Abstract
In bacterial pathogenesis, virulence gene regulation is controlled by two-component regulatory systems. In Escherichia coli, the EnvZ/OmpR two-component system is best understood as regulating expression of outer membrane proteins, but in Salmonella enterica, OmpR activates transcription of the SsrA/B two-component system located on Salmonella pathogenicity island 2 (SPI-2). The response regulator SsrB controls expression of a type III secretory system in which effectors modify the vacuolar membrane and prevent its degradation via the endocytic pathway. Vacuolar modification enables Salmonella to survive and replicate in the macrophage phagosome and disseminate to the liver and spleen to cause systemic infection. The signals that activate EnvZ and SsrA are unknown but are related to the acidic pH encountered in the vacuole. Our previous work established that SsrB binds to regions of DNA that are AT-rich, with poor sequence conservation. Although SsrB is a major virulence regulator in Salmonella, very little is known regarding how it binds DNA and activates transcription. In the present work, we solved the structure of the C-terminal DNA binding domain of SsrB (SsrBC) by NMR and analyzed the effect of amino acid substitutions on function. We identified residues in the DNA recognition helix (Lys179, Met186) and the dimerization interface (Val197, Leu201) that are important for SsrB transcriptional activation and DNA binding. An essential cysteine residue in the N-terminal receiver domain was also identified (Cys45), and the effect of Cys203 on dimerization was evaluated. Our results suggest that although disulfide bond formation is not required for dimerization, dimerization occurs upon DNA binding and is required for subsequent activation of transcription. Disruption of the dimer interface by a C203E substitution reduces SsrB activity. Modification of Cys203 or Cys45 may be an important mode of SsrB inactivation inside the host.
Salmonella infections occur in a wide variety of vertebrate hosts and continue to be a major health problem worldwide for humans. Salmonella infection requires at least two pathogenicity islands, Salmonella pathogenicity islands 1 and 2 (SPI-1 and SPI-2),3 both of which encode type III secretion systems as well as secreted effectors, chaperones, and regulatory proteins (1–3). Genes located on SPI-1 are required for initial adherence to and invasion of intestinal epithelial cells (4). SPI-2 genes are required for intracellular survival, replication, and systemic infection of Salmonella (5, 6). SPI-2 consists of a 40-kb region located at 31 centisomes on the chromosome and contains ∼32 genes (7). SPI-2 genes are organized into functional clusters encoding regulatory, structural genes, effectors, and chaperones. To date 10 promoters of SPI-2 genes and SPI-2 co-regulated genes have been identified (8–10). These promoters, located upstream of the ssrA, ssrB, ssaB, sseA, ssaG, ssaM, sseI, srfN, sifA, and sifB genes, transcribe genes either individually or in large operons. Transcription from these promoters is activated by the SPI-2 encoded two-component signal transduction system, SsrA/SsrB (8, 11–13).
Two-component signal transduction systems regulate gene expression in response to specific environmental signals (for review see Ref. 14). These systems represent the major paradigm for signal transduction in prokaryotes. They are frequently involved in regulating expression of virulence genes in pathogenic bacteria and are also present in the archaea, lower eukaryotes, and plants. In its simplest form, a two-component system contains a sensor kinase, often a membrane protein that functions in trans-membrane signaling, and a response regulator, usually a DNA-binding protein that regulates transcription. In Salmonella enterica serovar typhimurium, the SsrA/SsrB two-component system controls expression of SPI-2 genes as well as several non-SPI-2-encoded virulence genes (8, 11–13). These genes encode effector proteins that are secreted through the SPI-2 type III secretion system or are structural components of the secretory apparatus (5, 11).
SsrA is a tripartite kinase composed of an ATP binding domain, a histidine phosphorylation domain, a receiver or phosphorylation domain also present in response regulators and a histidyl phosphotransfer domain. SsrA is located in the bacterial inner membrane and, based on homology to similar histidine kinases, has two transmembrane domains. These complex domain structures suggest a phosphorelay whereby the phosphoryl group is transferred via intramolecular reactions from the histidine to an aspartate, to a histidine, and then onto the conserved aspartate of the SsrB response regulator (8, 9, 13, 15).
SsrB is a two-domain response regulator in the NarL/FixJ subfamily. The N-terminal receiver domain of SsrB contains the conserved aspartic acid phosphorylation site and the C-terminal effector domain binds DNA (13). The receiver domains are highly conserved, whereas the effector domains are more tailored to their specialized output functions. NarL, a response regulator involved in nitrogen sensing, is the first structure of this subfamily to be solved and it is also the first structure of a full-length response regulator (16). The structure elucidates many details of the activation mechanism, as the recognition helix is physically blocked by the N terminus. Phosphorylation drives a conformational change that relieves this inhibitory effect, exposing the recognition helix and promoting dimerization (17). SsrB functions by a similar mechanism (13). To activate transcription, SsrB must be phosphorylated at Asp56 (13), yet surprisingly, overexpression can activate SPI-2 genes in the absence of the SsrA kinase (8, 13). This result indicates that alternative inputs in the form of additional kinases or small molecule phosphodonors such as acetyl phosphate must exist that can phosphorylate SsrB. In this manner the SsrA/SsrB system is uncoupled, as is expression of the ssrA/ssrB genes (9, 13).
SsrB is homologous at the primary sequence level to several other two-component regulators for which structures have been determined, including NarL, RcsB, and DosR (16, 18–21). Although this subfamily shares significant structural similarity, important functional differences exist. DosR forms a unique tetramer on the DNA, forming a dimer of dimers, although its relevance in vivo remains to be established (21). NarL binds to its DNA targets as a homodimer, whereas RcsB can bind to DNA either as a homodimer or as a heterodimer with RcsA (22). NarL binds to a well conserved DNA consensus sequence (23), whereas the RcsB binding site is more degenerate (22), and it is difficult to discern a specific binding motif for SsrBC (8). DosR binds to a pseudopalindrome that is GC-rich (19). The DNA binding domain of SsrBC alone can function as a transcription factor in vivo (13), but the DNA binding domain of NarL cannot, even though it is capable of DNA binding (17). It was therefore of interest to determine how these differences in function might be reflected as structural differences between the subfamily members.
In the present work, we solved the solution structure of SsrBC by NMR and examined the effect of amino acid substitution on DNA binding, dimerization, and transcription. The C-terminal 75 amino acid residues of SsrB (SsrBC) fold into a four-helix bundle, and the SsrBC dimerization surface is similar to that of DosR, NarL, and RcsB. SsrBC binds to regions of DNA that are AT-rich with poor sequence conservation. We identified residues in the DNA recognition helix and the dimerization interface that are important for SsrB transcriptional activation and DNA binding. An essential cysteine residue located in the N-terminal receiver domain was also identified, and the effect of Cys203 on dimerization was evaluated. Our results suggest that although disulfide bond formation is not required for dimerization, dimerization occurs upon DNA binding and is required for subsequent transcriptional activation. Disruption of the dimer interface by substitution of Cys203 with a negatively charged residue substantially reduces SsrB activity. Cys203 modification may represent an important mode of SsrB inactivation inside the host.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Plasmids, and Culture Conditions—A list of the bacterial strains and plasmids used in this study is included in supplemental Table 1. DH5α was used for routine cloning and BL21(DE3) for protein expression. Bacterial cultures were grown in LB broth or N9 minimal medium (8) as indicated. Antibiotics, where required, were used at the following concentrations: ampicillin 100 μg/ml, chloramphenicol 30 μg/ml, and kanamycin 50 μg/ml.
