Abstract
A key structural element in the ion translocating F-, A-, and V-ATPases is the peripheral stalk, an assembly of two polypeptides that provides a structural link between the ATPase and ion channel domains. Previously, we have characterized the peripheral stalk forming subunits E and H of the A-ATPase from Thermoplasma acidophilum and demonstrated that the two polypeptides interact to form a stable heterodimer with 1:1 stoichiometry (Kish-Trier, E., Briere, L. K., Dunn, S. D., and Wilkens, S. (2008) J. Mol. Biol. 375, 673–685). To define the domain architecture of the A-ATPase peripheral stalk, we have now generated truncated versions of the E and H subunits and analyzed their ability to bind each other. The data show that the N termini of the subunits form an α-helical coiled-coil, ∼80 residues in length, whereas the C-terminal residues interact to form a globular domain containingα- and β-structure. We find that the isolated C-terminal domain of the E subunit exists as a dimer in solution, consistent with a recent crystal structure of the related Pyrococcus horikoshii A-ATPase E subunit (Lokanath, N. K., Matsuura, Y., Kuroishi, C., Takahashi, N., and Kunishima, N. (2007) J. Mol. Biol. 366, 933–944). However, upon the addition of a peptide comprising the C-terminal 21 residues of the H subunit (or full-length H subunit), dimeric E subunit C-terminal domain dissociates to form a 1:1 heterodimer. NMR spectroscopy was used to show that H subunit C-terminal peptide binds to E subunit C-terminal domain via the terminal α-helices, with little involvement of the β-sheet region. Based on these data, we propose a structural model of the A-ATPase peripheral stalk.
The archaeal ATP synthase (A1A0-ATPase),2 along with the related F1F0- and V1V0-ATPases (proton pumping vacuolar ATPases), is a rotary molecular motor (1–4). The rotary ATPases are bilobular in overall architecture, with one lobe comprising the water-soluble A1, F1, or V1 and the other comprising the membrane-bound A0, F0, or V0 domain, respectively. The subunit composition of the A-ATPase is A3B3DE2FH2 for the A1 and CIKx for the A0. In the A1 domain, the three A and B subunits come together in an alternating fashion to form a hexamer with a hydrophobic inner cavity into which part of the D subunit is inserted. Subunits D and F comprise the central stalk connection to A0, whereas two heterodimeric EH complexes are thought to form the peripheral stalk attachment to A0 seen in electron microscopy reconstructions (5, 6). In the A0 domain (subunits CIKx), the K subunits (proteolipids) form a ring that is linked to the central stalk by the C subunit, whereas the cytoplasmic N-terminal domain of the I subunit probably mediates the binding of the EH peripheral stalks to A0, as suggested for the bacterial A/V-type enzyme (7). Although closer in structure to the proton-pumping V-ATPase, the A-ATPase functions in vivo as an ATP synthase, coupling ion motive force to ATP synthesis, most likely via a similar rotary mechanism as demonstrated for the bacterial A/V- and the vacuolar type enzymes (8, 9). During catalysis, substrate binding occurs sequentially on the three catalytic sites, which are formed predominantly by the A subunits. This is accompanied by conformation changes in the A3B3 hexamer that are linked to the rotation of the embedded D subunit together with the rotor subunits F, C, and the proteolipid ring. Each copy of K contains a lipid-exposed carboxyl residue (Asp or Glu), which is transiently interfaced with the membrane-bound domain of I during rotation, thereby catalyzing ion translocation. The EH peripheral stalks function to stabilize the A3B3 hexamer against the torque generated during rotation of the central stalk. Much work has been accomplished to elucidate the architectural features of the rotational and catalytic domains, especially in the related F- and V-type enzymes. However, the peripheral stalk complexes in the A- and V-type enzymes remain an area open to question. Although the stoichiometry of the peripheral stalks in the A/V-type and the vacuolar type ATPases have recently been resolved to two and three, respectively (6, 10), the overall structure of the peripheral stalk, including the nature of attachment to the A3B3 hexamer and I subunit (called subunit a in the F- and V-ATPase), is not well understood. Some structural information exists in the form of the A-ATPase E subunit C-terminal domain (11), although isolation from its binding partner H may have influenced its conformation.
Previously, our lab has characterized the Thermoplasma acidophilum A-ATPase E and H subunits individually and in complex (12). We found that despite their tendency to oligomerize when isolated separately, upon mixing, E and H form a tight heterodimer that was monodisperse and elongated in solution, which is consistent with its role as the peripheral stalk element in the A-ATPase. Here, we have expanded our study of the A-ATPase EH complex through the production of various N- and C-terminal truncation mutants of both binding partners. The data show that the EH complex is comprised of two distinct domains, one that contains both N termini interacting via a coiled-coil and a second that contains both C termini folded in a globular structure containing mixed secondary structure. Consistent with recent crystallographic data for the related A-ATPase from Pyrococcus horikoshii (11), we found that the isolated C-terminal domain of the E subunit exists as a stable homodimer in solution. However, the addition of subunit H or a peptide consisting of the 21 C-terminal residues of the subunit to the dimeric C-terminal domain of subunit E resulted in dissociation of the homodimer with concomitant formation of a 1:1 heterodimer containing the C termini of both polypeptides. This study delineates and characterizes the two domains of the EH complex and will aid in the further exploration of the nature of peripheral stalk attachment and function in the intact A1A0-ATPase.
