Abstract
Integrated cascades of protein tyrosine and serine/threonine phosphorylation play essential roles in transducing signals in response to growth factors and cytokines. How adaptor or scaffold proteins assemble signaling complexes through both phosphotyrosine and phosphoserine/threonine residues to regulate specific signaling pathways and biological responses is unclear. We show in multiple cell types that endogenous 14-3-3ζ is phosphorylated on Tyr179 in response to granulocyte macrophage colony-stimulating factor. Importantly, 14-3-3ζ can function as an intermolecular bridge that couples to phosphoserine residues and also directly binds the SH2 domain of Shc via Tyr179. The assembly of these 14-3-3:Shc scaffolds is specifically required for the recruitment of a phosphatidylinositol 3-kinase signaling complex and the regulation of CTL-EN cell survival in response to cytokine. The biological significance of these findings was further demonstrated using primary bone marrow-derived mast cells from 14-3-3ζ-/- mice. We show that cytokine was able to promote Akt phosphorylation and viability of primary mast cells derived from 14-3-3ζ-/- mice when reconstituted with wild type 14-3-3ζ, but the Akt phosphorylation and survival response was reduced in cells reconstituted with the Y179F mutant. Together, these results show that 14-3-3:Shc scaffolds can act as multivalent signaling nodes for the integration of both phosphoserine/threonine and phosphotyrosine pathways to regulate specific cellular responses.
The ability of a cell to respond to extrinsic stimuli critically hinges on its ability to regulate specific intracellular protein-protein interactions in a reversible manner. Such signals are relayed within the cell through the assembly of signaling complexes that are built using protein scaffolds. One important mechanism by which this occurs is via the binding of Src homology 2 (SH2)5 or phosphotyrosine-binding (PTB) domains to phosphotyrosine residues (1, 2). Importantly, the ability of individual SH2 or PTB domains to recognize specific phosphotyrosine motifs in different proteins enables the assembly of purpose-built signaling complexes that promote signaling via specific pathways (3). In some cases, signaling proteins not only contain more than one SH2 and/or PTB domain but are also themselves tyrosine-phosphorylated, leading to a network of phosphotyrosine-mediated protein-protein interactions.
Although less well studied, phosphoserine/threonine-binding proteins are also important for the assembly of signaling complexes. For example, the 14-3-3 family of proteins is able to bind phosphoserine/threonine residues in a sequence-specific context (RSX(S/T)XP and RXXX(S/T)XP, where (S/T) is phosphoserine/threonine) (4, 5). The 14-3-3 proteins have been proposed to function as “modifiers” or “sequestrators”; however, because of their dimeric structure, they have also been proposed to function as “adaptor” or “scaffold” proteins through their ability to bring together two serine/threonine phosphorylated proteins (4–7). Additionally, a number of phosphoserine/threonine-binding modules such as tryptophan-tryptophan (WW), Forkhead-associated (FHA), Polo box (PBD), and BRCA1 C-terminal (BRCT) domains have been shown to interact with phosphoserine/threonine residues within a sequence-specific context and have also been proposed to be important for the assembly of multi-protein signaling complexes (8).
The genes/cassettes encoding each phosphotyrosine- and phosphoserine/threonine-binding protein/module arose as a separate evolutionary event, and the DNA encoding these modules has been subject to frequent duplication and shuffling. For example, the 14-3-3 family of proteins is ubiquitously expressed in mammalian tissues and is composed of seven different isoforms, each encoded by a separate gene (6). In addition, duplication and shuffling of SH2, PTB, WW, FHA, PBD, and BCRT cassettes has led to their wide distribution among signaling proteins. Yet, despite the frequent duplication and shuffling of the DNA encoding these domains throughout evolution, proteins that contain both a phosphotyrosine-binding cassette (e.g. SH2 or PTB) and a phosphoserine/threonine-binding cassette (e.g. 14-3-3, WW, FHA, PBD, and BCRT) have not been identified. This is perhaps surprising given the highly integrated nature of phosphotyrosine and phosphoserine/threonine signaling and would suggest that alternative strategies to regulate integration are at play.
We show here that 14-3-3ζ is tyrosine-phosphorylated, enabling it to interact with Shc and provide a scaffold for the assembly of signaling complexes via both phosphoserine/threonine and phosphotyrosine residues. Our results show that Tyr179 of 14-3-3ζ directly binds to the SH2 domain of Shc and that this interaction is critical for the assembly of a phosphatidylinositol (PI) 3-kinase signaling complex in response to granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulation. Moreover, we show that Tyr179 of 14-3-3ζ is necessary and sufficient for the ability of GM-CSF to regulate PI 3-kinase and cell survival in the CTL-EN line. Furthermore, reconstitution of primary mast cells derived from 14-3-3ζ-/- mice with wild type (wt) or mutant 14-3-3ζ demonstrated an important role for Tyr179 in cytokine-mediated Akt phosphorylation and cell survival. These multivalent 14-3-3:Shc scaffolds provide a novel mechanism by which phosphoserine/threonine and phosphotyrosine pathways can be integrated for the regulation of specific cellular responses.
EXPERIMENTAL PROCEDURES
Cell Culture—HEK 293 T cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% (v/v) fetal calf serum (FCS) (Commonwealth Serum Laboratories) and transfected using calcium phosphate. Factor-dependent CTL-EN cells expressing the human GM-CSF α subunit and either the wt common β subunit (βc) or the βcS585G mutant were cultured in RPMI and 10% FCS supplemented with mouse IL-2 (9). The myelomonocytic UT7 factor-dependent cell line was cultured in RPMI and 10% FCS supplemented with 2 ng/ml GM-CSF. Mononuclear cells were purified from normal donors as previously described (10). CTL-EN and UT7 cells were electroporated with constructs for the expression of 14-3-3ζ-Myc (20 μg), Akt-HA (5 μg), and/or ERK-HA (5 μg). The pRcCMV-14-3-3ζ-Myc construct was generated by PCR-cloning of C-terminally Myc-tagged 14-3-3ζ cDNA into pRcCMV (Invitrogen). Mutants of this construct were generated using the QuikChange site-directed mutagenesis kit (Stratagene) using oligonucleotides (Geneworks).
Purification of Recombinant Proteins and in Vitro Kinase Assays—Recombinant glutathione S-transferase (GST), GST-SH2Shc, GST-14-3-3ζ, GST-14-3-3ζ-T179F were purified from Escherichia coli using glutathione-Sepharose (GE Healthcare) as previously described (11). 14-3-3ζ (10 μg) was cleaved from GST using 2 units of thrombin (MP Biochemicals) in 150 mm NaCl, 2.5 mm CaCl2, 2 mm dithiothreitol, 50 mm Tris-HCl, pH 8.0, at room temperature overnight. Released 14-3-3ζ was then further purified by anion exchange chromatography. 14-3-3 was applied to the column in 20 mm ethanolamine, pH 10.5, washed with 200 mm NaCl, 20 mm pyridine, pH 6.0, and eluted in 20 mm pyridine pH 6.0 buffer containing 1 m NaCl. Dialyzed recombinant wt 14-3-3ζ or 14-3-3ζ-T179F (3 μg) was phosphorylated in vitro with 0.1 μg of c-Src (Upstate) in kinase buffer (10 mm MgCl2, 10 mm Tris, pH 7.4) in the presence or absence of 4 mm ATP at 30 °C for 4 h. PI 3-kinase assays were performed as previously described (12).
Immunoblotting—The anti-p85 polyclonal antibody (pAb) (Upstate Biotechnology Inc.) was used at 1:2000. The anti-Shc pAb (Transduction Laboratories), anti-phospho-Akt (Thr308) pAb (Cell Signaling), anti-phospho-Akt (Ser473) pAb (Cell Signaling), anti-phosphotyrosine pAb (Biomol), anti Raf-1 pAb (Santa Cruz), anti-Bad pAb (Cell Signaling), Ser(P) 14-3-3 binding motif antibody (Cell Signaling or AbCam), and anti-14-3-3ζ EB1 pAb (9) were used at 1:1000. The anti-βc 8E4 and 1C1 monoclonal antibodies (mAb), 4G10 anti-phosphotyrosine mAb (Upstate Biotechnology Inc.), anti-EE pAb (AbCam), anti-Myc 9E10 mAb, and the anti-HA 12CA5 mAb were used at 1 μg/ml. The anti-active-ERK pAb (Promega) was used at 1:5000. The anti-Tyr(P)179 antibody was generated by coupling KKSVFY(pY)EILNSC to keyhole limpet hemocyanin and injecting into New Zealand White rabbits (9, 12) and used at 1:1000.