Site-directed Mutagenesis—Site directed mutagenesis was performed using QuikChange (Stratagene). Plasmids pKF43 (full-length SsrB) and pKF104 (C-terminal domain) were used as templates (13). Oligonucleotide primers ordered from Sigma are listed in supplemental Table 2. Primer pairs C45Sf and C45Sr, K179Af and K179Ar, E182Af and E182Ar, T183Af and T183Ar, M186Af and M186Ar, V197Af and V197Ar, T198Af and T198Ar, L201Af and L201Ar, N202Af and N202Ar, C203Af and C203Ar, C203Ef and C203Er, and C203Sf and C203Sr were used to introduce the C45S, K179A, E182A, T183A, M186A, V197A, T198A, L201A, N202A, C203A, C203E, and C203S substitutions. Substitutions introduced into full-length SsrB in plasmid pKF43 resulted in plasmids pRC32 (C45S), pRC24 (K179A), pRC31 (E182A), pRC22 (T183A), pRC25 (M186A), pRC27 (V197A), pRC28 (T198A), pRC29 (L201A), and pRC30 (N202A). Substitutions introduced into SsrBc in plasmid pKF104 resulted in plasmids pRC39 (C203A), pRC44 (K179A), pRC45 (M186A), pRC47 (V197A), pRC48 (T198A), pRC49 (L201A), and pRC50 (N202A). The resulting plasmids were verified by sequencing.
β-Galactosidase Assays—Plasmids expressing SsrB and substituted SsrB proteins were transformed into strain MJW604, and β-galactosidase assays were performed as described previously (13). Briefly, cells were grown to A600 ∼ 1.0, at which time arabinose was added to a final concentration of 0.1%. Following a further incubation of 2 h, samples were taken and β-galactosidase assays performed according to the method of Miller (24). Results shown are the average of four independent cultures.
Transdominance Analysis—Plasmids expressing wild-type SsrB and substituted SsrB proteins were transformed into strain MJW704, which contains an intact chromosomal copy of the ssrB gene. Strains were grown overnight in LB and subsequently subcultured into N9 minimal medium. Following 7 h of growth in N9 at 37 °C, arabinose was added (0.1% w/v), and the cultures were incubated at 37 °C for an additional 1 h. At this point samples were taken for β-galactosidase assays, performed as described above.
Protein Induction and Purification—SsrBC and its substituted derivatives used in DNA mobility shift assays and BMH cross-linking was purified using the QIAexpressionist™ protein miniprep system (Qiagen). Plasmids expressing SsrBc were transformed into BL21(DE3), grown to an A600 ∼ 0.6, and induced with arabinose (0.1%) for 3 h at 37 °C. 50 ml of culture was pelleted and stored at –20 °C until required. For protein purification, pellets were resuspended in 3 ml of lysis buffer (50 mm NaH2PO4, 300 mm NaCl, 10 mm imidazole, pH 8.0) containing 100 μg/ml lysozyme and sonicated (Fisher Scientific Sonic Dismembrator, model 100, 3 × 30 s, setting 7 with 2 min on ice between each pulse) to ensure complete lysis and shearing of DNA. Lysates were then incubated with 100 μl of nickel-nitrilotriacetic acid resin for 1 h at room temperature with shaking. The resin was pelleted, washed five times with wash buffer (50 mm NaH2PO4, 300 mm NaCl, 20 mm imidazole, pH 8.0), and eluted in 300 μl of elution buffer (50 mm NaH2PO4, 300 mm NaCl, 250 mm imidazole, pH 8.0). Glycerol was added to a final concentration of 5%, and proteins were stored at –70 °C until required.
Electrophoretic Mobility Shift Assay—Electrophoretic mobility shift assays (EMSAs) were performed using the Lightshift chemiluminescence EMSA kit (Pierce) according to the manufacturer's instructions. A 300-bp fragment of the sseI promoter containing the SsrB binding site was amplified using biotinylated oligos B-srfHf and B-srfHr (supplemental Table 2). 20 fmol of biotinylated sseI DNA was used in a 15-μl reaction containing binding buffer (10 mm Tris, pH 7.5, 50 mm KCl) along with 2.5% glycerol, 5 mm MgCl2, 50 ng/μl poly(dI-dC), and 0.05% Nonidet P-40. SsrBC protein at the concentrations indicated was added, and samples were separated by electrophoresis on 5% nondenaturing acrylamide gels run in 0.5× Tris acetate buffer with EDTA. Following electrophoresis, DNA was electro-transferred to a nylon membrane and detected using the biotin detection system (Pierce).
BMH Cross-linking—BMH cross-linking was performed as described previously (25). Approximately 10 μm SsrBC protein was used in the reactions with 50 μm BMH. We also added sseI promoter DNA (1:1 molar ratio) to determine whether the presence of DNA enhanced or interfered with dimer formation. Samples were separated by PAGE on 12% gels and stained with Coomassie Brilliant Blue.
Protein Expression and Purification for NMR—SsrBC protein was uniformly labeled with 13C and 15N while being overexpressed in Escherichia coli strain BL21(DE3) at 37 °C in M9 minimal media containing [15N]NH4Cl and [13C]glucose. Expression of SsrBC protein was induced by the addition of 0.1% arabinose and grown overnight as described previously (13). The cells were harvested by centrifugation, and the His-tagged protein was purified using nickel-nitrilotriacetic acid resin under denaturing conditions according to the manufacturer's instructions (Qiagen). Purified SsrBc protein was renatured by dialysis against a buffer containing 50 mm Na2HPO4, pH 6.5, 50 mm NaCl, and 5 mm Na2S2O4. The protein concentrations used for NMR analysis ranged from 0.4 to 1 mm as measured by the Bio-Rad protein assay using bovine serum albumin as the standard. Western blots were performed as described previously (13).
NMR Spectroscopy and Data Processing—All NMR experiments were performed at 35 °C on a Bruker Avance 800 MHz equipped with a cryoprobe and pulsed-field gradients. The spectra were processed using the software package Triad. The backbone and side-chain resonance assignments were obtained through standard double and triple nuclear experiments with the Topspin software supplied with the instrument. A mixing time of 120 ms was used in all NOESY experiments to generate distance constraints for structure calculations. The assignment of the sequence-specific backbone resonance assignment of 69 amino acid residues of SsrBC was achieved through a combination of standard two-dimensional and three-dimensional double and triple resonance NMR experiments (26). More than 75% of all side-chain resonances were assigned for SsrBC.
Structure Determination—The structure determination was based on NOE-derived distances, dihedral angles, and hydrogen bond distance constraints. The NOE distance constraints were obtained from standard three-dimensional 15N- and 13C-edited NOESY-HSQC experiments. The backbone dihedral angle constraints were determined using the program TALOS (27). Hydrogen bond distance constraints were obtained from the hydrogen-deuterium exchange experiments in conjunction with the intermediate range NOESY and the secondary structural information. The structures of SsrBC were calculated with the DYANA program (28). Initial structures were calculated using unambiguous NOEs derived from 13C-, 15N-, and 1H-enriched SsrBC. Based on the initial structures, further assignments of the NOE peaks were assigned. A total of 200 structures were calculated using DYANA by the standard simulated annealing protocol and NOE distance constraints, dihedral angles, and hydrogen bond distance constraints. In the final analysis, 20 structures with the lowest DYANA energy and constraints violation energy were selected and examined by PRO-CHECK-NMR (29). The structures were analyzed using the MOLMOL program (30).
RESULTS
Resonance Assignment and the Structure of SsrBC—We solved the solution structure of SsrBC by high-resolution 1H, 15N, and 13C NMR spectroscopy. Initial studies with the full-length SsrB protein were complicated because of its extreme insolubility. We had shown previously that the isolated C-terminal domain SsrBC could activate transcription (13), and we used it in DNase I protection assays to identify SPI-2 genes that are regulated by SsrB (8). Because of its ability to substitute functionally for the full-length protein in vivo, we used the C-terminal domain for the NMR structural studies reported herein.
