Abstract
N-Glycosylation of integrin α5β1 plays a crucial role in cell spreading, cell migration, ligand binding, and dimer formation, but the detailed mechanisms by which N-glycosylation mediates these functions remain unclear. In a previous study, we showed that three potential N-glycosylation sites (α5S3–5) on the β-propeller of the α5 subunit are essential to the functional expression of the subunit. In particular, site 5 (α5S5) is the most important for its expression on the cell surface. In this study, the function of the N-glycans on the integrin β1 subunit was investigated using sequential site-directed mutagenesis to remove the combined putative N-glycosylation sites. Removal of the N-glycosylation sites on the I-like domain of the β1 subunit (i.e. the Δ4-6 mutant) decreased both the level of expression and heterodimeric formation, resulting in inhibition of cell spreading. Interestingly, cell spreading was observed only when the β1 subunit possessed these three N-glycosylation sites (i.e. the S4-6 mutant). Furthermore, the S4-6 mutant could form heterodimers with either α5S3-5 or α5S5 mutant of the α5 subunit. Taken together, the results of the present study reveal for the first time that N-glycosylation of the I-like domain of the β1 subunit is essential to both the heterodimer formation and biological function of the subunit. Moreover, because the α5S3-5/β1S4-6 mutant represents the minimal N-glycosylation required for functional expression of the β1 subunit, it might also be useful for the study of molecular structures.
Integrin is a heterodimeric glycoprotein that consists of both an α and a β subunit (1). The interaction between integrin and the extracellular matrix is essential to both physiologic and pathologic events, such as cell migration, development, cell viability, immune homeostasis, and tumorigenesis (2, 3). Among the integrin superfamily, β1 integrin can combine with 12 distinct α subunits (α1–11, αv) to form heterodimers, thereby acquiring a wide variety of ligand specificity (1, 4). Integrins are thought to be regulated by inside-out signaling mechanisms that provoke conformational changes, which modulate the affinity of integrin for the ligand (5). However, an increasing body of evidence suggests that cell-surface carbohydrates mediate a variety of interactions between integrin and its extracellular environment, thereby affecting integrin activity and possibly tumor metastasis as well (6–8).
Guo et al. (9) reported that an increase in β1–6-GlcNAc sugar chains on the integrin β1 subunit stimulated cell migration. In addition, elevated sialylation of the β1 subunit, because of Ras-induced STGal-I transferase activity, also induced cell migration (10, 11). Conversely, cell migration and spreading were reduced by the addition of a bisecting GlcNAc, which is a product of N-acetylglucosaminyltransferase III (GnT-III),2 to the α5β1 and α3β1 integrins (12, 13). Alterations of N-glycans on integrins might also regulate their cis interactions with membrane-associated proteins, including the epidermal growth factor receptor, the galectin family, and the tetraspanin family of proteins (14–19).
In addition to the positive and negative regulatory effects of N-glycan, several research groups have reported that N-glycans must be present on integrin α5β1 for the αβ heterodimer formation and proper integrin-matrix interactions. Consistent with this hypothesis, in the presence of the glycosylation inhibitor, tunicamycin, normal integrin-substrate binding and transport to the cell surface are inhibited (20). Moreover, treatment of purified integrin with N-glycosidase F blocked both the inherent association of the subunits and the interaction between integrin and fibronectin (FN) (21). These results suggest that N-glycosylation is essential to the functional expression of α5β1. However, because integrin α5β1 contains 26 potential N-linked glycosylation sites, 14 in the α subunit and 12 in the β subunit, identification of the sites that are essential to its biological functions is key to understanding the molecular mechanisms by which N-glycans alter integrin function. Recently, our group determined that N-glycosylation of the β-propeller domain on the α5 subunit is essential to both heterodimerization and biological functions of the subunit. Furthermore, we determined that sites 3–5 are the most important sites for α5 subunit-mediated cell spreading and migration on FN (22). The purpose of this study was to clarify the roles of N-glycosylation of the β1 subunit. Therefore, we performed combined substitutions in the putative N-glycosylation sites by replacement of asparagine residues with glutamine residues. We subsequently introduced these mutated genes into β1-deficient epithelial cells (GE11). The results of these mutation experiments revealed that the N-glycosylation sites on the I-like domain of the β1 subunit, sites number 4–6 (S4-6), are essential to both heterodimer formation and biological functions, such as cell spreading.
EXPERIMENTAL PROCEDURES
Reagent—The Phoenix cell line was purchased from ATCC (Manassas, VA). A monoclonal antibody (mAb) against the human integrin β1 subunit (P5D2), which was obtained from Developmental Studies Hybridoma Bank, University of Iowa, was purified from the hybridoma supernatant using a protein A-Sepharose™ 4 Fast Flow column (GE Healthcare). For Western blot analysis, mAbs against amino acids 82–87 of the human integrin β1 subunit (MAB1965, JB1A) and α-tubulin (DM1A) were obtained from Chemicon (Temecula, CA) and Sigma, respectively. Polyclonal rabbit antibodies against the carboxyl terminus of human β1 integrin (AB1952P), α5 integrin (H-104), α5 integrin (4705S), and calnexin (SPA-860) were purchased from Chemicon, Santa Cruz Biotechnology (Santa Cruz, CA), Cell Signaling Technology, Inc. (Danvers, MA), and StressGen (Ann Arbor, MI), respectively. A goat antibody against the green fluorescent protein (GFP) was obtained from Rockland Immunochemicals, Inc. (Gilbertsville, PA). Peroxidase-conjugated anti-rabbit and anti-goat antibodies were obtained from Cell Signaling Technology, Inc. A peroxidase-conjugated goat anti-mouse IgG antibody was obtained from Chemicon (Temecula, CA). A rat antibody against mouse α5 integrin subunit (Sc-23941) was purchased form Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Cultures—Epithelial GE11 cells, derived from β1 integrin knock-out embryonic stem cells (23), were maintained at 37 °C in Dulbecco's modified Eagle's medium (DMEM; Invitrogen), supplemented with 10% fetal bovine serum (FBS), penicillin/streptomycin, and 20 nm trichostatin A. Phoenix cells and integrin α5-deficient cells (CHO-B2) (22) were grown in DMEM supplemented with 10% FBS, penicillin/streptomycin. CHO Lec 3.2.8.1 cells, a GnT-I-deficient cell line, were a gift from Dr. Pamela Stanly (Albert Einstein College of Medicine, New York) (24).