Immunoprecipitations and Pulldowns—CTL-EN cells expressing the GM-CSF receptor (9) were factor-deprived overnight in RPMI containing 0.5% FCS and then stimulated with 50 ng/ml human GM-CSF or 1 mm sodium pervanadate. The cells were lysed in either RIPA buffer or Nonidet P-40 lysis buffer as previously described (9). Immunoprecipitations were performed using 1 μg of antibody absorbed to protein A-Sepharose (Amersham Biosciences). Pulldown experiments were performed using 1 μg of peptide absorbed to 30 μl of ImmunoPure immobilized Streptavidin (Pierce). Peptides (Mimotopes) encompassing Tyr179 of 14-3-3ζ were: Biotin-NHS-SGSGRASVFY(pY)EILNSK (Tyr(P)179) and Biotin-NHSSGSGRASVFYYEILNSK (non-phospho-Tyr179). Peptides encompassing Ser585 of the βc subunit of the GM-CSF receptor were: Biotin-NHS-KGGFDFNGPYLGPPHSR(pS)LPDGG (Ser(P)585) and Biotin-NHS-KGGFDFNGPYLGPPHSRSLPDGG (non-phospho-Ser585). 14-3-3ζ-Myc association with the SH2 domain of Shc was examined in pulldown experiments using either GST-SH2Shc or GST as previously described (12).
Cell Survival and Proliferation Assays—CTL-EN cells expressing the wt GM-CSF receptor (GMRα and βc) or mutant receptor (GMRα and βcS585G) were co-electroporated with constructs for the expression of enhanced green fluorescent protein (EGFP) (2 μg) and either wt or mutant 14-3-3ζ-Myc (20 μg) and then placed into RPMI, 10% FCS, and IL-2. Quantification of several experiments indicated that wt 14-3-3ζ and the T179F mutant were expressed at an average of 81% (standard deviation, ±25%) of the total endogenous 14-3-3 proteins (supplemental Fig. S1). Thus, the transfected 14-3-3ζ-Myc proteins are expressed at essentially endogenous levels. After 24 h, the cells were plated at 2 × 105 cells/ml in RPMI containing 10% FCS and 50 ng/ml GM-CSF. After 48 h, the cells were stained with annexin V-PE (Caltag). Analysis was performed by flow cytometry for the detection of GFP-positive cells that were also annexin V-PE-positive. For proliferation, CTL-EN cells were co-electroporated with constructs for the expression of enhanced cyan fluorescent protein (2 μg) and either wt or mutant 14-3-3ζ-Myc (20 μg). The cells were further cultured for 48 h in RPMI containing 10% FCS and IL-2, following which cyan fluorescent protein-positive cells were purified by fluorescence-activated cell sorting and were plated at 1.5 × 105 cells/ml in RPMI containing 10% FCS and 50 ng/ml of GM-CSF for 24 h, and the cells were pulsed with 10 μm 5-bromo-2-deoxyuridine (BrdUrd) (Roche Applied Science) for the last 4 h. The cells were fixed and stained with anti-BrdUrd-fluorescein isothiocyanate antibodies (Roche Applied Science). BrdUrd incorporation was assessed by flow cytometry.
Structural Analysis—Three-dimensional coordinates for 14-3-3 (Protein Data Bank code 2C1N) and SH2 domain of Shc (Protein Data Bank code 1TCE) were downloaded from the Protein Data Bank. The phospho-peptide bound to Shc was used to dock Shc onto the surface of 14-3-3 by superposing the equivalent atoms in the phospho-peptide with the 179YEIL residues in 14-3-3 using LSQMAN (13). Molecular figures were produced with PYMOL (14).
14-3-3ζ Gene Targeted Mice and Preparation of Bone Marrow-derived Mast Cells—Embryonic stem cells of normal karyotype with a single retroviral gene trap insertion in the second intron of the 14-3-3ζ gene were purchased from the Lexicon Genetics Inc. These cells were used to generate 14-3-3ζ-/- SV129 mice. The mice were maintained in 12-h light/dark cycle and were provided food and water ad libitum. All of the studies were in accordance with institutional ethics committee guidelines. The mice were genotyped by PCR using oligonucleotides: GAACTTCAGATCTGGTGAC, GATTGTACTCAAAATGGTGGAC, and GCGTTACTTAAGCTAGCTTGC. Primers used for the detection of specific 14-3-3 isoforms are shown in supplemental Table S1. As previously described (15), bone marrow-derived mast cells were obtained by culturing bone marrow cells from femurs of wt and 14-3-3ζ-/- SV129 mice in DMEM, 10% FCS, and 20% WEHI-3 conditioned medium for 4–12 weeks, at which time >89% of the cells were identified as mast cells by May Grunwald-Giemsa staining and by flow cytometric analysis for cell surface expression of c-kit and FcεRI (supplemental Fig. S2).
RESULTS
Cytokine-mediated Interaction of Endogenous 14-3-3 and Shc—We have previously shown that there are two distinct cell survival pathways emanating from the βc subunit of the GM-CSF receptor: one that promotes βcSer585 phosphorylation and 14-3-3 binding and the other that promotes βcTyr577 phosphorylation and Shc binding (9, 10). This raised the possibility that 14-3-3 and Shc may form part of a βc receptor-proximal signaling scaffold involved in regulating cytokine function. We therefore examined the possibility that 14-3-3 and Shc interact in response to cytokine. GM-CSF stimulation of primary human mononuclear cells purified from peripheral blood induced the interaction of endogenous 14-3-3 and Shc (Fig. 1A, left panel). Similar regulation of endogenous 14-3-3:Shc interaction in response to GM-CSF was also observed in CTL-EN cells (Fig. 1A, right panel). However, examination of p46 and p52 Shc sequences did not reveal conserved canonical 14-3-3 phosphoserine/threonine-binding sites (4, 6). Furthermore, we have shown that recombinant purified GST-14-3-3ζ was unable to precipitate p46 or p52 Shc from HEK 293T or CTL-EN cells, suggesting that the interaction observed in Fig. 1A was not mediated via the binding of 14-3-3 to phosphoserine/threonine residues in Shc (data not shown). We therefore considered an alternative possibility that the SH2 domain of Shc may interact with tyrosine-phosphorylated 14-3-3. We performed pulldown experiments using recombinant SH2 domain of Shc (GST-SH2Shc) to precipitate 14-3-3 from CTL-EN and UT7 factor-dependent hemopoietic cell lines stimulated with GM-CSF. GST-SH2Shc precipitated 14-3-3 from both CTL-EN (Fig. 1B) or UT7 (data not shown) cells following GM-CSF stimulation, suggesting that endogenous 14-3-3 is tyrosine-phosphorylated and can interact with the SH2 domain of Shc following cytokine stimulation.
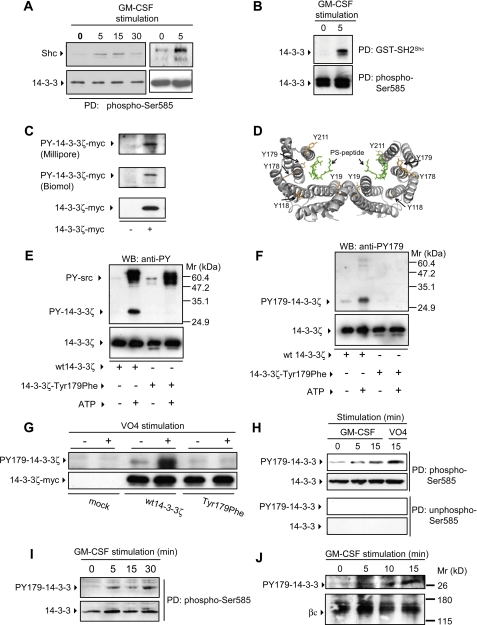
FIGURE 1.