EXPERIMENTAL PROCEDURES
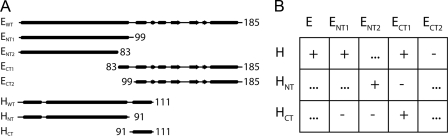
Protein Expression and Purification—All of the constructs used in this study were expressed as N-terminal fusions with Escherichia coli maltose-binding protein (MBP), which were isolated by amylose resin affinity chromatography and cleaved with protease. A summary of the constructs analyzed in this study is shown in Fig. 1A. Constructs ENT1(2–99), ENT2(2–83), ECT1(83–185), ECT2(99–185), HNT(2–91), and HCT(91–111) were subcloned, using conventional techniques, into the modified pMal-c2e plasmid using 5′-KpnI and 3′-HindIII sites. The naturally occurring cysteine at position 121 in the E subunit was replaced by alanine as described (12). All of the resulting constructs were confirmed by DNA sequencing (Upstate Medical University DNA sequencing core facility). Plasmid DNA was expressed in Rosetta pLacI E. coli cells (Novagen), and fusion protein was purified and cleaved as previous (12). ENT1, ENT2, and ECT2 tend to aggregate when cleaved from MBP during purification, although ENT1 and ENT2 were found to be soluble when in complex with H or HNT (see below). Separation of target proteins from cleaved MBP fusion was as follows: E and H were as described previously (12); HNT-cleaved fractions were pooled, and most of the MBP was removed by a 60% ammonium sulfate precipitation and subsequent centrifugation at 13,000 × g for 30 min. Redissolved protein was further purified by gel filtration chromatography (Superdex 75; GE Healthcare; 16/50 column) in 25 mm sodium phosphate (pH 7), 0.5 mm EDTA, 0.02% sodium azide. ECT1-cleaved fractions were pooled, concentrated to ∼5 ml by ultrafiltration (5,000 molecular weight cut-off; VivaSpin) and dialyzed against 20 mm Tris-HCl (pH 8.0), 25 mm NaCl, 0.5 mm EDTA overnight. The sample was then passed over a 5-ml DEAE column (Sigma), which was then washed with 5CV of the dialysis buffer. MBP was captured by the column, and ECT1 was in the flow through and wash fractions. HCT-cleaved fractions were pooled and boiled for 5 min, with subsequent centrifugation at 13,000 × g for 30 min. The supernatant was dialyzed against 25 mm Tris-HCl (pH 7.4), 10 mm NaCl, 0.5 mm EDTA (Buffer A). The sample was then passed over a 1-ml MonoQ column (GE Healthcare), washed with 10 CV Buffer A, and eluted with 0–80% of 20 mm Tris-HCl (pH 7.4), 0.5 mm EDTA, 500 mm NaCl in 20 CV (Buffer B). The samples analyzed by CD spectroscopy were prepared as follows: ENT2HNT-cleaved ENT2 and HNT were mixed at a ∼1:1.2 molar ratio. A majority of MBP and excess HNT was removed by a 65% ammonium sulfate precipitation, followed by resuspension of the pellets in 25 mm Tris-HCl (pH 7.4), 10 mm NaCl, 0.5 mm EDTA and overnight dialysis against the same. Dialyzed sample was passed over a 1-ml MonoQ column (GE Healthcare), washed with 14 CV, and eluted in a step gradient (0–20% 2 CV; 20% 5 CV; 20–40% 15 CV). ENT2HNT containing fractions were identified by SDS-PAGE, concentrated to ∼1 ml by ultrafiltration (5,000 molecular weight cut-off; VivaSpin), and passed over a gel filtration column (Superdex 75; GE Healthcare; 16/50 column) equilibrated in 25 mm Tris-HCl (pH 7.4), 0.5 mm EDTA. ECT1HCT-cleaved ECT1 was pooled with a ∼1:1.2 molar ratio of cleaved HCT. The sample was then dialyzed against 20 mm Bis-Tris-HCl (pH 6.5), 25 mm NaCl, 0.5 mm EDTA and passed over a 5-ml DEAE column as described above. As in the ECT1 prep described above, ECT1HCT flowed through the column; protein-containing fractions were concentrated and further purified by gel filtration as described above.
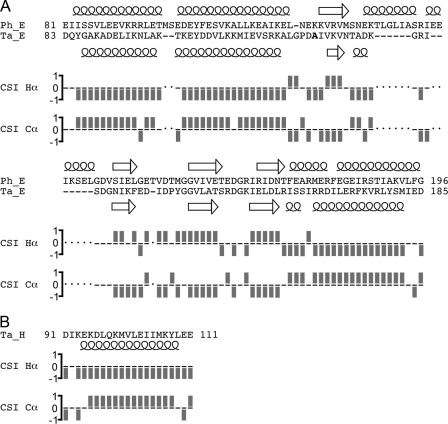
FIGURE 1.
Summary of the EH truncation mutants and their interaction. A, schematic of full-length E and H subunits and truncation mutants used in this study. Secondary structure prediction was obtained from the PSIPRED prediction server. The arrows, cylinders, and lines denote predicted β-sheet, α-helix, and random coil content, respectively. B, table of interaction assay results. Positive and negative interactions are designated with (+ and -), indicating the ability/inability to co-purify by either ion exchange or gel filtration. Interactions not assessed in this study are marked (···).
Size Exclusion Chromatography—The Superdex 75 HR 16/50 column, attached to an ÄKTA fast protein liquid chromatography (GE Healthcare) was calibrated with insulin (5.8 kDa), RNase A (15.2 kDa), green fluorescent protein (27 kDa), albumin (61.6 kDa), and blue dextran (∼2 × 103 kDa).
CD Spectroscopy—Concentrated proteins were exchanged into 25 mm sodium phosphate (pH 7) and CD wavelength and temperature melt spectra were collected as previous (12). The protein concentrations of ENT2HNT, ECT1HCT, and EH were determined by absorbance at 277 nm of GdHCl denatured samples (13). A 0.2-cm cuvette was used for ENT2HNT and ECT1HCT, whereas a 0.1-cm cuvette was used for EH. Secondary structure content was estimated by K2D2 (14, 15) and is summarized in supplemental Fig. S1.
Analysis of Complex Formation—The various E and H constructs were assayed for the ability to co-purify by ion exchange or co-elute by gel filtration in a manner equivalent to that of the full-length EH complex (12). The complexes characterized by CD (ENT2HNT and ECT1HCT) were prepared as described above. When the target(s) were known not to remain in solution upon cleavage from MBP, the intact fusion was used to probe the interaction (full-length E and H can readily form a complex as MBP fusions; data not shown). Additionally, the technique taking advantage of the most resolved characteristic between the putative binding partners, predicted charge dispersion (ion exchange) or elution volume (gel filtration), was utilized. In the next section, “gel filtration” refers to the passage of 1 ml of ultrafiltered (5,000 molecular weight cut-off; VivaSpin) sample over a Superdex 75 HR 16/50 column attached to an ÄKTA fast protein liquid chromatography (GE Healthcare), which was equilibrated in either 25 mm sodium phosphate (pH 7), 0.5 mm EDTA, 0.02% azide, or 25 mm Tris-HCl (pH 7.4), 0.5 mm EDTA; “ion exchange” refers to sample dialysis against 25 mm Tris-HCl (pH 7.4), 10 mm NaCl, 0.5 mm EDTA, followed by ultraconcentration (5,000 molecular weight cut-off; Vivaspin) to 2 ml and passage over a 1-ml MonoQ column (GE Healthcare), which was washed with 10 CV of the dialysis buffer as Buffer A and eluted in 20 CV by a gradient of 0–80% 25 mm Tris-HCl (pH 7.4), 500 mm NaCl, 0.5 mm EDTA as Buffer B (buffers differ for ENT1 and H as noted). The resultant peak elution fractions from both techniques were analyzed by SDS-PAGE. The following complexes were analyzed for complex formation: ENT1 and H, MBP-ENT1 fusion was combined ∼1:1.2 with purified H, followed by protease cleavage, a majority of MBP was removed by 60% ammonium sulfate precipitation, and the redissolved pellet was dialyzed and analyzed by ion exchange, with 20 mm BisTris-HCl (pH 6.5), 25 mm NaCl, 0.5 mm EDTA as Buffer A, and 20 mm BisTris-HCl (pH 6.5), 500 mm NaCl, 0.5 mm EDTA as Buffer B; ENT1 and HCT, MBP-ENT1 fusion was combined ∼1:1.2 with purified HCT and analyzed by ion exchange; ENT2 and HCT, MBP-ENT2 fusion was combined ∼1:1.2 with purified HCT and analyzed by ion exchange; ECT1 and H, purified ECT1 was combined ∼1:1.2 with purified H and analyzed by gel filtration; ECT1 and HNT, MBP-HNT fusion was combined ∼1:1.2 with ECT1 and analyzed by ion exchange; and ECT2 and H, MBP-ECT2 fusion was combined ∼1:1.2 with purified H and analyzed by gel filtration.