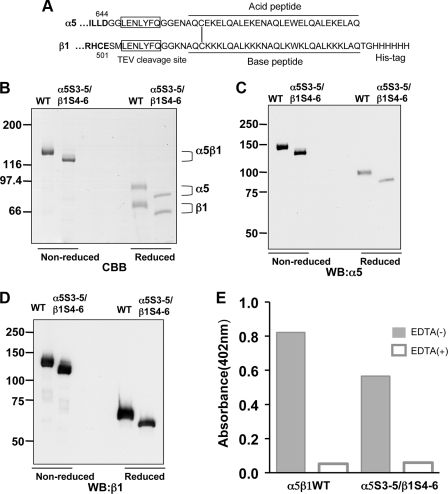
cDNA Constructs—The cDNA of integrin β1 subunit was amplified by PCR from the reverse-transcribed product of human placenta total RNA (OriGene Technologies, Inc., Rockville, MD) and then was inserted into a cloning vector (pENTR-D-Topo, Invitrogen), according to the manufacturer's protocol. Mutations were introduced into the β1 cDNA using a Quick-Change site-directed mutagenesis kit (Stratagene, La Jolla, CA.) according to the manufacturer's instructions. The retrovirus vector, pBabe-Puro plasmid (25), was made to be Gateway-compatible using the Gateway Conversion System kit (Invitrogen), resulting in pBabe-puro-Rfa. The LR clonase enzyme (Invitrogen) was used to transfer the cDNA of either β1 integrin or GFP from the cloning vectors. The coding regions of all cDNA constructs were sequenced using an ABI PRISM 3130 sequencer (Applied Biosystems Japan Ltd., Tokyo, Japan) and compared with the human gene ITGB1 (GenBank™ BC020057.1) to confirm both the presence of the desired mutations (Fig. 1) and the absence of any additional mutations. The cDNA constructs of soluble α5 and β1 subunits were designed according to previous studies (26–29). The soluble expression system of α5β1 integrin was clearly described by Takagi et al. (27, 28). Briefly, the expression vectors of α5 (pEF1-α5-Δ664-tev-AHCys) and β1 (pEF1-puro-β1-Δ501-tev-BHCys-6XHis) subunits were kind gifts provided by Dr. Junichi Takagi (Institute for Protein Research, Osaka University, Japan). We further shorten the extracellular domain of α5 to residue Asp644 based on the extensive study carried by Coe et al. (26). These cDNAs were then cloned into the pENTR-D-topo cloning vector, resulting in α5-Δ644-tev-AHCys (residues from Met1 to Asp644) and β1-Δ501-tev-BHCys-6XHis (residues from Met1 to Glu501) in pENTR as shown in Fig. 7A. These constructs of soluble integrin α5β1 contained nine putative N-glycosylation sites on each subunit. Cloning vectors for the N-glycosylation mutants of the soluble α5 and β1 subunits were prepared using overlap extension PCR. The wild type (WT) and mutants constructs were recombined with a lentivirus vector plasmid, CSII-EF-Rfa using LR clonase (30).
FIGURE 1.
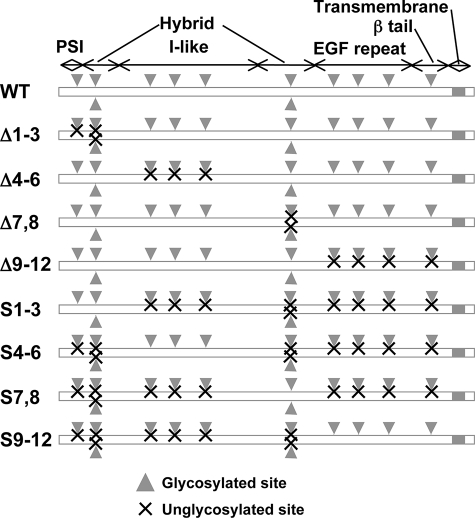
Schematic illustration of the potential N-glycosylation sites on the integrin β1 subunit. The sites corresponding to the putative N-glycosylation sites on the β1 subunit (Asn50, Asn94, Asn97, Asn212, Asn269, Asn363, Asn406, Asn417, Asn481, Asn520, Asn584, and Asn669) are shown by gray arrowheads. The crosses represent the removal of glycosylation at each N-glycan site by site-directed mutagenesis, i.e. Asn to Gln. EGF, epidermal growth factor.
FIGURE 7.
Characterization of WT and underglycosylated mutant of solubleα5β1 integrin. A, extracellular portions of α5 (1–644) and β1 (1–501) subunits (boldface) were fused to acid and base peptides, respectively, and the boxed seven amino acids indicate the recognition sequence for tobacco etch virus (TEV) protease as described (27). SDS-PAGE and immunoblot analyses of purified recombinant integrins are shown. The purified WT and underglycosylated mutant of the integrin α5β1, prepared as described under “Experimental Procedures,” were subjected to 6% SDS-PAGE for visualization using Coomassie Brilliant Blue R-250 (CBB) (B), immunoblotting with anti-α5 (4705S) (C), or β1 mAb (JB1A) (D) under reducing (left side) and nonreducing (right side) conditions. WB, Western blot. The brackets indicate the positions of WT and the α5S3-5/β1S4-6 underglycosylated mutant of the recombinant integrin. E, comparison of ligand binding abilities between WT and the α5S3-5/β1S4-6 underglycosylated mutant of the integrin. Equivalent amounts of the recombinant integrins were added to microtiter plates that had been coated with FN (5 μg/ml). The plates were then incubated for 3 h at 37 °C in the presence of 1 mm MnCl2 (gray bar) or 10 mm EDTA (open bar). The bound integrins were quantified using anti-α5 antibody (HA5) and alkaline phosphatase-conjugated streptavidin as described under “Experimental Procedures.”
Retrovirus Production and Transduction—The pBabe-Puro-based vectors described above were transfected into the Phoenix ecotropic retrovirus-producing cells using Lipofectamine 2000 (Invitrogen) for the production of viral supernatants. The retroviruses for CHO-B2 cells were produced by cotransfection of both the pBabe-puro-based vector and pLP/VSVG (Invitrogen) into Phoenix cells. The lentivirus vectors were cotransfected with pCAG-HIVgp and pCMV-VSV-G-RSV-Rev into 293T cells. Forty eight hours after transfection, retrovirus and lentivirus supernatants were filtered and then incubated with target cells in the presence of 10 μg/ml Polybrene (Sigma) at 32 °C overnight, respectively. Two days after infection, GE11 cells and CHO-B2 cells were selected using puromycin. Pooled stable cell lines were used in this study.
Immunoprecipitation and Western Blot—Cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in lysis buffer containing 20 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1% (w/v) Triton X-100, and Protease Inhibitor Mixture (Nacalai Tesque, Kyoto, Japan). The supernatants were collected, and protein concentrations were determined using the BCA protein assay kit (Pierce). Samples containing equal amounts of protein were incubated with 2 μg of each antibody and 15 μl of protein G-Sepharose™ 4 Fast Flow (GE Healthcare) for 2 h at 4 °C. The immunoprecipitates were washed three times with lysis buffer and subjected to 6% SDS-PAGE. After electrophoresis, the proteins were transferred to a nitrocellulose membrane (Whatman). The membrane was incubated with primary and secondary antibodies, each for 1 h; detection was performed using an ECL kit (GE Healthcare), according to the manufacturer's instructions.
Flow Cytometric Analysis—Flow cytometric analysis was performed as described previously (12, 22) with minor modifications. Briefly, semi-confluent cells were detached from the culture dishes using trypsin containing 1 mm EDTA and were subsequently stained with or without the primary mouse anti-β1 or rat anti-α5 antibody, followed by incubation with Alexa Fluor 488 goat anti-mouse IgG and Alexa Fluor 633 goat anti-rat IgG (Invitrogen), respectively. After washing three times with PBS, flow cytometric analysis was performed using an FACSCalibur flow cytometer and Cell Quest Pro software (BD Biosciences).