14-3-3 proteins undergo tyrosine phosphorylation in response to GM-CSF stimulation. A, primary human mononuclear cells from peripheral blood (left panel) or CTL-EN cells (right panel) were stimulated with GM-CSF. Endogenous 14-3-3 was precipitated with a Ser(P)585 peptide (Biotin-NHS-KGGFDFNGPYLGPPHSR(pS)LPDGG; to precipitate total endogenous 14-3-3), and precipitates were immunoblotted with anti-Shc and anti-14-3-3 pAbs. B, factor-dependent CTL-EN cells expressing the human GM-CSF receptor were factor-deprived overnight in medium containing 0.5% FCS and then stimulated with GM-CSF. The cells were lysed, and pulldown experiments were performed using either GST-SH2Shc (to precipitate tyrosine-phosphorylated endogenous 14-3-3) or the Ser(P)585 peptide (to precipitate total endogenous 14-3-3), and precipitates were immunoblotted with anti-14-3-3 pAb. C, HEK 293T cells were transfected with a construct for the expression of wt 14-3-3ζ-Myc. After 48 h, the cells were lysed and 14-3-3ζ-Myc immunoprecipitated using the 9E10 mAb. Immunoprecipitates were subjected to immunoblot analysis with either the 4G10 mAb (Millipore) (top panel), an anti-phosphotyrosine pAb (Biomol) (middle panel) or the 9E10 mAb (bottom panel). D, the crystal structure of human 14-3-3ζ indicates that it is composed of 2 monomers that dimerize to form a large central groove that binds to phosphoserine peptides (PS-peptide) (16). The location of tyrosines 19, 118, 178, 179, and 211 of 14-3-3ζ are indicated. E and F, recombinant purified 14-3-3ζ and 14-3-3ζ-T179F were phosphorylated in vitro using c-Src and then subjected to immunoblot analysis using the 4G10 anti-phosphotyrosine mAb (anti-PY) (E, top panel), anti-Tyr(P)179-14-3-3ζ pAb (anti-PY179) (F, top panel) or anti-14-3-3ζ pAb to indicate loading (E and F, bottom panels). G, HEK-293T cells were transfected with constructs for the expression of wt 14-3-3ζ-Myc or the T179F mutant. After 48 h, the cells were stimulated for 15 min with sodium pervanadate (VO4) (+) or left unstimulated (-). The cells were then lysed and subjected to immunoprecipitation using the 9E10 mAb. Immunoprecipitated proteins were subjected to immunoblot analysis using the anti-Tyr(P)179-14-3-3ζ pAb (G, top panel) and the 9E10 mAb (G, bottom panel). H, CTL-EN cells expressing the human GM-CSF receptor were factor-deprived overnight. The cells were then stimulated with 50 ng/ml GM-CSF or sodium pervanadate (VO4), following which the cells were lysed, and endogenous 14-3-3 was precipitated (PD) using either a Ser(P)585 peptide (Biotin-NHS-KGGFDFNGPYLGPPHSR(pS)LPDGG) or an Ser585 (non-phospho-Ser585) control peptide (Biotin-NHS-KGGFDFNGPYLGPPHSRSLPDGG) adsorbed to streptavidin-Sepharose resin. Pulldowns were subjected to immunoblot analysis using the anti-Tyr(P)179-14-3-3ζ pAb or anti-14-3-3 pAb. I, primary human mononuclear cells were stimulated with GM-CSF and total endogenous 14-3-3 was precipitated as in A. Precipitates were blotted with anti-Tyr(P)179-14-3-3ζ pAb or anti-14-3-3 pAb. J, the UT7 factor-dependent cell line was factor-deprived overnight and then stimulated with 50 ng/ml GM-CSF, following which the cells were lysed, and the βc subunit of the GM-CSF receptor was immunoprecipitated using the 8E4 and 1C1 mAbs. Immunoprecipitates were then subjected to immunoblot analysis with either anti-Tyr(P)179 pAb (top panel) or 1C1 anti-βc mAb (bottom panel). The results are typical of at least two experiments. WB, Western blotting.
Tyr179 of 14-3-3 Is Phosphorylated in a Cytokine-dependent Manner—Although the 14-3-3 proteins are proposed to regulate diverse cellular processes via their ability to bind phosphoserine/threonine residues within a sequence-specific context (4, 6), their ability to directly participate in phosphotyrosine pathways has not been previously studied. We therefore examined whether 14-3-3ζ could be tyrosine-phosphorylated. As shown in Fig. 1C, tyrosine-phosphorylated 14-3-3ζ-Myc was clearly detected in transfected cells using two different anti-phosphotyrosine antibodies. We therefore examined specific 14-3-3 tyrosines for their 1) surface accessibility in the crystal structure of 14-3-3ζ (16), 2) potential as SH2-binding sites, or 3) potential as kinase phosphorylation sites. This analysis showed that Tyr179 of 14-3-3ζ 1) resides in a surface accessible loop between helices 7 and 8 (Fig. 1D), 2) lies within a suitable motif (179YEIL) for the binding of the SH2 domain of Shc (Y(I/E/Y/L)X(I/L/M)) (3), and 3) is a potential phosphorylation site for the Src kinases (17). Furthermore, Tyr179 is present in all seven isoforms of 14-3-3 and is conserved throughout evolution from Xenopus to humans (supplemental Fig. S3). We first undertook a mass spectrometry approach to determine whether 14-3-3ζ-Myc was phosphorylated. As shown in supplemental Fig. S4, the observed mass of 14-3-3ζ-Myc was +77 consistent with a covalent addition of a single phosphate group (theoretical +80 mass units). To determine whether Tyr179 of 14-3-3 was phosphorylated, we performed both tryptic and chymotryptic digests on 9E10 immunoprecipitates of 14-3-3ζ-Myc expressed in HEK 293T cells, and the immunoprecipitates were subjected to matrix-assisted laser desorption ionization time-of-flight/time-of-flight mass spectrometry. Using this approach, we were unable to detect Tyr(P)179 tryptic peptides. However, chemical synthesis of a peptide equivalent to the Tyr(P)179 tryptic peptide demonstrated that it was insoluble in aqueous buffers including trypsin digestion buffer and 4G10 chromatography running buffer (data not shown) and consequently not detectable by mass spectrometry.
We therefore generated anti-Tyr(P)179 pAb and confirmed that these antibodies specifically recognize Tyr(P)179 in recombinant 14-3-3ζ phosphorylated in vitro using c-Src, but not a 14-3-3ζ-T179F mutant (Fig. 1, E and F). Importantly, the anti-Tyr(P)179 pAb did not recognize autophosphorylated c-Src (Fig. 1F), which was clearly detected by the 4G10 anti-phosphotyrosine antibodies (Fig. 1E). To determine whether Tyr179 phosphorylation could be regulated, we transfected HEK 293T cells with wt 14-3-3ζ-Myc and the T179F mutant and treated these cells with sodium pervanadate to increase global tyrosine phosphorylation. As shown in Fig. 1G, Tyr179 phosphorylation was detected in cells expressing the wt 14-3-3ζ-Myc following sodium pervanadate treatment but not in cells expressing the T179F mutant. Using these anti-Tyr(P)179 phosphospecific antibodies, we then examined the regulation of Tyr179 phosphorylation in the CTL-EN and UT7 factor-dependent cells in response to GM-CSF. To examine the phosphorylation of Tyr179 in endogenous 14-3-3 proteins, we performed pulldown experiments with a phosphoserine peptide and immunoblotted these pulldowns with the anti-Tyr(P)179 antibodies. As shown in Fig. 1H, increased Tyr179 phosphorylation of endogenous 14-3-3 was observed following GM-CSF stimulation as well as with pervanadate treatment of CTL-EN cells. In line with the results in Fig. 1A, Tyr179 of endogenous 14-3-3 was also phosphorylated in primary human mononuclear cells following GM-CSF stimulation (Fig. 1I). Furthermore, endogenous 14-3-3 recruited to βcof the GM-CSF receptor following cytokine stimulation of UT7 cells was phosphorylated on Tyr179 (Fig. 1J).
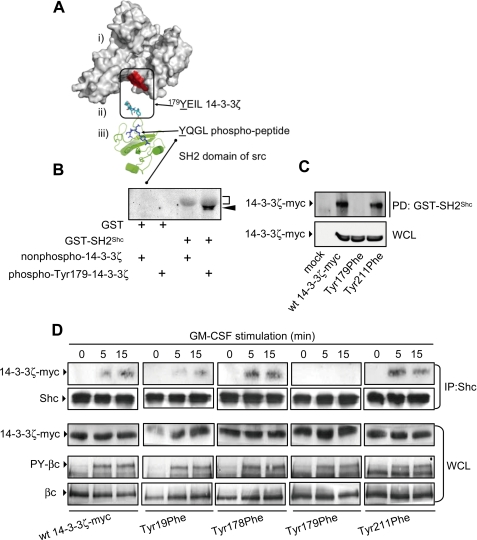
Shc Interacts with Tyr179 of 14-3-3ζ in a Cytokine-dependent Manner—Tyr179 lies within a suitable context for the binding of the SH2 domain of Shc (Y(I/E/Y/L)X(I/L/M)) (3). Examination of the three-dimensional structures of 14-3-3 and the SH2 domain of Shc indicate that the residues of 179YEIL are located on the surface of 14-3-3ζ in such an orientation that the SH2 domain of Shc could readily bind 14-3-3ζ (Fig. 2A). Furthermore, when 14-3-3ζ dimerizes, the 179YEIL motifs of each subunit are exposed on the surface, allowing the binding of two SH2 domains. We therefore examined the ability of the SH2 domain of Shc to directly interact with recombinant purified 14-3-3ζ phosphorylated on Tyr179 in vitro. As shown in Fig. 2B, GST-SH2Shc was able to precipitate purified recombinant 14-3-3ζ phosphorylated on Tyr179 but not nonphosphorylated 14-3-3ζ. In addition, a Tyr(P)179 peptide was able to precipitate recombinant purified SH2Shc in vitro, further indicating that Tyr179 is able to directly interact with the SH2 domain of Shc (data not shown). We have also shown that GST-SH2Shc was able to precipitate wt 14-3-3ζ-Myc and a Y211F mutant from HEK 293T lysates, but precipitation of the T179F mutant was greatly reduced (Fig. 2C).
