Abstract
The Src homology phosphotyrosyl phosphatase 2 (SHP2) plays a positive role in HER2-induced signaling and transformation, but its mechanism of action is poorly understood. Given the significance of HER2 in breast cancer, defining a mechanism for SHP2 in the HER2 signaling pathway is of paramount importance. In the current report we show that SHP2 positively modulates the Ras-extracellular signal-regulated kinase 1 and 2 and the phospoinositide-3-kinase-Akt pathways downstream of HER2 by increasing the half-life the activated form of Ras. This is accomplished by dephosphorylating an autophosphorylation site on HER2 that serves as a docking platform for the SH2 domains of the Ras GTPase-activating protein (RasGAP). The net effect is an increase in the intensity and duration of GTP-Ras levels with the overall impact of enhanced HER2 signaling and cell transformation. In conformity to these findings, the HER2 mutant that lacks the SHP2 target site exhibits an enhanced signaling and cell transformation potential. Therefore, SHP2 promotes HER2-induced signaling and transformation at least in part by dephosphorylating a negative regulatory autophosphorylation site. These results suggest that SHP2 might serve as a therapeutic target against breast cancer and other cancers characterized by HER2 overexpression.
The Src homology phosphotyrosyl phosphatase 2 (SHP2)2 functions as a positive effector of cell growth and survival (1–4), migration and invasion (5–8), and morphogenesis and transformation (9–11). In receptor-tyrosine kinase signaling (12–14), SHP2 positively transduces the Ras-extracellular signal-regulated kinase 1 and 2 (ERK1/2) and the phosphoinositide-3-kinase-Akt (or protein kinase B) signaling pathways. SHP2 also promotes cell transformation induced by the constitutively active form of fibroblast growth factor receptor 3 and v-Src (9, 11). The discovery of germline-activating SHP2 mutations in Noonan and LEOPARD syndrome patients (15–18) and the subsequent experimental demonstration of these phenotypes in knockin and transgenic mice expressing these mutants (19, 20) has led to the conclusion that disregulation of SHP2 is responsible for these disease states. Furthermore, somatic activating SHP2 mutations were discovered in juvenile myelomonocytic leukemia, acute myelogenous leukemia, and chronic myelomonocytic (18, 21) and are suggested to play a causative role.
SHP2 possesses two Src homology 2 (SH2) domains in the N-terminal region that allow the protein to localize to substrate microdomains after tyrosyl phosphorylation of interacting proteins. The phosphotyrosyl phosphatase (PTP) domain in the C-terminal region is responsible for dephosphorylation of target substrates (13, 22). Mutation of the critical Cys residue in the active site of SHP2 abolishes its phosphatase activity, leading to the production of a dominant-negative protein (23). The activity of SHP2 is regulated by an intramolecular conformational switch. SHP2 assumes a “closed conformation” when inactive and an “open conformation” when active. In the closed conformation the N-SH2 domain interacts with the PTP domain, physically impeding the activity of the enzyme. Upon engagement of the SH2 domains with phosphotyrosine, the PTP domain is relieved of autoinhibition and dephosphorylates target substrates (23–26). Interaction between specific residues on the N-SH2 and the PTP domains mediates the closed conformation. Mutation of these residues leads to a constitutively active SHP2, and the occurrence of such mutations in humans causes the development of Noonan syndrome and associated leukemia (16–18).
Recently, we have shown that inhibition of SHP2 in the HER2-positive breast cancer cell lines abolishes mitogenic and cell survival signaling and reverses transformation, leading to differentiation of malignant cells into a normal breast epithelial phenotype (27). Given the significance of HER2 in breast cancer, the finding that SHP2 plays a positive role was very interesting. We, thus, sought to investigate the molecular mechanism that underlies the positive role of SHP2 in HER2-induced signaling and transformation. To do so, it was first necessary to decipher the identity of SHP2 substrates whose dephosphorylation promotes the oncogenic functions of HER2. Using the recently developed substrate-trapping mutant of SHP2 as a reagent (28), we have identified HER2 itself as an SHP2 substrate. We have further shown that SHP2 dephosphorylates an autophosphorylation site on HER2 that serves as a docking site for the SH2 domains of the Ras GTPase-activating protein (Ras-GAP), the down-regulator of Ras. This effect of SHP2 increases the intensity and duration of GTP-Ras levels with the overall impact of enhanced HER2 signaling and cell transformation.
MATERIALS AND METHODS
Cells, Cell Culture, and Reagents—The cells used in this study were the BT474 breast cancer cell line, the mouse embryo fibroblast (MEF) cell line, and MCF-10A, the immortalized breast epithelial cell line, which were all purchased from American Tissue Culture Collection (ATCC). The BT474 and the MEF cells were grown in RPMI 1640 and Dulbecco's modified Eagle's medium, respectively, both supplemented with 10% fetal calf serum. The MCF-10A were grown in Dulbecco's modified Eagle's medium supplemented with 10 μg/ml recombinant human insulin, 20 ng/ml EGF (PeproTech), 0.5 μg/ml hydrocortisone, 100 ng/ml cholera toxin (Sigma), and 5% horse serum as described previously (29). All cell types were maintained at 37 °C with 5% CO2. The anti-HER2, the anti-PTP1D (SHP2), and the anti-Ras monoclonal antibodies were purchased from Pharmingen, the anti-β-actin and the anti-FLAG-tag monoclonal antibodies were from Sigma, the anti-EGFR antibody was a kind gift from Dr. Mike Hayman (Stony Brook University), and the anti-phospho-ERK1/2 and the anti-phospho-Akt antibodies were from Cell Signaling. Horseradish peroxidase-conjugated secondary antibodies were from Amersham Biosciences, and Alexa fluor 488 and rhodamine-labeled secondary antibodies were from Molecular Probes.
Plasmid Construction and Site-directed Mutagenesis—Development of the SHP2 substrate-trapping mutant (D425A/C459S-SHP2, termed double mutant (DM)-SHP2) and the corresponding GST fusion forms were reported previously (10, 28). In this study the full-length wild type SHP2 (WT-SHP2) and DM-SHP2, FLAG-tagged at the C terminus, were subcloned into REBNA/IRES/GFP retroviral vector using the same strategy described previously (9). The HER2 cDNA in the pCMV-SPORT6 plasmid was purchased from ATCC. The Tyr-1023 to Phe mutation was introduced using the Stratagene site-directed mutagenesis kit and protocol with the following complementary primers; the sense primer was 5′-GGGACCTGGTGGATGCTGAGGAGTTTCTGGTACCCCAGCAGGGCTTCTTCTG-3′, and the antisense primer was 5′-CAGAAGAAGCCCTGCTGGGGTACCAGAAACTCCTCAGCATCCACCAGGTCCC-3′. For expression in the MEF and MCF-10A cells, both the wild type and the mutant HER2 cDNA were Myc-tagged at the C terminus and subcloned into the same retroviral vector as in SHP2 at XhoI and NotI sites with the forward primer, 5′-CTCGAGCGCCACCATGCGACCCTCCGGGAC-3′, and the Myc-tagged reverse primer, 5′-GCGGCCGCTCACAGATCCTCTTCAGAGATGAGTTTCTGCTCCATTGCTCCAATAAATTACTGCTTTG-3′.