Metabolic Labeling—The pulse-chase experiments were performed as described previously with minor modifications (22). 5 × 105 cells on 6-well dishes were washed three times with PBS and then starved for 30 min in DMEM by excluding methionine and cysteine (Sigma). After starvation, the cells were pulse-labeled in 500 μl of DMEM containing 200 μCi of [35S]methionine and -cysteine (Express protein labeling mix, PerkinElmer Life Sciences) for 30 min and then chased with complete DMEM containing 10% FBS at the indicated times. The cells were lysed, and the cell lysates were immunoprecipitated with β1 subunit antibody (JB1A). The immunoprecipitates were separated on 6% SDS-PAGE. After drying the gels, radioactive bands were visualized with a Fuji BAS 5000 Bio-Image Analyzer.
Immunofluorescence Microscopy—Cells on the glass coverslips (Iwaki, Tokyo, Japan) were fixed with ice-cold methanol. The fixed cells were permeabilized by incubation in 0.05% Triton X-100 in PBS. After blocking with 3% BSA, cells were incubated with primary antibodies. Cells were visualized with Alexa 488- or Alexa 546-conjugated secondary antibody. Fluorescence images were observed by confocal microscopy using a FluoView FV1000 (Olympus, Tokyo, Japan).
Cell Adhesion and Spreading—Cell spreading assays were performed as described previously with minor modifications (12, 22). Briefly, 96-well flat bottom microtiter plates (Corning Glass, Corning, NY) were coated with a solution of 5 μg/ml purified human FN (Sigma) in 50 μl of PBS overnight at 4 °C and were subsequently blocked with 1% bovine serum albumin (BSA). The cells were detached with trypsin containing 1 mm EDTA, washed with FBS-containing DMEM, and then suspended in serum-free DMEM with 0.1% BSA at 1 × 104 cells/ml. After a 20-min incubation, representative fields were observed using a phase-contrast microscopy. Cell adhesion was monitored dynamically using real-time cell electronic sensing (RT-CES) apparatus (ACEA, San Diego) (31, 32). The cells were resuspended in serum-free DMEM with 0.1% BSA and then were seeded at 5 × 104 cells/ml in the electrode sensors (16X E-plate, ACEA), which had been coated with 10 μg/ml purified human FN and blocked with 1% BSA. Cell adhesion that occurred during the subsequent 3-h period was continually monitored by taking a measurement with the RT-CES apparatus operated with RT-CES SP Software every 5 min.
Expression and Purification of WT and Underglycosylated Mutant of the Recombinant Integrins—Recombinant integrins were produced using the CHO LEC 3.2.8.1 cells and were purified from cell culture supernatant as described previously with minor modifications (27, 28). Briefly, CHO LEC 3.2.8.1 cells were coinfected with the lentiviruses for the expression of both the soluble α5 and β1 subunits and were subsequently grown in serum-free ASF 104 media (Ajinomoto Co., Inc., Tokyo, Japan). The conditioned media were collected and clarified by centrifugation and then precipitated with a saturated solution of ammonium sulfate (80%). Concentrated culture media were dialyzed against Tris-buffered saline (TBS), and then passed through a gelatin-Sepharose column (GE Healthcare). The eluted fractions were subjected to affinity chromatography using nickel-nitrilotriacetic acid-agarose (Qiagen Inc., Valencia, CA). The columns were washed with Tris-buffered saline containing 1 mm CaCl2 and 1 mm MgCl2 (TBS(+)), and the bound proteins were eluted with TBS(+) containing 250 mm imidazole.
Integrin Binding Assay—The integrin binding assay was performed as described previously (12), with minor modifications. Briefly, 96-well flat bottom microtiter plates were coated with a solution of 5 μg/ml purified human FN (Sigma) in 50 ml of PBS overnight at 4 °C. Nonspecific binding was then blocked with 1% BSA for 1 h at room temperature. Integrins were added to the plates and allowed to bind for 3 h in the presence of either 1 mm MnCl2 or 10 mm EDTA at 37 °C. Next, the plates were washed with TBS containing 1 mm MnCl2, 0.1% BSA, and 0.02% Tween 20 or TBS containing 0.1% BSA, 0.02% Tween 20, and 10 mm EDTA, and the bound integrins were quantified using an enzyme-linked immunosorbent assay. The plates were incubated with the mouse anti-α5 antibody (HA; diluted 1:1000) for 30 min at room temperature in TBS containing 1 mm MnCl2, and then were washed three times followed by incubation with the biotinylated anti-mouse antibody (Vector Laboratories, Inc., Burlingame, CA). After three washes, the wells were incubated with avidin D-conjugated alkaline phosphatase (Vector Laboratories, Inc.). The bound antibodies were quantified by measurement of the absorbance at 402 nm after incubation with p-nitrophenyl phosphate (Calbiochem).
RESULTS
Construction of Various Integrin β1 Mutants by the Mutagenesis of Potential N-Glycosylation Sites—The human integrin β1 subunit contains 12 potential N-glycosylation sites, as shown in Fig. 1. Reportedly, 10 of these sites are normally N-glycosylated, whereas sites 2 and 11 do not normally carry N-linked glycans (33). However, which of the 12 N-glycosylation sites are important for biological function of the subunit remains unknown. Recently, we used a site-directed mutagenesis to determine that the N-glycans on the β-propeller domain of the α5 subunit are essential to α5β1 heterodimerization, cell surface expression, and biological function (22). In this study, we mutated the asparagine residues within the NX(S/T) glycosylation consensus sequence to glutamine in various domains of β1 subunit (Fig. 1) to glutamine residues. These mutated cDNAs were then introduced into integrin β1 subunit-deficient GE11 cells. The expression of the constructs was detected using Western blotting. We hypothesized that the N-glycosylation sites on the β1 subunit could also be divided into indispensable and dispensable sites, as observed previously for the α5 subunit. The underglycosylated mutant constructs of β1 integrin used in this study are listed in Fig. 1 as follows: Δ1-3, Δ4-6, Δ7,8, and Δ9-12. These constructs correspond to the following unglycosylated sites on the PSI and in the upstream region of the hybrid domains: the I-like domain, the downstream region of the hybrid domain, epidermal growth factor repeat, and β-tail domains of the β1 subunit, respectively.