NMR Spectroscopy—Uniformly 15N-labeled ECT1 and ECT1HCT were produced in M9 medium with 15N-NH4Cl as the sole nitrogen source. 15N and 13C double-labeled samples were produced in a similar fashion, with the exception of 0.2% [13C]glucose as the sole carbon source. [15N]NH4Cl and [13C6]glucose was from Isotec, Inc. The proteins were purified as described above and exchanged into 25 mm sodium phosphate, 0.5 mm EDTA, 0.02% azide, 7% D2O. All of the spectra were recorded at 25 °C on a Bruker Avance 600-MHz spectrometer equipped with a HCN triple-axis gradient probe. Sequential assignment of the 15N HSQC spectra was achieved with the HNCACB experiment supplemented with 15N HSQC-nuclear Overhauser enhancement spectroscopy/total correlation spectroscopy experimental data. All of the NMR data were processed in Felix2004. Proton resonances were referenced by setting the 2,2-dimethyl-2-silapentane-5-sulfonate frequency as 0 ppm and extrapolating the 15N and 13C zero point frequencies as described (16). The secondary structure of ECT1HCT was determined from α carbon and α proton chemical shift values using the chemical shift index protocol (17). Briefly, upfield or downfield changes in chemical shift from random coil values of greater than ±0.1 ppm (for 1Hα) and +0.8/-0.5 ppm (for 13Cα) are scored as +1 or -1, respectively. The α-helix is assigned when four or more residues are -1 for 1Hα and/or +1 for 13Cα. The β-strand is assigned when the inverse holds true for three or more residues.
Secondary Structure Prediction—For secondary structure and coiled-coil prediction, the PSIPRED (18) and parcoil2 (19) servers were used.
RESULTS AND DISCUSSION
Previously, we have shown that the T. acidophilum A-ATPase E and H subunits form a tight, monodisperse heterodimer with 1:1 stoichiometry (12). In the process of this study, it was found that the individual subunits tend to oligomerize and that formation of the EH heterodimer out-competes this tendency. Moreover, analytical ultracentrifugation and gel filtration experiments suggested an extended conformation for the EH complex, which is consistent with its role as the peripheral stalk element in the T. acidophilum A-ATPase. Here we expand on our study of the EH complex by dissecting out individual domains for analysis.
Domain Interactions in the EH Complex—To probe the regions of E and H that are required to form the EH heterodimer, N- and C-terminal truncation mutants were constructed and assayed for their ability to interact (Fig. 1). Putative binding partners were probed for their ability to either co-elute via gel filtration or co-purify via ion exchange in a manner consistent with that of the full-length EH complex (12). Fig. 1B summarizes the probed interactions and resulting observations. Overall, the binding studies revealed that the EH heterodimer is organized in two major domains: a coiled-coil domain comprised by the N termini of the two subunits and a C-terminal domain containing the globular C terminus of the E subunit bound to the C-terminal α-helix of H. Because the N- and C-terminal parts of the EH peripheral stalk complex appeared to represent stable entities on their own, the two domains were characterized independently as presented in the following sections.
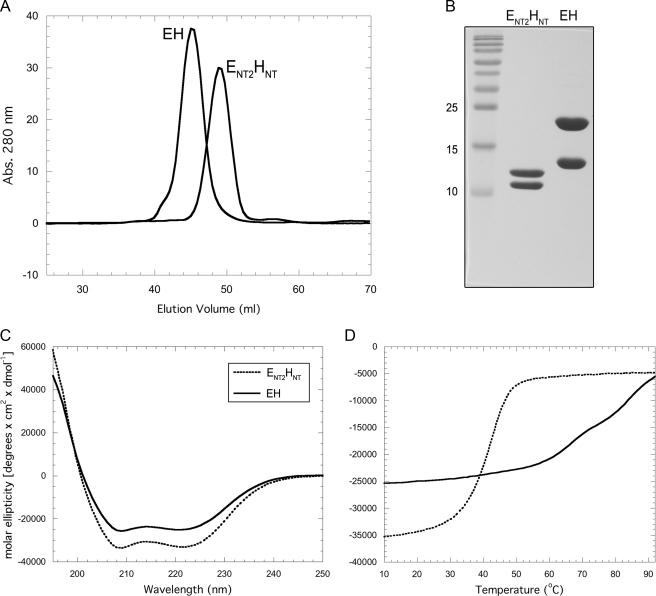
Characterization of ENT2HNT by CD Spectroscopy—The EH N-terminal coiled-coil domain was reconstituted by mixing individually expressed ENT2 (residues 2–83) and HNT (residues 2–91), and the resulting ENT2HNT complex was purified by ion exchange and size exclusion chromatography as described under “Experimental Procedures.” Fig. 2A shows the elution profile of ENT2HNT and intact EH. As can be seen, ENT2HNT eluted with a lower apparent molecular mass than EH but still larger than its expected mass of ∼22 kDa, suggesting that the N-terminal domain is also elongated as previously shown for intact EH (12). Fig. 2B shows SDS-PAGE of purified ENT2HNT and full-length EH complex. To compare the secondary structure content of ENT2HNT with that of the full-length complex, we carried out CD wavelength experiments (Fig. 2C). As can be seen, ENT2HNT (dotted line in Fig. 2C) contains a greater per residue ellipticity than the full-length complex. Additionally, the ratio of the minima at 222 and 208 nm (θ222/θ208) is close to unity (0.99), an indication that ENT2HNT contains a significant amount of coiled-coil secondary structure (20). A similar observation has been reported for the full-length EH complex (12) as well as the related EG heterodimer from yeast V-ATPase (21), indicating that the CD spectrum of the intact peripheral stalk complex is dominated by its N-terminal domain, which appears to comprises the majority, if not all, of the full-length EH complex coiled-coil content. This is consistent with an estimate of the secondary structure content for the two complexes based on the CD data by K2D2 (14, 15), which indicated an α helical content of 76% versus 63% for ENT2HNT and the intact EH complex, respectively (supplemental Fig. S1). The coiled-coil content of EH and ENT2HNT, as indicated by the CD measurements, is also consistent with the parcoil2 algorithm (19) that predicts coiled-coil formation with high probability for residues ∼20–80 of subunit H and with somewhat lower certainty for residues 2–40 of subunit E. Interestingly, ENT2 and HNT display more than 30% sequence identity (12), suggesting a similarity to other peripheral stalks that are known to have homodimeric coiled-coil domains (22). In particular, the handedness of the E. coli b subunit dimerization domain has been suggested to form either a left-handed (23) or right-handed coiled-coil (24) with varying degrees of offset. It has been speculated that the unusual arrangement of a right-handed coiled-coil with offset helices may function to counteract the torque generated during rotational catalysis in both the synthesis and hydrolysis direction (25). However, analysis of the primary sequences of the T. acidophilum A-ATPase E and H subunit N termini for the presence of the signature motifs characteristic for the two stranded left- or right-handed coiled-coil (heptad or hendecad repeats, respectively) is not conclusive, and it is therefore not known at this point which handedness the EH coiled-coil domain adopts (12).