Cell Transfection, Retrovirus Production, and Production of Stable Cell Lines—We used the FuGENE 6 reagent (Roche Applied Science) for transfection of the SHP2 and HER2 retroviral constructs into appropriate packaging cells. The BT474, MEF, and MCF-10A cell lines stably expressing the SHP2 and/or the HER2 proteins were produced by infection with respective retroviruses in the presence of 1 μg/ml Polybrene (Sigma). After incubation in growth medium for ∼48 h, cells were treated with 1 μg/ml Blasticidine, the selection antibiotic expressed by the viral vector, to remove non-expressing cells. Blasticidin-resistant cell populations were used for the various experiments described in the relevant sections.
Preparation of Cell Lysates and Immunoblotting Analysis—All cell lysates were prepared in a buffer containing 20 mm Tris-HCl, pH 7.4, 150 mm NaCl, 50 mm NaF, 1 mm EDTA, 10% glycerol, 1% Triton-X-100, 1 mm sodium orthovanadate, and a protease inhibitor mixture. Lysates were briefly sonicated to break chromosomal DNA that could interfere with polyacrylamide gel electrophoresis separation of proteins. After clearing by centrifugation at 12,000 rpm for 10 min, the lysates were analyzed as described in the respective experiments. For electrophoresis gel separation of proteins, samples were denatured by boiling with Laemmli sample buffer and run on an 8% denaturing polyacrylamide gel. After transfer onto a nitrocellulose membrane and blocking with 3% bovine serum albumin, the membranes were stained with primary antibodies overnight at 4 °C, washed 3 times with Tris-buffered saline containing 1% Triton-X-100, stained with secondary antibodies for 1 h at room temperature, and detected using the chemiluminescence method.
Substrate-trapping and Affinity Precipitation Studies—For substrate trapping, the BT474 breast cancer cells expressing vector alone, WT-SHP2, and DM-SHP2 were grown to about 80% confluency, serum-starved overnight, and stimulated with 10 ng/ml EGF for 10 min or left unstimulated. Lysates prepared from these cells were subjected to immunoprecipitation with anti-FLAG and immunoblotting with anti-phosphotyrosine (Tyr(P)) and anti-HER2 antibodies. The immunoprecipitation studies were further corroborated by affinity precipitation with the GST fusion constructs. The GST fusions of the PTP domains of the WT- and the DM-SHP2 and the SH2 domains of RasGAP were reported previously (12, 30). For affinity precipitation, GST fusion proteins expressed in bacteria (∼2 μg per reaction tube) were first captured on glutathione-Sepharose beads by incubation of cleared lysates for 30 min at 4 °C and then washing 3 times with eukaryotic cell lysis buffer. Cell lysates prepared from BT474 and MEF cells expressing WT-HER2 and Y203F-HER2 were then added to the beads, incubated overnight at 4 °C, washed 3 times with cell lysis buffer, eluted by boiling for 10 min with Laemmli sample buffer, and analyzed by immunoblotting as described above.
Immunocomplex PTPase Assay—In vitro dephosphorylation of HER2 by SHP2 was tested as described previously by us and others (28, 31). Briefly, HER2 was immunoprecipitated from the BT474 cell lysates and washed 2 times with lysis buffer and 1 times with phosphatase buffer (25 mm Tris-HCl, pH 7.0, 50 mm NaCl, 10 mm dithiothreitol, and 2 mm EDTA). Beads were resuspended in 100 μl of the phosphatase buffer and then incubated at 30 °C for 10 min after the addition of purified WT- or DM-PTP. The level of HER2 tyrosine phosphorylation was determined by immunoblotting with an anti-Tyr(P) antibody.
Analysis of Ras Activation—State of Ras activation was determined by affinity precipitation with the GST fusion of the Ras binding domain of Raf-1 (GST-RBD) as described previously (12, 32). Briefly, the GST-RBD was captured on glutathione-Sepharose beads by incubation of cleared bacterial lysates containing the fusion protein for 1 h at 4 °C and then washing 3 times with cell lysis buffer described above. Cleared test cell lysates prepared from MEF and MCF-10A cell-expressing vector, WT-HER2, and Y1023F-HER2 were incubated with immobilized GST-RBD for 4 h at 4 °C, washed 3 times with lysis buffer, and eluted by boiling with Laemmli sample buffer. Polyacrylamide gel electrophoresis and immunoblotting protocols were as described above. Finally, the level of GTP-Ras bound to the GST-RBD was determined by immunoblotting with anti-Ras monoclonal antibody. Note that Raf-1 binds only to the activated f6torm of Ras (GTP-Ras).
Anchorage-independent Growth in Soft Agar—Anchorage-independent growth in the soft agar was used as one of the assays to determine state of cell transformation (33). First, 6-cm cell culture plates were overlaid with 0.3% agar in the growth medium, and next, cells suspended in 3 ml of growth medium were mixed with melted agar to a final concentration of 0.3% and immediately poured onto the agar overlay. After 5 min of incubation at room temperature for the gar to solidify, the cells were transferred to a 37 °C and 5% CO2 incubator for 7 days. During this time the cells were fed with soft agar medium every 3 days. Colony formation was visualized under a microscope, and phase contrast pictures were taken using an Olympus IX71 microscope equipped with Olympus DP30BW digital camera.
Cell Differentiation in Laminin-rich Basement Membrane Cultures—Laminin-rich basement membrane (LRBM) cultures were performed as described previously (29, 34). Briefly, 80 μl of growth factor reduced LRBM medium (BD Biosciences) was overlaid onto 4-well chamber slides (Falcon) and allowed to solidify for 15 min at 37 °C in a cell culture incubator. Approximately 103 cells resuspended in 250 μl of assay medium (Dulbecco's modified Eagle's medium supplemented with 2% horse serum, 10 μg/ml recombinant human insulin, 0.5 μg/ml hydrocortisone, 5 ng/ml EGF, and 100 ng/ml cholera toxin) were plated on a solidified LRBM and cultured at 37 °C in a 5% CO2 incubator. The cells were re-fed with assay medium every 4 days, and phase contrast pictures were taken after 15 days.
Immunofluorescence Microscopy—Cells in LRBM cultures were processed for immunofluorescence microscopy as described previously (29, 35) with slight modifications. Briefly, cells in the LRBM cultures were fixed with 4% paraformaldehyde for 20 min, washed 3 times with phosphate-buffered saline (PBS), permeabilized with 0.2% Triton-X-100 in PBS for 30 min, and blocked with 3% bovine serum albumin in PBS for 1 h. The samples were then stained with primary antibodies at 4 °C overnight, washed 3 times with phosphate-buffered saline containing 0.2% Triton-X-100, and incubated with fluorescent-labeled secondary antibodies for 1 h at room temperature. After washing three times, the chambers were removed, and slides were covered with appropriate size coverslips and sealed. Finally, slides were inverted and scanned with laser scanning microscope (LSM 510, Zeiss), and pictures were collected.