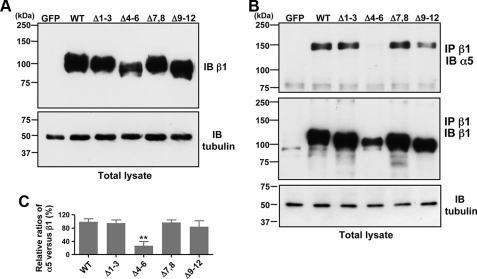
Effects of the Removal of N-Glycosylation Sites on the β1 Subunit on Cellular Expression, Heterodimer Formation, and Expression on Cell Surface—As described above, although N-glycosylation of integrin α5β1 is essential to its function (20, 21, 34), the distinct roles of N-glycosylation of each domain of the β1 subunit are largely unknown. To examine the effects of the underglycosylated mutations shown in Fig. 1 on cellular expression, heterodimer formation, and expression on the cell surface, we introduced these mutated cDNAs into GE11 cells. First, we compared the level of expression in total cell lysates among the mutants. As shown in Fig. 2A, the expression of Δ4-6 mutant was less than that of other mutants, as evaluated using the anti-β1 antibody (JB1A). Similar results were observed using another anti-β1 antibody, which recognizes the carboxyl-terminal epitope of the β1 subunit (data not shown). These results suggest that N-glycans on the I-like domain of β1 integrin are required for efficient expression of the β1 subunit. We verified equivalent amounts of proteins were loaded by blotting with the α-tubulin antibody (Fig. 2A, lower panel). It should be noted that the similar mobility on the SDS-PAGE between Δ1-3 and Δ7,8 mutants, or between Δ4-6 and Δ9-12 mutants, further supported the notion that site 2 and site 11 do not carry N-linked glycans as reported by Seales et al. (33). The integrin β1 subunit associates with multiple α subunits to form various heterodimers, which may stabilize its expression. To determine whether underglycosylated mutation in a specific domain of the β1 subunit affects integrin αβ heterodimerization, we performed Western blotting of immunoprecipitated samples prepared from GE11 cells expressing WT or an underglycosylated β1 subunit. Reportedly, the α5 subunit is the main constituent in β1 integrin-containing dimers in β1 subunit-rescued GE11 cells (35). Therefore, we evaluated α5β1 heterodimerization. In the cells expressing WT β1 integrin, the α5 subunit was clearly detected in the β1 immunocomplexes, which were immunoprecipitated with anti-β1 antibody (Fig. 2B). However, expression of the α5 subunit in the β1 immunocomplexes prepared from Δ4-6 was considerably less than that in cells expressing WT, Δ1-3, Δ7,8, or Δ9-12 mutations (Fig. 2, B and C). These results suggest that N-glycosylation on the I-like domain of β1 integrin is essential for efficient α/β heterodimer formation.
FIGURE 2.
Effects of underglycosylation onβ1 subunit expression and association of theβ1 subunit with the α subunit. A, total cell lysates from GFP-expressing cells (control), WT, and several underglycosylated mutants as indicated (Δ1-3, Δ4-6, and Δ9-12) were blotted with the anti-integrin β1 antibody (JB1A) and anti-α-tubulin antibody (DM1A) as a loading control. B, equivalent amounts of cell lysate were immunoprecipitated (IP) with the anti-β1 integrin (P5D2). The immunoprecipitates were then subjected to 6% SDS-PAGE and blotted (IB) with the anti-α5 (H-104) and anti-β1 integrin (JB1A) antibodies. Loading of equivalent amounts of protein in total cell lysates was verified using the anti α-tubulin antibody. C, quantitative data were expressed as the relative ratios of α5/β1 subunit in the anti-β1 immunoprecipitate. The optical densities of the α5 and β1 subunit bands were measured using ImageJ software. The ratio of α5 versus β1 of wild type was set at 100. Data were obtained from three independent experiments (mean ± S.D.). **, p < 0.01 according to Student's two-tailed t test.
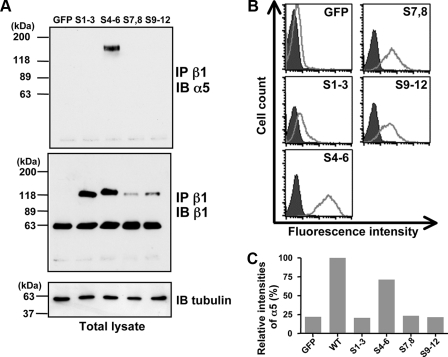
Of particular interest, the α5 subunit was only expressed in the β1 immunocomplexes of cells expressing the S4-6 mutation, which was devoid of all glycosylation sites, other than those in the I-like domain (Fig. 3A). Although the level of S1-3 expression was similar to that in the S4-6 mutant (Fig. 3A, middle panel), the α5/β1 heterocomplex was hardly detected in S1-3-expressing cells (Fig. 3A, upper panel). Furthermore, the level of cell-surface expression on these mutants was investigated using flow cytometric analysis. FACS analysis showed that the level of cell-surface expression by the S4-6 mutant was the highest among the removal mutants (Fig. 3B). Similarly, the level of cell-surface expression by the Δ4-6 mutant was considerably less than that of WT (43% of WT), whereas expression by the Δ1-3, Δ7,8, and Δ9-12 mutants were 91, 104, and 79% of WT, respectively. On the other hand, FACS analysis showed that the expression of α5 was clearly observed only in S4-6, but not in either S1-3, S7,8 or S9-12, as shown in Fig. 3C. Taken together, these results further support our hypothesis that N-glycosylation on the I-like domain is indispensable for both α/β dimerization and efficient expression on the cell surface.
FIGURE 3.
Comparison of cell-surface expression and association with the α subunit among the underglycosylated mutants. A, equivalent amounts of cells lysates were immunoprecipitated (IP) with the anti-β1 integrin (P5D2), and then the immunoprecipitates were subjected to 6% SDS-PAGE and blotted (IB) with the anti-α5 (H-104) or anti-β1 (JB1A) antibody. Loading of equivalent amounts of protein in the total cell lysate was verified using the anti α-tubulin antibody. B, expression of β1 integrin on the cell surface was examined using FACS analysis. Prior to analysis, the cells were incubated with anti-β1 antibody (P5D2), followed by incubation with Alexa Fluor 488 goat anti-mouse IgG as described under “Experimental Procedures.” Negative control staining (shaded histogram) was done without the first antibody. C, relative expression levels of α subunit on the cell surface. The cells were stained with rat anti-α5 antibody and followed by incubation with Alexa Fluor 633 goat anti-rat IgG. The expression levels of α5 subunit were expressed as relative fluorescence intensities examined using FACS analysis. The fluorescence intensity of wild type of α5 subunit was set at 100.
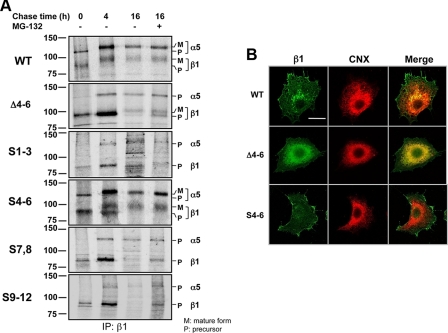
Effects of N-Glycosylation on I-like Domain of β1 on Post-translational Processing, Stability, and Cellular Localization—To elucidate the underlying mechanisms of impaired αβ heterodimeric formation and the decreased expression levels in the β1 subunits of underglycosylated mutants, such as Δ4-6, the biosynthetic kinetics of the α5β1 integrin in WT and in underglycosylated mutant transfectants were examined using a pulse-chase method. When chased at 0 h, the precursors for the β1 subunit were observed in all of these transfectants. However, the precursors for α5 were clearly detected only in the WT and S4-6 (Fig. 4A). It seems that there were no significant differences in total expression levels of the nascent β1 subunits among these transfectants (chased at 0 or 4 h). Interestingly, the contents of the mature forms of the β1 subunit were clearly observed, even when chased at 4 h, in the WT and S4-6 transfectants but obviously not in the other mutants. Coincidentally, the mature forms of the α5 subunit were also clearly observed only in the WT and S4-6 transfectants. On the other hand, the protein degradation rates were much faster in Δ4-6, S7,8, and S9-12, compared with that of the WT or the S4-6, as shown the chase data at 4 and 16 h, although it remains unclear why S1-3 was stable even in its precursor form. The degradation of β1 proteins might be partially mediated through a proteasome pathway as described previously (36), because treatment with MG-132, a proteasome inhibitor, increased expression levels of β1 in all mutants, with the noted exception of the S1-3 mutant (Fig. 4A).