FIGURE 2.
Tyr179 of 14-3-3ζ is necessary for GM-CSF-mediated recruitment of Shc. A, structural analysis of 14-3-3ζ and the SH2 domain of Src shows the solvent-accessible surface of 14-3-3ζ (i) and the location of the 179YEIL is indicated as a patch of red on the surface, the 179YEIL motif (cyan) from 14-3-3 in the orientation required to bind to the SH2 domain of Shc (ii), and a cartoon of the SH2 domain of Shc (green) with phosphopeptide (blue) bound (iii). B, purified recombinant GST or GST-SH2Shc bound to glutathione-Sepharose was incubated with either purified recombinant 14-3-3ζ phosphorylated on Tyr179 or a nonphosphorylated 14-3-3ζ control for 1 h in Nonidet P-40 lysis buffer. Pulldowns were washed and subjected to immunoblot analysis with 4G10 mAb. The diffuse gray band corresponds to GST-SH2Shc (bracket), and tyrosine-phosphorylated 14-3-3ζ (arrowhead) lies immediately below. C, HEK 293T cells were transfected with constructs expressing either the wt 14-3-3ζ-Myc or the indicated mutants. After 48 h, the cells were lysed, and lysates were incubated with either the GST-SH2Shc or a GST control. Pulldowns (PD) and whole cell lysates (WCL) were subjected to immunoblot analysis using the 9E10 mAb. D, CTL-EN cells expressing the human GM-CSF receptor were electroporated with constructs for the expression of either wt 14-3-3ζ-Myc or the indicated mutants and factor-deprived overnight. The cells were then stimulated with 50 ng/ml GM-CSF followed by lysis and anti-Shc immunoprecipitation. Immunoprecipitates (IP) were subjected to immunoblot analysis using the 9E10 mAb or anti-Shc pAb. Tyrosine phosphorylation of the βc subunit of the GM-CSF receptor in response to GM-CSF was examined in lysates (WCL) using the 4G10 mAb (PY-βc). WCL were also immunoblotted using the 1C1 anti-βc mAb and the 9E10 mAb to demonstrate equal loading. The results are typical of two experiments.
We next examined the cytokine-dependent interaction of Shc using specific 14-3-3ζ-Myc tyrosine mutants. As shown in Fig. 2D, wt 14-3-3ζ-Myc as well as the Y19F, Y178F, and Y211F mutants were able to co-immunoprecipitate with Shc following GM-CSF stimulation, whereas the T179F mutant was unable to associate with Shc. This lack of association was not due to any overall defect in receptor function, because βc tyrosine phosphorylation (PY-βc) in cells expressing the T179F mutant was essentially equivalent to that observed in cells expressing wt 14-3-3ζ-Myc (Fig. 2D).
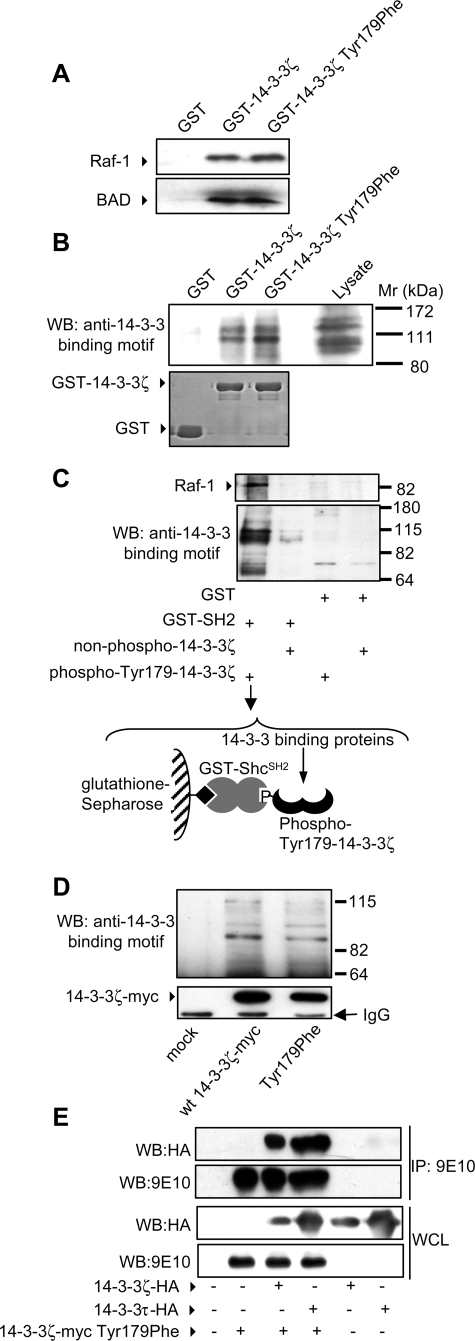
We then confirmed that the lack of interaction between the T179F mutant and Shc following cytokine stimulation was not due to a disruption in the phosphoserine binding ability of the mutant 14-3-3. First, we were able to precipitate both wt 14-3-3ζ and the T179F mutant using a phosphoserine peptide (see Fig. 5). Second, we performed pulldown experiments with GST-14-3-3ζ and the GST-14-3-3ζ-T179F mutant and showed that they were able to precipitate two widely described 14-3-3-binding proteins, Raf-1 and BAD (Fig. 3A). Third, GST-14-3-3ζ and the GST-14-3-3ζ-T179F mutant were able to precipitate a similar cohort of 14-3-3-binding proteins (Fig. 3B). Fourth, binding recombinant Tyr179-phosphorylated 14-3-3ζ to GST-SH2Shc did not interfere with the ability of 14-3-3 within the complex to interact with a range of 14-3-3-binding proteins including Raf-1 (Fig. 3C). Fifth, and most importantly, we were able to show that both wt 14-3-3ζ and the T179F mutant interacted with a similar panel of 14-3-3-binding proteins in cells (Fig. 3D). Thus, by each of the criteria above, the T179F mutant displays normal phosphoserine/phosphothreonine binding ability. In addition, we have also shown in co-immunoprecipitation experiments that the ability of the T179F mutant to homodimerize with 14-3-3ζ or heterodimerize with other 14-3-3 isoforms was not affected (Fig. 3E).
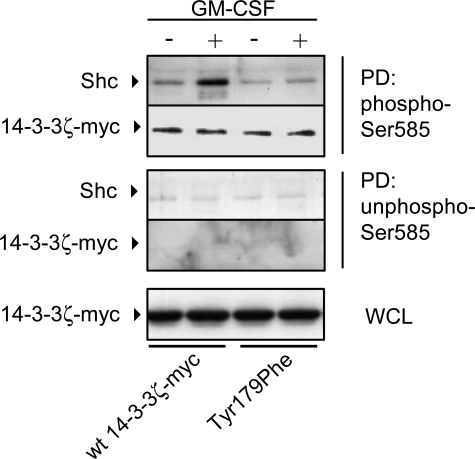
FIGURE 5.
14-3-3ζ can simultaneously bind phosphoserine residues and Shc. CTL-EN cells were electroporated with either wt 14-3-3ζ-Myc or the T179F mutant following which the cells were factor-deprived overnight and then stimulated with 50 ng/ml GM-CSF for 5 min. The cells were then lysed and pulldowns (PD) were performed using either a Ser(P)585 peptide (Biotin-NHS-KGGFDFNGPYLGPPHSR(pS)LPDGG) or a non-phospho-Ser585 control peptide (Biotin-NHS-KGGFDFNGPYLGPPHSRSLPDGG) adsorbed to streptavidin-Sepharose. PD and whole cell lysates (WCL) were subjected to SDS-PAGE and immunoblot analysis using anti-Shc pAb and the 9E10 mAb. The results are typical of two experiments.
FIGURE 3.