RESULTS
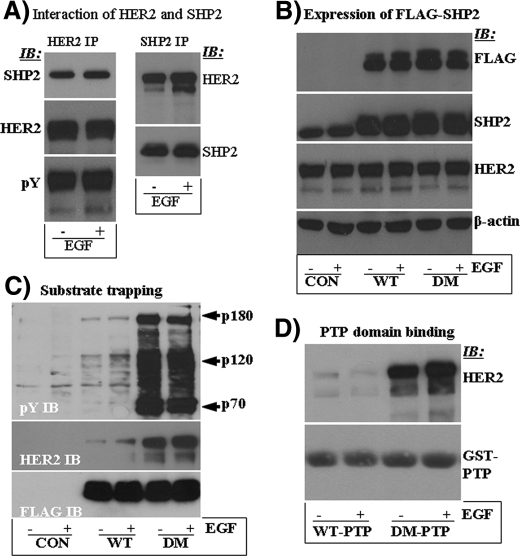
SHP2 Interacts with the HER2 Signaling Complex—SHP2 interacts directly or indirectly with receptor-tyrosine kinase through its SH2 domains (23, 36). This interaction enables SHP2 to localize to substrate microdomains and positively modulate cell growth and survival signaling by PTP domain-mediated dephosphorylation of target substrates (10, 24, 37). It was, thus, logical to think that SHP2 interacts with the HER2 signaling complex to play its positive role. This possibility was tested by reciprocal immunoprecipitation studies on lysates prepared from the BT474 breast cancer cell line that overexpresses HER2 (38) but has very low amount of the EGFR. SHP2 coprecipitated with HER2 in both the absence and the presence of EGF (Fig. 1A, left panel), suggesting constitutive tyrosine phosphorylation of HER2 under conditions of overexpression. Indeed, anti-Tyr(P) immunoblotting showed that HER2 was highly autophosphorylated even in the absence of EGF. Reciprocal experiments also showed that HER2 coprecipitates with SHP2 (Fig. 1A, right panel). Therefore, SHP2 forms a stable signaling complex with HER2.
FIGURE 1.
A, analysis of SHP2 interaction with the HER2 signaling complex by reciprocal immunoprecipitation (IP) with anti-HER2 and anti-SHP2 antibodies and immunoblotting (IB) with the indicated antibodies. B, Western blot analysis of total cell lysates for HER2, ectopically expressed SHP2 (FLAG-tagged), and endogenous SHP2. The β-actin blot was used as a loading control. C, intracellular substrate-trapping analysis by immunoprecipitation with anti-FLAG and Western blotting first with anti-Tyr(P) (top panel) and second with anti-HER2 antibodies (middle panel). Further reblotting with anti-FLAG antibody (bottom panel) showed that comparable amounts of ectopically expressed WT- and DM-SHP2 proteins were recovered during the immunoprecipitation reactions. D, confirmation of PTP domain-mediated substrate trapping by in vitro affinity precipitation with the GST fusion of the PTP domains of WT-SHP2 (WT-PTP) and DM-SHP2 (DM-PTP).
Identification of SHP2 Substrates in the BT474 Cells—The phosphatase function of SHP2 is critical for its positive role in receptor-tyrosine kinase signaling (39). We, thus, decided to identify proteins whose dephosphorylation by SHP2 positively impacts HER2-induced signaling and transformation. The substrate-trapping mutant of SHP2, the DM-SHP2 (28), which forms a stable complex with target substrates through its PTP domain, was used as a reagent to isolate and identify such proteins. First, BT474 cells stably expressing vector alone, WT-SHP2, and DM-SHP2 were obtained by retroviral transduction as described by us recently (10). To discriminate between endogenous and ectopically expressed SHP2, a FLAG tag was added to the C terminus. Analysis of input total cell lysates is shown in Fig. 1B. Ectopic expression of both SHP2 proteins was comparable, and it was approximately four times that of the endogenous. The level of the HER2 protein was similar as was the total protein concentration.
In anti-FLAG immunoprecipitation studies, the DM-SHP2 brought down highly enriched phosphotyrosyl (Tyr(P)) proteins; major bands of ∼180 and 120 kDa (Fig. 1C). Very low amounts of corresponding proteins were also brought down by the WT-SHP2 protein, suggesting SH2 domain-mediated interactions. Based on size, we suspected that the 180-kDa protein could be HER2 itself. Indeed, reblotting with anti-HER2 antibody showed that it was HER2 (middle panel). EGF stimulation did not have a significant effect on substrate trapping, confirming the observations in Fig. 1A where HER2 was constitutively autophosphorylated in the BT474 cells. Further reblotting with anti-FLAG antibody showed that comparable amounts of the WT- and the DM-SHP2 proteins were recovered during the immunoprecipitation reaction (lower panel). The identity of the 120-kDa phosphotyrosyl protein remains unknown. The 70-KDa Tyr(P) protein was SHP2 as revealed by the anti-FLAG immunoblotting, but only the DM-SHP2 was phosphorylated. Note that SHP2 becomes tyrosine-phosphorylated upon growth factor stimulation and is able to autodephosphorylate itself (40). Mutational inactivation as in the DM-SHP2 abolishes this function, leading to a sustained tyrosine phosphorylation state. To confirm PTP domain-mediated trapping of HER2, in vitro binding studies with the GST fusions of the PTP domains of both the WT-SHP2 and the DM-SHP2 were performed. The GST-DM-PTP affinity precipitated HER2, whereas the GST-WT-PTP did not (Fig. 1D). These results were consistent with previous reports where the substrate-trapping mutant of SHP2 forms an active site-mediated stable complex with target substrates, whereas the wild type protein dephosphorylates and releases them (28, 30). Therefore, HER2 is a potential SHP2 substrate. Because SHP2 plays a positive role in receptor-tyrosine kinase-induced signaling and transformation, the discovery of HER2 as an SHP2 substrate was surprising. We were intrigued by these findings and focused on HER2 to see why its dephosphorylation by SHP2 promotes the signaling and cell transformation functions.