FIGURE 4.
Effects of N-glycosylation on I-like domain of β1 on post-translational processing, stability, and cellular localization. A, after metabolic labeling with [35S]methionine and -cysteine for 30 min, cells were washed with fresh medium with or without MG-132, a proteasome inhibitor, in a final concentration at 8 μm, and then chased at the indicated times. The cells were lysed, and the same amounts of cell lysate were immunoprecipitated (IP) with anti-β1 (JB1A) antibody at the indicated times. The visualizations were performed as described under “Experimental Procedures.” M and P indicate the migrated position of mature and precursor form of α5 and β1 subunit, respectively. B, cells were stained with mouse antibody against the β1 subunit (JB1A) and rabbit antibody against calnexin (CNX) (SPA-860), followed by visualization with goat antibodies against mouse IgG-conjugated Alexa 488 and rabbit IgG-conjugate Alexa 546, respectively. The bar denotes 20 μm.
Next, we examined the localization of WT, S4-6, and Δ4-6 of β1 by immunostaining. Interestingly, both the WT and the S4-6 of the β1 subunit were expressed mainly on the cell surface as usual, whereas the Δ4-6 accumulated mainly in the endoplasmic reticulum colocalized with calnexin (Fig. 4B). These results partly support the notion that β1 can be associated with a de novo synthesized α subunit, otherwise the excess of the non-complexed β1 would either be degraded immediately or would remain in the endoplasmic reticulum (36, 37). On the other hand, based on results shown in Fig. 3C and Fig. 4B, without association with α5, the β1 subunit appeared to be marginally expressed on the cell surface in these underglycosylated mutants. In fact, Meng et al. (38) reported the existence of a monomer β1 subunit on the cell surface detected by a specific anti-β1 monomer antibody. Therefore, the expression levels of integrin on the cell surface may not always match total expression levels, because these levels could be influenced by several factors, such as α/β assembly, maturation, and degradation, as described above.
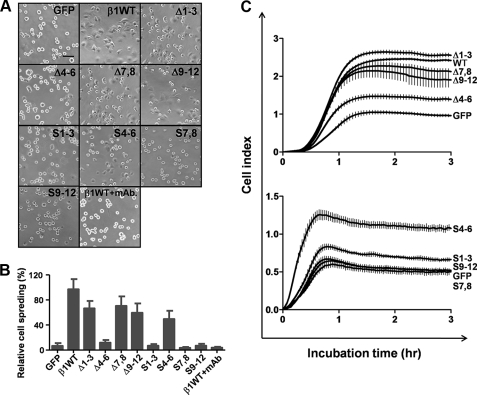
Effects of the Removal of N-Glycosylation Sites on the Integrin β1 on FN-mediated Cell Spreading—Integrin-mediated biological functions, such as cell spreading and cell migration, can be modulated by aberrant changes in the N-glycosylation of integrins. In a study, we reported that N-glycosylation of the β-propeller, but not the other domains, of the integrin α5 subunit is essential to integrin-mediated biological functions. In this study, we compared cell spreading among the N-glycosylation mutants. Cell spreading was assayed within 20 min after replating the cells on FN-coated dishes. As expected, overexpression of the WT β1 subunit gene, but not the GFP control, largely rescued cell spreading on FN (Fig. 5, A and B), providing GE11 cells are a useful cell model for studies of integrin β1 function. Cell spreading was completely inhibited by an anti-β1 function-blocking antibody (P5D2) but not by normal IgG. This result indicates that the initial cell spreading on FN is mediated mainly by integrin α5β1. On the other hand, overexpression of the underglycosylated mutant, Δ4-6, did not result in cell spreading at the same time point. In contrast, overexpression of the Δ1-3, Δ7,8, and Δ9-12 mutants induced cell spreading on FN-coated dishes, as did the overexpression of the WT gene. Interestingly, cells expressing the S4-6, but not the S1-3, S7,8, or S9-12 mutant, exhibited significant cell spreading. Furthermore, a cell adhesion kinetics assay was performed using the RT-CES system (Fig. 5C). Cell adhesion kinetics, evaluated using RT-CES, was greatly suppressed in the Δ4-6 mutant compared with other mutants, such as Δ1-3 and Δ7,8 mutants (Fig. 5C, upper panel). On the other hand, overexpression of the S4-6 mutant significantly increased cell adhesion. Cell adhesion was not enhanced by overexpression of S1-3, S7,8, or S9-12 (Fig. 5C, lower panel). Collectively, these results strongly suggest that the N-glycosylation of the I-like domain of the β1 subunit is essential to biological function of the subunit.
FIGURE 5.
Comparison of cell spreading on FN among various underglycosylated mutants of the β1 subunit. A, cells were detached and replated on culture dishes pre-coated with 5 μg/ml FN. After incubation for 20 min, the cells were fixed with 3.7% paraformaldehyde, and representative fields were photographed using a 200-fold phase contrast microscope. The bar denotes 200 μm. B, quantification of cell spreading on FN expressed as the means ± S.D. of three independent experiments. C, cell adhesion kinetics assay using RT-CES system as described under “Experimental Procedures.” Cell index means extent of cell adhesion. The bars show standard deviation.
