing list of signaling pathways. These positive signaling functions are often mediated by the ability of β-arrestin to serve as an adaptor or scaffold molecule, bringing elements of diverse signaling pathways into proximity with one another and the receptors and thereby facilitating their activation. This new paradigm for understanding the previously unrecognized signaling properties of the β-arrestin-GRK system has been explored in a wide variety of transfected cultured cell systems.
However, to date, relatively little investigation of these novel signaling pathways has been carried out in primary cell culture systems expressing endogenous levels of 7TM receptors. In seeking such a system in which to characterize and compare β-arrestin and G protein-mediated signaling pathways from a typical 7TM receptor, our attention was drawn to cultured rat vascular smooth muscle cells (VSMCs). Several features of rat VSMCs suggest this to be a relevant system for these purposes. Rat VSMCs express a variety of physiologically important 7TM receptors including the angiotensin II type 1A receptor (AT1R) (3). This receptor has been the focus of extensive study in transfected cell systems with respect to its β-arrestin-mediated signaling to a variety of pathways, most particularly extracellular signal-regulated kinase (ERK). Moreover, the AT1R mediates the physiologically important effects of angiotensin II (ANG) on vascular tone as well as on proliferation and chemotaxis (4, 5). Pathophysiologically, ANG stimulation of this receptor has been implicated in VSMC proliferation and chemotaxis, which are thought to play an important role in such important disease processes as atherosclerosis and restenosis after angioplasty (6, 7). Moreover, a ligand has been characterized [Sar1,Ile4,Ile8](SII)-angiotensin (SII), a triply mutated angiotensin octapeptide that, in transfected cell systems, acts as a specific agonist for β-arrestin-mediated signaling, although not activating G protein-mediated signaling (8).
Accordingly, in the studies described here, we set out to investigate the characteristics of activation of ERK in rat VSMCs that might be mediated through G protein as well as β-arrestin signaling. The results not only demonstrate the importance of β-arrestin-mediated signaling in ERK-mediated proliferative responses of these cells, but also shed new light on the molecular mechanisms and interrelationships between the β-arrestin and classical G protein-mediated activation of these pathways.
EXPERIMENTAL PROCEDURES
Materials—[Sar1,Ile4,Ile8]-Angiotensin II was synthesized in the Cleveland Clinic core synthesis facility. Valsartan was obtained from Novartis and PD 123319 from RBI was used. Angiotensin II was purchased from Sigma. Human recombinant epidermal growth factor (EGF) was purchased from Roche Molecular Biochemicals. The EGFR-specific inhibitor tyrphostin AG1478, the Src-specific inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-D]pyrimidine (PP2), the MEK inhibitor PD98059, and N-phenylsulfonyl hydroxamic acid derivative (2R-[(4-biphenylsulfonyl)amino]-N-hydroxy-3-phenylpropinamide) (BiPS), and PKC inhibitors (GFX, Gö6976, and Gö6983) were from Calbiochem. Polyclonal anti-EGFR and anti-phospho-EGFR (Tyr845) antibodies used for immunoprecipitation and immunoblotting were from Cell Signaling and Upstate Biotechnology, Inc. Polyclonal rabbit β-arrestin 1(A1CT) and polyclonal rabbit anti-β-arrestin 2 (A2CT) antibodies were used for detecting β-arrestin1 and -2, respectively (9). Polyclonal PKCζ antibody was from Santa Cruz. Polyclonal anti-phospho-ERK antibody (Cell Signaling) and polyclonal anti-ERK2 antibody (Millipore) were used for ERK activation assay. [3H]Thymidine was purchased from Amersham Biosciences. Lipofectamine 2000 transfection reagents were from Invitrogen.
Synthesis of Small Interfering RNAs (siRNAs)—Chemically synthesized, double-stranded siRNAs, with 19-nucleotide duplex RNA and 2-nucleotide 3′-dTdT overhangs were purchased from Dharmacon in deprotected and desalted forms. The siRNA sequence targeting β-arrestin1 is 5′-AGCCUUCUGUGCUGAGAAC-3′, corresponding to position 431–459 relative to the start codon. The siRNA sequences targeting β-arrestin2 are 5′-GGACCGCAAAGUGUUUGUG-3′ and 5′-CCAACCTCATTGAATTCGA-3′, corresponding to positions 150–168 and 1115–1133 relative to the start codon (10). siGENOME ON-TARGET plus set of four rat PRKCZ was purchased from Dharmacon. A non-silencing RNA duplex (5′-AAUUCUCCGAACGUGUCACGU-3′), as the manufacturer indicated, was used as a control.
Cell Culture and RNA Transfection—VSMCs were prepared from aorta of male Sprague-Dawley rats by enzymatic digestion (11) and maintained in Medium 199 (M199) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Eighty to 90% confluent, slow growing early passage (<5) cells in 100-mm dishes were transfected with 20 μg of siRNA using 60 μl of Lipofectamine 2000 transfection reagent (11). Forty-eight hours after transfection, cells were starved with media with 0.1% bovine serum albumin for at least 48 h prior to stimulation.
Calcium Fluorimetry—Cells were loaded with the dye Fura-2 as per the manufacturer's instructions (Invitrogen), and treated with either ANG (100 nm) or SII (10 μm), in the presence of either 1 μm valsartan (Angiotensin receptor type 1 blocker, AT1RB) or 300 nm PD123319 (Angiotensin receptor type 2 blocker, AT2RB). The instantaneous 340/380-nm excitation ratio for Fura-2 was calculated and plotted as a function of time.
DNA Synthesis—DNA synthesis was determined as incorporation of [3H]thymidine into trichloroacetic acid-insoluble material as described (11). Cells were seeded 10,000 cells/well in 12-well plates and grown to subconfluence (1 day). Cells were rendered quiescent by 48 h in serum-free M199 supplemented with 0.1% bovine serum albumin and stimulated by addition of fresh medium containing the agonists and/or inhibitors. [3H]Thymidine (0.5 μCi/ml) was added to the medium at the time of stimulation. Cells were washed twice with phosphate-buffered saline and three times with 0.5% trichloroacetic acid, and then cells were lysed with 1 n NaOH. Extent of cell growth was determined by counting incorporation of [3H]thymidine into nascent DNA strands.
Immunoprecipitation and Western Blotting—Immunoprecipitation was performed by a modification of the methods of Daub et al. (12). Cells were serum-starved in M199 with 0.1% bovine serum albumin for 48 h after siRNA transfection. Stimulation of serum-starved rat VSMC in 100-mm dishes with appropriate agonists was done at 37 °C for 2 min. After stimulation, monolayers were washed once with ice-cold phosphate-buffered saline and lysed in buffer containing 50 mm HEPES (pH 7.5), 150 mm NaCl, 1% Triton X-100, 1 mm EDTA, 10% glycerol, 10 mm sodium pyrophosphate, 2 mm sodium orthovanadate, 10 mm sodium fluoride, 1 mm phenylmethylsulfonyl fluoride, and 10 μg/ml aprotinin. The EGFR was immunoprecipitated using 4 μg of anti-EGFR antibody plus 25 μl of 50% slurry of Protein G Plus/Protein A-agarose and agitated overnight at 4 °C. Western blotting was performed to detect phosphorylation residues at Tyr-845 of the EGFR with antibody (Cell Signaling).