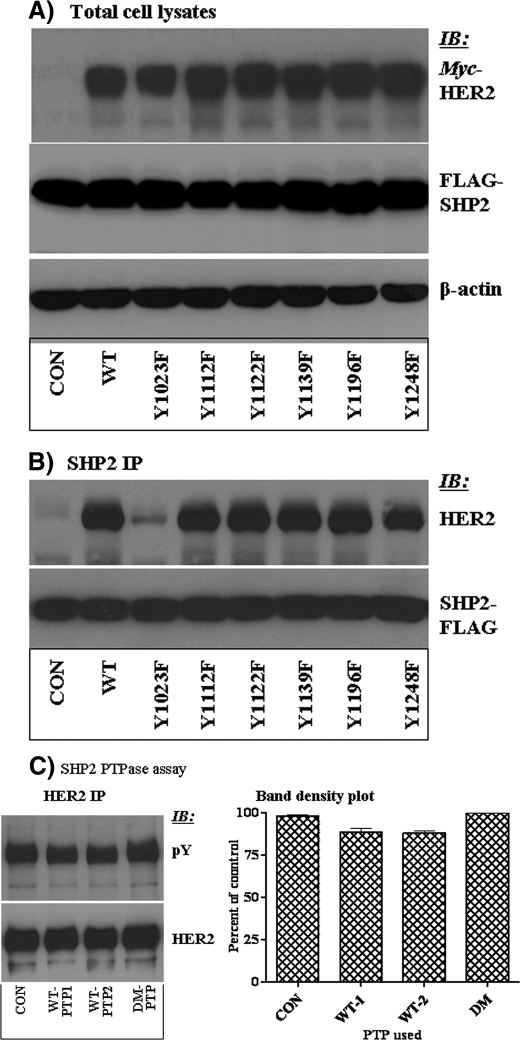
SHP2 Targets the Tyr-1023 Autophosphorylation Site in HER2—The next logical step was to identity the autophosphorylation site(s) on HER2 targeted by SHP2. Reportedly, HER2 has six tyrosine (Tyr) autophosphorylation sites in the C-terminal region, including Tyr-1023 with unknown function, Tyr-1112 and Tyr-1139 that serve as docking sites for CBL and Grb2, respectively, and Tyr-1196, Tyr-1122, and Tyr-1248 that provide docking sites for Shc proteins (41). Each of these Tyr residues were mutated to phenylalanine and stably co-expressed with the DM-SHP2 in the MEF cell line that has undetectable amounts of EGFR and HER2. Analysis of total cell lysates showed that all the HER2 proteins and the DM-SHP2 were expressed comparably (Fig. 2A). The same lysates were analyzed by immunoprecipitation with anti-FLAG and immunoblotting with anti-HER2 antibodies. The rationale was that mutation of SHP2 target autophosphorylation sites could lead to loss of trapping. Only the Y1023F-HER2 protein was inefficiently trapped by the DM-SHP2, whereas the rest of the mutants and the WT-HER2 proteins were efficiently trapped (Fig. 2B). The efficient trapping of HER2 mutants that lack the Grb2 and Shc binding sites by the DM-SHP2 suggests that removal of a non-target autophosphorylation site could not affect trapping by the DM-SHP2.
FIGURE 2.
Identification of an SHP2 target HER2 autophosphorylation site. A, the DM-SHP2 was co-expressed with the vector alone, the WT-HER2, and the indicated HER2 mutants in the MEF cells. The DM-SHP2 and the different HER2 proteins were tagged with FLAG and Myc, respectively. Total cell lysate analysis showed that the DM-SHP2 and the HER2 proteins were expressed comparably in the expected lanes. Anti-β-actin Western blot (WB) showed that total protein concentration was comparable in all lanes. B, the same total cell lysates were subjected to immunoprecipitation (IP) with anti-FLAG antibody and immunoblotting with anti HER2 antibody. As shown, the DM-SHP2 brought down all, but not Y1023F-HER2. Reblotting with anti-FLAG antibody showed that comparable amount of the DM-SHP2 protein was recovered in all lanes. C, immunocomplex phosphotyrosyl phosphatase (PTPase) assay to confirm that SHP2 dephosphorylates HER2. The left panel is anti-Tyr(P) immunoblotting, whereas the right panel is a plot of anti-Tyr(P) band densities obtained from three independent experiments. Note that SHP2 dephosphorylates HER2 only partially.
To confirm that SHP2 indeed dephosphorylates HER2, in vitro PTPase assays were performed as described by us recently (30). HER2 was immunoprecipitated from the BT474 cell lysates and then treated with two different concentrations (1 and 2 μg/ml) of purified WT-PTP; the DM-PTP at 2 μg/ml concentration was used as a negative control for dephosphorylation reactions. The WT-PTP partially dephosphorylated HER2, and doubling the PTP concentration did not reduce the HER2 phosphorylation level further (Fig. 2C, left panel). Band density measurements revealed that SHP2 reduced the level of HER2 phosphorylation by ∼10% (Fig. 2C, right panel). As expected, the PTP-dead DM-PTP did not dephosphorylate HER2. These results show that SHP2 dephosphorylates HER2 at few sites, supporting the site-directed mutagenesis experiments where a single autophosphorylation site, Tyr-1023, was targeted by SHP2. We, thus, concentrated on the WT- and the Tyr-1023-HER2 in the ensuing studies.
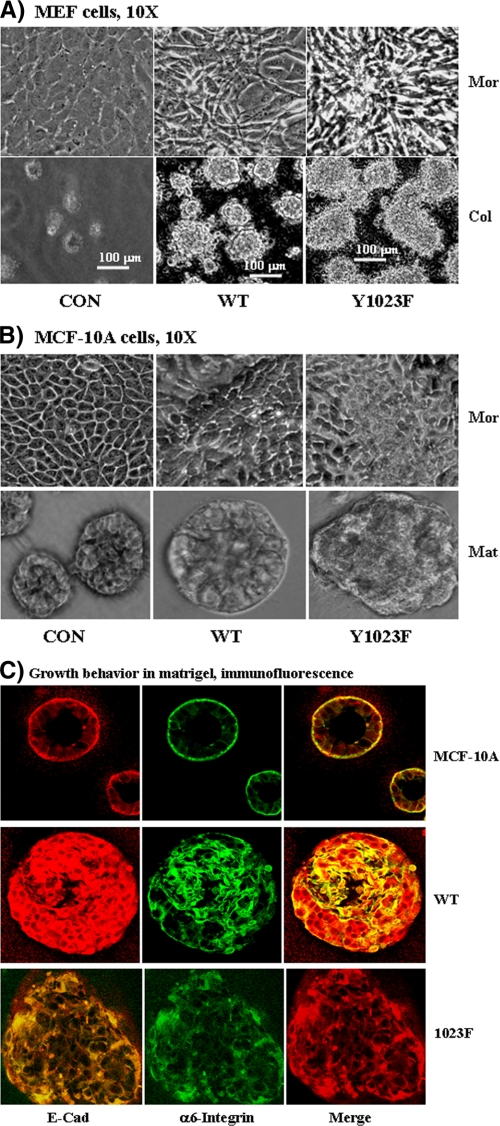
Y1023F-HER2 Is More Transforming Than the Wild Type Counterpart—Confluent MEF cells form a monolayer sheet of flattened cells that stop growth due to contact inhibition. Expression of the WT- and the Y1023F-HER2 in these cells induced morphological changes, including loss of the typical flattened morphology and acquisition of a highly refractive spindle-shaped appearance. Interestingly, these phenotypes were more pronounced in cells expressing the Y1023F-HER2 as was also evidenced by formation of a dome-like multicellular mass (Fig. 3A, top). Anchorage-independent growth assay in soft agar demonstrated that both the WT-HER2 and the Y1023F-HER2 cells form viable colonies with no significant difference in total number of colonies (Fig. 3A, bottom). However, the size of colonies formed by the Y1023F-HER2 cells was 1.5–2 times larger in size than those formed by the WT-HER2 cells (see the measurement inset). As expected, the vector control cells were unable to form viable colonies.
FIGURE 3.