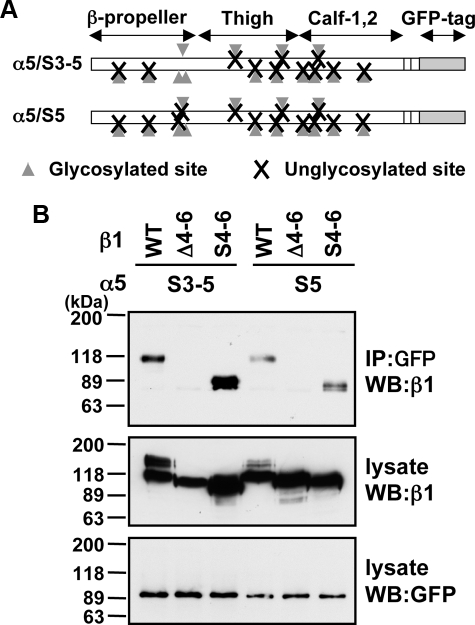
S4-6 Mutant of β1 Can Form Dimers with the Minimally N-Glycosylated Mutants of the α5 Subunit—N-Glycosylation is essential to integrin α5β1 heterodimer formation, and, as such, it plays an important role in the biological function of the integrin. Integrin α5β1 modified by GnT-III demonstrated reduced cell adhesion and cell migration on FN (12). Unlike GnT-III, GnT-V specifically modified the β1 subunit, not the α5 subunit, and up-regulated integrin α5β1-mediated cell migration (9). Recently, we found that three N-glycosylation sites on the α5 subunit, i.e. α5S3-5, were essential to its biological functions, such as cell adhesion and cell migration on FN, and heterodimerization. Furthermore, we also found that the α5 subunit N-glycosylation site, α5S5, is the most important site for cell-surface expression, although this site has no effect on biological function (22). To examine whether the S4-6 mutant of the β1 subunit could form heterodimer with the α5 mutants shown in Fig. 6A, we introduced either WT or the β1 mutants into CHO-B2 cells expressing N-glycosylation mutants of the α5 subunit. As expected, the WT β1 subunit efficiently formed heterodimer with S3-5 of the α5 subunit, as reported previously. Of particular interest, the S4-6, but not the Δ4-6 mutant, associated with S3-5 of the α5 subunit (Fig. 6B). Furthermore, both WT and S4-6 formed heterodimer with the S5 mutant, which has only one N-glycosylation site on the α5 subunit. These mutants in cell-surface expression were confirmed by FACS analysis (data not shown). These results, taken together, suggest that N-glycosylation on both the β-propeller of the α5 subunit and I-like domain of the β1 subunit is the minimal requirement for αβ dimer formation.
FIGURE 6.
Minimal N-glycosylation requirement for α5β1 dimer formation. A, schematic illustration of two underglycosylation mutants of the α5 subunit containing a GFP tag. The sites corresponding to putative N-glycosylation sites (Asn84, Asn182, Asn297, Asn307, Asn316, Asn524, Asn530, Asn593, Asn609, Asn675, Asn712, Asn724, Asn773, and Asn868) on the α5 subunit are shown by the gray arrowheads. The crosses represent the removal of glycosylation at each N-glycan site by site-directed mutagenesis. α5S3-5 and α5S5 indicate that the removal of all sites other than the indicated sites, i.e. Asn297/Asn307/Asn316, and Asn316, respectively. IP, immunoprecipitation; WB, Western blot. B, cells expressing the indicated N-glycosylation mutants of α5 (α5S3-5 and α5S5) were infected with viruses containing either WT or N-glycosylation mutants (Δ4-6 and S4-6) of the β1 subunit as described under “Experimental Procedures.” The cell lysates were immunoprecipitated with anti-GFP antibody, and the immunocomplexes were subjected to SDS-PAGE and blotted with anti-β1 subunit (JB1A) (upper panel). The total cell lysates were blotted using an anti-β1 subunit antibody (JB1A) (middle panel) and anti-GFP antibody (lower panel).
Purification and Characterization of Recombinant Underglycosylated Mutant of the Integrin—Because cells deficient in both the α5 and the β1 subunits are not currently available, we constructed secret expression vectors to avoid interference with endogenous expression of α5 or β1 subunit. In addition, to minimize the influence of complex N-glycan structures on integrin function, we introduced the expression vectors into CHO LEC 3.2.8 cells, which do not expression GnT-I, because GnT-I catalyzes the GlcNAc to the terminal α-1,3-linked Man in Man5GlcNAc2Asn to initiate the synthesis of hybrid and complex N-glycans in multicellular organisms (39). The WT and α5S3-4/β1S4-6 mutant integrins were purified from conditioned media using nickel-nitrilotriacetic acid affinity chromatography. As expected, the purified integrins gave a single homogeneous band under nonreducing conditions, although two bands were observed in the presence of 2-mercaptoethanol (Fig. 7A). These results suggest that the α and β subunits were covalently linked through a disulfide bond, because α and β subunits each contain a carboxyl-terminal cysteine as described (27). These purified integrins were also confirmed by immunoblot analysis with antibodies against the α5 (Fig. 7B) and β1 subunit (Fig. 7C) under both nonreducing and reducing conditions, respectively. Furthermore, we performed a solid-phase binding assay between the recombinant integrins and FN. The equivalent amounts of the recombinant integrins were added to FN-coated microtiter plates. Interestingly, the binding capacity of the α5S3-4/β1S4-6 underglycosylated mutant was comparable with that of WT, suggesting that N-glycosylations of both the β-propeller of the α5 subunit and the I-like domain of the β1 subunit are important for biological function, such as ligand binding. The binding specificity between α5β1 integrin and FN was confirmed by treatment with 10 mm EDTA, which completely blocked integrin-FN interactions. These results strongly suggest that N-glycosylation of a specific domain is important for both dimer formation and functional expression.
DISCUSSION
This study examined the effects of underglycosylation of the β1 subunit. We determined that only the N-glycan on the I-like domain of the β1 subunit is essential to its biological functions, such as β1 integrin-mediated initial cell spreading and dimer formation. Furthermore, we proposed that the α5S3-5/β1S4-6 mutant presents the minimal N-glycosylation requirement for functional α5β1 integrin expression. In fact, the putative N-glycosylation sites on the I-like domain are completely conserved in human, mouse, rat, Xenopus, and chicken. Recently, Liu et al. (40), using a molecular dynamic modeling approach, determined that alteration in the glycosylation of the I-like domain of β1 might affect interactions between oligosaccharides and the I-like domain, which in turn alters the accessibility of the specificity-determining loop to ligands. In addition, we found that N-glycosylation site 4 on the β-propeller of the α5 subunit was specifically modified by GnT-III, thereby regulating α5β1-mediated cell spreading and cell migration.3 These observations suggest that site-specific modulation of N-glycans on integrin affects its biological functions. Therefore, a detailed mutagenesis study of N-glycans on integrins is indispensable for the elucidation of the complicated mechanisms that are involved in its functional regulation by glycosyltransferases, such as GnT-III, α2,6-sialyltransferase, and GnT-V (9, 12, 13, 41).
It is well known that integrin-mediated cell adhesion functions cooperatively with growth factor receptors to control cell proliferation, differentiation, survival, and migration of epithelial cells and fibroblasts (42). The association of integrins with growth factor receptors has been demonstrated in coclustering and coprecipitation studies (43, 44). In fact, integrins enable growth factor signaling in many cases, as normal growth factor signaling does not occur unless cells are adhered to the extracellular matrix or to other cells through integrins. Hakomori and co-workers (45) reported that the formation of integrin-tetraspanin complexes was affected by the N-glycosylation of both integrin and tetraspanins, as well as by gangliosides in the microdomain. For example, CD82 with complete N-glycosylation demonstrated a reduction in its association with α3 or α5 integrin, whereas CD82 with incomplete N-glycosylation exhibited enhanced association (46). Conversely, the association of CD9 with either the α3 or α5 integrin subunits was not influenced by N-glycosylation, as CD9 contains no N-glycosylation sites. Based on these observations, we postulate that N-glycosylation of integrin may participate in supramolecular complex formation on the cell surface, which controls intracellular signal transduction. Recently, we demonstrated, using biochemical visualization method and antibody array, that many receptor tyrosine kinases, such as epidermal growth factor receptor, formed clusters with β1 integrin in living cells (47). We believe that these underglycosylated mutants of the β1 and α5 subunits will prove very useful in clarification of the molecular mechanisms of supramolecular complex formation proximal to the cell membrane, which in turn might both positively and negatively regulate cellular biological functions by modulation of N-glycans.