For Western blotting for ERK activation, VSMCs on 6-well plates were starved for at least 48 h in serum-free medium prior to stimulation. After stimulation, cells were solubilized by directly adding the 2× SDS sample buffer followed by sonication. Aliquoted cells after transfection were solubilized in a lysis buffer, as described previously (13), to measure the protein concentration. Equal amounts of cellular extracts were separated on 4–20% Tris glycine polyacrylamide gels and transferred to nitrocellulose membranes for immunoblotting. Phosphorylated ERK1/2, total ERK1/2, and β-arrestins were detected by immunoblotting with rabbit polyclonal anti-phospho-ERK (Cell Signaling, 1:2,000), anti-ERK (Upstate Technology Inc, 1:10,000), and anti-β-arrestin (A1CT, 1:3,000 and A2CT, 1:3000) antibodies, respectively (9). Chemiluminescent detection was performed using the SuperSignal West Pico reagent (Pierce), and all the immunoblots were visualized and quantified by Bio Imaging system (Syngene). The levels of ERK phosphorylation was normalized to a loading control (total ERK). Statistical analysis was done using a one-way analysis of variance (ANOVA) (PRISM software) to correct for multiple comparisons (Bonferroni's multiple comparison test) with post test.
RESULTS
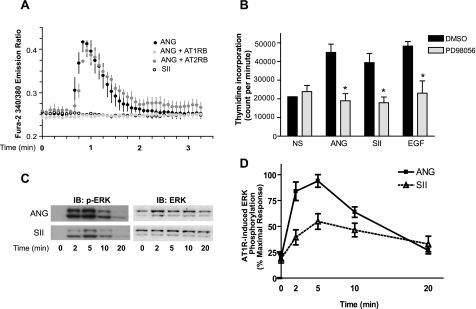
Intracellular Calcium Mobilization and Its Effect on DNA Synthesis and ERK Activation Stimulated with ANG or SII—ANG regulates vascular tone and induces mitogenic responses. To understand the functions of β-arrestins in endogenously expressed AT1R-mediated mitogenic pathways, we utilized a triply mutated angiotensin octapeptide (SII) that acts as a specific agonist for β-arrestin-mediated signaling while not activating G protein-mediated signaling (8). To validate the utility of SII as an activator of only β-arrestin-dependent signaling pathways in primary cultured rat VSMCs (as is the case with receptor-transfected HEK 293 cells (8)), calcium influx in response to ANG and SII was measured. As shown in Fig. 1A, VSMCs treated with ANG display robust calcium mobilization, whereas cells treated with SII shows no calcium mobilization, implying that SII cannot activate G-protein pathways in these cells. The ANG response is completely blocked in the presence of an angiotensin receptor type 1 blocker (AT1RB, Valsartan) but not by an angiotensin receptor type 2 blocker (AT2RB, PD 123319) (Fig. 1A).
FIGURE 1.
Mobilization of calcium, DNA synthesis, and phosphorylation of ERK stimulated by ANG and SII in VSMCs. A, VSMCs were loaded with the calcium-binding dye Fura-2, and stimulated either with ANG (100 nm) or SII (10 μm) in the presence or absence of pretreatment with the AT1R antagonist (AT1RB) valsartan (50 μm) or AT2R antagonist (AT2RB) PD123319 (30 μm). Calcium fluorimetric traces are shown with the 340/380 nm excitation ratio (y axis) plotted as a function of time (x axis). Results displayed are mean ± S.E. of three independent experiments. B, VSMCs were serum starved for 24 h to arrest cycling and pretreated with dimethyl sulfoxide (DMSO) or MEK inhibitor PD98056 (20 μm). ANG (100 nm), SII (10 μm), or EGF (10 ng/ml) along with 3H-labeled thymidine were then added to the media, and 24 h later cells were harvested as described under “Experimental Procedures.” NS indicates no stimulation. Results depicted represent the mean ± S.E. of count per minute (cpm) values from four independent experiments. Statistical analysis was done using a one-way ANOVA (PRISM software) to correct for multiple comparisons (Bonferroni's multiple comparison test) with post test. The PD98056-pretreated condition shows significant reduction compare with the dimethyl sulfoxide-pretreated condition for each stimulant (*, p < 0.05). C, VSMCs with endogenous AT1R were treated with 100 nm ANG or 10 μm SII for the indicated times. Equal amounts of cell lysate were separated by SDS-PAGE and analyzed for phosphorylated ERK (p-ERK) and total ERK (ERK) by Western blotting. IB, immunoblot. D, signals were quantified by densitometry and p-ERK was normalized to a loading control (ERK). p-ERK activation was expressed as percentage of the maximal phosphorylated ERK obtained by ANG stimulation at 5 min. Each data point represents the mean ± S.E. from eight independent experiments.
It is well known that ERK activation plays an important role in cell proliferation. To investigate the contribution of β-arrestin-mediated ERK activation to VSMC proliferation, we studied ANG- and SII-mediated DNA synthesis in VSMCs as an indicator of cell proliferation. VSMCs were pretreated or not with a pharmacological inhibitor (PD98059) of mitogen-activated protein kinase kinase (MEK) followed by stimulation with 100 nm ANG, 10 μm SII, or 10 ng/ml EGF. Although SII cannot activate classical G-protein-dependent Ca2+ fluxes (Fig. 1A), it significantly increased DNA synthesis (thymidine incorporation) to a level similar to that provoked by ANG or EGF stimulation in VSMCs (Fig. 1B). Pretreatment with PD98059 ablated virtually all activation over basal levels (n = 5; p < 0.05), indicating that ERK activation regulates DNA synthesis in VSMCs (Fig. 1B). These data indicate that a G-protein-independent ERK activation mechanism that can be stimulated by SII can mediate AT1R-regulated DNA synthesis in rat VSMCs.
In transfected HEK 293 cells it has been demonstrated that SII elicits AT1R signaling to ERK activation without activating the Gq protein (8). Fig. 1 demonstrates the inability of SII to activate calcium fluxes, a classic Gq response in VSMCs, whereas retaining the ability to induce DNA synthesis, which requires ERK activation. Next, we tested SII-mediated ERK activation mediated by the AT1R-expressed endogenously in VSMCs. Fig. 1, C and D, show that ERK activation by SII in VSMCs reaches maximal levels by 5 min, and amounted to a 2-fold increase over basal, which was about 50% of ANG-mediated ERK activation. Both ANG- and SII-stimulated ERK activation is completely sensitive to AT1RB pretreatment in VSMCs (data not shown). These results demonstrate that a G protein-independent pathway contributes to ERK activation in primary VSMCs through endogenously expressed AT1R.
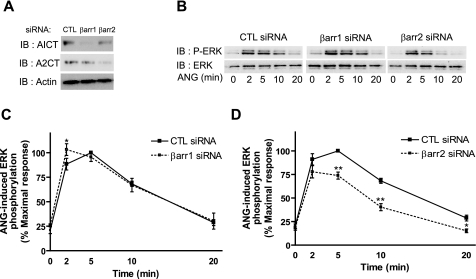
Effect of β-Arrestin Depletion on ANG- and SII-mediated ERK Activation—As shown in Figs. 1, G protein-independent signaling is involved in AT1R-mediated DNA synthesis via ERK activation in VSMCs. In HEK 293 cells, we have previously demonstrated that β-arrestins play critical roles in G protein-independent ERK activation (13). Therefore, we examined the effects of RNA interference (RNAi)-mediated suppression of β-arrestin1 and -2 expression on the kinetics of ERK activation following stimulation with ANG and SII in VSMCs. Transfection with siRNAs targeting β-arrestin1 or -2 effectively and specifically silence the expression of each β-arrestin (Fig. 2A). In β-arrestin1 siRNA-transfected cells, ANG stimulation evokes a kinetic pattern of ERK activation similar to control but with significantly elevated levels of activation at the 2-min time point, compared with control siRNA-transfected cells (Fig. 2, B and C). In contrast, depletion of β-arrestin2 leads to decreased ERK activation (by over 30%) at the later time points (5 to 20 min) (Fig. 2, B and D).