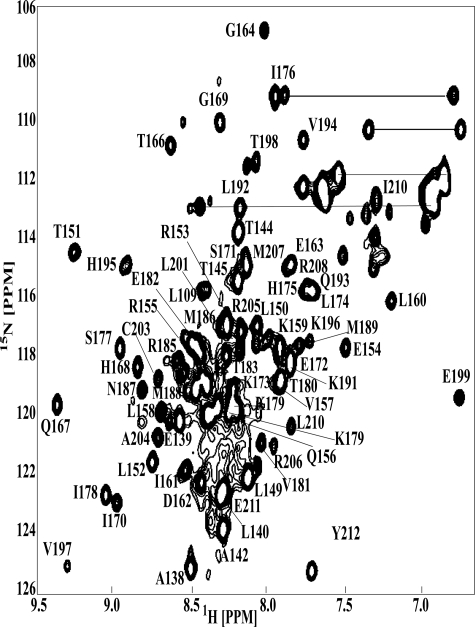
A 1H-15N HSQC spectrum of 15N-labeled SsrBC with corresponding residue assignments indicated is shown in Fig. 1. In this spectrum, expected correlation peaks for the backbone amide resonances were assigned. The pattern of short and medium range NOEs and 3JHNHα coupling constants served to identify the secondary structural elements of SsrBC (Fig. 2). The pattern of dNN(i, i+2), dαN(i, i+3), and dαN(i, i+4) NOE connectivities with 3JHNHα coupling constants smaller than 6 Hz indicated the presence of four α-helices (Leu150–Ile161, α-1; Gly169–Lys173, α-2; Ser177–Met186, α-3; and Val197–Arg205, α-4).
FIGURE 1.
An assigned HSQC spectrum of SsrBC. The spectrum was acquired at 35 °C and pH 6.5. The resonance assignments are indicated in the figure. The horizontal bars connect the side chain NH2 groups to Asn and Glu.
FIGURE 2.
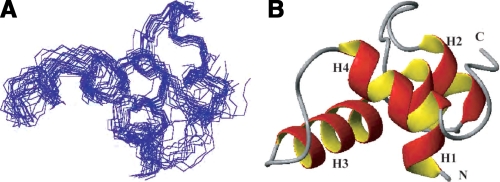
A, an ensemble of the 20 final DYANA structures is shown. The backbone heavy atom traces superimposed for residues 15–75 are displayed. B, ribbon diagram of SsrBC. The location of the four α-helices from the SsrB three-dimensional structure are labeled H1–H4. The N and C termini are indicated.
After resonance assignment, the three-dimensional structure was calculated using the program DYANA. A total of 814 interproton restraints and 37 dihedral angle constraints were used in the final round of the structural calculation. For the final structural calculation, the N-terminal 14 residues from the interdomain linker were excluded because of their disordered nature. At the last stage, 100 conformers were calculated, and of these, 20 DYANA conformers with the lowest target function were selected to represent the structure (Fig. 2A). The coordinates have been deposited in the Research Collaboratory for Structural Bioinformatics Protein Data Bank with accession code 2JPC. The r.m.s.d. of the well structured regions of the 20 best structures was 0.62 ± 0.12 Å for backbone atoms and 1.61 ± 0.22 Å for all non-hydrogen atoms. The quality of the structures is high, with no dihedral angles in the disallowed regions. The structural statistics are summarized in Table 1.
TABLE 1.
Data collection and refinement statistics
| NMR-derived restraints | |
| Total interproton restraints | 814 |
| Intra-residue (|i - j| = 0) | 118 |
| Sequential (|i - j| = 1) | 249 |
| Medium range (1 < |i - j| < 5) | 196 |
| Long range (|i - j| > 4) | 191 |
| Hydrogen bonds | 60 |
| Dihedral angles (ϕ, ψ) | 37 |
| Residual violationsa | |
| DYANA target function | 0.34 |
| Upper limit | |
| Sum (Å) | 2.7 |
| Maximum (Å) | 0.11 |
| Van der Waals | |
| Sum (Å) | 2.2 |
| Maximum (Å) | 0.12 |
| Average r.m.s.d. to mean structureb | |
| Backbone atoms N, Cα, C′ (Å) | 0.62 ± 0.12 |
| All heavy atoms (Å) | 1.61 ± 0.22 |
| Ramachandran plot (% residues) | |
| Residues in most favored regions | 73.0 |
| Residues in additional allowed regions | 19.3 |
| Residues in generously allowed regions | 7.7 |
| Residues in disallowed regions | 0.0 |
Under residual violations, the values are means ± S.D.
Residues are in secondary elements.
The structure of SsrBC consists of a compact bundle of four well folded α-helices encompassing residues 143–212 (labeled H1–H4 in the ribbon diagram in Fig. 2B). The three-dimensional structure of SsrBC is stabilized by hydrophobic residues, which are conserved among the NarL subfamily members (Fig. 3). The averaged structure from the current NMR study aligns well with the x-ray structure of NarLC with an r.m.s.d. value of 1.62 Å (Fig. 4A) and with DosR of 1.4 Å (Fig. 4B).
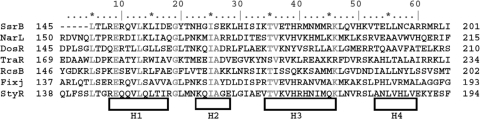
FIGURE 3.
An alignment of the primary structure of the DNA binding domains of SsrB and its homologues. References for these homologues are as follows (in parentheses): NarL (17, 23), DosR (21), TraR (18), RcsB (20), FixJ (36) and StyR (35). Amino acids indicated in gray are conserved, and the secondary structure is indicated below the sequence.
FIGURE 4.
A, overlay of NarL structure (gray) with SsrBc (blue). B, overlay of DosR (red) and SsrB (green). N = amino terminus, C = carboxyl terminus; helices 1–4 are labeled H1–H4. The r.m.s.d. for Cα for SsrB and DosR is 1.4 Å and for SsrB and NarL is 1.62 Å.
SsrBC-DNA Interactions and Contact Residues—Thus far, we have identified 10 genes that are directly activated by SsrB; they are AT-rich but do not contain a well conserved consensus site (8, 10, 12, 13). This may be in part because, at many promoters, SsrB acts primarily to counter the effects of gene silencing by H-NS, rather than as a direct transcriptional activator (8, 32). The primary structures of SsrB, NarL, and RcsB DNA binding domains are highly homologous (Fig. 3). Their three-dimensional structures are also highly homologous, with an r.m.s.d. less than 1.6 Å between SsrBC and either NarLC, RcsBC, or DosRC. As shown in Fig. 2B, SsrBC contains four α-helices (H1–H4; H6–H10 in the full-length protein). H2 and H3 comprise the helix-turn-helix DNA binding domain, whereas H4 corresponds to the dimerization domain of both NarL and RcsB (17, 20) as well as SsrB (this work).