The 14-3-3ζ-T179F mutant is able to bind phosphoserine/threonine target proteins and heterodimerize with other isoforms of 14-3-3. HEK 293T cells were lysed and whole cell lysates subjected to pulldown experiments using either GST-Sepharose, GST-14-3-3ζ-Sepharose, or GST-14-3-3ζ T179F-Sepharose. Pulldowns were examined by immunoblot analysis using anti-Raf-1 and anti-BAD pAb (A) or a phospho-specific antibody that recognizes phosphorylated 14-3-3 binding motifs (Cell Signaling) (B). C, recombinant purified 14-3-3ζ was phosphorylated with c-Src (Tyr(P)179-14-3-3ζ) or left nonphosphorylated (non-phospho-14-3-3ζ) and then incubated with either GST-SH2Shc or GST alone bound to glutathione-Sepharose resin. The resin was washed to remove unbound 14-3-3ζ, and the assembled complexes (the complex assembled in lane 1 and used in the pulldown experiment is illustrated) were then used to pulldown 14-3-3-binding proteins from HEK 293T cell lysates. The pulldowns were washed in Nonidet P-40 lysis buffer and subjected to immunoblot analysis with phospho-specific antibodies that recognize phosphorylated 14-3-3 binding motifs or anti-Raf-1 pAb. D, HEK-293T cells were either mock transfected or transfected with constructs for the expression of wt 14-3-3ζ-Myc or the T179F mutant. After 48 h, the cells were lysed in Nonidet P-40 lysis buffer and subjected to immunoprecipitation using the 9E10 mAb. Immunoprecipitated proteins were subjected to SDS-PAGE and immunoblot analysis with phospho-specific antibodies that recognize phosphorylated 14-3-3 binding motifs (top panel) or the 9E10 mAb (bottom panel). E, HEK-293T cells were co-transfected with the indicated combinations of 14-3-3 isoforms. After 48 h, the cells were lysed, and lysates were immunoprecipitated (IP) with the 9E10 mAb. Immunoprecipitates and whole cell lysates (WCL) were subjected to SDS-PAGE and immunoblotted using the 9E10 mAb or the 12CA5 mAb. The 14-3-3ζ-Myc-T179F mutant was able to co-immunoprecipitate with 14-3-3γ-EE (data not shown), 14-3-3ζ-HA, and 14-3-3τ-HA. WB, Western blotting.
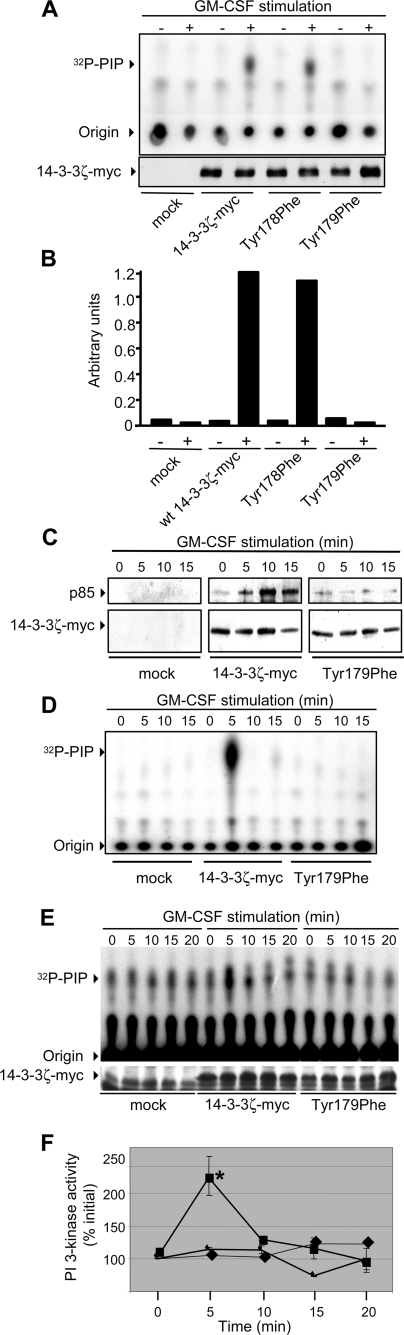
Tyr179 of 14-3-3ζ Is Important for the Assembly of a PI 3-Kinase Signaling Complex in Response to Cytokine—Our findings, as well as those of others, have shown that both 14-3-3 and Shc are important for the regulation of PI 3-kinase signaling in response to GM-CSF (10, 12, 18). We therefore examined the possibility that the binding of Shc to Tyr179 of 14-3-3ζ in response to GM-CSF stimulation may be necessary for the assembly of an active PI 3-kinase signaling complex. As shown in Fig. 4A, GM-CSF stimulation of CTL-EN cells resulted in increased PI 3-kinase activity associated with wt 14-3-3ζ-Myc, the Y178F mutant but not the T179F mutant. To examine the kinetics of PI 3-kinase recruitment and activation, we performed time course experiments. Although increased recruitment (Fig. 4C) and activation (Fig. 4D) of PI 3-kinase was observed over 15 min following cytokine stimulation, no significant recruitment or activation was observed for the T179F mutant (Fig. 4, C and D). Importantly, a key role for Tyr179 of 14-3-3ζ was also observed in the UT7 cell line where PI 3-kinase activity was recruited to wt 14-3-3ζ-Myc but not the T179F mutant (Fig. 4, E and F).
FIGURE 4.
Tyr179 of 14-3-3ζ is necessary for the GM-CSF-mediated recruitment and activation of PI 3-kinase. A, CTL-EN cells expressing the human GM-CSF receptor were electroporated with constructs for the expression of either the wt 14-3-3ζ-Myc or the indicated mutants, and cells were factor-deprived overnight. The cells were then stimulated with 50 ng/ml GM-CSF for 5 min and lysed, and 14-3-3ζ-Myc was immunoprecipitated using the 9E10 mAb. Immunoprecipitates were then washed, and PI 3-kinase activity was measured in vitro using 32P-γATP and phosphatidylinositol (PIP) as substrates. Extracted 32P-lipids were subjected to thin layer chromatography, and indicated are 32P-PIP and the origin. Quantification of these PhosphorImager signals (Typhoon) is shown in B. C, CTL-EN cells were electroporated without plasmid (mock), wt 14-3-3ζ-Myc, or the T179F mutant, following which the cells were factor-deprived and stimulated with GM-CSF as for A. 14-3-3ζ-Myc was immunoprecipitated with the 9E10 mAb with ⅓ of the immunoprecipitate used for PI 3-kinase assays (D) and 2/3 used for immunoblot analysis with anti-p85 pAb or 9E10 mAb (C). E, UT7 factor-dependent cells were electroporated with the indicated plasmids and PI 3-kinase assays were performed as in A and D. Quantification of PhosphorImager signals from two experiments is shown, and the error bars indicate the standard deviations (F). PI 3-kinase activity associated with the T179F mutant (♦) at 5 min was significantly decreased in comparison to the activity associated with wt 14-3-3ζ (▪) (*, p < 0.03). Mock transfected cells are indicated by the ▴.
The results in Fig. 3C suggested that 14-3-3 could function as an intermolecular bridge between phosphoserine/threonine 14-3-3 binding partners and the SH2 domain of Shc. To further test this possibility, we examined the ability of wt 14-3-3ζ-Myc and the T179F mutant to bind a phosphoserine peptide and Shc simultaneously. Using a phospho-peptide encompassing Ser585 of the GM-CSF receptor that we have previously shown is able to bind 14-3-3 with high affinity (9, 12), we performed pulldown experiments from CTL-EN cells. The Ser(P)585 peptide was able to bind wt 14-3-3ζ-Myc, whereas Shc was only recruited to the Ser(P)585-peptide/wt 14-3-3ζ-Myc complex following cytokine stimulation (Fig. 5, second lane). Although equivalent amounts of 14-3-3ζ-T179F mutant were precipitated by the Ser(P)585 peptide (indicating that its ability to bind Ser(P) peptides is not impaired), the ability of the Ser(P)585-peptide/14-3-3ζ-T179F complex to recruit Shc in response to cytokine was reduced (Fig. 5, compare second and fourth lanes). These results indicate that 14-3-3ζ is able to form an intermolecular bridge that, on the one hand is able to bind Ser(P) residues and on the other binds Shc via Tyr179. Although Shc is known to be able to recruit PI 3-kinase via Grb2 and Gab2 (18), we did not detect the presence of these proteins in our 14-3-3:Shc complexes, suggesting that they constitute a different pool of Shc to the Shc/Grb2/Gab2 to that identified by others (18).