FIGURE 2.
Purification and characterization of ENT2HNT. A, comparison of EH and ENT2HNT gel filtration profiles. EH, which has been shown to be monodisperse and elongated in solution (12), elutes with an apparent molecular mass of ∼52 kDa (35.5 kDa actual). ENT2HNT elutes with a smaller apparent molecular mass of ∼43 kDa (21.5 kDa actual), consistent with ENT2HNT, like intact EH, being elongated and monodisperse. B, 15% SDS-PAGE gel of ENT2HNT and EH as visualized by Coomassie staining. Individual subunits were produced as fusion proteins and then reconstituted as described under “Experimental Procedures.” C, CD wavelength scan of ENT2HNT and EH in 10 mm sodium phosphate (pH 7) at 25 °C. Although both samples exhibit a union of the minima at 222 and 208 nm, indicating coiled-coil α-helical content, ENT2HNT exhibits ∼30% more molar ellipticity overall, suggesting that it represents the isolated coiled-coil domain. D, CD signal monitored at 222 nm as a function of increasing temperature (Tmelt). EH, as previously reported, exhibits several cooperative transitions, whereas ENT2HNT shows only a single strong transition, which may indicate it contains a single domain of EH, namely the coiled-coil domain. The reduced thermal stability may result from the isolation of the domain from the remainder of the complex.
Additionally, we probed the thermal stability of ENT2HNT by observing the CD signal at 222 nm as a function of temperature. The resultant graph shown in Fig. 2D (dotted line) reveals a single, strongly cooperative transition at ∼40 °C, which is unlike the several weaker transitions observed for the full-length complex (solid line in Fig. 2D). The presence of a single transition suggests that ENT2HNT is composed of a single cooperative element, which, from the above CD wavelength scan data, is probably represented by the coiled-coil domain. The reduced temperature of this transition, compared with any observed for intact EH, may result from the isolation of the domain from the remainder of the complex.
The unequal length of ENT2 (83 residues) and HNT (91 residues) suggests that HNT has an ∼10-residue overhang on either the N- or C-terminal end of ENT2. To determine which end of HNT represents the overhang, we subjected ENT2HNT and full-length EH complex to limited proteolysis by trypsin followed by mass spectrometry (supplemental Fig. S2). The data indicate that trypsin is able to quickly cleave the N terminus of H after the arginine at position 5, resulting in a comparably stable EH″ or ENT2HNT″ complex with an intact H or HNT C terminus. Because there are two trypsin cleavage sites in the C-terminal 10 residues of HNT, we conclude that the HNT overhang occurs at the N-terminal end of subunit H.
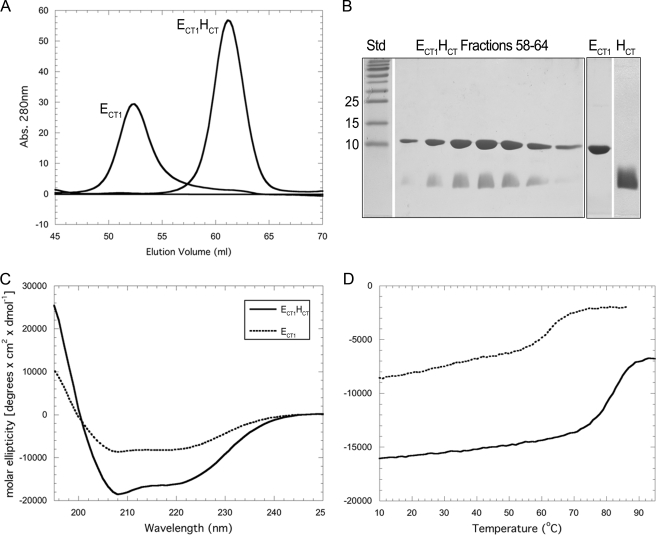
Characterization of ECT1 and Interaction of ECT1 with HCT—Guided by two-hybrid interaction studies in the related V-ATPase from yeast (26)3 and in an effort to generate suitable domains of the peripheral stalk subunits for structural studies, we initially generated a series of E subunit truncations including the ECT1 construct comprised of residues 83–185 (Fig. 1A). ECT1 was expressed in E. coli as an MBP fusion and purified via affinity and size exclusion chromatography as described under “Experimental Procedures.” Fig. 3A shows the size exclusion chromatography profile of the purified ECT1 domain. As can be seen, isolated ECT1 elutes at a volume corresponding to an apparent molecular mass of 35 kDa (∼52 ml), suggesting that ECT1 behaves as a dimer in solution. This observation is consistent with a recent crystal structure of the C-terminal domain (residues 81–198) of the E subunit of the related A-ATPase from P. horikoshii that showed two subunit E C-terminal domains interacting via their N- and C-terminal α-helices to form a dimer (11). However, as we have previously reported, no E subunit dimerization is seen in the full-length EH complex, suggesting that dimer formation in the subunit E C-terminal domain is a result of the absence of its binding partner, subunit H. We therefore initially tested whether ECT1 dimer was able to interact with full-length subunit H by performing gel filtration and NMR titration experiments. Both approaches showed a dramatic change in the behavior of ECT1, indicating a high affinity interaction between the two proteins (for NMR titration, see supplemental Fig. S3). No interaction was observed between ECT1 and HNT, indicating that the interaction between ECT1 and full-length subunit H was mediated by the C-terminal 21 residues of subunit H (Fig. 1). We therefore expressed the C-terminal 21 residues of subunit H (HCT) as N-terminal fusion with MBP and purified the peptide after removal of MBP as described under “Experimental Procedures.” Fig. 3A shows the gel filtration profile of ECT1 upon binding of HCT. As can be seen, a shift in peak elution from 52 ml for free ECT1 to 60.5 ml for ECT1HCT complex is observed, corresponding to a change in apparent molecular mass from 35 kDa (for ECT1) to 22 kDa (for ECT1HCT), which is close to the predicted molecular mass of 15 kDa for the heterodimer (Fig. 3B). The change in apparent molecular weight indicates that binding of HCT occurs with sufficient affinity to disrupt the ECT1-ECT1 homodimer interface upon heterodimer formation.