FIGURE 2.
Effects of β-arrestin RNAi on ANG-mediated phosphorylation of ERK. VSMCs were transfected with the control (CTL), β-arrestin1 and -2 siRNAs using Lipofectamine 2000. A, representative Western blot for expression of β-arrestins after siRNA transfection was shown. β-Arrestin1 and -2 were detected by immunoblotting (IB) with rabbit polyclonal anti-β-arrestin1 (A1CT) and anti-β-arrestin2 (A2CT) antibodies, respectively. Immunoblotting with actin was shown as a loading control. B–D, serum-starved cells were treated with 100 nm ANG for the indicated times and cell lysates were analyzed for phosphorylated ERK (p-ERK) and total ERK (ERK). B, representative Western blot for ANG induced p-ERK and ERK in siRNA-transfected VSMCs was shown. C and D, signals were quantified by densitometry and p-ERK was normalized to a loading control (ERK). The signal at each point is expressed as percentage of the maximal p-ERK signal (5 min) in CTL siRNA-transfected cells. The graphs represent mean ± S.E. from six independent experiments. Statistical analysis was done using a one-way ANOVA (PRISM software) to correct for multiple comparisons (Bonferroni's multiple comparison test) with post test. The siRNA transfected condition shows significant differences compared with control siRNA-transfected cells at each time point (*, p < 0.05; **, p < 0.01).
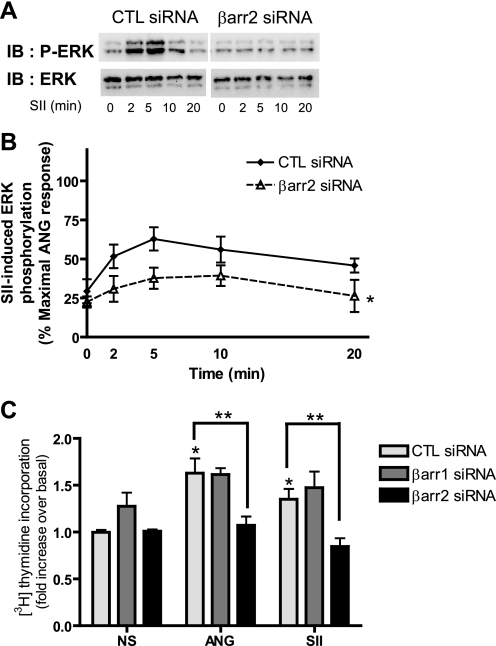
SII-stimulated ERK activation is completely lost in β-arrestin2 siRNA-transfected VSMCs (Fig. 3, A and B), suggesting that SII-stimulated ERK activation is entirely β-arrestin2-mediated. To further validate the contribution of β-arrestin2 to VSMC proliferation, we measured ANG- and SII-induced thymidine incorporation as an indicator of DNA synthesis and cell proliferation in VSMCs after depletion of β-arrestin1 or -2. ANG- and SII-induced DNA synthesis was significantly inhibited only by deletion of β-arrestin2 (Fig. 3C). Taken together, these results indicate that β-arrestin2 has a major role in AT1R-mediated ERK signaling and cell proliferation in VSMCs.
FIGURE 3.
Effects of β-arrestin RNAi on SII-mediated phosphorylation of ERK. A and B, VSMCs were transfected with control (CTL) or β-arrestin2 siRNAs using Lipofectamine 2000. Serum-starved cells were treated with 10 μm SII for the indicated times and cell lysates were analyzed for phosphorylated ERK (p-ERK) and total ERK (ERK). A, representative Western blot for SII-induced p-ERK and total ERK in siRNA-transfected VSMCs was shown. B, signals were quantified by densitometry and p-ERK was normalized to a loading control (ERK). Signal at each point is expressed as percentage of ANG stimulated the maximal p-ERK signal at 5 min in control (CTL) siRNA-transfected cells as depicted in Fig. 2B. The graph represents mean ± S.E. from six independent experiments. Statistical analysis in each time point of kinetic graphs were determined by using a two-way ANOVA (Bonferroni's post test) between β-arrestin2 and control (CTL) siRNA-transfected cells (*, p < 0.05). C, VSMCs were transfected with control, β-arrestin1 or -2 siRNAs using Lipofectamine 2000 and then serum starved for 24 h to arrest cycling. ANG (100 nm) or SII (10 μm) along with 3H-labeled thymidine was then added to the media, and 24 h later cells were harvested as described under “Experimental Procedures.” NS indicates no stimulation. Results depicted represent the mean ± S.E. of fold-increase of basal counts per minute (cpm) values from three independent experiments. Statistical analysis was done using a one-way ANOVA (PRISM software) to correct for multiple comparisons (Bonferroni's post test). ANG and SII significantly increase thymidine incorporation compared with NS control (*, p < 0.05). Depletion of β-arrestin2 shows significant reduction on both ANG- and SII-mediated thymidine incorporation compared with its own agonist stimulation in the CTL siRNA-transfected condition (**, p < 0.05). IB, immunoblot.
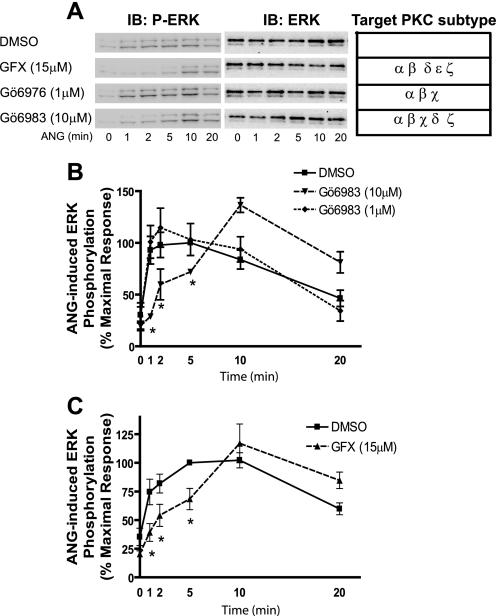
Role of PKC in AT1R-mediated ERK Activation in VSMCs—Upon agonist binding, AT1R activates Gq protein, which activates phospholipase C. Active phospholipase C generates inositol triphosphate and diacyglycerol, which increase intracellular calcium influx and PKC, respectively (14). We have previously found that in HEK 293 cells AT1R-mediated G protein-dependent ERK activation requires PKC activity. This process is rapid (peak < 2 min) and quite transient (∼2 min) (13). Therefore, we assessed the role of PKC in mediating ANG-stimulated ERK activation in VSMCs by using PKC inhibitors. We tested several PKC inhibitors including GFX (bisindolylmaleimide I), Ro-31-8425, Gö6976, and Gö6983, which inhibit the activities of various combinations of conventional (α, β, or γ), novel (δ, ε, or μ), and atypical (ζ, ι) isoforms of PKC in a concentration-dependent way. There was no significant inhibition of ERK activation by either 1 μm Gö6976, a selective inhibitor of PKC α, β, and χ (Fig. 4A), or by 1 μm Ro-31-8425 (data not shown), a selective inhibitor of PKC α, β, χ, and ε. ANG-mediated ERK activation was also not inhibited by either 1 μm Gö6983 (Fig. 4B) or 5 μm GFX (data not shown), which at the concentrations tested only inhibit the activities of PKC isoforms α, β, δ, and/or χ. In contrast, pretreatment with PKC inhibitors (15 μm GFX and 10 μm Gö6983) resulted in dramatic decreases (∼75%) in ERK activation at early time points (1 and 2 min) after ANG stimulation, but little inhibition was observed at longer time points (10 and 20 min) (Fig. 4). The general PKC activator, phorbol 12-myristate 13-acetate induced ERK activation and this was abolished by all of the PKC inhibitors used in VSMCs (data not shown). The kinetic pattern of ERK activation in the presence of the PKC inhibitors GFX and Gö6983 in VSMCs (Fig. 4) is similar to that observed previously for ANG-stimulated, β-arrestin2-dependent ERK activation in HEK 293 cells expressing AT1R (13). Only early activity (i.e. prior to 5–10 min of stimulation) was inhibited. Thus, the temporal patterns of G protein-dependent (PKC dependent) and β-arrestin2-dependent ERK activation through endogenous AT1Rs in VSMCs are distinct from each other. The majority of early activity (≤2 min) is elicited via the G protein-dependent pathway (PKC inhibitor sensitive), whereas late activity (>5 min) is predominantly mediated by the β-arrestin2-dependent pathway.