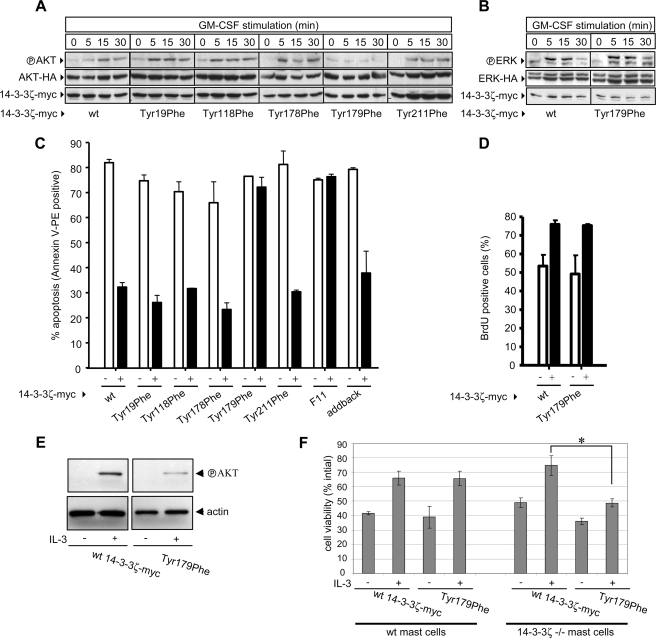
Tyr179 of 14-3-3ζ Is Necessary for the Phosphorylation of Akt (Protein Kinase B) and Cell Survival—We then examined the role of Tyr179 of 14-3-3ζ in regulating Akt phosphorylation downstream of PI 3-kinase. GM-CSF stimulation of cells expressing wt 14-3-3ζ-Myc resulted in increased phosphorylation of Akt-HA (Fig. 6A). Similar induction of Akt-HA phosphorylation was observed in cells expressing the Y19F, Y118F, Y178F, and Y211F mutants. However, no phosphorylation of Akt-HA was observed in response to GM-CSF in cells expressing the T179F mutant (Fig. 6A). Significantly, no defect in ERK-HA phosphorylation was observed in cells expressing the T179F mutant, indicating that Tyr179 of 14-3-3ζ was selectively involved in PI 3-kinase signaling (Fig. 6B).
FIGURE 6.
Tyr179 of 14-3-3ζ required for the phosphorylation of Akt and is necessary and sufficient for regulating GM-CSF-mediated cell survival. CTL-EN cells expressing the GM-CSF receptor were co-electroporated with constructs expressing Akt-HA (A) or ERK-HA (B) and either wt 14-3-3ζ-Myc or the indicated 14-3-3ζ mutants. Co-transfection of Akt-HA or ERK-HA reporter plasmids allowed the specific examination of Akt and ERK phosphorylation in response to GM-CSF in transfected cells only. The cells were factor-deprived overnight and then stimulated with GM-CSF. The cells were then lysed, and the lysates were subjected to immunoblot analysis using the anti-phospho-Akt pAb (Thr308 and Ser473) (A), anti-active-ERK pAb (B) or the 9E10 or 12CA5 mAb. C, CTL-EN cells expressing the βcS585G mutant of GM-CSF receptor were co-electroporated with constructs expressing EGFP (to allow detection of transfected cells) and either wt 14-3-3ζ-Myc or the indicated mutants. After 24 h, the cells were washed and plated in medium containing either no factor (-) or 50 ng/ml GM-CSF (+). After a further 48 h, the cells were stained with annexin V-PE and analyzed by flow cytometry. Shown is the percentage of EGFP-positive cells that are annexin V-PE-positive. Cell proliferation was also examined (D). CTL-EN cells expressing the βcS585G mutant of GM-CSF receptor were co-electroporated with constructs expressing enhanced cyan fluorescent protein and either wt 14-3-3ζ-Myc or the T179F mutant. After 24 h, cyan fluorescent protein-positive cells were purified by fluorescence-activated cell sorting and plated in either no factor (-) or 50 ng/ml GM-CSF (+) for 24 h with a BrdUrd pulse for the last 4 h. The cells were then fixed, stained for BrdUrd incorporation, and analyzed by flow cytometry. E, primary bone marrow-derived mast cells from 14-3-3ζ-/- mice were electroporated with either wt 14-3-3ζ-Myc or the T179F mutant. After 24 h, the cells were washed and plated at 1.5 × 105/ml in serum-free DMEM containing 0.1% bovine serum albumin overnight and then stimulated with 1000 pm IL-3 and Akt phosphorylation was examined as in A. F, primary bone marrow-derived mast cells from wt and 14-3-3ζ-/- mice were electroporated with either wt 14-3-3ζ-Myc or the T179F mutant together with EGFP to allow identification of transfected cells. After 24 h, the cells were washed and plated at 1.5 × 105/ml in serum-free DMEM containing 0.1% bovine serum albumin and 1000 pm IL-3. The number of viable GFP-positive cells was determined by flow cytometry and forward/side scatter characteristics of mast cells at 48 h as a percentage of the initial GFP-positive population to account for differences in transfection efficiency. A significant reduction in IL-3-mediated survival of 14-3-3ζ-/- bone marrow-derived mast cells expressing the T179F mutant was observed when compared with knock-out cells expressing the wt 14-3-3ζ-Myc (*, p < 0.0007 from two separate experiments). All of the results are representative of at least two experiments.
Because the PI-3 kinase pathway has been widely observed to play important roles in cell survival and proliferation, we examined the ability of the T179F mutant to regulate these biological responses. For these experiments, we took advantage of specific GM-CSF receptor mutants previously identified in our laboratory as having an important role in mediating PI 3-kinase activation as well as cell survival and proliferation. Our previous results showed that the βc subunit of the GM-CSF receptor is able to promote PI 3-kinase activation via two pathways; one pathway is regulated via Tyr577 of βc, which binds Shc, and the other pathway is regulated via Ser585 of βc, which binds 14-3-3 (9, 10, 12). Importantly, although no reduction in cell survival or proliferation in response to high concentrations of cytokine (>10 pm) was observed in either the Y577F or the S585G single mutants, these biological responses were defective in the Y577F/S585G double mutant (9, 10, 12). Thus, our results indicated that Tyr577 could compensate for the loss of Ser585, whereas Ser585 could compensate for the loss of Tyr577. We therefore specifically examined the ability of 14-3-3:Shc scaffolds bound to Tyr577 in βc of the GM-CSF receptor to promote cell survival and proliferation by performing experiments within the context of the βcS585G GM-CSF receptor mutant. As shown in Fig. 6C, GM-CSF was able to promote cell survival in CTL-EN cells expressing wt 14-3-3ζ-Myc. No defect in GM-CSF-mediated cell survival was observed for the Y19F, Y118F, Y178F, or Y211F mutants, whereas expression of the T179F mutant reduced the ability of GM-CSF to promote cell survival (Fig. 6C). Importantly, a 14-3-3ζ-Myc mutant in which all 11 tyrosine residues were mutated to phenylalanine (F11) was also defective in transducing survival signals, whereas an add-back mutant in which Tyr179 was restored mediated cell survival (Fig. 6C), showing that Tyr179 of 14-3-3ζ is both necessary and sufficient for regulating cytokine-mediated survival.
Although cell proliferation was significantly reduced at later time points (48 h) where overt cell death was observed (data not shown), analysis at earlier time points (24 h) prior to the effector phase of apoptosis indicated that the T179F mutant had no significant effect on cytokine-mediated proliferation (Fig. 6D). Thus, although cells were able to commit to cell cycle progression, they were not able to activate cell survival pathways.
To further examine the physiological role of Tyr179 in regulating cell survival, we employed primary bone marrow-derived mast cells from 14-3-3ζ-/- mice. These cells are unique in that although most cell types and tissues express multiple 14-3-3 isoforms, 14-3-3ζ constitutes nearly 90% of the total 14-3-3 in mast cells as determined by both reverse transcription-PCR and Western blotting (supplemental Fig. S5). Furthermore, IL-3, which signals via the same βc subunit as GM-CSF, is a potent regulator of mast cell survival. No defect in differentiation was observed in mast cells derived from 14-3-3ζ-/- mice as determined by morphology and the expression of the differentiation markers, c-Kit and FCεR1 (supplemental Fig. S2). However, although cells derived from 14-3-3ζ-/- mice and reconstituted with wt 14-3-3ζ were able to promote Akt phosphorylation and survive in response to IL-3, cells reconstituted with the Tyr179 mutant showed reduced Akt phosphorylation and viability (Fig. 6, E and F). Together, our results identify a novel 14-3-3:Shc signaling axis critical in the architecture of the signosome that controls PI 3-kinase activation and cell survival and reveals a new function for 14-3-3 proteins in integrating cytokine-mediated phosphotyrosine signaling.