FIGURE 3.
Purification and characterization of ECT1 and ECT1HCT. A, overlay of ECT1 and ECT1HCT elution profiles collected during passage of the samples over a16 × 500 mm Superdex 75 gel filtration column. Binding of HCT to ECT1 resulted in an elution volume shift from 52 to 60.5 ml. This corresponds to a change in estimated molecular mass (spherical protein) from 35 to 22 kDa, compared with actual molecular masses of 12. kDa and 15 kDa for ECT1 and ECT1HCT, respectively. The change in elution volume suggests that HCT binding effectively reduced the spherical volume of ECT1, likely by out-competing homodimer formation. B, 15% SDS-PAGE gel of the ECT1HCT elution peak and individually purified components visualized by Coomassie staining. ECT1 and HCT were produced individually as fusion proteins and reconstituted as described under “Experimental Procedures.” C, CD wavelength scan of ECT1 and ECT1HCT in 10 mm sodium phosphate (pH 7) at 25 °C. Although retaining a shape indicative of mixed secondary structure, ECT1HCT exhibits a greater molar ellipticity than ECT1. Although this may be partially explained by the additional α-helical content contributed by HCT, it is also likely that HCT stabilizes ECT1 fold. This is further supported by the increase in thermal stability observed for ECT1HCT versus ECT1 alone (D) and additionally studied in Fig. 4.
Characterization of ECT1HCT by CD Spectroscopy and Comparison with ECT1—To assess the conformational changes occurring in ECT1 upon the binding of HCT, we performed CD wavelength and temperature melt experiments. The CD spectrum of ECT1HCT shows a greater molar ellipticity compared with the spectrum of isolated ECT1 domain (Fig. 3C). Specifically, as expected from the prediction of HCT as helical, the minima at 208 nm is more pronounced for ECT1HCT than ECT1. However, it is unlikely that this contribution alone provides a complete explanation for the increase in the magnitude of the signal intensity. Instead, the binding of HCT likely stabilizes a particular conformation of ECT1. This is further supported by the respective thermal stabilities of ECT1 versus ECT1HCT as observed by monitoring the CD signal at 222 nm as a function of temperature (Fig. 3D). Both ECT1 and ECT1HCT exhibit single, cooperative transitions, although the transition for ECT1HCT is both at a higher temperature and more pronounced (82 °C for ECT1HCT compared with 63 °C for ECT1). These observations imply that ECT1HCT is more stable than isolated, homodimeric ECT1.
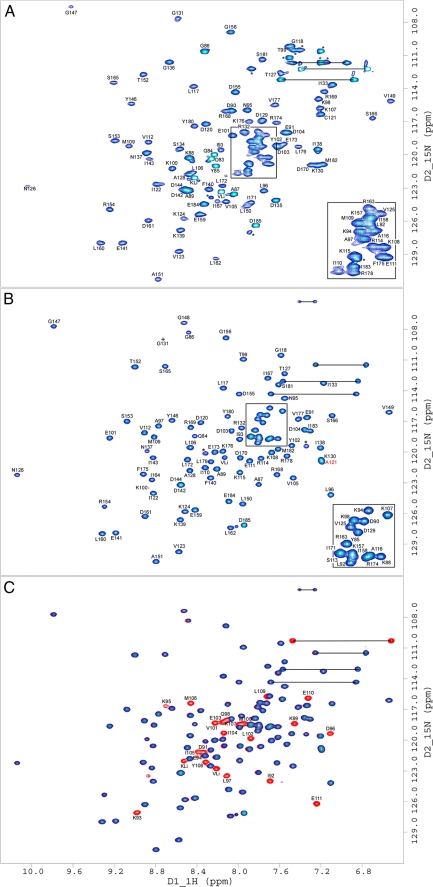
NMR Spectroscopy of ECT1 and ECT1HCT—To further explore the binding of ECT1 to HCT, we analyzed and compared 15N HSQC spectra of 15N-labeled ECT1 alone and in complex with labeled and unlabeled HCT (Fig. 4). Fig. 4A shows a 1H/15N HSQC experiment for the uniformly 15N-labeled ECT1 domain. As can be seen, the spectrum shows generally good chemical shift dispersion of the backbone amide resonances, typical for a protein with mixed secondary structure. Yet some of the peaks appear broadened and of unequal intensity, indicative of dimer/oligomer formation at the concentrations used for NMR spectroscopy. However, a dramatic change in the appearance of the ECT1 spectrum is observed upon the addition of full-length H or HCT peptide. A titration of 15N-labeled ECT1 with unlabeled H leads to the appearance of additional resonances at substoichiometric ratios, indicative of high affinity binding of H to ECT1 (supplemental Fig. S3). Fig. 4B shows a 1H/15N HSQC spectrum of purified ECT1HCT complex (15N-ECT, 14N-HCT), which is more resolved and homogenous in regards to peak intensity when compared with that of ECT1 alone (Fig. 4A). This effect is global and suggests a gain in structural stability of ECT1 in response to the binding of HCT. This is consistent with a reduction in overall volume and, again, supports the breakup of ECT1 dimers by the addition of HCT. In Fig. 4C we assessed the status of HCT when bound to ECT1 by superimposing the spectrum of fully 15N-labeled ECT1HCT (red) with the spectrum of ECT1HCT where ECT1 alone was 15N labeled (blue). The overall chemical shift dispersion of the HCT resonances indicates a stable conformation of HCT when bound to ECT1. For both isolated ECT1 (Fig. 4A) and ECT1HCT complex (Fig. 4, B and C), amide resonances were sufficiently resolved to allow for the unambiguous assignment of the majority of peaks, which are notated in the respective spectra.
FIGURE 4.