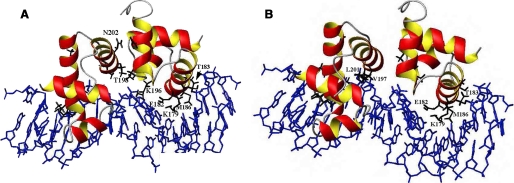
Although the structures of NarL and RcsB are highly homologous, they exhibit very different DNA binding patterns. NarL binds as a homodimer to highly conserved, specific DNA sites exhibiting anti-parallel symmetry (17). RcsB can bind to diverse DNA targets as either a homodimer or as a heterodimer with RcsA (20). DosR binds as a dimer of dimers, forming a unique tetramer and recognizing a conserved DNA binding site (21). An alignment of the recognition helix (H3) of SsrB and its homologues, NarL and RcsB, is shown in Fig. 5A along with other NarL family members. Known or predicted DNA contact residues are underlined. In the best studied member of this family, NarL, Lys188 accommodates DNA sequence variation in the three different complexes that have been examined thus far (23). It provides “flexible specificity,” recognizing the major groove floor directly and/or via bridging waters. Val189 is a highly conserved NarL residue that enforces significant DNA base distortion (17, 23, 33). Lys192 of NarL is also important for recognition, by hydrogen bonding to guanines at regions of high DNA helical writhe. It is noteworthy that whereas RcsB has only one of these residues (corresponding to Lys192 of NarL), none of them are conserved in SsrB (Fig. 5A). In place of the conserved Val189 of NarL, SsrB has a threonine and RcsB a serine residue. In SsrB, a negatively charged glutamic acid residue replaces Lys188 of NarL, whereas in RcsB, it is replaced by a serine. However, both SsrB and RcsB have a lysine further upstream that corresponds to Lys179 of DosR, a known DNA contact residue (21). Lys192 of NarL, although conserved in RcsB, is replaced with a methionine in SsrB. Altogether, these alignments raise interesting questions with respect to DNA binding specificity in these NarL homologues. RcsB and NarL both share one conserved DNA contact residue, as do RcsB and SsrB. SsrB and NarL share no conserved residues, raising the possibility that SsrB recognizes DNA differently from NarL, although our NMR results suggest that the DNA contact surface of SsrB is similar to that of NarL. Thus, we assume that the α-3 helix of SsrBC is the major groove DNA contact surface, as has been shown in co-crystal structures of the other regulators listed here. Based on the structures of the NarL-DNA, TraR-DNA, and DosR-DNA complexes, we modeled SsrBC bound to DNA (Fig. 6). The residues predicted to be involved in SsrB-DNA interactions are Lys179, Thr183, and Met186 (highlighted in Fig. 6A). Glu182 is predicted to make nonspecific contacts with the phosphate backbone. In another view, Val197 and Leu201 may also be DNA contact residues, although they are in the loop and the beginning of the dimerization helix H4 (Fig. 6B).
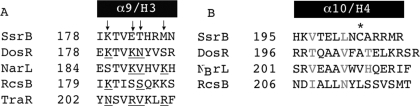
FIGURE 5.
Sequence alignment of the DNA contact helix and dimerization helix of SsrB with NarL family members. A, primary sequence alignment of DNA contact helix (α9/H3) of SsrB with NarL family members for which the structure is known. Amino acid residues of NarL family members known or predicted to be involved in DNA contact are underlined. Predicted DNA contact residues of SsrB are indicated by arrows. B, primary sequence alignment of the dimerization helix of SsrB with NarL, RcsB, and DosR. TraR was not included because its dimerization interface is different (18, 31, 41). Potential amino acid residues involved in dimerization are indicated in red (NarL) and blue (RcsB). A unique cysteine residue is indicated by an asterisk.
FIGURE 6.
A, three-dimensional model of SsrBC bound to DNA. Predicted base contacts Lys179, Glu182, and Thr183 are indicated as are the likely dimerization residues Thr198 and Asn202. B, in a different view, the potential DNA contact with amino acids Val197 and Leu201 are indicated. See “Results” for details.
Substitution of Predicted DNA Contact Residues Disrupts Transcription—To identify which of the predicted DNA contact residues are important for SsrB function, we performed site-directed mutagenesis and determined the ability of the mutant proteins to activate transcription. Lys179, Glu182, Thr183, and Met186 were each substituted with alanine, and the ability of the mutant proteins to activate expression of a sseI(srfH)-lacZ fusion strain was determined. Previous studies have shown that sseI is highly dependent on SsrB for transcriptional activation (11, 13), and DNase I footprinting has identified an SsrB binding site between –122 and –95 upstream of the transcriptional start site (13). SsrB and its mutant derivatives were expressed from an arabinose-inducible promoter in strain MJW604, and β-galactosidase assays were performed to determine the transcriptional activation of sseI-lacZ (Fig. 7). In the absence of SsrB, essentially no transcription from the sseI promoter was detected (Fig. 7, column 1), whereas expression of SsrB strongly induced sseI (Fig. 7, column 2, set to 100%). Transcriptional activation was completely dependent on the phosphorylated Asp56 (Fig. 7, column 3) as shown previously (13). The mutant Lys179A completely abolished sseI transcription (Fig. 7, column 4), whereas Met186A resulted in an ∼4-fold decrease in activity compared with the wild type (Fig. 7, column 7). In contrast, substitution of Thr183 with alanine improved the ability of SsrB to activate sseI-lacZ expression (Fig. 7, column 6), resulting in 50% stimulation over the activity of the wild type. The Glu182 substitution reduced activity to 60%, consistent with a weak effect from a residue predicted to make nonspecific interactions with the phosphate backbone (Fig. 7, column 5). These results demonstrate that Lys179 and Met186 are both important for sseI promoter activation by SsrB, whereas Thr183 is not required. Similar results were obtained with the mutants expressed only as an SsrBC fragment, except for Met186A (data not shown; see “Discussion”).
FIGURE 7.
DNA recognition helix substitutions affect SsrB activity. β-Galactosidase assays were performed in strain MJW604 to determine activity from the sseI promoter while expressing SsrB from an arabinose-inducible plasmid. MJW604 expressing wild-type SsrB was used as a positive control, and MJW604 not expressing SsrB, or expressing SsrB containing a D56A substitution, was used as the negative control. Results are shown for substitutions K179A, E182A, T183A, and M186A.
Analysis of the SsrBC Dimerization Helix—Sequence alignment of SsrBC helix 4 (H4) with the dimerization helices of DosRC, NarLC, and RcsBC is shown in Fig. 5B. In the co-crystal structure of the NarLC-DNA complex, two hydrophobic residues, Val204 and Val208, reside on the surface of the dimerization helix and engage in intermolecular interactions to form a homodimer on the DNA binding site (17). In the RcsB structure, Ile199 and Asn203 occupy the corresponding positions of the two valines of NarL. RcsB can bind to DNA as a homodimer, but it also binds to DNA as a heterodimer with RcsA (20). The interaction surface and residues involved in RcsB-RcsA heterodimerization are unknown but are presumed to be via interactions in the N terminus.4
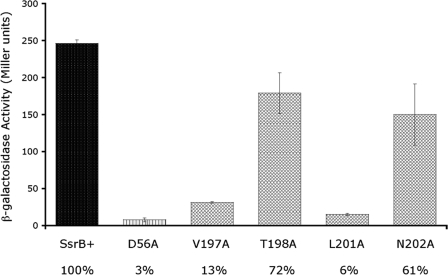
Our NMR structures indicate that the residues on the surface of H4 are different from the other SsrB family members. To determine which residues in H4 are important for SsrB dimerization, the potential dimerization residues were substituted independently with alanine and the transcriptional activity of the mutant proteins determined in the sseI-lacZ fusion strain (Fig. 8). Alanine substitutions at positions Val197 and Leu201 resulted in mutant SsrB proteins that were unable to activate transcription, e.g. activity was 13 and 6%, respectively (Fig. 8, columns 3 and 5; where wild type = 100%). In a different alignment, it was suggested that Thr198 and Asn202 may correspond to Ile208 and Asn212 of RcsB; thus, these residues were also substituted and their transcriptional activity determined. Substitutions at positions Thr198 or Asn202 had only a moderate effect on SsrB activity, reducing sseI transcription to 72 and 61%, respectively, compared with the wild type (Fig. 8, columns 4 and 6). The absolute requirement for Val197 and Leu201 indicates that they are important for SsrB activity, and their alignment with dimerization positions in DosR, NarL, and RcsB suggests they are involved in dimerization of SsrB (see “Discussion”). This result also suggests that dimerization is required for SsrB activation of the sseI promoter. Western blots were performed to determine SsrB protein levels in each strain. Comparable levels of SsrB protein were detected with each mutant (see example Fig. 9, inset). In addition, HSQC spectra were acquired on Lys179A and Leu201A mutant proteins, indicating that the proteins are well folded (except for helix 4 of Leu201A; see below).
FIGURE 8.
The effect of dimerization helix substitutions on SsrB activity. β-Galactosidase assays to determine activity from the sseI promoter were performed in strain MJW604 expressing SsrB from an arabinose-inducible plasmid. Wild-type SsrB and SsrB D56A were used as the positive and negative control, respectively.