Based on both previous studies (21, 22) and the results of the present study, it is clear that N-glycosylation is essential to α5β1 heterodimer formation. However, this may not always be the case. Purified α5β1 integrin, but not αLβ2, treated with N-glycosidase F blocked the inherent association of both subunits. In addition, the specificity-determining loop within the I-like domain of the β2 subunit, an essential conserved region required for αβ dimer formation, does not contain potential N-glycosylation sites (48, 49), indicating N-glycosylation may not participate in β2-containing integrin assembly. In contrast, the specificity-determining loop segment is not required for formation of α5β1, suggesting that use of subunit interface residues is variable among integrins (48). On the other hand, both the binding and killing of target cells by cytotoxins, such as CyaA, LtxA, and HlyA, depended on recognition of the N-glycans on β2 integrin (50).
In the crystal structure of integrin αVβ3, the main contact between the αV and β3 subunit is the β-propeller on αV and the I-like domain of β3 with hydrophobic, ionic, and mixed type interactions (29, 51). Mould et al. (52) determined the structure of α5β1 using homology modeling. Based on the model, the interfaces of the α5β1 dimer are surrounded by N-glycans. Furthermore, based on homology modeling of the I-like domain of the β1 subunit, the interfaces seem to be influenced by the surrounding N-glycans (data not shown). In fact, artificial introduction of an N-glycan edge at the dimer interface prevented the association of the two subunits in the case of the GABA receptor, which is a G-protein-coupled receptor. This result suggests that N-glycans act at the dimer interface (53). These studies strongly support the notion that the structural environment of the αβ interface can be affected by the presence of N-glycans. Currently, the atomic resolution structure of integrin α5β1 has not been determined for the following reasons: only small amounts of purified protein are available; the large size of the molecule; the conformational flexibility; and the presence of both transmembrane domains and heavily N-glycosylated regions in both subunits. Therefore, we believe that integrin mutants, such as α5S3-5 or α5S5 and β1S4-6, might greatly facilitate future studies of the crystal structure.
Acknowledgments
We thank Dr. Arnoud Sonnenberg (Division of Cell Biology, Netherlands Cancer Institute, Amsterdam, Netherlands), Dr. Pamela Stanly (Albert Einstein College of Medicine, New York), Dr. Hiroyuki Miyoshi (BioResource Center, RIKEN, Japan), and Wako Pure Chemical Industries, Ltd. (Osaka, Japan) for providing the GE11 cells, CHO Lec 3.2.8.1 cells, lentivirus vectors, and RT-CES apparatus, respectively. We also thank Dr. Junichi Takagi (Institute for Protein Research, Osaka University, Japan) for providing cDNA of the soluble integrin α5β1 and for helpful discussions.
This work was supported in part by the Core Research for Evolutional Science and Technology, the Japan Science and Technology Agency, the “Academic Frontier” Project for Private Universities from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the core to core program and a grant-in-aid for young scientists (B) (to T. I.), Japan Society for the Promotion of Science, and Takeda Science Foundation, Japan.
Footnotes
The abbreviations used are: GnT, N-acetylglucosaminyltransferase; FN, fibronectin; CHO, Chinese hamster ovary; mAb, monoclonal antibody; GFP, green fluorescence protein; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; PBS, phosphate-buffered saline; BSA, bovine serum albumin; WT, wild type; mAb, monoclonal antibody; FACS, fluorescence-activated cell sorter; TBS, Tris-buffered saline.
Sato, Y., Isaji, T., Tajiri, M., Yoshida-Yamamoto, S., Yoshinaka, T., Somehara, T., Fukuda, T., Wada, Y., and Gu, J. (March 9, 2009) J. Biol. Chem. 10.1074/jbc.M807660200.
References
- 1.Hynes, R. O. (2002) Cell 110 673-687 [DOI] [PubMed] [Google Scholar]
- 2.Giancotti, F. G., and Ruoslahti, E. (1999) Science 285 1028-1032 [DOI] [PubMed] [Google Scholar]
- 3.Arnaout, M. A. (1990) Immunol. Rev. 114 145-180 [DOI] [PubMed] [Google Scholar]
- 4.Takagi, J. (2007) Curr. Opin. Cell Biol. 19 557-564 [DOI] [PubMed] [Google Scholar]
- 5.Arnaout, M. A., Goodman, S. L., and Xiong, J. P. (2007) Curr. Opin. Cell Biol. 19 495-507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao, Y., Sato, Y., Isaji, T., Fukuda, T., Matsumoto, A., Miyoshi, E., Gu, J., and Taniguchi, N. (2008) FEBS J. 275 1939-1948 [DOI] [PubMed] [Google Scholar]
- 7.Zhao, Y. Y., Takahashi, M., Gu, J. G., Miyoshi, E., Matsumoto, A., Kitazume, S., and Taniguchi, N. (2008) Cancer Sci. 99 1304-1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellis, S. L. (2004) Biochim. Biophys. Acta 1663 52-60 [DOI] [PubMed] [Google Scholar]
- 9.Guo, H. B., Lee, I., Kamar, M., Akiyama, S. K., and Pierce, M. (2002) Cancer Res. 62 6837-6845 [PubMed] [Google Scholar]
- 10.Seales, E. C., Jurado, G. A., Brunson, B. A., Wakefield, J. K., Frost, A. R., and Bellis, S. L. (2005) Cancer Res. 65 4645-4652 [DOI] [PubMed] [Google Scholar]
- 11.Seales, E. C., Jurado, G. A., Singhal, A., and Bellis, S. L. (2003) Oncogene 22 7137-7145 [DOI] [PubMed] [Google Scholar]
- 12.Isaji, T., Gu, J., Nishiuchi, R., Zhao, Y., Takahashi, M., Miyoshi, E., Honke, K., Sekiguchi, K., and Taniguchi, N. (2004) J. Biol. Chem. 279 19747-19754 [DOI] [PubMed] [Google Scholar]
- 13.Zhao, Y., Nakagawa, T., Itoh, S., Inamori, K., Isaji, T., Kariya, Y., Kondo, A., Miyoshi, E., Miyazaki, K., Kawasaki, N., Taniguchi, N., and Gu, J. (2006) J. Biol. Chem. 281 32122-32130 [DOI] [PubMed] [Google Scholar]
- 14.Gu, J., Zhao, Y., Isaji, T., Shibukawa, Y., Ihara, H., Takahashi, M., Ikeda, Y., Miyoshi, E., Honke, K., and Taniguchi, N. (2004) Glycobiology 14 177-186 [DOI] [PubMed] [Google Scholar]