15N HSQC NMR spectra of ECT1 and ECT1HCT. Two-dimensional 1H/15N HSQC NMR spectra taken at 298 K in 25 mm sodium phosphate (pH 7), 0.5 mm EDTA, and 0.02% NaN3. Spectra presented are: ECT1 (A), ECT1HCT where HCT is unlabeled for clarity (B), and where ECT1HCT is fully labeled (red) and is overlaid by the partially labeled spectrum from B in blue (C). The observed change in ECT1 behavior upon HCT binding, explored in Fig. 3, is further evidenced here by the global improvement in backbone amide peak signal dispersion and homogeneity indicative of ECT1 fold stabilization by HCT binding. Side chain amide peaks are linked by black horizontal lines. Resonances marked with an asterisk belong to amino acid side chains (e.g. Arg) or residues that could not be assigned because of overlap/poor signal to noise. KLi and VLi are part of the N-terminal linker (GPKVP). For ECT1 and ECT1HCT, Ser113, Gly148, Ile164, Ser134, Asp135, and Gly136 could not be assigned, respectively. The mutation Ta_E C121A has been described previously (Ref. 12; indicated by the red label in B). The concentration of NMR samples was between 0.5 and 1 mm.
Secondary Structure Analysis of ECT1HCT—In the available P. horikoshii E subunit C terminus (Ph_ECT) crystal structure, dimerization is mediated by a domain swap, whereby the N-terminal α-helix of one monomer interacts with the C-terminal α-helix of the other monomer and vice versa (11). To assess whether Ph_ECT is an appropriate model for T. acidophilum E subunit C-terminal domain (Ta_ECT1) bound to Ta_HCT, we analyzed the secondary structure of Ta_ECT1 and compared it with the crystal structure of Ph_ECT. Fig. 5 summarizes the secondary structure content of the P. horikoshii ECT dimer and the T. acidophilum ECT1HCT complex. The secondary structure assignment of Ta_ECT1HCT is based on Cα proton and carbon chemical shift analysis by the chemical shift index protocol (Ref. 17 and supplemental Fig. S4 for the differences of 1Hα and 13Cα chemical shifts from random coil values). As can be seen, the secondary structure content of the C-terminal domains of the two related E subunits is very similar, indicating that Ph_ECT is an appropriate model structure for Ta_ECT1 bound to HCT. The similar secondary structure content further suggests that binding of Ta_HCT to Ta_ECT1 does not alter the overall secondary structure but rather results in repacking of the N- and C-terminal α-helices of E subunit C-terminal domain to accommodate the C-terminal α-helix of subunit H. The data also show that residues ∼95–108 of subunit H are folded as an α-helix, and it seems likely that this α-helix together with the N- and C-terminal α-helices of ECT1 form a three α-helix bundle.
FIGURE 5.
Secondary structure analysis of ECT1HCT as carried out by the chemical shift index protocol. A, the sequence alignment of the T. acidophilum ECT1 (Ta_ECT1) and the P. horikoshii ECT (Ph_ECT) was performed manually and refined by secondary structure comparison. Note that Ph_ECT has two α-helices connecting β-strands 1 and 2 that are significantly shorter in Ta_ECT1. The secondary structure for Ph_ECT (from the crystal structure; Ref. 11) and Ta_ECT1 (from the Hα and Cα chemical shift values using the chemical shift index (CSI) protocol as described under “Experimental Procedures”). The overall high degree of secondary structure similarity between the two proteins suggests that Ph_ECT is a good model for Ta_ECT1. Furthermore, the retention of secondary structure in Ta_ECT1 when bound to HCT, as shown here, indicates that the break up of Ta_ECT1 dimers by the addition of HCT (Figs. 3 and 4) is accompanied by a rearrangement of secondary structure elements rather than a change in composition of the same. B, the secondary structure analysis of HCT indicates α-helix for residues ∼95–108. See supplemental Fig. S4 for absolute deviations of 1Hα and 13Cα chemical shift values from random coil values.
Amide-Proton Chemical Shift Changes (Δ1Hδ) of ECT1 upon Binding of HCT—Our observation of ECT1HCT complex formation and the lack of HCT binding to ECT2 suggests that HCT disrupts ECT1 dimerization by interacting with its N-terminal α-helix. However, ENT1, which contains this helix on its C terminus, cannot form a similarly tight complex with HCT (Fig. 1B). Additionally, Fig. 5 demonstrates that HCT binding to ECT1 does not confer major changes in secondary structure content but rather a reordering of terminal helices. To probe this further we assessed the backbone amide-proton chemical shift change of ECT1 residues upon binding of HCT (Fig. 6A). ECT1 residues undergoing an amide 1H chemical shift change upon HCT binding of greater than 0.75, 0.5, and 0.25 ppm are highlighted in the crystal structure of Ph_ECT in red, orange, and yellow, respectively. As can be seen in Fig. 6B, the residues experiencing the greatest chemical shift perturbation lie in the N- and C-terminal α-helices of ECT1, indicating that both N- and C-terminal α-helices of ECT1 are involved in binding HCT.
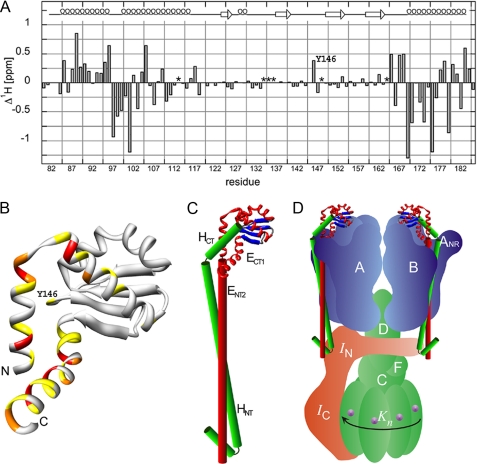
FIGURE 6.
Analysis of ECT1 chemical shift change upon the addition of HCT and a model of the proposed domain arrangement in the full-length EH peripheral stalk complex. Assignment of the 15N HSQC spectra presented in Fig. 4 permitted the calculation of ECT1 amide-proton chemical shift changes (Δ1Hδ) upon binding HCT. A, as can be seen, the majority of residues experiencing the most significant chemical shift perturbation upon HCT binding are located in the N- and C-terminal helices of ECT1 (residues for which no assignments could be obtained in either the ECT1 (Ser113, Gly148, and Ile164) or ECT1HCT (Ser134, Asp135, and Gly136) spectra are marked with an asterisk). B, the positions analogous to the Ta_ECT1 residues undergoing the largest chemical shift changes are highlighted in the available crystal structure of Ph_ECT with one dimer partner removed (Protein Data Bank code 2dma; Ref. 11). The largest change in chemical shift in the middle portion of the structure is experienced by Tyr146, which can be seen located close to the N- and C-terminal α-helices of Ph_ECT in the crystal structure (Ta_E Y146 corresponds to Ph_E M157). C, HCT, modeled as an α-helix and guided by the data presented here, is docked onto the Ph_ECT crystal structure monomer. Continuing below the globular ECT1HCT domain, the N-terminal domain, ENT2HNT, is modeled as a pair of parallel helices, representing the coiled-coil domain. We speculate that the N-terminal α-helix of HNT is folded back to interact with and stabilize the N-terminal ends of the coiled-coil domain. D, the resulting EH model placed into a schematic model of the T. acidophilum A-ATPase. The stoichiometry of the EH peripheral stalks has recently been determined for the related A/V-ATPase from T. thermophilus (6).