FIGURE 9.
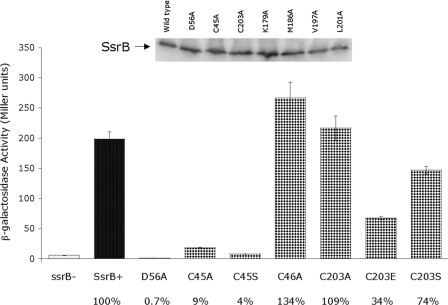
Cysteine substitutions alter SsrB-dependent transcription. β-Galactosidase assays were performed in strain MJW604 expressing SsrB from an arabinose-inducible plasmid to determine the affect of cysteine substitutions on SsrB activity. Inset: representative Western blot performed on cultures grown identically to those used in β-galactosidase assays. Equivalent amounts of wild-type SsrB and its substituted derivatives were detected in each sample. All samples were normalized by A600, and an equivalent amount was loaded in each lane prior to SDS-PAGE.
Role of Cysteine Residues in SsrB Function—Our analysis of the dimerization helix of SsrBC identified two residues important for SsrB activity, Val197 and Leu201. Another interesting feature of helix 4 is the presence of a cysteine residue located at position 203. This cysteine in SsrB is not conserved in other family members (Fig. 5B). Sequence analysis of SsrB identified two additional cysteines located in the N terminus at positions 45 and 46. This vicinal configuration and distribution of cysteine residues is unique to SsrB response regulator proteins in Salmonella serovars typhimurium and typhi.5 The presence of a cysteine residue within H4 raised the possibility that it could play a role in SsrB dimerization and function. We therefore investigated the role of all three cysteine residues in SsrB transcriptional activity.
We initially substituted each of the cysteines with alanine and examined the ability of the mutant proteins to bind DNA and to activate transcription. We used alanine substitutions, even though serine is considered a more conservative replacement, because substitution of the lone cysteine in OmpR with serine prevented phosphorylation (34). β-Galactosidase assays were performed using the sseI-lacZ fusion with either wild-type SsrB or SsrB with a D56A substitution as positive and negative controls, respectively (Fig. 9). Induction of SsrB expression resulted in activation of the sseI promoter, whereas expression of SsrB D56A did not activate sseI (Fig. 9, columns 1–3). Substitution of cysteine at position 203 with alanine had no effect on SsrB activity, indicating that disulfide bond formation is not required for activity (Fig. 9, column 7). Similarly, the substitution C46A had a modest effect on SsrB-dependent transcription (Fig. 9, column 6, 34% higher than wild type). In contrast, the C45A mutant was significantly impaired (9% of wild-type activity) in its ability to activate expression of the sseI-lacZ fusion (Fig. 9, column 4). A serine substitution at Cys45 was also not functional (Fig. 9, column 5). This result demonstrates an important role for Cys45 in SsrB function and indicates that neither Cys46 nor Cys203 is required for SsrB activity (see “Discussion”). To further investigate the role of Cys203, additional substitutions were constructed at this position. Although serine substitution had only a modest effect (74% of wild type), a glutamic acid substitution reduced activity to 34%. In the NMR structure, the side chain of Cys203 interacts with Leu192 located in the loop between H3 and H4. When Cys203 is substituted with an alanine or a serine, this interaction is likely maintained, whereas a residue with a negative charge at this position can destabilize the helix, disrupting the dimer as well as DNA binding (see Fig. 9 and also “Discussion”). To establish that the differences in sseI activity among all substituted forms of SsrB was not due to altered expression levels of the mutant proteins, Western blots were performed to determine SsrB protein levels in each strain. Comparable levels of SsrB protein were detected with each mutant (a representative blot is shown in Fig. 9, inset).
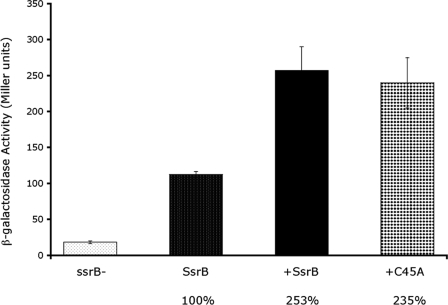
Transdominance Effect of Cys45—To further explore the essential role of Cys45 in the N-terminal receiver domain on SsrB activity, we expressed the SsrB mutant in a strain containing the sseI-lacZ fusion in the presence of the wild-type chromosomal ssrB gene. The experiment was performed under SsrB-inducing conditions so that both forms of the protein were present in the cell. If the mutant form of SsrB can dimerize with the wild-type SsrB but cannot bind to DNA, the resulting “faulty dimer” will not activate transcription of sseI, and β-galactosidase activity will be reduced compared with the wild-type strain not expressing mutant SsrB. Alternatively, expression of a mutant form of SsrB that is unable to dimerize will not result in the formation of “faulty dimers,” and sseI transcription should not be affected. As expected, an increase in sseI-lacZ expression was observed in the wild-type strain compared with the ssrB null (Fig. 10, columns 1 and 2; wild type = 100%). Overexpression of wild-type SsrB in the wild-type background resulted in a further 2.5-fold increase in expression (Fig. 10, column 3), indicating that normally, chromosomal levels of SsrB are limiting. Similarly, expression of the SsrB mutant C45A in the wild-type background also resulted in a >2-fold increase in transcription (Fig. 10, column 4), similar to that seen when overexpressing wild-type SsrB. Yet when C45A was the only form of SsrB present, it was unable to activate sseI transcription (see Fig. 9). This result suggests that a C45A monomer can dimerize with a wild-type SsrB monomer and that the heterodimer exhibits enhanced wild-type activity (Fig. 10, compare columns 3 and 4). Thus, it would appear that the defect in C45A transcription is a defect in its ability to dimerize, not in its ability to bind to DNA (see “Discussion”). In contrast, overexpressed SsrB mutants D56A, K179A, M186A, V197A, and L201A in the wild-type background all exhibited reduced transcriptional activity at the level of the empty vector control (data not shown), suggesting that the mutants form faulty dimers with wild-type SsrB, which significantly reduce its activity.
FIGURE 10.
Transdominance assay using chromosomal wild-type SsrB and plasmid-encoded SsrB or SsrB-C45A. Wild-type or mutant SsrB was expressed from an arabinose-inducible plasmid in strain MJW704, which contains a wild-type chromosomal ssrB gene. Assays were performed under ssrB-inducing conditions to ensure expression of chromosomal SsrB. β-Galactosidase assays were performed to determine the sseI-lacZ activity with both forms of SsrB in the cell. The results are expressed as a percent, where wild-type chromosomal SsrB is 100%.
H4 Substitutions Affect Dimer Formation—To further investigate the role of amino acids in the dimerization helix, we performed a series of cross-linking experiments with the homobifunctional reagent BMH and purified SsrBC and SsrBC mutants C203A, K179A, M186A, V197A, T198A, L201A, and N202A. SsrBC formed a disulfide bond in vitro, which was reduced by the addition of DTT (Fig. 11A, lanes 1 and 2). The C203A mutant did not form a disulfide bond, and addition of BMH did not result in a cross-linked dimeric form of the protein, confirming that the disulfide bond was formed through Cys203 (Fig. 11B). Thus, although SsrBC can form a disulfide via Cys203, disulfide bond formation is not required for transcription, as C203A and C203S mutants are active (Fig. 9). SsrBC recognition helix mutants K179A and M186A cross-linked with BMH to the same extent as the wild-type protein (Fig 11, C and D), indicating that substitutions in the recognition helix do not alter the ability of SsrBC to dimerize. Furthermore, most substitutions in the dimerization helix also did not alter the BMH cross-linking pattern, including V197A, T198A, and N202A (Fig. 11E, F, and H). Although substitution at all of these positions affects transcription (Figs. 7 and 8), the result shown in Fig. 11 suggests that substitution does not affect SsrB dimerization via Cys203. V197A is right at the beginning of H4 and the end of the loop between recognition H3 and H4. In the DosR co-crystal structure, this position is involved in DNA contact rather than dimerization (see Fig. 6A), so its effect on transcription is not surprising (Fig. 8). In contrast, SsrBC L201A was severely impaired in its ability to form a dimer when cross-linked with BMH (Fig. 11G). This result indicates that substitution of Leu201 greatly reduces the ability of SsrBC to dimerize and is the likely cause of its inability to activate sseI transcription (Fig. 8). L201A and V197A are on the same face of the dimerization helix, opposite Cys203 (see Fig. 11, structure). NMR analysis of the L201A mutant indicated that the substitution leads to unfolding of H4, which would substantially reduce its ability to dimerize (data not shown). Analytical ultracentrifugation measurements confirmed the presence of a disulfide-linked dimer, which was present at a ratio of ∼50:50 monomer/dimer at all concentrations examined. Reduction of the disulfide produced a single monomeric species (data not shown).