FIGURE 4.
Effects of PKC inhibitors on ANG-stimulated phosphorylation of ERK. Serum-starved VSMCs were pretreated with vehicle (dimethyl sulfoxide, DMSO) or the indicated PKC inhibitors for 30 min, and then stimulated with 100 nm ANG for the indicated times. Western blots for phosphorylated ERK (p-ERK) and total ERK (ERK) were performed. A, representative p-ERK Western blots and targeted PKC subtypes for PKC inhibitors were presented. B and C, signals were quantified by densitometry and p-ERK was normalized to a loading control (ERK). The percentage of p-ERK was calculated for each time point and plotted as percentage of the maximal p-ERK signal with dimethyl sulfoxide at 5 min. Data represent the mean ± S.E. of four independent experiments. Statistical analysis was done using a one-way ANOVA (PRISM software) to correct for multiple comparisons (Bonferroni's post test). Pretreated conditions with PKC inhibitors show the significant differences compared with dimethyl sulfoxide-treated cells at the indicated time point (*, p < 0.05). IB, immunoblot.
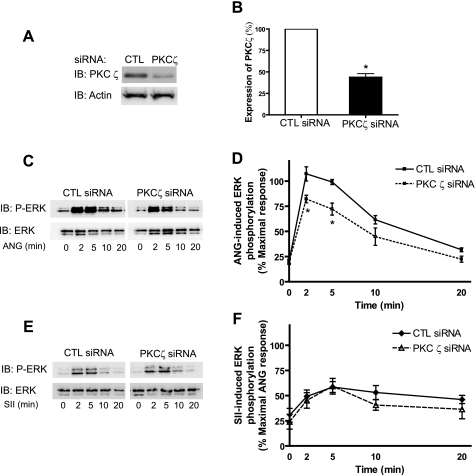
PKCζ Mediates G-protein-dependent ERK Activation Upon Stimulation with ANG—Three isoforms of PKC, α, δ, and ζ, have been shown to be expressed in VSMCs (15). Among them, PKCζ has been implicated in ANG-mediated mitogenic responses and cell growth in VSMCs (16), although different isoforms of PKC may play roles in ERK activation in different cell types (17, 18). In Fig. 4, we observed inhibition of ERK activation at the early time points when two PKC inhibitors capable of blocking PKC activity were used. Furthermore, blockade of PKCα and δ appears not to inhibit this ERK activation. Thus, we next examined the role of PKCζ in ERK activation in VSMCs utilizing the RNAi technique. Transfection with PKCζ-specific siRNA reduces the expression of PKCζ by 70% in VSMCs (Fig. 5, A and B). ANG-induced ERK activation at early time points (<5 min) was significantly decreased in cells transfected with PKCζ siRNA compared with that in control siRNA-transfected VSMCs (Fig. 5, C and D). To further validate the role of PKCζ in β-arrestin-dependent (or G protein-dependent) pathways, we also stimulated VSMCs transfected with PKCζ siRNA with SII. As shown in Fig. 5, E and F, SII leads to activation of ERK at the same magnitude in both control and PKCζ siRNA-transfected cells. Taken together, these data suggest that PKCζ, at least partially, mediates ANG-induced early ERK activation, whereas not affecting β-arrestin-dependent ERK activation in VSMCs.
FIGURE 5.
Effects of PKCζ RNAi on AT1R-mediated phosphorylation of ERK. VSMCs were transfected with control (CTL) siRNA or siGENOME ON-TARGET plus a set of four rat PKCζ siRNAs (Dharmacon) using Lipofectamine 2000. A, representative Western blot for expression of PKCζ and actin after siRNA transfection was shown. B, the expression levels of PKCζ after siRNA transfection were measured by Western blot and normalized with its loading control (actin). Results are shown as % of expression of PKCζ in CTL siRNA cells. Data represent mean ± S.E. from four independent experiments (*, p < 0.05). C–F, siRNA-transfected and serumstarved VSMCs were stimulated by 100 nm ANG or 10 μm SII for the indicated times and cell lysates were analyzed for phosphorylated ERK (p-ERK) and total ERK (ERK). C and E, representative p-ERK and ERK Western blots were presented for ANG (C) and SII (E) stimulation. D and F, signals were quantified by densitometry and p-ERK was normalized to a loading control (ERK). Data represent mean ± S.E. from four independent experiments. Signal at each point is expressed as percentage of the maximal p-ERK signal-stimulated ANG for 5 min in CTL siRNA VSMCs. D, statistical analysis was done using a one-way ANOVA (PRISM software) to correct for multiple comparisons (Bonferroni's post test) for ANG-induced ERK activation in PKCζ siRNA-transfected cells compared with CTL siRNA-transfected cells at each time point (* p < 0.05). F, SII-induced ERK activation was not significantly different between PKCζ siRNAs and CTL siRNA-transfected VSMCs. IB, immunoblot.