Structural Model of Intact EH Complex—In the full-length EH complex, the N-terminal ends of both ECT1 and HCT are joined with their N-terminal domains, which, as shown in Fig. 2, appear to be folded as a coiled-coil for most of their length. Fig. 6C shows our current working model of the domain architecture of the A-ATPase peripheral stalk, which is based on the crystal structure of P. horikoshii A-ATPase E subunit C-terminal domain and the results presented here. Additional experimental support for the interaction of the N termini of E and H subunits has recently been obtained from peptide binding studies, which indicated an interaction between residues H1–47 and E41–60 for the related A-ATPase from Methanocaldococcus janaschii (27). Secondary structure prediction (Fig. 1 and Ref. 12) predicts that residues 5–19 at the N terminus of subunit H are folded as an α-helix followed by a turn (residues 20–22). Based on this prediction and the proteolysis experiment mentioned above, we speculate that the N-terminal α-helix of subunit H is folded back to interact with, and stabilize, the two N-terminal ends of the coiled-coil domain. The resulting EH heterodimer model is ∼150 Å long, consistent with its role as peripheral stalk. The arrangement of the EH peripheral stalk in the schematic model of the A-ATPase is shown in Fig. 6D. As mentioned above, two EH (EG) peripheral stalks have been found in the related bacterial A/V-type ATPase from Thermus thermophilus (6). We speculate that both peripheral stalks interact via their N-terminal domains with the elongated N-terminal domain of the I subunit, whereas their C-terminal domains are bound to the N-terminal regions of two of the three B subunits. Such an arrangement would be consistent with chemical cross-linking data that place the related EG heterodimer of the vacuolar ATPase along the surface of the B subunit (28). These results together with cross-linking experiments conducted with the A-ATPase from Methanosarcina mazei Gö1, which place the C-terminal region of the H subunit near the N-terminal region of one of the A subunits (29), suggest that the binding region for the EH (or EG) heterodimer is near a subunit AB interface as shown in Fig. 6D. Interestingly, three (subunit EG) peripheral stalks have been found in the related eukaryotic V-ATPase (10), and based on our recent three-dimensional reconstruction of the same enzyme (30), we have proposed that the third EG peripheral stalk is anchored to the membrane-bound a subunit via the V-ATPase C subunit (Vma5p), a subunit not found in the A- or A/V-type enzyme.
Concluding Remarks—It has been suggested that the progenitor rotary ATPase most likely resembled the bacterial F1F0-ATPase (31) and that the A-type, followed by the V-type, arose sometime later. This is supported by the so-called tree of life, which indicates a branching off of bacteria, before archaea, on the way to the eukaryotic “limb” (32). The gross morphological similarities of the F-, A-, and V-type motors and the nature of the A-type as being a “chimera” of F- and V-type attributes have been noted (33). Specifically, the peripheral stalks, although differing in subunit composition and complexity, carry out a similar role of stabilizing the hexamer against the torque generated during central stalk rotation. In the simplest case, the F-type peripheral stalk is composed of two copies of the b subunit and a single copy of the δ subunit (nomenclature of the bacterial enzyme). The N-terminal transmembrane α-helices of the b dimer anchor the stalk to subunit a of the F0 sector, whereas the C terminus of one of the b subunits (bCT) connects to the C terminus of δ (δCT). It is the N terminus of δ, which provides the connection to the hexamer via the N-terminal α-helix of one of the α subunits (34). Interestingly, there is evidence to support a fusion event between the respective C termini of δ and b to form the A/V-type peripheral stalk subunit E as well as the related FliH subunit of the T3SS secretion system. Namely, there is significant sequence similarity to b in the N termini of subunits E and FliH, in addition to similarity to δCT in their C termini (35, 36). Additionally, we have detected ∼30% identity between ENT2 and HNT (12), which may be a vestige of the b homodimer, and G (H of the A-ATPase is G in the V-ATPase) has been reported to have weak identity to bacterial b (37). However, the lack of a counterpart for the N terminus of δ and the presence of multiple stalks in the A/V-type enzymes imply that the connection to the hexamer may be unlike that observed in the F-type enzyme (34).
Our work here further resolves the A-ATPase EH peripheral stalk complex through the identification and characterization of the ENT2HNT coiled-coil domain and the ECT1HCT globular domain. In the coiled-coil ENT2HNT domain, the subunits are parallel in orientation with HNT being shifted down about 10 residues with respect to the N terminus of ENT2. This domain is likely analogous to the coiled-coil region of the bacterial F-type b dimer and may provide a unique opportunity to study the possible function of the peripheral stalk coiled-coil domain in the transient storage of energy during catalysis (38). The ECT1HCT complex is of particular interest in the light of our observations, which suggest a significant repacking of the terminal helices of ECT1 in response to binding HCT.ECT1HCT, as opposed to the dimer form of ECT1, likely represents the physiologically relevant form of the EH complex C terminus and will provide a valuable system in which to study the possibly unique interaction of an A/V-type peripheral stalk with the A3B3 hexamer.
Supplementary Material
Acknowledgments
We thank Dave Kiemle from the SUNY-ESF Analytical Instrumentation Facility for assistance with the NMR spectroscopy experiments, Lee Parsons for help with the mass spectrometry analysis, and Dr. Stewart Loh for help with the CD spectroscopy.
This work was supported, in whole or in part, by National Institutes of Health Grants GM58600 and CA100246 (to S. W.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1–S4.
Footnotes
The abbreviations used are: A1, water-soluble domain of the A-ATPase; A0, membrane-bound domain of the A-ATPase; MBP, maltose-binding protein; HSQC, heteroatom single quantum coherence.