A, Y1023F-HER2 is more transforming to MEF cells than the WT counterpart. Shown are morphological changes induced by HER2 expression at full cell density (top panel). The vector control (CON) cells exhibit typical MEF morphology with flattened monolayer sheet, whereas the WT-HER2 and the Y1023F-HER2 cells are elongated, refractive, and do not form a typical monolayer sheet. In addition, the Y1023F-HER2 cells formed dome-like cellular aggregate suggesting loss of contact-induced growth inhibition. Bottom panel, the indicated cells were grown in soft agar as described under “Materials and Methods.” As shown, the control cells could not, whereas the WT-HER2 and the Y1023F-HER2 cells were able to form colonies with the Y1023F-HER2 forming larger ones than the WT-HER2 (∼1.5–2 times). B, top panel, Y1023F-HER2 is also more transforming to MCF-10A cells than the WT protein. Expression of either the WT-HER2 or the Y1023F-HER2 disrupts the typical cobblestone-like appearance of the MCF-10A cells at full cell density. Both HER2 proteins induce ridges and convolutions to the monolayer sheet with the Y1023F-HER2 cells showing a more pronounced phenotype. Bottom panel, growth behavior of MCF-10A cells expressing the indicated HER2 proteins in LRBM culture. Shown are phase-contrast pictures where the CON cells form acini-like structures, whereas the WT-HER2 and the Y1023F cells could not. Note that the WT-HER2 cells for a spheroid, whereas the Y1023F cells form an amorphous structure. C, cells in LRBM cultures were analyzed by immunofluorescence confocal microscopy by co-staining with anti-E-cadherin (E-cad) and anti-α6-integrin antibodies. Note that the control cells form acini-like structures with hollow lumen, WT-HER2 cells form spheroid structures with no hollow lumen, whereas the Y1023F-HER2 cells form an amorphous cellular mass with no hollow lumen. Mor, morphology; Col, colony; Mat, Matrigel.
The observations made in MEF cells were further validated in biologically relevant MCF-10A cells, the immortalized but non-malignant breast epithelial cell line. This was important because overexpression of HER2 in breast tumors is known to promote an aggressive disease state and poor prognosis for patient survival (42, 43). We, thus, expressed the HER2 proteins in MCF-10A cells by retroviral transduction and studied their impact on cellular phenotypes.
The vector control cells exhibited a typical MCF-10A morphology characterized by a cobblestone-like appearance at confluency, whereas the WT- and the Y1023F-HER2 cells exhibited divergent phenotypes, including loss the cobblestone appearance, formation of undulating ridges and convolutions, and an apparent multilayered cellular mass; these phenotypes were more pronounced in the Y1023F-HER2 cells (Fig. 3B, top). The anchorage-independent growth property of the MCF-10A cells expressing the WT- and the Y102F-HER2 proteins was similar to that of the MEF cells (data not shown).
The HER2-induced MCF-10A transformation was further corroborated by a three-dimensional LRBM Matrigel assay. MCF-10A cells form polarized acini-like structures in LRBM cultures, whereas breast cancer cell lines form a disorganized cellular mass that continues to grow as long as they are supplied with nutrients (27, 29). As expected, the control cells formed acini-like spheroid structures with apparent hollow lumen, whereas the WT-HER2 cells were able to form rounded but a larger cellular mass with no indication of hollow lumen. On the other hand, the Y1023F-HER2 cells formed an amorphous cellular mass that continued to grow even after 20 days (Fig. 4B, bottom). Anti-α6 integrin and anti-E-cadherin immunofluorescence microscopy confirmed that the control cells had polarized acini as evidenced by a single layer of extracellular matrix-integrin interaction, adherens junction-mediated lateral cell-cell adhesion, and a central hollow lumen (Fig. 3C). Consistent with phase contrast pictures, the WT-HER2 cells formed large and rounded structures with no hollow lumen as evidenced by intense α6-integrin and E-cadherin staining throughout the structure. The structures formed by the Y1023F-HER2 cells were even more drastic as depicted by the amorphous shape and α6-integrin and E-cadherin staining throughout the cellular mass. These results further confirm that both the WT-HER2 and the Y1023F-HER2 proteins are transforming to MCF-10A, but the Y1023F-HER2 transforms better than the WT counterpart. Together, these results demonstrate that SHP2 enhances the transformation potential of HER2 by dephosphorylating Tyr-1023 both in fibroblasts and breast epithelial cells.
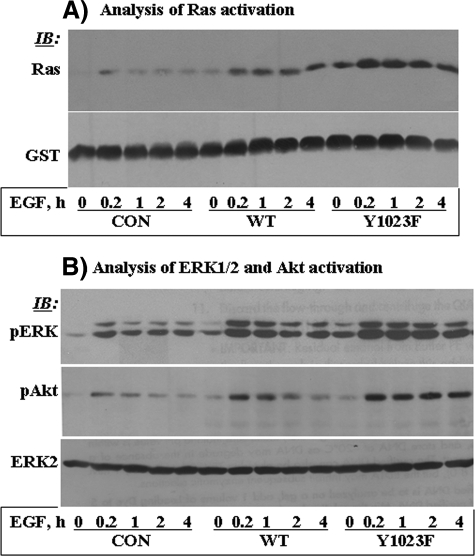
FIGURE 4.
Y1023F-HER2 exhibits robust signaling potency than the WT counterpart. A, the GTP-Ras level was determined by affinity precipitation with the GST fusion of the Ras binding domain of Raf-1 (GST-RBD) and then detected by Western blotting (IB) with anti-Ras antibody. Reblotting with anti-GST antibody showed that the amount of GST-RBD used in each lane was comparable. B, corresponding total cell lysates were analyzed by Western blotting with anti-phospho-ERK1/2 (pERK) and anti-phospho-Akt (pAkt) antibodies. Anti-ERK2 reblotting showed that the total protein level in each lane was comparable.
Y1023F-HER2 Is More Robust in Signaling Potency Than the WT Protein—Given the positive role of SHP2 in receptor-tyrosine kinase-induced cell growth and survival signaling, we reasoned that the augmented transformation potency of the SHP2 target HER2 mutant might be related to its enhanced signaling potency. We, thus, determined the state of Ras, ERK1/2, and Akt activation in MCF-10A cells expressing vector alone, WT-HER2, and Y1023F-HER2. Because these cells express a modest amount of the EGFR, we investigated the impact of HER2 expression on both basal and EGF-induced signaling. As such, cells were treated with 10 ng/ml EGF for varying time points ranging from 10 min to 4 h. Basal state GTP-Ras was undetectable in the controls, detectable in the WT-HER2, and more enhanced in the Y1023F-HER2 cells. EGF-induced Ras activation was very low and short-lived in the control cells but prolonged and enhanced in the WT- and the Y1023F-HER2 cells, with the Y1023F-HER2 cells showing a more enhanced activation. Consistent with Ras activation, basal state ERK1/2 and Akt activation was significant in the WT-HER2 and enhanced in the Y1023F-HER2 cells. EGF-induced activation was short-lived in the controls, moderately enhanced and sustained in the WT-HER2 cells, and highly enhanced and sustained in the Y1023F-HER2 cells (Fig. 4B). Therefore, the SHP2 target HER2 mutant is more robust in inducing cell growth and survival signaling than the WT counterpart, which might explain the enhanced transformation potential of Y1023F-HER2.