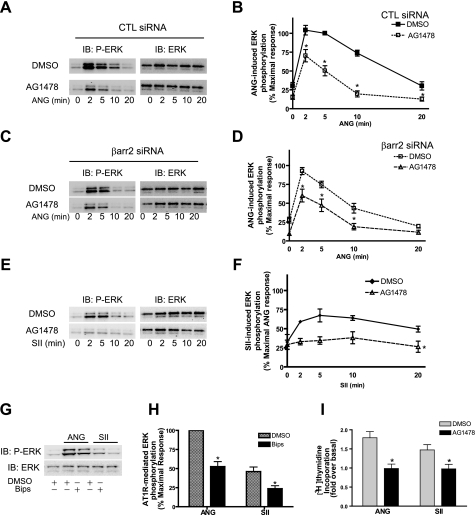
ANG- and SII-mediated ERK Activation Is Dependent on EGFR Kinase and Metalloprotease 2/9 in VSMCs—It is known that AT1R-mediated EGFR transactivation plays an important role in proliferation of various cell types including VSMCs (19). ANG promotes activation of the EGFR pathway through release of the EGFR ligand “heparin-binding EGF” in various cell types (12, 19). However, it is not clear whether EGFR transactivation is required for β-arrestin-dependent ERK activation in VSMCs. To answer this question, we examined ERK activation after ANG and SII stimulation in the presence of various pharmacological inhibitors of components thought to be involved in the EGFR transactivation pathway. First we tested the requirement for EGFR kinase activity in ANG-induced ERK activation. In the presence of AG1478, an EGFR-specific kinase inhibitor, ANG-induced ERK activation is dramatically inhibited at all tested time points (Fig. 6, A and B). Next, we investigated the contribution of EGFR kinase activity particularly for the β-arrestin2-dependent pathway by testing ANG-mediated ERK activation in the presence of AG1478 after depletion of β-arrestin2. In VSMCs transfected with β-arrestin2 siRNA, ERK activation was further reduced by AG1478 as shown in Fig. 6, C and D. ANG-stimulated ERK activation was inhibited by AG1478 in both control and β-arrestin2 siRNA-transfected VSMCs (reduced to ∼40–50% of maximal ANG stimulation (Fig. 6, B and D). These data indicate that, without β-arrestin2, the remaining G protein-dependent component was also affected by the EGFR kinase inhibitor. Thus, both G protein- and β-arrestin2-dependent components required EGFR transactivation for AT1R-mediated ERK activation.
FIGURE 6.
EGFR-dependent activation of ERK mediated by ANG and SII stimulation. A–F, VSMCs were transfected with control (CTL) or β-arrestin2 siRNAs using Lipofectamine 2000. Serum-starved VSMCs were pretreated with dimethyl sulfoxide (DMSO) or 250 nm AG1478 and then stimulated with 100 nm ANG or 10 μm SII for the indicated times. Equal amounts of cell lysate were separated by SDS-PAGE and analyzed for phosphorylated ERK (p-ERK) and total ERK (ERK) by Western blotting. Signals were quantified by densitometry and p-ERK was normalized to a loading control (ERK). Signal at each point is expressed as percentage of the maximal p-ERK signal (ANG for 5 min) in a dimethyl sulfoxide (DMSO)-treated CTL siRNA-transfected condition. A and C, representative Western blots of p-ERK and ERK from ANG-stimulated CTL siRNA (A) and β-arrestin2 siRNAs-transfected VSMCs (C) are presented. B and D, data represent mean ± S.E. from six independent experiments in panels A and C. Statistical analysis was done using a one-way ANOVA (PRISM software) to correct for multiple comparisons (Bonferroni's post test). *, p < 0.05 compared with the dimethyl sulfoxide-treated condition at each time point. E, representative SII-induced p-ERK and ERK Western blots from VSMCs pretreated with DMSO or AG1478. F, data represent mean ± S.E. from six independent experiments. Statistical analysis in each time point of kinetic graphs were determined by using a two-way ANOVA (Bonferroni's post test) between dimethyl sulfoxide and AG1478-treated cells (*, p < 0.05). G and H, serum-starved VSMCs were pretreated with dimethyl sulfoxide or 10μm BiPS and then stimulated with 100 nm ANG or 10 μm SII for 5 min. Equal amounts of cell lysate were separated by SDS-PAGE and analyzed for p-ERK and ERK by Western blotting. Signals were quantified by densitometry and p-ERK was normalized to a loading control (ERK). Signals at each condition were expressed as percentage of the maximal p-ERK obtained at 5 min for ANG stimulation in DMSO-treated VSMCs. Each data point represents the mean ± S.E. from four independent experiments. Statistical analysis was done using a one-way ANOVA (PRISM software) to correct for multiple comparisons (Bonferroni's post test). *, p < 0.05, compared with the DMSO-pretreated condition for each stimulant. I, VSMCs were serum starved for 24 h to arrest cycling and pretreated with dimethyl sulfoxide or 250 nm AG1478. ANG (100 nm) or SII (10 μm) along with 3H-labeled thymidine was then added to the media, and 24 h later cells were harvested as described under “Experimental Procedures.” Results depicted represent the mean ± S.E. of fold-increase over basal counts per minute (cpm) from four independent experiments. Statistical analysis was done using a one-way ANOVA (PRISM software) to correct for multiple comparisons (Bonferroni's post test). The AG1478-pretreated condition shows significant reduction compared with the dimethyl sulfoxide-pretreated condition for each stimulant (*, p < 0.05). IB, immunoblot.
In addition, SII-mediated ERK activation was virtually completely abolished by AG1478 as shown in Fig. 6, E and F. Because SII only activates β-arrestin-dependent pathways, this is clear evidence that EGFR kinase activity is required for β-arrestin2-dependent ERK activation in VSMCs. Our results utilizing RNA interference and pharmacological inhibitors reveal that β-arrestin-dependent ERK activation requires EGFR kinase activity, suggesting that EGFR transactivation is required for both G protein and β-arrestin2-dependent ERK activation by AT1R.
ANG is known to induce transactivation of the EGFR through activation of metalloproteases that mediate cleavage of heparin-binding EGF from its precursor. It has been reported that BiPS, previously known as matrix metalloprotease-2/9 inhibitor, blocks ANG-induced EGFR transactivation (20). We examined the effect of BiPS on SII-mediated ERK activation in VSMCs. Pretreatment with BiPS completely inhibits SII-induced ERK activation; whereas it inhibits ANG-induced ERK activation by about 50% (Fig. 6, G and H). EGF-induced ERK activation is not affected by pretreatment with BiPS (data not shown). Furthermore, we observed that both ANG- and SII-induced DNA synthesis were significantly inhibited by pretreatment with AG1478 (Fig. 6I). These data imply that ERK activation stimulated by ANG in VSMCs may consist of several distinct components. Our data suggest that the AT1R-mediated G-protein- and β-arrestin-dependent signaling pathways converge by promoting EGFR transactivation-dependent ERK activation, which results in VSMC proliferation.
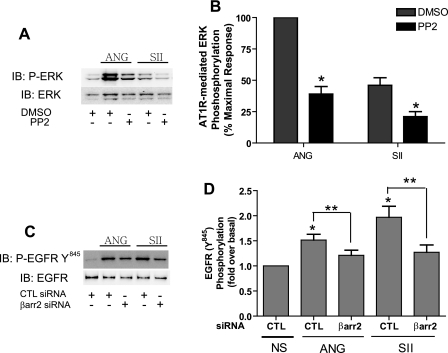
Role of Src Kinase in β-Arrestins-dependent ERK Activation and EGFR Transactivation—In recent years, Src tyrosine kinase has emerged as a key player in ANG-mediated cellular effects including the Ras/ERK activation pathway (21, 22). Dominant negative mutants of c-Src lead to decreases in ANG-induced ERK activation in VSMCs (23). Furthermore, it has been reported that ERK activation by ANG is reduced in VSMCs derived from Src knock-out mice (24). Therefore, we examined the function of Src kinase on AT1R-mediated, β-arrestin-dependent ERK activation in VSMCs. Fig. 7, A and B, shows that in the presence of PP2, a Src kinase inhibitor, AT1R-mediated ERK stimulation by either ANG or SII is significantly reduced. These levels are similar to those obtained with the EGFR kinase inhibitor, shown in Fig. 6, A and E. These data demonstrate that both ANG- and SII-stimulated ERK activation require Src kinase activity. We also found that EGF-mediated ERK itself is sensitive to PP2 being reduced by 45% (data not shown). This further suggests that AT1R-mediated Src kinase activation may play multiple roles in regulating ERK activation both prior to and/or subsequent to EGFR transactivation.