DISCUSSION
The regulation of protein-protein association through tyrosine phosphorylation and the binding of SH2 or PTB domains as well as through serine/threonine phosphorylation and the binding of 14-3-3, WW, FHA, PBD, and BRCT proteins is a central mechanism by which cytokines and growth factors regulate intracellular signaling pathways and cell function (1–4, 6, 8). We now show that phosphotyrosine and phosphoserine pathways can be directly integrated through the assembly of 14-3-3:Shc scaffolds (Fig. 7). We have shown that endogenous 14-3-3 and Shc interact in response to GM-CSF (Fig. 1). Furthermore, this interaction is mediated by Tyr179 phosphorylation of 14-3-3 and the binding of the SH2 domain of Shc (Figs. 1 and 2). Using phosphospecific antibodies, we have shown that Tyr179 of endogenous 14-3-3 is phosphorylated in response to cytokine in multiple cell types and that Tyr(P)179-14-3-3 is recruited to the GM-CSF receptor (Fig. 1). In addition, we show that the interaction of Shc with Tyr179 of 14-3-3ζ is involved in the assembly of a PI 3-kinase signaling complex in response to cytokine (Fig. 4), the phosphorylation of Akt, and the regulation of cell survival (Fig. 6). These 14-3-3:Shc scaffolds are specifically involved in PI 3-kinase signaling and survival because expression of the T179F mutant does not interfere with cytokine-mediated ERK phosphorylation or proliferation (Fig. 6). Significantly, the add-back mutant of 14-3-3ζ in which all tyrosines, except for Tyr179, were substituted for phenylalanine was able to transduce GM-CSF-mediated cell survival signals, indicating that Tyr179 is both necessary and sufficient for this response (Fig. 6). The physiological significance of Tyr179 phosphorylation in regulating cell survival was further demonstrated using primary bone marrow-derived mast cells from 14-3-3ζ-/- mice. We demonstrate that IL-3 was able to promote Akt phosphorylation and viability of primary mast cells derived from 14-3-3ζ-/- mice when reconstituted with wt 14-3-3ζ, but not the T179F mutant (Fig. 6). Thus, although 14-3-3ζ clearly exerts its biological functions through the binding of phosphoserine/threonine residues, we have identified a new activity for 14-3-3ζ as a phosphotyrosine scaffold that is important in regulating PI 3-kinase signaling and cell survival (Fig. 7).
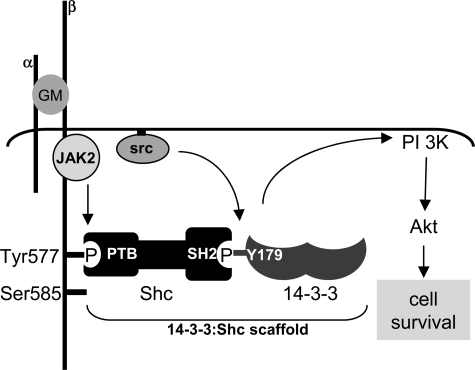
FIGURE 7.
Model for the assembly of 14-3-3:Shc scaffolds. Stimulation with 50 ng/ml GM-CSF (GM) results in the activation of tyrosine kinases including JAK2 and Src family of kinases. Although we have shown that the activated receptor constitutes a high order dodecamer (33), only two subunits of the receptor are illustrated. JAK2 can phosphorylate Tyr577 of βc, whereas Src family kinases can phosphorylate Tyr179 of 14-3-3ζ. The phosphorylation of 14-3-3ζ on Tyr179 provides a docking site for the direct binding of the SH2 domain of Shc. The 14-3-3:Shc scaffold bound to Tyr577 of the βc subunit of the GM-CSF receptor is specifically required for the activation of the PI 3-kinase/Akt pathway and the regulation of cell survival.
In keeping with their ability to regulate diverse cellular processes (4, 6), the 14-3-3 proteins have been found to interact with an impressive array of proteins. Several recent reports using proteomic approaches have identified over 200 binding partners for the 14-3-3 proteins (19–21). However, these studies may not have found important SH2-mediated interactions with 14-3-3 because the experimental approaches used were designed to specifically identify phosphoserine/threonine binding partners (19–21). The 14-3-3 proteins have also been frequently identified in two-hybrid screens as interacting partners for diverse bait proteins. Similarly, standard two-hybrid techniques do not allow the identification of clones that interact via phosphotyrosine-mediated SH2 domains, possibly explaining why such an important class of 14-3-3 binding partners has been overlooked. Thus, our results would suggest that the interactive proteome for 14-3-3 would extend beyond phosphoserine/threonine-dependent interactions to include SH2 domain proteins, and their role in signaling is likely to be much wider than previously envisaged.
The 14-3-3 proteins have been widely described as playing essential roles in cell survival through their ability to bind phosphoserine/threonine target proteins involved in the regulating apoptosis. For example, whereas 14-3-3 has been shown to suppress ASK1 (apoptosis signal-regulated kinase 1)-mediated cell death, expression of a K49E mutant that is defective in binding phosphoserine/threonine target proteins enhances ASK1-mediated apoptosis (22). Similarly, expression of a R56A/R60A mutant of 14-3-3ζ, which is unable to bind phosphoserine/threonine target proteins, renders fibroblasts more sensitive to apoptotic insults such as UV-C irradiation, serum deprivation, and tumor necrosis factor α stimulation (23). In each case, the 14-3-3 mutants that are unable to bind phosphoserine/threonine target proteins are proposed to exert a dominant-negative effect through their heterodimerization with endogenous wt 14-3-3 proteins, thereby blocking their interaction with target proteins (22, 23). However, our results now show that the 14-3-3 proteins can also mediate cell survival signals via tyrosine phosphorylation. In this respect, it is important to point out that the dominant-negative activity of the T179F mutant of 14-3-3ζ observed in our studies is quite distinct from that of the previously described K49E and R56A/R60A dominant-negative mutants in that the T179F mutant is able to bind phosphoserine/threonine target proteins (Fig. 3). Thus, the defect in the T179F mutant is due to its inability to couple to phosphotyrosine signaling pathways and recruit Shc leading to defective cytokine-mediated PI 3-kinase signaling and cell death.
Our results show that the stoichiometry of Tyr179 phosphorylation following cytokine stimulation is low (supplemental Fig. S6). Although these results indicate that only a small percentage of 14-3-3 is phosphorylated on Tyr179, our findings are not unexpected. The 14-3-3 proteins serve a wide variety of functions within the cell, and only a fraction of the total 14-3-3 would be devoted to any one specific function at any one time. Furthermore, compared with the 14-3-3 proteins, the βc subunit of the GM-CSF receptor is not highly expressed and would therefore be limiting.
It is becoming clear that the activities of the 14-3-3 proteins can be regulated through a number of post-translational modifications. For example, 14-3-3ζ is phosphorylated on Ser184 by c-Jun N-terminal kinase (JNK) in response to DNA damage leading to the nuclear targeting of c-Abl and apoptosis (24). In addition, 14-3-3ζ has been shown to be phosphorylated on Ser58 by a number of kinases, and this can lead to monomerization of the 14-3-3 subunits and the release of bound proteins (25). Other serine and threonine residues have also been reported to be phosphorylated in the 14-3-3 proteins; however, the physiological significance of these are not yet known (26). In addition, Giacometti et al. (27) have shown that Tyr137 of plant 14-3-3 GF14-6 can be phosphorylated by the tyrosine kinase domain of the insulin-like growth factor receptor. The kinase that phosphorylates Tyr179 remains to be determined. Although the GM-CSF receptor is a potent activator of both Jak and Src family of kinases (28), we were only able to phosphorylate Tyr179 in vitro with Src and Lyn, but not in two independent preparations of Jak2 (Fig. 1 and data not shown), suggesting a possible role for the Src kinases in phosphorylating Tyr179.
The discovery that SH2 and PTB domains are able to bind phosphotyrosine residues and are widely distributed among signaling proteins established an important paradigm for the assembly of signaling complexes in response to cytokines and growth factors (1, 2). More recently, this paradigm has been extended by the discovery of phosphoserine/threonine-binding domains/proteins such as the WW, FHA, PBD, and the BRCT domains as well as the 14-3-3 family of proteins (4, 6, 8). Phosphoserine/threonine and phosphotyrosine signaling pathways are widely viewed as being transduced via overlapping and highly integrated pathways. One mechanism by which phosphoserine/threonine and phosphotyrosine signaling pathways can be integrated is via dual specificity kinases and phosphatases (29, 30). However, little is known regarding how adaptor or scaffold proteins assemble signaling complexes through both phosphotyrosine and phosphoserine/threonine residues to regulate specific signaling pathways and biological responses. In particular, adaptor or scaffold proteins that contain both a phosphotyrosine-binding domain (e.g. SH2 or PTB) and a phosphoserine/threonine binding cassette (e.g. 14-3-3, WW, FHA, PBD, or BRCT) have not been identified. This would suggest that other strategies have evolved to allow the assembly of signaling complexes via phosphotyrosine and phosphoserine/threonine residues. Although IRS1 and Cbl have been shown to bind both SH2 domain proteins as well as the 14-3-3 proteins (31, 32), a specific biological role for 14-3-3 binding and their ability to simultaneously integrate phosphotyrosine and phosphoserine/threonine pathways, to our knowledge, has not been reported. Our findings demonstrating the ability of the 14-3-3 proteins to bind phosphoserine/threonine residues and SH2 domains suggest a new role as multifunctional adaptors that act as nodes for the assembly of signaling complexes via both phosphoserine/threonine and phosphotyrosine residues. The studies presented herein demonstrating that 14-3-3 proteins can be regulated by site-specific tyrosine phosphorylation illustrate a novel and dynamic mechanism for the specific transduction of signals via the PI 3-kinase pathway to promote cell survival. This molecular plasticity would allow 14-3-3:Shc scaffolds to couple with phosphoserine/threonine (via 14-3-3) and phosphotyrosine (via Shc) and thereby assemble signaling complexes with different valences and ultimately integrate specific signaling pathways and biological activities.