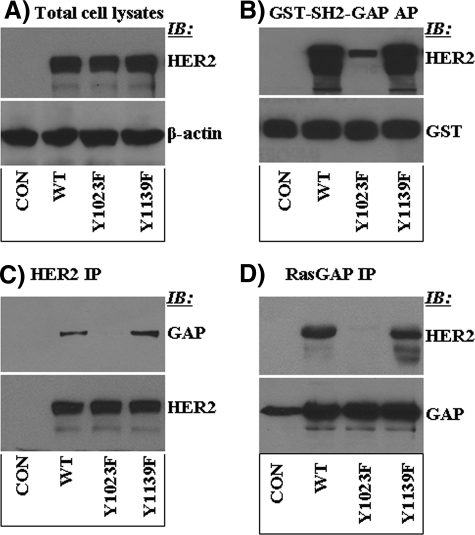
Tyr-1023 Is a RasGAP Docking Site—Next, we investigated why dephosphorylation of Tyr-1023 by SHP2 enhances HER2-induced signaling and transformation. Based on the results so far, it was logical to think that phosphorylated Tyr-1023 (Tyr(P)-1023) might serve as a docking scaffold for negative regulatory proteins to the Ras-ERK and the phosphoinositide-3-kinase-Akt signaling pathways. Interestingly, the primary amino acid sequence C-terminal to the target tyrosine (Y1023LIP) perfectly fits to the previously described RasGAP SH2 domain binding consensus sequence, the YXXP motif (44, 45). This possibility was tested by in vitro binding studies using the GST fusion of the SH2 domains of RasGAP hereinafter named GST-SH2-GAP. The MEF cells expressing vector alone, WT-HER2, Y1023F-HER2, and Y1139F-HER2, were used for these experiments. As shown in Fig. 5A, all the three HER2 proteins were expressed comparably. The inclusion of Y1139F-HER2 was to see if mutation of a non-target site in HER2 could affect interaction with RasGAP. Affinity precipitation with the GST-SH2-GAP and immunoblotting with anti-HER2 antibody showed that the WT-HER2 and the Y1139F-HER2 proteins were efficiently precipitated, whereas the Y1023F-HER2 protein was not (Fig. 5B). Reblotting with anti-GST antibody confirmed that a comparable amount of the GST-SH2-GAP protein was used in each precipitation reaction. To assess the biological relevance of the in vitro binding results, the occurrence of intracellular interaction was investigated by reciprocal immunoprecipitation studies. The WT- and the Y1139F-HER2 proteins coprecipitated with RasGAP, whereas the Y1203F-HER2 did not. Similarly, RasGAP coprecipitated with the WT- and the Y1139F-HER2 proteins but only insignificantly with the Y1023F-HER2 (Fig. 5C). These results are consistent with phosphorylated Tyr-1023 acting as a RasGAP docking site. In turn, they suggest that SHP2 promotes HER2-induced signaling by dephosphorylating a negative regulatory autophosphorylation site.
FIGURE 5.
Tyr-1023 is essential for binding of RasGAP to HER2. A, analysis of total cell lysates. The WT-, the Y1023F-, and the Y1139F-HER2 proteins were expressed comparably. Anti-β-actin Western blotting (IB) showed that total protein content in each lane was similar. B, affinity precipitation (AP) of the same lysates with the GST fusion of the SH2 domains of RasGAP (GST-SH2-GAP). As shown, GST-SH2-GAP could not precipitate Y1023F-HER2, suggesting that this site is important for interaction of RasGAP with HER2. On the other hand, mutation of the Grb2 binding site (Y1139F) did not affect binding. C, testing intracellular interaction of RasGAP with HER2 by immunoprecipitation (IP) with anti-HER2 and immunoblotting with anti-RasGAP antibodies. D, testing intracellular interaction of HER2 with RasGAP by immunoprecipitation with anti-RasGAP and immunoblotting with anti-HER2 antibodies.
DISCUSSION
Inhibition of SHP2 in HER2-positive breast cancer cell lines leads to mesenchymal to epithelial transition (27). Because of the significance of HER2 in breast cancer, we sought to investigate the molecular mechanism of SHP2 in promoting HER2-induced signaling and transformation. Previous studies have shown that the positive role of SHP2 in receptor-tyrosine kinase signaling starts with SH2 domain-mediated interaction (23, 36) that enables SHP2 to localize to substrate microdomains and dephosphorylate target substrates (10, 24, 37). It was, thus, reasoned that the positive role of SHP2 in HER2-induced signaling and transformation must involve its interaction with the HER2 signaling complex. Indeed, SHP2 interacts with HER2 as demonstrated by reciprocal immunoprecipitation reactions (Fig. 1A). The EGF-independent interaction was suggestive of the constitutive tyrosine phosphorylation of HER2, whereas the minimal effect of EGF on the interaction is indicative of the low level of EGFR in the BT474 breast cancer cell lines.
To define the molecular mechanism of SHP2, it was essential to first identify target substrates whose dephosphorylation drives HER2-induced signaling and transformation. The development of an efficient substrate-trapping mutant of SHP2 (DM-SHP2) has provided opportunities for isolating and identifying target substrates and for characterizing how dephosphorylation by SHP2 positively impacts cellular signaling and transformation (10, 28, 46). The DM-SHP2 lacks the nucleophilic Cys-459 and the proton donor Asp-425 of the PTP domain, and as a result, it forms a stable complex with substrates (12, 28, 30). We, thus, utilized the DM-SHP2 to identify SHP2 substrates in the HER2 signaling pathway.
By a combination of substrate trapping and Western blot analysis, we have discovered for the first time that HER2 itself is an SHP2 substrate. Given that SHP2 is a positive effector of HER2 signaling and that autophosphorylation of HER2 is important for signal transduction, the identification of HER2 as an SHP2 substrate looked counterintuitive at the beginning. Site-directed mutagenesis of all known autophosphorylation sites and subsequent substrate-trapping studies revealed that SHP2 targets only Tyr-1023. It was very remarkable to note that SHP2 does not target the Grb2 and the Shc binding sites that were previously described as important for HER2-induced mitogenic and cell survival signaling. In conformity to single autophosphorylation site targeting, only partial dephosphorylation of HER2 (∼10%) was observed in immunocomplex PTPase experiments (Fig. 2C). Therefore, SHP2 specifically targets a single autophosphorylation site in HER2.
The next logical step was to prove how dephosphorylation of Tyr(P)-1023 of HER2 by SHP2 positively impacts HER2-induced signaling and transformation. Surprisingly, we have discovered that Y1023F-HER2 was more transforming to MEF cells than the wild type counterpart as determined by morphological changes and anchorage-independent growth assays. Morphologically, MEF cells expressing Y1023F-HER2 exhibited a highly refractile appearance with more interspersed and elongated shape. More often than not the Y1023F-HER2 cells formed a aggregated multicellular mass reminiscent of focus formation, a commonly used assay system for evaluating cell transformation. Formation of larger colonies by Y1023F-HER2 cells in soft agar further confirmed that mutation of Tyr-1023 confers enhanced transforming potential to the HER2 protein.