P. M. Kane, personal communication.
References
- 1.Müller, V., and Grüber, G. (2003) Cell. Mol. Life. Sci. 60 474-494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forgac, M. (2007) Nat. Rev. Mol. Cell Biol. 8 917-929 [DOI] [PubMed] [Google Scholar]
- 3.Boyer, P. D. (1997) Annu. Rev. Biochem. 66 717-749 [DOI] [PubMed] [Google Scholar]
- 4.Wilkens, S. (2005) Adv. Prot. Chem. 71 345-382 [DOI] [PubMed] [Google Scholar]
- 5.Coskun, U., Chaban, Y. L., Lingl, A., Müller, V., Keegstra, W., Boekema, E. J., and Grüber, G. (2004) J. Biol. Chem. 279 38644-38648 [DOI] [PubMed] [Google Scholar]
- 6.Esteban, O., Bernal, R. A., Donohoe, M., Videlar, H., Sharon, M., Robinson, C. V., and Stock, D. (2007) J. Biol. Chem. 283 2595-2603 [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto, M., Unzai, S., Saijo, S., Ito, K., Mizutani, K., Suno-Ikeda, C., Yabuki-Miyata, Y., Terada, T., Toyama, M., Shirouzu, M., Kobayashi, T., Kakinuma, Y., Yamato, I., Yokoyama, S., Iwata, S., and Murata, T. (2008) J. Biol. Chem. 283 19422-19431 [DOI] [PubMed] [Google Scholar]
- 8.Imamura, H., Nakano, M., Noji, H., Muneyuki, E., Ohkuma, S., Yoshida, M., and Yokoyama, K. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 2313-2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirata, T., Iwamotot-Kihara, A., Sun-Wada, G.-H., Okajima, T., Wada, Y., and Futai, M. (2003) J. Biol. Chem. 277 23714-23719 [DOI] [PubMed] [Google Scholar]
- 10.Kitagawa, N., Mazon, H., Heck, A. J., and Wilkens, S. (2008) J. Biol. Chem. 283 3329-3337 [DOI] [PubMed] [Google Scholar]
- 11.Lokanath, N. K., Matsuura, Y., Kuroishi, C., Takahashi, N., and Kunishima, N. (2007) J. Mol. Biol. 366 933-944 [DOI] [PubMed] [Google Scholar]
- 12.Kish-Trier, E., Briere, L. K., Dunn, S. D., and Wilkens, S. (2008) J. Mol. Biol. 375 673-685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill, S. C., and von Hippel, P. H. (1989) Anal. Biochem. 182 319-326 [DOI] [PubMed] [Google Scholar]
- 14.Andrade, M. A., Chacon, P., Merelo, J. J., and Moran, F. (1993) Protein Eng. 6 383-390 [DOI] [PubMed] [Google Scholar]
- 15.Perez-Iratxeta, C., and Andrade-Navarro, M. A. (2007) BMC Struct. Biol. 8 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wishart, D. S., Bigam, C. G., Yao, J., Abildgaard, F., Dyson, H. J., Oldfield, E., Markley, J. L., and Sykes, B. D. (1995) J. Biomol. NMR 6 135-140 [DOI] [PubMed] [Google Scholar]
- 17.Wishart, D. S., and Sykes, B. D. (1994) Methods Enzymol. 239 363-392 [DOI] [PubMed] [Google Scholar]
- 18.Jones, D. T. (1999) J. Mol. Biol. 292 195-202 [DOI] [PubMed] [Google Scholar]
- 19.McDonnell, A. V., Jiang, T., Keating, A. E., and Berger, B. (2006) Bioinformatics 22 356-358 [DOI] [PubMed] [Google Scholar]
- 20.Lau, S. Y., Taneja, A. K., and Hodges, R. S. (1984) J. Biol. Chem. 259 13253-13261 [PubMed] [Google Scholar]
- 21.Féthière, J., Venzke, D., Diepholz, M., Seybert, A., Geerlof, A., Gentzel, M., Wilm, M., and Böttcher, B. (2004) J. Biol. Chem. 279 40670-40676 [DOI] [PubMed] [Google Scholar]
- 22.Dunn, S. D., Revington, M., Cipriano, D. J., and Shilton, B. H. (2000) J. Bioenerg. Biomembr. 32 347-355 [DOI] [PubMed] [Google Scholar]
- 23.Wise, J. G., and Vogel, P. D. (2008) Biophys. J. 94 5040-5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood, K. S., and Dunn, S. D. (2007) J. Biol. Chem. 282 31920-31927 [DOI] [PubMed] [Google Scholar]
- 25.Del Rizzo, P., Bi, Y., and Dunn, S. D. (2006) J. Mol. Biol. 364 735-746 [DOI] [PubMed] [Google Scholar]
- 26.Ohira, M., Smardon, A. M., Charsky, C. M., Liu, J., Tarsio, M., and Kane, P. M. (2006) J. Biol. Chem. 281 22752-22760 [DOI] [PubMed] [Google Scholar]
- 27.Gayen, S., Balakrishna, A. M., Biukovic, G., Yulei, W., Hunke, C., and Grüber, G. (2008) FEBS J. 275 1803-1812 [DOI] [PubMed] [Google Scholar]
- 28.Arata, Y., Baleja, J. D., and Forgac, M. (2002) J. Biol. Chem. 277 3357-3363 [DOI] [PubMed] [Google Scholar]
- 29.Schäfer, I., Rössle, M., Biukovic, G., Müller, V., and Grüber, G. (2006) J. Bioenerg. Biomembr. 38 83-92 [DOI] [PubMed] [Google Scholar]
- 30.Zhang, Z., Zheng, Y., Mazon, H., Milgrom, E., Kitagawa, N., Kish-Trier, E., Heck, A. J. R., Kane, P. M., and Wilkens, S. (2008) J. Biol. Chem. 283 35983-35995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cross, R. L., and Müller, V. (2004) FEBS Lett. 576 1-4 [DOI] [PubMed] [Google Scholar]
- 32.Woese, C. R. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 8392-8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schäfer, G., and Meyering-Vos, M. (1992) Biochim. Biophys. Acta 1101 232-235 [DOI] [PubMed] [Google Scholar]
- 34.Wilkens, S., Borchardt, D., Weber, J., and Senior, A. E. (2005) Biochemistry 44 11786-11794 [DOI] [PubMed] [Google Scholar]
- 35.Lane, M. C., O'Toole, P. W., and Moore, S. A. (2006) J. Biol. Chem. 281 508-517 [DOI] [PubMed] [Google Scholar]
- 36.Pallen, M. J., Bailey, C. M., and Bearson, S. A. (2006) Protein Sci. 15 935-941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Supeková, L., Supek, F., and Nelson, N. (1995) J. Biol. Chem. 270 13726-13732 [DOI] [PubMed] [Google Scholar]
- 38.Neukirch, S., Goriely, A., and Hausrath, A. C. (2008) Phys. Rev. Lett. 100 038105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.