FIGURE 7.
Roles of Src in ANG- and SII-induced activation of ERK and EGFR. A and B, VSMCs were pretreated with DMSO or 10 μm PP2 (Src inhibitor) and then treated with 100 nm ANG or 10 μm SII for 5 min. Equal amounts of cell lysate were separated by SDS-PAGE and analyzed for phosphorylated ERK (p-ERK) and total ERK (ERK) by Western blotting. Signals were quantified by densitometry and p-ERK was normalized to a loading control (ERK). Signal at each point is expressed as percentage of the maximal p-ERK signal (ANG for 5 min) in dimethyl sulfoxide (DMSO)-treated VSMCs. A, representative p-ERK and ERK Western blots in dimethyl sulfoxide or PP2-pretreated VSMCs were presented. B, each data point represents the mean ± S.E. from four independent experiments. Statistical analysis was done using a one-way ANOVA (PRISM software) to correct for multiple comparisons (Bonferroni's post test). *, p < 0.05, compared with dimethyl sulfoxide treatment for each stimulant. C and D, VSMCs were transfected with the indicated siRNAs using Lipofectamine 2000. Serum-deprived VSMCs were incubated with 100 nm ANG and 10 μm SII for 2 min and immunoprecipitated with EGFR antibody. EGFRY845 phosphorylation was determined by Western analysis with Tyr(P)-845-specific antibody and EGFR antibody. EGFRY845 phosphorylation was quantified by densitometry and normalized to a loading control (EGFR). C, representative Western blots for EGFRY845 and EGFR in siRNA-transfected VSMCs were presented. D, data are presented as fold-increase over basal (the mean ± S.E.) from six independent experiments. Statistical analysis was done using a one-way ANOVA (PRISM software) to correct for multiple comparisons (Bonferroni's post test). ANG and SII significantly increase phosphorylation of EGFR at Tyr-845 compared with no stimulation (NS) control (*, p < 0.05). Depletion of β-arrestin2 shows a significant reduction on both ANG and SII mediated phosphorylation of EGFR at Tyr-845 compared with its own agonist stimulation in the control siRNA-transfected condition (**, p < 0.05).
Proteins in the Src tyrosine kinase family have been suggested previously to function as upstream mediators of EGFR transactivation, particularly in response to the stimulation of 7TM receptors (25). Thus we hypothesized that Src can be recruited to the AT1R upon agonist binding by β-arrestin2 and play a role in AT1R-mediated EGFR transactivation. To test this hypothesis, we examined the phosphorylation of EGFR at Tyr-845 upon ANG and SII stimulation in control or β-arrestins siRNA-transfected VSMCs. This site is known to be phosphorylated by Src kinase (26). Both ANG and SII significantly stimulate phosphorylation of EGFR at Tyr-845 in control cells (Fig. 7, C and D). Interestingly, SII is equally effective as ANG in inducing phosphorylation at this site, which is a 1.9 ± 0.2-fold increase over basal. Both ANG- and SII-induced EGFR phosphorylation at Tyr-845 is dramatically reduced by depletion of β-arrestin2 expression by siRNA. On the contrary, depletion of β-arrestin1 expression had no effect on AT1R-mediated phosphorylation of EGFR (data not shown). Consistent with other findings, stimulation with the PKC activator phorbol 12-myristate 13-acetate failed to induce phosphorylation of EGFR on Tyr-845 (data not shown). Our results suggest that β-arrestin2 regulates AT1R-mediated EGFR transactivation and subsequent ERK activation by recruiting downstream signaling molecules such as Src kinase to the receptors.
DISCUSSION
ANG-mediated signaling in VSMCs plays important roles in the development of pathological conditions such as restenosis, atherosclerosis, and hypertension. Abnormal regulation of 7TM receptor-mediated VSMC proliferation and migration are the major forces inducing such pathological conditions in a variety of vascular diseases. Mechanisms of VSMC proliferation induced by G protein signaling induced by 7TM receptor systems have been extensively studied. Here we investigated the novel roles of β-arrestins in 7TM receptor-mediated signaling in VSMCs. Classically, β-arrestins terminate signals emanating from agonist-bound 7TM receptors by physically interfering with receptor coupling to its cognate G protein (desensitization), and also removing receptors from the cell surface through initiating interaction with several elements of the clathrin-coated pit endocytic machinery (internalization). Recent data, however, have revealed that β-arrestins also act as signal transducers and adaptors that scaffold a variety of signaling molecules, leading to mitogen-activated protein kinase activation, DNA synthesis, protein translation, and cell migration (2).
Roles of β-arrestins as signal transducers have been extensively studied for AT1R-mediated ERK activation in receptor-transfected HEK 293 cells (13, 27). Here, we studied ANG-stimulated ERK activation in a physiologically relevant cell type, rat VSMCs, and compared the results to those observed previously in HEK 293 cells transiently transfected with AT1R. Whereas, the overall patterns were similar, several notable differences were observed. AT1R-mediated ERK activation in VSMCs is more transient than that in AT1R-transfected HEK 293 cells. In the latter, ANG-stimulated ERK activation reached maximal levels rapidly, remained stable for up to 10 min, and decreased very slowly (over 1 h) (13). On the other hand, ANG- and SII-induced ERK activation in VSMCs peaks at 5 min and is quite transient, decreasing to background levels by 30 min. This may be due to the very low levels of endogenously expressed AT1R in VSMCs (∼15 fmol/mg). By utilizing β-arrestin RNAi and PKC inhibitors, two pathways for ERK activation by the AT1R expressed in HEK 293 cells have been distinguished previously; rapid and transient G protein-dependent activation, which is sensitive to PKC inhibitors versus slow and prolonged β-arrestin2-dependent activation, which is sensitive to β-arrestin2 RNAi (13). In VSMCs, pretreatment with PKC inhibitors also specifically inhibits early activation of ERK (1–2 min) but not that at later time points, indicating that early activation of ERK requires G-protein-mediated second messenger production. Furthermore, depletion of β-arrestin2, but not of β-arrestin1, using RNAi leads to inhibition of ANG-mediated ERK activation at later time points in VSMCs. However, ERK activation at later time points in VSMCs was not completely abolished as was previously observed in AT1R-transfected HEK 293 cells, possibly due to incomplete depletion of β-arrestin2 (∼70%) in primary cultured VSMCs.
Functions of β-arrestins as signal transducers have been demonstrated for many different 7TM receptors including angiotensin II type 1A (AT1A) (13), β2-adrenergic (28), neurokinin 1 (29), proteinase-activated (30), vasopressin 2 (31), CXCR4 chemokine (32), and parathyroid hormone (33) receptors, as well as for other types of receptors including the insulin-like growth factor 1-receptor (34), ion channels (35), nicotinic acetylcholine receptor (36), and transforming grow factor β receptor (37). In the case of 7TM receptor-mediated ERK activation, the later phase of β-arrestin-dependent ERK activation is sometimes blocked by depletion of either β-arrestin, by depletion of β-arrestin2, but not β-arrestin1, or vice versa depending on receptor and cell type. For example, both β-arrestin1 and -2 are required for β-arrestin-dependent ERK activation mediated by β2-adrenergic (28) and parathyroid hormone (33) receptors. On the other hand, β-arrestin1 and -2 show reciprocal functions in AT1R (27) and V2 (31) receptor-mediated ERK activation; β-arrestin1 acts as a physiological “dominant-negative” of β-arrestin2-mediated ERK activation by these receptors. In the present study, ANG-mediated ERK activation in β-arrestin1 siRNA-transfected VSMCs was not enhanced compared with that from control siRNA-transfected cells except at the 2-min time point, and SII-mediated ERK activation was not changed in β-arrestin1 siRNA-transfected cells. Thus the role of β-arrestin1, if any, in modulating β-arrestin2-mediated ERK activation stimulated via the AT1R in VSMCs is not clear.