Because of the significance of HER2 in breast cancer, the transforming potential of Y1023F-HER2 was also investigated in the biologically relevant MCF-10A cells, the immortalized breast epithelial cell line. Consistent with the observations in MEF cells, the Y1023F-HER2 was also more transforming to MCF-10A cells than the wild type protein. Expression of both the wild type and the Y1023F-HER2 proteins disrupted the typical cobblestone appearance of the MCF-10A monolayer sheet, but the changes induced by the Y1023F-HER2 protein were more drastic as evidenced by formation of an enhanced multicellular mass, suggesting a continued cell proliferation even after confluency. Anchorage-independent growth assays further confirmed that MCF-10A cells expressing Y1023F-HER2 form larger colonies than cells expressing the wild type counterpart.
Final conformation as to the enhanced transformation potential of Y1023F-HER2 came from three-dimensional Matrigel assays. In this assay system MCF-10A cells form acini-like structures reminiscent of a lactating mammary gland. This growth behavior was disrupted by expression of WT-HER2 where cells still formed spherical structures but without hollow lumen. Apparently, expression WT-HER2 does not interfere with spheroid formation but with the process of autophagy that creates the hollow lumen, which is consistent with previous reports (47). On the other hand, the disruptions induced by the Y1023F-HER2 were more drastic, as MCF-10A cells expressing this mutant could form neither a hollow lumen nor a spherical structure. Therefore, Y1023F-HER2 is more transforming to MCF-10A than the wild type protein. A previous work by William Muller's group (48) has shown that mutation of Tyr-1027 in Neu, the residue that corresponds to Tyr-1023 in HER2, leads to a more transforming protein. Therefore, our results are consistent with this report and further imply that Tyr-1027 of Neu might be an SHP2 target. It will be interesting to prove this possibility in future studies.
The enhanced signaling and transforming property of the Y1023F-HER2 protein was a clear indication of Tyr-1023 being a negative regulatory autophosphorylation site. Given that the Ras-ERK and the phosphoinositide-3-kinase-Akt signaling pathways downstream of HER2 are essential for cell transformation, it was logical to think that Tyr(P)-1023 might act as a docking scaffold to proteins that negatively regulate these pathways. Based on our work on the EGFR (12) and previous other work on the Torso-Cork Screw (49) signaling axis, we speculated that Tyr(P)-1023 could be a docking site for RasGAP, the down-regulator of Ras. Affinity precipitation with the SH2 domains of RasGAP and reciprocal anti-RasGAP and anti-HER2 immunoprecipitation studies clearly showed that Tyr(P)-1023 is indeed a RasGAP docking site on HER2 (Fig. 5). In conformity with these findings, Tyr-1023 exists in 1023YXXP motif, a consensus sequence previously described as a RasGAP SH2 domain binding motif (44, 45). Together, our results suggest that the enhanced biological activity of the Y1023F-HER2 is due to loss of RasGAP binding. Therefore, SHP2 promotes HER2-induced signaling and transformation at least in part by dephosphorylating Tyr(P)-1023, a RasGAP docking site on HER2. In this connection, caution must be exercised not to conclude that HER2 is the sole substrate for SHP2 in promoting HER2-induced signaling and transformation. It is possible that dephosphorylation of other as yet unidentified substrates by SHP2 might also contribute to this molecular and cellular event. The SHP2 substrate-trapping mutant will continue to be a valuable reagent to delineate the molecular mechanism of SHP2 in HER2 and other signaling pathways where SHP2 is known to positively impact.
This work was supported, in whole or in part, by National Institutes of Health Grant CA124940 (NCI, to Y. M. A.).
Footnotes
The abbreviations used are: SHP2, Src homology phosphotyrosyl phosphatase 2; SH2, Src homology 2; PTP, phosphotyrosyl phosphatase; Ras-GAP, Ras GTPase-activating protein; MEF, mouse embryo fibroblast; RBD, Ras binding domain; LRBM, laminin-rich basement membrane; DM, double mutant; ERK, extracellular signal-regulated kinase; EGFR, epidermal growth factor (EGF) receptor; GST, glutathione S-transferase; WT, wild type.
References
- 1.Chatterjee-Kishore, M., van den Akker, F., and Stark, G. R. (2000) Trends Cell Biol. 10 106-111 [DOI] [PubMed] [Google Scholar]
- 2.DeMali, K. A., Balciunaite, E., and Kazlauskas, A. (1999) J. Biol. Chem. 274 19551-19558 [DOI] [PubMed] [Google Scholar]
- 3.Di Fiore, P. P., Pierce, J. H., Fleming, T. P., Hazan, R., Ullrich, A., King, C. R., Schlessinger, J., and Aaronson, S. A. (1987) Cell 51 1063-1070 [DOI] [PubMed] [Google Scholar]
- 4.Honegger, A. M., Schmidt, A., Ullrich, A., and Schlessinger, J. (1990) Mol. Cell. Biol. 10 4035-4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inagaki, K., Noguchi, T., Matozaki, T., Horikawa, T., Fukunaga, K., Tsuda, M., Ichihashi, M., and Kasuga, M. (2000) Oncogene 19 75-84 [DOI] [PubMed] [Google Scholar]
- 6.Kodama, A., Matozaki, T., Fukuhara, A., Kikyo, M., Ichihashi, M., and Takai, Y. (2000) Mol. Biol. Cell 11 2565-2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manes, S., Mira, E., Gomez-Mouton, C., Zhao, Z. J., Lacalle, R. A., and Martinez, A. C. (1999) Mol. Cell. Biol. 19 3125-3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu, D. H., Qu, C. K., Henegariu, O., Lu, X., and Feng, G. S. (1998) J. Biol. Chem. 273 21125-21131 [DOI] [PubMed] [Google Scholar]
- 9.Agazie, Y. M., Movilla, N., Ischenko, I., and Hayman, M. J. (2003) Oncogene 22 6909-6918 [DOI] [PubMed] [Google Scholar]
- 10.Burks, J., and Agazie, Y. M. (2006) Oncogene 25 7166-7179 [DOI] [PubMed] [Google Scholar]
- 11.Hakak, Y., Hsu, Y. S., and Martin, G. S. (2000) Oncogene 19 3164-3171 [DOI] [PubMed] [Google Scholar]
- 12.Agazie, Y. M., and Hayman, M. J. (2003) Mol. Cell. Biol. 23 7875-7886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng, G. S., Hui, C. C., and Pawson, T. (1993) Science 259 1607-1611 [DOI] [PubMed] [Google Scholar]