FIGURE 11.
BMH cross-linking of wild-type and substituted SsrB. Purified SsrB protein (lane 1) was treated with either DTT (lane 2), BMH followed by DTT (lane 3), or BMH followed by DTT in the presence of sseI DNA (lane 4). A, wild-type SsrBC. B, SsrBC C203A. C, SsrBC K179A. D, SsrBC M186A. E, SsrBC V197A. F, SsrBC T198A. G, SsrBC L201A. H, SsrBC N202A.
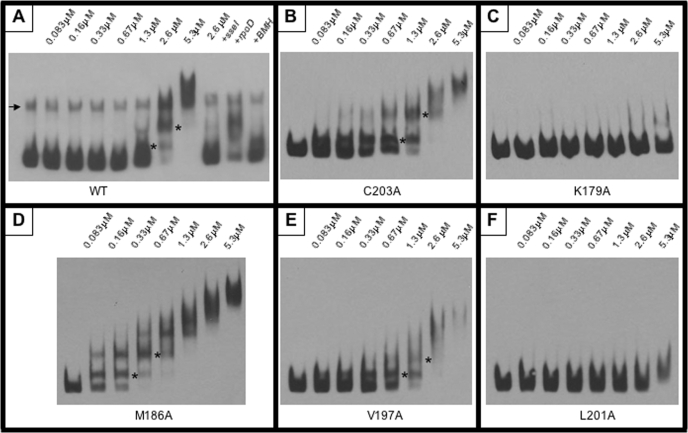
Substitutions in the Recognition Helix Disrupt DNA Binding—BMH cross-linking analysis suggested a role for Leu201 in dimerization, thus accounting for the inability of L201A to activate transcription. SsrBC mutants K179A, M186A, and V197A cross-linked similarly to the wild type, suggesting that these amino acids play a different role in SsrB function (Fig. 11). Lys179 and Met186 are in the DNA recognition helix of SsrB and are predicted to be DNA contact residues. Based on the co-crystal structure of DosR, Val197, despite its position within H4, is also predicted to contact DNA (Fig. 6A). Thus, the inability of these mutant proteins to activate transcription likely resulted from an inability to bind to DNA. To examine the DNA binding properties of the SsrBC mutants, we performed electrophoretic mobility shift assays (Fig. 12). A 300-bp fragment of the sseI promoter containing an SsrB binding site (13) was amplified using primers that were biotinylated on the 5′-ends. The resulting biotinylated DNA fragment was used in the assay with purified SsrBC and the various mutant proteins. Increasing concentrations of wild-type SsrBC resulted in the formation of an SsrBC-DNA complex that migrated through the gel with a higher apparent molecular weight than the free DNA probe (Fig. 12A). Two distinct species are clearly visible as the concentration of SsrBC is increased beginning at 83 nm (the lowest concentration employed). The formation of this protein-DNA complex was not affected by the addition of 40-fold excess nonspecific competitor DNA (Fig. 12A, lane 10). In contrast, the addition of 40-fold excess unlabeled sseI DNA resulted in the release of the labeled probe (Fig. 12A, lane 9). These results confirm that the complex formed is due to a specific interaction of SsrBC with its target DNA. The SsrBC mutants showed varying abilities to bind to the sseI promoter. C203A exhibited a DNA binding pattern similar to the wild type (Fig. 12B), consistent with its lack of effect on transcription (Fig. 9). Similarly, V197A showed a similar pattern of binding but was shifted to higher protein concentrations (e.g. compare shifted bands at 1.3 μm; Fig. 12, B and E), indicating a more apparent binding defect. In contrast, the K179A and L201A mutants were completely disrupted in their ability to bind DNA, even at >5 μm SsrBC (Fig. 12, C and F). This result is consistent with the lack of transcriptional activation shown in Figs. 7 and 8. In contrast, M186A exhibited an increased affinity for the sseI promoter compared with wild type (∼2–3 fold; Fig. 12D). Although M186A bound sseI DNA with higher affinity, it only activated sseI transcription by 24% compared with wild type (Fig. 7). This result suggests that M186A is capable of binding to DNA but is unable either to effectively counter the effects of H-NS silencing or to form a productive interaction with RNA polymerase (see “Discussion”).
FIGURE 12.
Electrophoretic mobility shift assay. Binding of purified SsrBC and its substituted derivatives to the sseI promoter was determined using an EMSA. Increasing concentrations of protein (as indicated above each lane) were incubated with sseI promoter DNA and separated by PAGE. Protein-DNA complexes migrated more slowly through the gel resulting in a “shift.” EMSAs were performed using identical concentrations of wild-type SsrBC (WT (A)), SsrBC C203A (B), SsrBC K179A (C), SsrBC M186A (D), SsrBC V197A (E), and SsrBC L201A (F). Discrete shifts are indicated by asterisks, and the arrow (in A) indicates the presence of contaminating single-stranded DNA (not present in the other reactions). A, lane 11, indicates that the BMH cross-linked protein is capable of binding to sseI DNA but with reduced affinity (compare with lane 7).
DISCUSSION
SsrB is the major virulence factor in S. enterica, regulating expression of genes within and outside of SPI-2 required for systemic infection. Yet surprisingly little is known about how it recognizes DNA and relieves H-NS silencing to activate transcription. In the present work, we used NMR spectroscopy to determine the structure of the C-terminal DNA binding domain of the response regulator SsrB (SsrBC). Our initial attempts to use full-length protein were thwarted because of its rapid insolubility. As our previous studies had demonstrated that the isolated C terminus of SsrB was stable in solution, bound DNA, and activated transcription of SsrB target genes, we used the isolated C terminus in the structural analysis reported herein.
The three-dimensional structure of SsrBC is highly homologous to both NarL and RcsB. It consists of four α-helices and contains a helix-turn-helix DNA binding domain (helices 2 and 3) and a dimerization helix (helix 4). Based upon primary sequence alignments of SsrB with its homologues for which structures are known, we identified amino acid residues in the DNA contact helix and dimerization helix that we predicted to be involved in DNA binding and dimerization. We tested these predictions by introducing alanine substitutions at each position and determining the ability of the substituted proteins to activate transcription. Four C-terminal substitutions were found to disrupt SsrB activity. Two substitutions (K179A and M186A) were located within the DNA contact helix, and two (V197A and L201A) were within the loop between H3 and H4 and the dimerization helix. The dimerization and DNA binding abilities of each of the substituted proteins were then examined. SsrBC mutant L201A was unable to dimerize or bind to the sseI promoter, and its structure was substantially disrupted by the substitution. Clearly this is an important hydrophobic residue, because alanine substitution destabilized H4. K179A could dimerize but was unable to bind DNA, even at high protein concentrations. Neither protein was able to activate expression of the sseI promoter, indicating that both dimerization and DNA binding are essential for transcription.