AT1R is a typical Gq-coupled 7TM receptor that initiates generation of inositol triphosphate and diacyglycerol second messengers by activation of phosphatidylinositol-specific phospholipase C. These second messengers in turn trigger mobilization of intracellular calcium and activation of PKC, which has been known to play important roles in AT1R-mediated ERK activation (14). In VSMCs, PKCδ has been known to be activated upon ANG stimulation leading to AKT activation (38) and PKCζ has been suggested to play a major role in AT1R-mediated ERK/Elk-1 activation via the Ras/MEK pathway (24). We showed that inhibition of PKCζ activity using chemical inhibitors or by depletion of its expression by RNAi inhibited G protein-dependent (early time point) AT1R-mediated ERK activation in VSMCs.
Numerous 7TM receptors have been known to utilize EGFR transactivation mechanisms to activate ERK in various cell types. Moreover, it has been observed that AT1R-mediated ERK activation requires EGFR kinase activity in several cell types including C9 hepatocytes and COS-7 cells transfected with the AT1R as well as in VSMCs (19, 39). It has been shown that G protein activation plays a role in AT1R-mediated EGFR transactivation through an heparin binding-EGF shedding mechanism (40). Here we found that the β-arrestin-biased analogue SII-ANG induced ERK activation and subsequent DNA synthesis also require EGFR kinase activity in VSMCs. Moreover, SII-mediated ERK activation was also inhibited in the presence of an matrix metalloprotease inhibitor, which is known to block heparin binding-EGF shedding in VSMCs, suggesting that β-arrestin2 as well as G proteins can mediate an heparin binding-EGF shedding mechanism in VSMCs. We also found that stimulation with either ANG or SII induced EGFR phosphorylation at Tyr-845 by Src kinase, which is known to activate EGFR. It has been shown that activation of many 7TM receptors recruits activated c-Src to the receptor complex in a β-arrestin-dependent manner (41). It thus appears that multiple signaling mechanisms can mediate AT1R and EGFR cross-talk in VSMCs. It will be of interest to determine whether distinct physiological outcomes of AT1R-mediated EGFR transactivation are associated with these different signaling mechanisms.
Several physiologic consequences of β-arrestin-dependent signaling have been discovered in recent years. For example, β-arrestin-mediated signal transduction via the AT1R results in positive inotropic and lusitropic effects in isolated adult mouse cardiomyocytes (42). β-Arrestin-dependent ERK signaling regulates mRNA translation and protein synthesis via Mnk1, a protein that both physically interacts with and is activated by β-arrestin (43). β1-Adrenergic receptors have been shown to mediate β-arrestin-dependent transactivation of the EGFR thus promoting activation of the cardioprotective pathway in the heart that counteracts the effects of catecholamine toxicity (44). In addition, atherosclerosis and restenosis in an animal model are aggravated by β-arrestin2 through mechanisms involving VSMC proliferation and migration (11). Taken together, these findings indicate the physiological and pathological importance of β-arrestins as transducers regulating mitogenic signals through 7TM receptors.
In summary, our findings indicate that for the ATIR in VSMCs G protein-dependent second messenger-mediated and β-arrestin2-dependent signaling pathways converge on transactivation of the EGFR and subsequent ERK activation. Each pathway contributes to ANG-mediated regulation of cardiovascular function. These results underscore the complexity and multiplicity of signaling mechanisms available to 7TM receptors and add to the growing recognition of the importance of β-arrestins as signal transducers.
Acknowledgments
We thank Donna Addison and Elizabeth Hall for excellent secretarial assistance and Drs. J. J. Kovacs and M. Hara for careful reading of the manuscript and comments. We also thank Dr. S. M. DeWire for technical advice.
Author's Choice—Final version full access.
This work was supported, in whole or in part, by National Institutes of Health Grants HL 16037 and HL 70631 (to R. J. L.).
Footnotes
The abbreviations used are: 7TM, seven transmembrane; AT1R, angiotensin type 1 receptor; ANG, angiotensin II; SII, [Sar1,Ile4,Ile8]-angiotensin II; ERK, extracellular signal-regulated kinase; PKC, protein kinase C; siRNA, small interfering RNA; HEK, human embryonic kidney; VSMC, vascular smooth muscle cell; EGFR, epidermal growth factor receptor; GRK, G protein-coupled receptor kinases; BiPS, 2R-[(4-biphenylsulfonyl)amino]-N-hydroxy-3-phenylpropinamide; MEK, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase; RNAi, RNA interference; GFX, bisindolylmaleimide I; ANOVA, analysis of variance.
References
- 1.Drake, M. T., Shenoy, S. K., and Lefkowitz, R. J. (2006) Circ. Res. 99 570–582 [DOI] [PubMed] [Google Scholar]
- 2.DeWire, S. M., Ahn, S., Lefkowitz, R. J., and Shenoy, S. K. (2007) Annu. Rev. Physiol. 69 483–510 [DOI] [PubMed] [Google Scholar]
- 3.Demoliou-Mason, C. D. (1998) Biol. Signals Recept. 7 90–97 [DOI] [PubMed] [Google Scholar]
- 4.Nozawa, Y., Matsuura, N., Miyake, H., Yamada, S., and Kimura, R. (1999) Life Sci. 64 2061–2070 [DOI] [PubMed] [Google Scholar]
- 5.Wassmann, S., and Nickenig, G. (2006) J. Hypertens. 24 S15–S21 [DOI] [PubMed] [Google Scholar]
- 6.Yu, P. J., Ferrari, G., Pirelli, L., Gulkarov, I., Galloway, A. C., Mignatti, P., and Pintucci, G. (2007) Cell. Signal. 19 1359–1371 [DOI] [PubMed] [Google Scholar]
- 7.Rudijanto, A. (2007) Acta Med. Indonesia 39 86–93 [PubMed] [Google Scholar]
- 8.Wei, H., Ahn, S., Shenoy, S. K., Karnik, S. S., Hunyady, L., Luttrell, L. M., and Lefkowitz, R. J. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 10782–10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attramadal, H., Arriza, J. L., Aoki, C., Dawson, T. M., Codina, J., Kwatra, M. M., Snyder, S. H., Caron, M. G., and Lefkowitz, R. J. (1992) J. Biol. Chem. 267 17882–17890 [PubMed] [Google Scholar]