- 14.Frearson, J. A., and Alexander, D. R. (1998) J. Exp. Med. 187 1417-1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tartaglia, M., Kalidas, K., Shaw, A., Song, X., Musat, D. L., van der Burgt, I., Brunner, H. G., Bertola, D. R., Crosby, A., Ion, A., Kucherlapati, R. S., Jeffery, S., Patton, M. A., and Gelb, B. D. (2002) Am. J. Hum. Genet. 70 1555-1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tartaglia, M., Martinelli, S., Iavarone, I., Cazzaniga, G., Spinelli, M., Giarin, E., Petrangeli, V., Carta, C., Masetti, R., Arico, M., Locatelli, F., Basso, G., Sorcini, M., Pession, A., and Biondi, A. (2005) Br. J. Haematol. 129 333-339 [DOI] [PubMed] [Google Scholar]
- 17.Tartaglia, M., Mehler, E. L., Goldberg, R., Zampino, G., Brunner, H. G., Kremer, H., van der Burgt, I., Crosby, A. H., Ion, A., Jeffery, S., Kalidas, K., Patton, M. A., Kucherlapati, R. S., and Gelb, B. D. (2001) Nat. Genet. 29 465-468 [DOI] [PubMed] [Google Scholar]
- 18.Tartaglia, M., Niemeyer, C. M., Fragale, A., Song, X., Buechner, J., Jung, A., Hahlen, K., Hasle, H., Licht, J. D., and Gelb, B. D. (2003) Nat. Genet. 34 148-150 [DOI] [PubMed] [Google Scholar]
- 19.Araki, T., Mohi, M. G., Ismat, F. A., Bronson, R. T., Williams, I. R., Kutok, J. L., Yang, W., Pao, L. I., Gilliland, D. G., Epstein, J. A., and Neel, B. G. (2004) Nat. Med 10 849-857 [DOI] [PubMed] [Google Scholar]
- 20.Mohi, M. G., Williams, I. R., Dearolf, C. R., Chan, G., Kutok, J. L., Cohen, S., Morgan, K., Boulton, C., Shigematsu, H., Keilhack, H., Akashi, K., Gilliland, D. G., and Neel, B. G. (2005) Cancer Cell 7 179-191 [DOI] [PubMed] [Google Scholar]
- 21.Loh, M. L., Reynolds, M. G., Vattikuti, S., Gerbing, R. B., Alonzo, T. A., Carlson, E., Cheng, J. W., Lee, C. M., Lange, B. J., and Meshinchi, S. (2004) Leukemia 18 1831-1834 [DOI] [PubMed] [Google Scholar]
- 22.Feng, G. S., and Pawson, T. (1994) Trends Genet 10 54-58 [DOI] [PubMed] [Google Scholar]
- 23.Feng, G. S., Shen, R., Heng, H. H., Tsui, L. C., Kazlauskas, A., and Pawson, T. (1994) Oncogene 9 1545-1550 [PubMed] [Google Scholar]
- 24.Bennett, A. M., Hausdorff, S. F., O'Reilly, A. M., Freeman, R. M., and Neel, B. G. (1996) Mol. Cell. Biol. 16 1189-1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saxton, T. M., Ciruna, B. G., Holmyard, D., Kulkarni, S., Harpal, K., Rossant, J., and Pawson, T. (2000) Nat. Genet. 24 420-423 [DOI] [PubMed] [Google Scholar]
- 26.Saxton, T. M., Henkemeyer, M., Gasca, S., Shen, R., Rossi, D. J., Shalaby, F., Feng, G. S., and Pawson, T. (1997) EMBO J. 16 2352-2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou, X. D., and Agazie, Y. M. (2008) Cell Death Differ. 15 988-996 [DOI] [PubMed] [Google Scholar]
- 28.Agazie, Y. M., and Hayman, M. J. (2003) J. Biol. Chem. 278 13952-13958 [DOI] [PubMed] [Google Scholar]
- 29.Debnath, J., Muthuswamy, S. K., and Brugge, J. S. (2003) Methods 30 256-268 [DOI] [PubMed] [Google Scholar]
- 30.Merritt, R., Hayman, M. J., and Agazie, Y. M. (2006) Biochim. Biophys. Acta 1763 45-56 [DOI] [PubMed] [Google Scholar]
- 31.Klinghoffer, R. A., and Kazlauskas, A. (1995) J. Biol. Chem. 270 22208-22217 [DOI] [PubMed] [Google Scholar]
- 32.Taylor, S. J., and Shalloway, D. (1996) Curr. Biol. 6 1621-1627 [DOI] [PubMed] [Google Scholar]
- 33.Agazie, Y., Ischenko, I., and Hayman, M. (2002) Oncogene 21 697-707 [DOI] [PubMed] [Google Scholar]
- 34.Bissell, M. J., Radisky, D. C., Rizki, A., Weaver, V. M., and Petersen, O. W. (2002) Differentiation 70 537-546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw, K. R., Wrobel, C. N., and Brugge, J. S. (2004) J. Mammary Gland Biol. Neoplasia 9 297-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hadari, Y. R., Kouhara, H., Lax, I., and Schlessinger, J. (1998) Mol. Cell. Biol. 18 3966-3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett, A. M., Tang, T. L., Sugimoto, S., Walsh, C. T., and Neel, B. G. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 7335-7339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasleau, F., Grooteclaes, M., and Gol-Winkler, R. (1993) Oncogene 8 849-854 [PubMed] [Google Scholar]
- 39.Deb, T. B., Wong, L., Salomon, D. S., Zhou, G., Dixon, J. E., Gutkind, J. S., Thompson, S. A., and Johnson, G. R. (1998) J. Biol. Chem. 273 16643-16646 [DOI] [PubMed] [Google Scholar]
- 40.Stein-Gerlach, M., Kharitonenkov, A., Vogel, W., Ali, S., and Ullrich, A. (1995) J. Biol. Chem. 270 24635-24637 [DOI] [PubMed] [Google Scholar]
- 41.Ono, M., and Kuwano, M. (2006) Clin. Cancer Res. 12 7242-7251 [DOI] [PubMed] [Google Scholar]
- 42.Badache, A., and Goncalves, A. (2006) J. Mammary Gland Biol. Neoplasia 11 13-25 [DOI] [PubMed] [Google Scholar]
- 43.Wolf-Yadlin, A., Kumar, N., Zhang, Y., Hautaniemi, S., Zaman, M., Kim, H. D., Grantcharova, V., Lauffenburger, D. A., and White, F. M. (2006) Mol. Syst. Biol. 2 1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Songyang, Z., and Cantley, L. C. (1995) Methods Enzymol. 254 523-535 [DOI] [PubMed] [Google Scholar]
- 45.Songyang, Z., Shoelson, S. E., Chaudhuri, M., Gish, G., Pawson, T., Haser, W. G., King, F., Roberts, T., Ratnofsky, S., Lechleider, R. J., Nee, B. J., Birge, R. B., Fajardo, J. E., Chou, M. M., Hanafusa, M., Schaffhausen, B., and Cantley, L. C. (1993) Cell 72 767-778 [DOI] [PubMed] [Google Scholar]
- 46.Kolli, S., Zito, C. I., Mossink, M. H., Wiemer, E. A., and Bennett, A. M. (2004) J. Biol. Chem. 279 29374-29385 [DOI] [PubMed] [Google Scholar]
- 47.Bentires-Alj, M., Gil, S. G., Chan, R., Wang, Z. C., Wang, Y., Imanaka, N., Harris, L. N., Richardson, A., Neel, B. G., and Gu, H. (2006) Nat. Med. 12 114-121 [DOI] [PubMed] [Google Scholar]
- 48.Dankort, D. L., Wang, Z., Blackmore, V., Moran, M. F., and Muller, W. J. (1997) Mol. Cell. Biol. 17 5410-5425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cleghon, V., Feldmann, P., Ghiglione, C., Copeland, T. D., Perrimon, N., Hughes, D. A., and Morrison, D. K. (1998) Mol. Cell 2 719-727 [DOI] [PubMed] [Google Scholar]