Supplementary Material
Acknowledgments
We thank Aruna Shivam and Dr. Christopher Bagley for technical assistance. We thank Ben Margolis (Howard Hughes Medical Institute, University of Michigan Medical School), Melanie Cobb (Department of Pharmacology, The University of Texas Southwestern Medical Center), and Philip N. Tsichlis (Molecular Oncology Research Institute, Tufts-New England Medical Center, Boston) for expression constructs. We thank Isabelle Lucet (Monash University, Clayton, Australia) for recombinant Jak2.
This work was supported by grants from the National Health and Medical Research Council of Australia and the Peter Nelson Foundation.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S6.
Footnotes
The abbreviations used are: SH, Src homology; wt, wild type; PI, phosphatidylinositol; PTB, phosphotyrosine-binding; WW, tryptophan-tryptophan; FHA, Forkhead-associated; PBD, Polo box domain; BRCT, BRCA1 C-terminal; GM-CSF, granulocyte-macrophage colony-stimulating factor; DMEM, Dulbecco's modified Eagle's medium; FCS, fetal calf serum; IL, interleukin; GST, glutathione S-transferase; pAb, polyclonal antibody; mAb, monoclonal antibody; GFP, green fluorescent protein; EGFP, enhanced GFP; BrdUrd, 5-bromo-2-deoxyuridine; ERK, extracellular signal-regulated kinase.
References
- 1.Pawson, T., and Scott, J. D. (1997) Science 278 2075-2080 [DOI] [PubMed] [Google Scholar]
- 2.Pawson, T. (2004) Cell 116 191-203 [DOI] [PubMed] [Google Scholar]
- 3.Songyang, Z., Shoelson, S. E., McGlade, J., Olivier, P., Pawson, T., Bustelo, X. R., Barbacid, M., Sabe, H., Hanafusa, H., and Yi, T. (1994) Mol. Cell Biol. 14 2777-2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaffe, M. B. (2002) FEBS Lett. 513 53-57 [DOI] [PubMed] [Google Scholar]
- 5.Aitken, A. (2006) Semin. Cancer Biol. 16 162-172 [DOI] [PubMed] [Google Scholar]
- 6.Dougherty, M. K., and Morrison, D. K. (2004) J. Cell Sci. 117 1875-1884 [DOI] [PubMed] [Google Scholar]
- 7.MacKintosh, C. (2004) Biochem. J. 381 329-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaffe, M. B., and Elia, A. E. H. (2001) Curr. Opin. Cell Biol. 13 131-138 [DOI] [PubMed] [Google Scholar]
- 9.Guthridge, M. A., Barry, E. F., Felquer, F. A., McClure, B. J., Stomski, F. C., Ramshaw, H., and Lopez, A. F. (2004) Blood 103 820-827 [DOI] [PubMed] [Google Scholar]
- 10.Guthridge, M. A., Powell, J. A., Barry, E. F., Stomski, F. C., McClure, B. J., Ramshaw, H., Felquer, F. A., Dottore, M., Thomas, D. T., To, B., Begley, C. G., and Lopez, A. F. (2006) EMBO J. 25 479-489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stomski, F. C., Dottore, M., Winnall, W., Guthridge, M. A., Woodcock, J., Bagley, C. J., Thomas, D. T., Andrew, R. K., Berndt, M. C., and Lopez, A. F. (1999) Blood 94 1933-1942 [PubMed] [Google Scholar]
- 12.Guthridge, M. A., Stomski, F. C., Barry, E. F., Winnall, W., Woodcock, J. M., McClure, B. J., Dottore, M., Berndt, M. C., and Lopez, A. F. (2000) Mol. Cell 6 99-108 [PubMed] [Google Scholar]
- 13.Kleywegt, G. J. (1996) Acta Crystallogr. Sect. D Biol. Crystallogr. 52 842-857 [DOI] [PubMed] [Google Scholar]
- 14.DeLano, W. L. (2005) Drug Discov. Today 10 213-217 [DOI] [PubMed] [Google Scholar]
- 15.Grimbaldeston, M. A., Nakae, S., Kalesnikoff, J., Tsai, M., and Galli, S. J. (2007) Nat. Immunol. 8 1095-1104 [DOI] [PubMed] [Google Scholar]
- 16.Rittinger, K., Budman, J., Xu, J., Volinia, S., Cantley, L. C., Smerdon, S. J., Gamblin, S. J., and Yaffe, M. B. (1999) Mol. Cell 4 153-166 [DOI] [PubMed] [Google Scholar]
- 17.Leitges, M., Gimborn, K., Elis, W., Kalesnikoff, J., Hughes, M. R., Krystal, G., and Huber, M. (2002) Mol. Cell Biol. 22 3970-3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu, H., Maeda, H., Moon, J. J., Lord, J. D., Yoakim, M., Nelson, B. H., and Neel, B. G. (2000) Mol. Cell Biol. 20 7109-7120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin, J., Smith, F. D., Stark, C., Wells, C. D., Fawcett, J. P., Kulkarni, S., Metalnikov, P., O'Donnell, P., Taylor, P., Taylor, L., Zougman, A., Woodgett, J. R., Langeberg, L. K., Scott, J. D., and Pawson, T. (2004) Curr. Biol. 14 1436-1450 [DOI] [PubMed] [Google Scholar]
- 20.Meek, S. E., Lane, W. S., and Piwnica-Worms, H. (2004) J. Biol. Chem. 279 32046-32054 [DOI] [PubMed] [Google Scholar]
- 21.Pozuelo, R. M., Geraghty, K. M., Wong, B. H., Wood, N. T., Campbell, D. G., Morrice, N., and MacKintosh, C. (2004) Biochem. J. 379 395-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, L., Chen, J., and Fu, H. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 8511-8515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing, H., Zhang, S., Weinheimer, C., Kovacs, A., and Muslin, A. J. (2000) EMBO J. 19 349-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida, K., Yamaguchi, T., Natsume, T., Kufe, D., and Miki, Y. (2005) Nat. Cell Biol. 7 278-285 [DOI] [PubMed] [Google Scholar]
- 25.Woodcock, J. M., Murphy, J., Stomski, F. C., Berndt, M. C., and Lopez, A. F. (2003) J. Biol. Chem. 278 36323-36327 [DOI] [PubMed] [Google Scholar]
- 26.Aitken, A., Baxter, H., Dubois, T., Clokie, S., Mackie, S., Mitchell, K., Peden, A., and Zemlickova, E. (2002) Biochem. Soc. Trans. 30 351-360 [DOI] [PubMed] [Google Scholar]
- 27.Giacometti, S., Camoni, L., Albumi, C., Visconti, S., De Michelis, M. I., and Aducci, P. (2004) Plant Biol. 6 422-431 [DOI] [PubMed] [Google Scholar]
- 28.Reddy, E. P., Korapati, A., Chaturvedi, P., and Rane, S. (2000) Oncogene 19 2532-2547 [DOI] [PubMed] [Google Scholar]
- 29.Guthridge, M. A., Bellosta, P., Tavaloni, N., and Basilico, C. (1997) Mol. Cell Biol. 17 5485-5498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owens, D. M., and Keyse, S. M. (2007) Oncogene 26 3203-3213 [DOI] [PubMed] [Google Scholar]
- 31.Ogihara, T., Isobe, T., Ichimura, T., Taoka, M., Funaki, M., Sakoda, H., Onishi, Y., Inuka, K., Anai, M., Fukushima, Y., Kikuchi, M., Yazaki, Y., Oka, Y., and Asano, T. (1997) J. Biol. Chem. 272 25267-25274 [DOI] [PubMed] [Google Scholar]
- 32.Liu, Y.-C., Elly, C., Yoshida, H., Bonnefoy-Berard, N., and Altman, A. (1996) J. Biol. Chem. 271 14591-14595 [DOI] [PubMed] [Google Scholar]
- 33.Hansen, G., Hercus, T. R., McClure, B. J., Stomski, F. C., Dottore, M., Powell, J., Ramshaw, H., Woodcock, J. M., Xu, Y., Guthridge, M., McKinstry, W. J., Lopez, A. F., and Parker, M. W. (2008) Cell 134 496-507 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.