- 10.Ahn, S., Nelson, C. D., Garrison, T. R., Miller, W. E., and Lefkowitz, R. J. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 1740–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, J., Zhang, L., Peppel, K., Wu, J. H., Zidar, D. A., Brian, L., DeWire, S. M., Exum, S. T., Lefkowitz, R. J., and Freedman, N. J. (2008) Circ. Res. 103 70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daub, H., Wallasch, C., Lankenau, A., Herrlich, A., and Ullrich, A. (1997) EMBO J. 16 7032–7044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn, S., Shenoy, S. K., Wei, H., and Lefkowitz, R. J. (2004) J. Biol. Chem. 279 35518–35525 [DOI] [PubMed] [Google Scholar]
- 14.de Gasparo, M., Catt, K. J., Inagami, T., Wright, J. W., and Unger, T. (2000) Pharmacol. Rev. 52 415–472 [PubMed] [Google Scholar]
- 15.Assender, J. W., Kontny, E., and Fredholm, B. B. (1994) FEBS Lett. 342 76–80 [DOI] [PubMed] [Google Scholar]
- 16.Parmentier, J. H., Zhang, C., Estes, A., Schaefer, S., and Malik, K. U. (2006) Am. J. Physiol. 291 H1602–H1613 [DOI] [PubMed] [Google Scholar]
- 17.Porreca, E., Ciccarelli, R., Di Febbo, C., and Cuccurullo, F. (1993) Atherosclerosis 104 137–145 [DOI] [PubMed] [Google Scholar]
- 18.Kariya, K., Karns, L. R., and Simpson, P. C. (1991) J. Biol. Chem. 266 10023–10026 [PubMed] [Google Scholar]
- 19.Eguchi, S., Iwasaki, H., Ueno, H., Frank, G. D., Motley, E. D., Eguchi, K., Marumo, F., Hirata, Y., and Inagami, T. (1999) J. Biol. Chem. 274 36843–36851 [DOI] [PubMed] [Google Scholar]
- 20.Saito, S., Frank, G. D., Motley, E. D., Dempsey, P. J., Utsunomiya, H., Inagami, T., and Eguchi, S. (2002) Biochem. Biophys. Res. Commun. 294 1023–1029 [DOI] [PubMed] [Google Scholar]
- 21.Zhou, C. H., Qian, Z. Y., Zheng, S. G., and Xiang, M. (2006) Eur. J. Pharmacol. 535 61–68 [DOI] [PubMed] [Google Scholar]
- 22.Seta, K., Nanamori, M., Modrall, J. G., Neubig, R. R., and Sadoshima, J. (2002) J. Biol. Chem. 277 9268–9277 [DOI] [PubMed] [Google Scholar]
- 23.Zou, Y., Komuro, I., Yamazaki, T., Kudoh, S., Aikawa, R., Zhu, W., Shiojima, I., Hiroi, Y., Tobe, K., Kadowaki, T., and Yazaki, Y. (1998) Circ. Res. 82 337–345 [DOI] [PubMed] [Google Scholar]
- 24.Godeny, M. D., and Sayeski, P. P. (2006) Am. J. Physiol. 291 C1297–C1307 [DOI] [PubMed] [Google Scholar]
- 25.Poppleton, H. M., Wiepz, G. J., Bertics, P. J., and Patel, T. B. (1999) Arch. Biochem. Biophys. 363 227–236 [DOI] [PubMed] [Google Scholar]
- 26.Xu, K. P., Yin, J., and Yu, F. S. (2006) Investig. Ophthalmol. Vis. Sci. 47 2832–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn, S., Wei, H., Garrison, T. R., and Lefkowitz, R. J. (2004) J. Biol. Chem. 279 7807–7811 [DOI] [PubMed] [Google Scholar]
- 28.Drake, M. T., Violin, J. D., Whalen, E. J., Wisler, J. W., Shenoy, S. K., and Lefkowitz, R. J. (2008) J. Biol. Chem. 283 5669–5676 [DOI] [PubMed] [Google Scholar]
- 29.Jafri, F., El-Shewy, H. M., Lee, M. H., Kelly, M., Luttrell, D. K., and Luttrell, L. M. (2006) J. Biol. Chem. 281 19346–19357 [DOI] [PubMed] [Google Scholar]
- 30.DeFea, K. A., Zalevsky, J., Thoma, M. S., Dery, O., Mullins, R. D., and Bunnett, N. W. (2000) J. Cell Biol. 148 1267–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren, X. R., Reiter, E., Ahn, S., Kim, J., Chen, W., and Lefkowitz, R. J. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 1448–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagane, B., Chow, K. Y., Balabanian, K., Levoye, A., Harriague, J., Planchenault, T., Baleux, F., Gunera-Saad, N., Arenzana-Seisdedos, F., and Bachelerie, F. (2008) Blood 112 34–44 [DOI] [PubMed] [Google Scholar]
- 33.Gesty-Palmer, D., Chen, M., Reiter, E., Ahn, S., Nelson, C. D., Wang, S., Eckhardt, A. E., Cowan, C. L., Spurney, R. F., Luttrell, L. M., and Lefkowitz, R. J. (2006) J. Biol. Chem. 281 10856–10864 [DOI] [PubMed] [Google Scholar]
- 34.Girnita, L., Shenoy, S. K., Sehat, B., Vasilcanu, R., Vasilcanu, D., Girnita, A., Lefkowitz, R. J., and Larsson, O. (2007) J. Biol. Chem. 282 11329–11338 [DOI] [PubMed] [Google Scholar]
- 35.Puckerin, A., Liu, L., Permaul, N., Carman, P., Lee, J., and Diverse-Pierluissi, M. A. (2006) J. Biol. Chem. 281 31131–31141 [DOI] [PubMed] [Google Scholar]
- 36.Dasgupta, P., Rastogi, S., Pillai, S., Ordonez-Ercan, D., Morris, M., Haura, E., and Chellappan, S. (2006) J. Clin. Investig. 116 2208–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen, W., Kirkbride, K. C., How, T., Nelson, C. D., Mo, J., Frederick, J. P., Wang, X. F., Lefkowitz, R. J., and Blobe, G. C. (2003) Science 301 1394–1397 [DOI] [PubMed] [Google Scholar]
- 38.Nakashima, H., Frank, G. D., Shirai, H., Hinoki, A., Higuchi, S., Ohtsu, H., Eguchi, K., Sanjay, A., Reyland, M. E., Dempsey, P. J., Inagami, T., and Eguchi, S. (2008) Hypertension 51 232–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah, B. H., Yesilkaya, A., Olivares-Reyes, J. A., Chen, H. D., Hunyady, L., and Catt, K. J. (2004) Mol. Endocrinol. 18 2035–2048 [DOI] [PubMed] [Google Scholar]
- 40.Prenzel, N., Zwick, E., Daub, H., Leserer, M., Abraham, R., Wallasch, C., and Ullrich, A. (1999) Nature 402 884–888 [DOI] [PubMed] [Google Scholar]
- 41.Luttrell, L. M., Hawes, B. E., van Biesen, T., Luttrell, D. K., Lansing, T. J., and Lefkowitz, R. J. (1996) J. Biol. Chem. 271 19443–19450 [DOI] [PubMed] [Google Scholar]
- 42.Rajagopal, K., Whalen, E. J., Violin, J. D., Stiber, J. A., Rosenberg, P. B., Premont, R. T., Coffman, T. M., Rockman, H. A., and Lefkowitz, R. J. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 16284–16289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeWire, S. M., Kim, J., Whalen, E. J., Ahn, S., Chen, M., and Lefkowitz, R. J. (2008) J. Biol. Chem. 283 10611–10620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noma, T., Lemaire, A., Naga Prasad, S. V., Barki-Harrington, L., Tilley, D. G., Chen, J., Le Corvoisier, P., Violin, J. D., Wei, H., Lefkowitz, R. J., and Rockman, H. A. (2007) J. Clin. Investig. 117 2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]