- 15.Goetz, J. G., Joshi, B., Lajoie, P., Strugnell, S. S., Scudamore, T., Kojic, L. D., and Nabi, I. R. (2008) J. Cell Biol. 180 1261-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preissner, K. T., Kanse, S. M., and May, A. E. (2000) Curr. Opin. Cell Biol. 12 621-628 [DOI] [PubMed] [Google Scholar]
- 17.Berditchevski, F. (2001) J. Cell Sci. 114 4143-4151 [DOI] [PubMed] [Google Scholar]
- 18.Todeschini, A. R., Dos Santos, J. N., Handa, K., and Hakomori, S. I. (2007) J. Biol. Chem. 282 8123-8133 [DOI] [PubMed] [Google Scholar]
- 19.Hemler, M. E. (2003) Annu. Rev. Cell Dev. Biol. 19 397-422 [DOI] [PubMed] [Google Scholar]
- 20.Chammas, R., Veiga, S. S., Line, S., Potocnjak, P., and Brentani, R. R. (1991) J. Biol. Chem. 266 3349-3355 [PubMed] [Google Scholar]
- 21.Zheng, M., Fang, H., and Hakomori, S. (1994) J. Biol. Chem. 269 12325-12331 [PubMed] [Google Scholar]
- 22.Isaji, T., Sato, Y., Zhao, Y., Miyoshi, E., Wada, Y., Taniguchi, N., and Gu, J. (2006) J. Biol. Chem. 281 33258-33267 [DOI] [PubMed] [Google Scholar]
- 23.Gimond, C., van Der Flier, A., van Delft, S., Brakebusch, C., Kuikman, I., Collard, J. G., Fassler, R., and Sonnenberg, A. (1999) J. Cell Biol. 147 1325-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanley, P. (1989) Mol. Cell. Biol. 9 377-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgenstern, J. P., and Land, H. (1990) Nucleic Acids Res. 18 3587-3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coe, A. P., Askari, J. A., Kline, A. D., Robinson, M. K., Kirby, H., Stephens, P. E., and Humphries, M. J. (2001) J. Biol. Chem. 276 35854-35866 [DOI] [PubMed] [Google Scholar]
- 27.Takagi, J., Erickson, H. P., and Springer, T. A. (2001) Nat. Struct. Biol. 8 412-416 [DOI] [PubMed] [Google Scholar]
- 28.Takagi, J., Petre, B. M., Walz, T., and Springer, T. A. (2002) Cell 110 599-611 [DOI] [PubMed] [Google Scholar]
- 29.Xiong, J. P., Stehle, T., Diefenbach, B., Zhang, R., Dunker, R., Scott, D. L., Joachimiak, A., Goodman, S. L., and Arnaout, M. A. (2001) Science 294 339-345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyoshi, H. (2004) Methods Mol. Biol. 246 429-438 [DOI] [PubMed] [Google Scholar]
- 31.Atienza, J. M., Zhu, J., Wang, X., Xu, X., and Abassi, Y. (2005) J. Biomol. Screen. 10 795-805 [DOI] [PubMed] [Google Scholar]
- 32.Solly, K., Wang, X., Xu, X., Strulovici, B., and Zheng, W. (2004) Assay Drug Dev. Technol. 2 363-372 [DOI] [PubMed] [Google Scholar]
- 33.Seales, E. C., Shaikh, F. M., Woodard-Grice, A. V., Aggarwal, P., McBrayer, A. C., Hennessy, K. M., and Bellis, S. L. (2005) J. Biol. Chem. 280 37610-37615 [DOI] [PubMed] [Google Scholar]
- 34.Gipson, I. K., Kiorpes, T. C., and Brennan, S. J. (1984) Dev. Biol. 101 212-220 [DOI] [PubMed] [Google Scholar]
- 35.Danen, E. H., Sonneveld, P., Brakebusch, C., Fassler, R., and Sonnenberg, A. (2002) J. Cell Biol. 159 1071-1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida, Y., Chiba, T., Tokunaga, F., Kawasaki, H., Iwai, K., Suzuki, T., Ito, Y., Matsuoka, K., Yoshida, M., Tanaka, K., and Tai, T. (2002) Nature 418 438-442 [DOI] [PubMed] [Google Scholar]
- 37.Heino, J., Ignotz, R. A., Hemler, M. E., Crouse, C., and Massague, J. (1989) J. Biol. Chem. 264 380-388 [PubMed] [Google Scholar]
- 38.Meng, X., Cheng, K., Krohkin, O., Mould, A. P., Humphries, M. J., Ens, W., Standing, K., and Wilkins, J. A. (2005) J. Cell Sci. 118 4009-4016 [DOI] [PubMed] [Google Scholar]
- 39.Kornfeld, R., and Kornfeld, S. (1985) Annu. Rev. Biochem. 54 631-664 [DOI] [PubMed] [Google Scholar]
- 40.Liu, Y., Pan, D., Bellis, S. L., and Song, Y. (2008) Proteins 73 989-1000 [DOI] [PubMed] [Google Scholar]
- 41.Semel, A. C., Seales, E. C., Singhal, A., Eklund, E. A., Colley, K. J., and Bellis, S. L. (2002) J. Biol. Chem. 277 32830-32836 [DOI] [PubMed] [Google Scholar]
- 42.Schwartz, M. A., and Ginsberg, M. H. (2002) Nat. Cell Biol. 4 E65-E68 [DOI] [PubMed] [Google Scholar]
- 43.Miyamoto, S., Teramoto, H., Gutkind, J. S., and Yamada, K. M. (1996) J. Cell Biol. 135 1633-1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sundberg, C., and Rubin, K. (1996) J. Cell Biol. 132 741-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawakami, Y., Kawakami, K., Steelant, W. F., Ono, M., Baek, R. C., Handa, K., Withers, D. A., and Hakomori, S. (2002) J. Biol. Chem. 277 34349-34358 [DOI] [PubMed] [Google Scholar]
- 46.Ono, M., Handa, K., Withers, D. A., and Hakomori, S. (2000) Biochem. Biophys. Res. Commun. 279 744-750 [DOI] [PubMed] [Google Scholar]
- 47.Kotani, N., Gu, J., Isaji, T., Udaka, K., Taniguchi, N., and Honke, K. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 7405-7409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takagi, J., DeBottis, D. P., Erickson, H. P., and Springer, T. A. (2002) Biochemistry 41 4339-4347 [DOI] [PubMed] [Google Scholar]
- 49.Huang, C., Lu, C., and Springer, T. A. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 3156-3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morova, J., Osicka, R., Masin, J., and Sebo, P. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 5355-5360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiong, J. P., Li, R., Essafi, M., Stehle, T., and Arnaout, M. A. (2000) J. Biol. Chem. 275 38762-38767 [DOI] [PubMed] [Google Scholar]
- 52.Mould, A. P., Symonds, E. J., Buckley, P. A., Grossmann, J. G., McEwan, P. A., Barton, S. J., Askari, J. A., Craig, S. E., Bella, J., and Humphries, M. J. (2003) J. Biol. Chem. 278 39993-39999 [DOI] [PubMed] [Google Scholar]
- 53.Rondard, P., Huang, S., Monnier, C., Tu, H., Blanchard, B., Oueslati, N., Malhaire, F., Li, Y., Trinquet, E., Labesse, G., Pin, J. P., and Liu, J. (2008) EMBO J. 27 1321-1332 [DOI] [PMC free article] [PubMed] [Google Scholar]