The specific contribution of Met186 and Val197 to SsrB function remains unclear. Substitution of either residue resulted in a decrease in sseI expression; however, both proteins were able to dimerize in vitro and bind DNA in vivo, although V197A exhibited reduced affinity for DNA (Fig. 12E). Val197 is the first residue in H4; this position is not conserved among SsrB family members. A positively charged residue (Arg or Lys) at this position in the crystal structures of the DNA complexes is shown to contact DNA and resides in the loop between H3 and H4 (17, 18, 20, 21, 23, 35, 36). Thus, we expect that this loop in SsrB also contacts DNA. The reduced activity of V197A and its lower affinity for DNA suggest that it may undergo a structural rearrangement when SsrB binds to DNA. Interestingly, the substitution M186A increased the affinity of SsrB for DNA (Fig. 12D). It is possible that the observed decrease in transcription could result from the formation of a tight protein-DNA complex at the promoter that inhibits RNA polymerase open complex formation or prevents interaction with RNA polymerase (a “positive control” mutant). A more detailed understanding of the specific mechanism with which SsrB activates transcription is required to distinguish between these possibilities, but our in vitro transcription assay suggests the former.6 SsrB has a dual role as a transcriptional activator and in relieving H-NS silencing (8). Understanding which activity predominates at the different SsrB-regulated promoters is crucial to our understanding of the role of SsrB in Salmonella virulence and will be a major focus of future experiments.
Despite extensive structural similarity, the two closest homologues to SsrB (NarL and RcsB) exhibit very distinct mechanisms of transcriptional activation. NarL activates genes by forming homodimers, which bind to a consensus DNA sequence and activate transcription. In contrast, RcsB can form homodimers or heterodimers with RcsA. RcsB homodimers and RcsB-RcsA heterodimers regulate distinct sets of genes, adding an additional layer of complexity to RcsB gene regulation (22, 23). We speculated that if SsrB were to dimerize similarly to NarL, then Val197 and Leu201 would be involved in the dimer interface and that both were important for SsrB activity (Fig. 8). However, NarL is distinct among the family members for which co-crystal structures have been solved by forming a dimer with H4 helices parallel to each other. In contrast, the others form more of an “X” with a more limited dimer interface (Fig. 6A). Thus, although the T198A and N202A substitutions had only minor effects on transcription (Fig. 8), they may still be important dimer contacts. A more radical substitution at this position might produce more dramatic effects, but this result may also indicate that the forces driving dimerization of H4 are weak.
A Role for Reduced DNA Specificity in Pathogenesis—Our analysis of the recognition helix of SsrB identified three residues we predicted to be involved in making direct contacts with the DNA. Two of these, Lys179 and Met186, turned out to be important for sseI activation, and Lys179A was shown to be unable to bind the sseI promoter (Fig. 12C). Unlike NarL and RcsB, no consensus DNA binding sequence has been identified for SsrB. Although Lys179 was shown to be important for SsrB binding to the sseI promoter, a direct role may still exist for Met186 and Thr183 in binding to other SsrB-activated genes, in particular those located within SPI-2. Our recent structural analysis of the global regulator OmpR clarified that OmpR is capable of global regulation precisely because it makes very few base contacts, binding to AT-rich DNA and making phosphate backbone contacts (37). Furthermore, OmpR contacts can vary at different promoters, i.e. DNA contacts are different at the porin genes than at the SPI-2 ssrA gene (37). This property of OmpR enables it to become a regulator of horizontally acquired genes during the course of evolution. Like OmpR, SsrB makes few base contacts, binds to AT-rich DNA, and likely makes numerous contacts with the phosphate backbone. Acquisition of an SsrB binding site has been demonstrated to be an important step in the evolutionary divergence of the human pathogen S. enterica and the reptile pathogen Salmonella bongori (10). It has recently been shown that H-NS represses horizontally acquired genes, including SPI-2 genes (32, 38). The mechanism by which SsrB overcomes H-NS repression is still unknown. Competition for binding or direct recruitment of RNA polymerase are two possible mechanisms, both of which are employed by SsrB at different promoters.7 If this is the case, the exact mechanism by which SsrB contacts DNA may vary from promoter to promoter, and Met186 and Thr183 may play more important roles at other promoters.
The Importance of Cysteines—Our investigation of SsrB structure and function also led us to examine the role of cysteine residues in SsrB activity. SsrB contains an unusual distribution of cysteine residues for a response regulator. One cysteine is located in the C terminus, within the dimerization helix, whereas two vicinal cysteines are located in the N-terminal receiver domain. Cys45 was shown to be required for function (Fig. 9), although its role in transcription is not yet clear. One interpretation is that C45A substitution causes an allosteric effect that reduces DNA binding in the C terminus. There are many examples in the case of OmpR where phosphorylation site substitutions alter DNA binding and vice versa (33, 34, 39). However, this cannot be the case with Cys45, because in the trans-dominance assay, there was an increase in transcription in the presence of wild-type SsrB, indicating that it is capable of DNA binding (Fig. 10). Cys45 might be involved in dimerization, perhaps by remodeling the dimer interface from an inactive form involving the N terminus to an active form involving H4-H4 interactions in the C terminus. Interestingly, Cys45 is located in α-helix 2 in the receiver domain, not in the typical α-4/β-5/α-5 dimer interface of many response regulators. Its role in SsrB function remains to be elucidated. Although substitution of Cys203 with alanine did not affect transcription (Fig. 9), our NMR results indicate that the Cys203 side chain interacts with Leu192. This interaction is likely maintained when Cys203 is substituted with alanine or serine, but the presence of a negatively charged glutamic acid residue at this position would reduce this interaction, destabilizing H4 and reducing SsrB dimerization, DNA binding, and transcription. Modification of Cys203 in vivo by NO when Salmonella is within the macrophage vacuole may have a similar effect and disrupt SsrB function. This could account for the decreased ability of Salmonella to survive inside activated macrophages (40). Disruption of SsrB function in this manner is likely to occur via destabilization of H4 and the loop between H3 and H4.
Supplementary Material
Acknowledgments
We are extremely grateful to Christine Schar, University of Illinois-Chicago (UIC), for help with the analytical ultracentrifugation experiments and their analysis. The analytical Ultracentrifuge was purchased using shared instrumentation Grant S10-RR22361 from the National Institutes of Health. The Bruker DRX800 was purchased with funds provided by Grant BIR0079604 from the National Science Foundation. X. L. thanks Dr. Benjamin Ramirez for assistance with NMR experiments. We are indebted to William Hendrickson, UIC, for suggestions with the EMSAs. We thank the Heffron Laboratory (Oregon Health and Sciences University, Portland) for the gift of MJW strains. We are grateful to Igor Zhulin (Oak Ridge National Laboratories, Knoxville, TN) for amino acid analysis of SsrB, and we thank Andres Vazquez-Torres and Maroof Husain (University of Colorado Health Science Center) for helpful discussions.
This work was supported, in whole or in part, by Grant GM058746 from the National Institutes of Health (to L. J. K.). This work was also supported by Grant MCB0613014 from the National Science Foundation.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2.
Footnotes
The abbreviations used are: SPI, Salmonella pathogenicity island; EMSA, electrophoretic mobility shift assay; r.m.s.d., root mean square deviation; BMH, bis-maleimidohexane; NOE, nuclear Overhauser effect; NOESY, NOE spectroscopy; HSQC, heteronuclear single quantum correlation; DTT, dithiothreitol.
F. Bernhard (Freie Universitat Berlin), personal communication.
I. Zhulin (Oak Ridge National Laboratory), personal communication.
D. Walthers and L. J. Kenney, unpublished results.
D. Walthers and L. J. Kenney, manuscript in preparation.
References
- 1.Mills, D. M., Bajaj, V., and Lee, C. A. (1995) Mol. Microbiol. 15 749–759 [DOI] [PubMed] [Google Scholar]
- 2.Ochman, H., Soncini, F. C., Solomon, F., and Groisman, E. A. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 7800–7804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shea, J. E., Hensel, M., Gleeson, C., and Holden, D. W. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 2593–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collazo, C. M., and Galan, J. E. (1997) Gene 192 51–59 [DOI] [PubMed] [Google Scholar]
- 5.Cirillo, D. M., Valdivia, R. H., Monack, D. M., and Falkow, S. (1998) Mol. Microbiol. 30 175–188 [DOI] [PubMed] [Google Scholar]
- 6.Hensel, M., Shea, J. E., Gleeson, C., Jones, M. D., Dalton, E., and Holden, D. W. (1995) Science 269 400–403 [DOI] [PubMed] [Google Scholar]
- 7.Hensel, M. (2000) Mol. Microbiol. 36 1015–1023 [DOI] [PubMed] [Google Scholar]
- 8.Walthers, D., Carroll, R. K., Navarre, W. W., Libby, S. J., Fang, F. C., and Kenney, L. J. (2007) Mol. Microbiol. 65 477–493 [DOI] [PubMed] [Google Scholar]
- 9.Feng, X., Oropeza, R., and Kenney, L. J. (2003) Mol. Microbiol. 48 1131–1143 [DOI] [PubMed] [Google Scholar]
- 10.Osborne, S., Walthers, D., Duong, N., Lowden, M. J., Tomljenovic, A. M., Wickham, M. E., Silphaduang, U., Waller, R. F., Kenney, L. J., and Coombes, B. K. (2009) Proc. Natl. Acad. U. S. A. 106 3892–3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worley, M. J., Ching, K. H., and Heffron, F. (2000) Mol. Microbiol. 36 749–761 [DOI] [PubMed] [Google Scholar]
- 12.Navarre, W. W., Halsey, T. A., Walthers, D., Frye, J., McClelland, M., Potter, J. L., Kenney, L. J., Gunn, J. S., Fang, F. C., and Libby, S. J. (2005) Mol. Microbiol. 56 492–508 [DOI] [PubMed] [Google Scholar]
- 13.Feng, X., Walthers, D., Oropeza, R., and Kenney, L. J. (2004) Mol. Microbiol. 54 823–835 [DOI] [PubMed] [Google Scholar]
- 14.Hoch, J. A., and Silhavy, T. J. (1995) Two-component Signal Transduction, ASM Press, Washington, D. C.
- 15.Hoch, J. A. (2000) Curr. Opin. Microbiol. 3 165–170 [DOI] [PubMed] [Google Scholar]
- 16.Baikalov, I., Schroder, I., Kaczor-Grzeskowiak, M., Grzeskowiak, K., Gunsalus, R. P., and Dickerson, R. E. (1996) Biochemistry 35 11053–11061 [DOI] [PubMed] [Google Scholar]
- 17.Maris, A. E., Sawaya, M. R., Kaczor-Grzeskowiak, M., Jarvis, M. R., Bearson, S. M., Kopka, M. L., Schroder, I., Gunsalus, R. P., and Dickerson, R. E. (2002) Nat. Struct. Biol. 9 771–778 [DOI] [PubMed] [Google Scholar]
- 18.Vannini, A., Volpari, C., Gargioli, C., Muraglia, E., Cortese, R., De Francesco, R., Neddermann, P., and Marco, S. D. (2002) EMBO J. 21 4393–4401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ducros, V. M., Lewis, R. J., Verma, C. S., Dodson, E. J., Leonard, G., Turkenburg, J. P., Murshudov, G. N., Wilkinson, A. J., and Brannigan, J. A. (2001) J. Mol. Biol. 306 759–771 [DOI] [PubMed] [Google Scholar]
- 20.Pristovsek, P., Sengupta, K., Lohr, F., Schafer, B., von Trebra, M. W., Ruterjans, H., and Bernhard, F. (2003) J. Biol. Chem. 278 17752–17759 [DOI] [PubMed] [Google Scholar]
- 21.Wisedchaisri, G., Wu, M., Rice, A. E., Roberts, D. M., Sherman, D. R., and Hol, W. G. (2005) J. Mol. Biol. 354 630–641 [DOI] [PubMed] [Google Scholar]
- 22.Wehland, M., Kiecker, C., Coplin, D. L., Kelm, O., Saenger, W., and Bernhard, F. (1999) J. Biol. Chem. 274 3300–3307 [DOI] [PubMed] [Google Scholar]
- 23.Maris, A. E., Kaczor-Grzeskowiak, M., Ma, Z., Kopka, M. L., Gunsalus, R. P., and Dickerson, R. E. (2005) Biochemistry 44 14538–14552 [DOI] [PubMed] [Google Scholar]
- 24.Miller, J. H. (1992) A Short Course in Bacterial Genetics, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 25.Maris, A. E., Walthers, D., Mattison, K., Byers, N., and Kenney, L. J. (2005) J. Mol. Biol. 350 843–856 [DOI] [PubMed] [Google Scholar]
- 26.Kanelis, V., Forman-Kay, J. D., and Kay, L. E. (2001) IUBMB Life 52 291–302 [DOI] [PubMed] [Google Scholar]
- 27.Cornilescu, G., Delaglio, F., and Bax, A. (1999) J. Biomol. NMR 13 289–302 [DOI] [PubMed] [Google Scholar]
- 28.Guntert, P., Mumenthaler, C., and Wuthrich, K. (1997) J. Mol. Biol. 273 283–298 [DOI] [PubMed] [Google Scholar]
- 29.Laskowski, R. A., Rullmannn, J. A., MacArthur, M. W., Kaptein, R., and Thornton, J. M. (1996) J. Biomol. NMR 8 477–486 [DOI] [PubMed] [Google Scholar]
- 30.Koradi, R., Billeter, M., and Wuthrich, K. (1996) J. Mol. Graph. 14 51–55, 29–32 [DOI] [PubMed] [Google Scholar]
- 31.Chen, G., Jeffrey, P. D., Fuqua, C., Shi, Y., and Chen, L. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 16474–16479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarre, W. W., Porwollik, S., Wang, Y., McClelland, M., Rosen, H., Libby, S. J., and Fang, F. C. (2006) Science 313 236–238 [DOI] [PubMed] [Google Scholar]
- 33.Tran, V. K., Oropeza, R., and Kenney, L. J. (2000) J. Mol. Biol. 299 1257–1270 [DOI] [PubMed] [Google Scholar]
- 34.Ames, S. K., Frankema, J., and Kenney, L. J. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 11792–11797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milani, M., Leoni, L., Rampioni, G., Zennaro, E., Ascenzi, P., and Bolognesi, M. (2005) Structure (Lond.) 13 1289–1297 [DOI] [PubMed] [Google Scholar]
- 36.Kurashima-Ito, K., Kasai, Y., Hosono, K., Tamura, K., Oue, S., Isogai, M., Ito, Y., Nakamura, H., and Shiro, Y. (2005) Biochemistry 44 14835–14844 [DOI] [PubMed] [Google Scholar]
- 37.Rhee, J. E., Sheng, W., Morgan, L. K., Nolet, R., Liao, X., and Kenney, L. J. (2008) J. Biol. Chem. 283 8664–8677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucchini, S., Rowley, G., Goldberg, M. D., Hurd, D., Harrison, M., and Hinton, J. C. (2006) PLoS Pathog. 2 e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattison, K., Oropeza, R., Byers, N., and Kenney, L. J. (2002) J. Mol. Biol. 315 497–511 [DOI] [PubMed] [Google Scholar]
- 40.McCollister, B. D., Bourret, T. J., Gill, R., Jones-Carson, J., and Vazquez-Torres, A. (2005) J. Exp. Med. 202 625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, R. G., Pappas, T., Brace, J. L., Miller, P. C., Oulmassov, T., Molyneaux, J. M., Anderson, J. C., Bashkin, J. K., Winans, S. C., and Joachimiak, A. (2002) Nature 417 971–974 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.