Abstract
Autophagy is a degradative process that recycles long-lived and faulty cellular components. It is linked to many diseases and is required for normal development. ULK1, a mammalian serine/threonine protein kinase, plays a key role in the initial stages of autophagy, though the exact molecular mechanism is unknown. Here we report identification of a novel protein complex containing ULK1 and two additional protein factors, FIP200 and ATG13, all of which are essential for starvation-induced autophagy. Both FIP200 and ATG13 are critical for correct localization of ULK1 to the pre-autophagosome and stability of ULK1 protein. Additionally, we demonstrate by using both cellular experiments and a de novo in vitro reconstituted reaction that FIP200 and ATG13 can enhance ULK1 kinase activity individually but both are required for maximal stimulation. Further, we show that ATG13 and ULK1 are phosphorylated by the mTOR pathway in a nutrient starvation-regulated manner, indicating that the ULK1·ATG13·FIP200 complex acts as a node for integrating incoming autophagy signals into autophagosome biogenesis.
Macroautophagy (herein referred to as autophagy) is a catabolic process whereby long-lived proteins and damaged organelles are shuttled to lysosomes for degradation. This process is conserved in all eukaryotes. Under normal growth conditions a housekeeping level of autophagy exists. Under stress, such as nutrient starvation, autophagy is strongly induced resulting in the engulfment of cytosolic components and organelles in specialized double-membrane structures termed autophagosomes. Following fusion of the outer autophagosomal membrane with lysosomes, the inner membrane and its cytoplasmic cargo are degraded and recycled (1–3). Recent work has implicated autophagy in many disease pathologies, including cancer, neurodegeneration, as well as in eliminating intracellular pathogens (4–8).
The morphology of autophagy was first described in mammalian cells over 50 years ago (9). However, it is only recently through yeast genetic screens, that multiple autophagy-related (ATG) genes have been identified (10–12). The yeast ATG proteins have been classified into four major groups: the Atg1 protein kinase complex, the Vps34 phosphatidylinositol 3-phosphate kinase complex, the Atg8/Atg12 conjugation systems, and the Atg9 recycling complex (13). Even though many ATG genes are now known, most of which have functional homologs in mammalian cells (14, 15), the molecular mechanism by which they sense the initial triggers and subsequently dictate autophagy-specific intracellular membrane events is far from understood.
In yeast, one of the earliest autophagy-specific events is believed to involve the Atg1 protein kinase complex. Atg1 is a serine/threonine protein kinase and a key autophagy-regulator (16). Atg1 is complexed to at least two other proteins during autophagy, Atg13 and Atg17, both of which are required for normal Atg1 function and autophagosome generation (17–19). Classical signaling pathways such as the cAMP-dependent kinase (PKA) pathway or the Tor kinase pathway appear to converge upon this complex, placing Atg1 at an early stage during autophagosome biogenesis (20–22). Atg1 phosphorylation by PKA blocks its association with the forming autophagosome (21), while the Tor pathway hyperphosphorylates Atg13 causing a reduced affinity of Atg13 for Atg1, resulting in repression of autophagy (17, 19). In contrast, nutrient starvation or inhibition of Tor leads to dephosphorylation of Atg13 thus increased Atg1 complex formation and kinase activity, resulting in stimulation of autophagy (19). Surprisingly, the physiological substrates of Atg1 kinase have not been identified; thus how Atg1 transduces upstream autophagic signaling is undefined. Recently, mammalian homologs of Atg1 have been identified as ULK1 and ULK2 (Unc-51-like kinase)2 (23–25). ULK1 and ULK2 are ubiquitously expressed and localize to the isolation membrane, or forming autophagosome, upon nutrient starvation (25); RNAi-mediated depletion of ULK1 in HEK293 cells compromises autophagy (23, 24). The exact role of ULK1 versus ULK2 in autophagy is unclear, and it is possible some redundancy exists between the two isoforms (26).
Given the conservation of autophagy from yeast to man, it is interesting to note that no mammalian counterpart to yeast Atg13 or Atg17 had been identified until very recently. The protein FIP200 (focal adhesion kinase family-interacting protein of 200 kDa) was identified as an autophagy-essential binding partner of both ULK1 and ULK2 (25), and it has been speculated that FIP200 might be the equivalent of yeast Atg17, despite low sequence similarity (25, 27).
In this study, we delve deeper into the molecular regulation of ULK1 to gain a better insight into how mammalian signaling pathways affect autophagy initiation. We describe here the identification of a triple complex consisting of ULK1, FIP200, and the mammalian equivalent of Atg13. This complex is required not only for localization of ULK1 to the isolation membrane but also for maximal kinase activity. In addition, both ATG13 and ULK1 are kinase substrates in the mTOR pathway and thus might function to sense nutrient starvation. Therefore, this study defines the role of mammalian ULK1-ATG13-FIP200 complex in mediating the initial autophagic triggers and to transduce the signal to the core autophagic machinery.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies—Rabbit anti-ULK1 (A7481) and mouse anti-FLAG (clone M2) were purchased from Sigma, rabbit anti-FIP200 from ProteinTech Inc., mouse anti-Myc clone 9E10 was obtained from the monoclonal antibody facility at Memorial Sloan-Kettering Cancer Center, mouse anti-GFP from Roche Applied Sciences, and mouse anti-T7 and mouse anti-S tag were from Novagen. Rapamycin was purchased from Sigma.
Cloning and Protein Expression—cDNAs were purchased ATCC: mouse ULK1 (clone ID 6834534) from OpenBiosystems: mouse LOC51897/ATG13 (clone ID 5359944), human FIP200 (clone ID 3908134). For expression in MEF cells, ULK1 was subcloned into pBabe/puro containing an N-terminal FLAG and S tag; ATG13 was subcloned into pBabe/blast containing an N-terminal GFP tag. For expression in 293T cells, ULK1 was subcloned into pCMV-Tag4 (Invitrogen) containing a C-terminal FLAG tag, and both ATG13 and FIP200 were subcloned into pCMV-Tag3 (Invitrogen) containing an N-terminal Myc tag. pIC194 (N-terminal mCherry) was a kind gift from Dr. Iain Cheeseman, Ludwig Institute for Cancer Research, San Diego, CA. Mammalian expression vectors encoding Myc-tagged mTOR and Myc-tagged kinase-dead mTOR were obtained from Addgene, courtesy of the Sabatini Laboratory, Whitehead Institute for Biomedical Research. For recombinant protein expression, ULK1 and FIP200 were subcloned into pFastBac containing N-terminal His tag, while ATG13 was subcloned into pFastBac containing N-terminal His and FLAG tag. Additionally ATG13 was subcloned into pET28a for expression in Escherichia coli.
Recombinant proteins for ULK1, FIP200, and ATG13 were expressed in Sf9 cells using the Bac-to-Bac expression system from Invitrogen. Cell pellets were resuspended in Buffer T (20 mm Tris, pH 8.0, 50 mm NaCl, 2 mm β-mercaptoethanol) and purified over a nickel column. Following extensive washing, proteins were eluted stepwise in Buffer T containing 10–250 mm imidazole. Pure fractions were pooled and dialyzed in 25 mm Tris, pH 7.5, 100 mm NaCl, and 1 mm DTT, before snap-freezing and storage at -80 °C. In the case of ULK1 and FIP200, protein was further purified by ion-exchange chromatography over an SP-Sepharose column and a Q-Sepharose column, respectively, followed by dialysis and snap-freezing. T7-ATG10 was expressed in bacteria and purified as described previously (28).
Cell Culture—All mammalian cells were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum and penicillin/streptomycin and cultured at 37 °C, 5% CO2. For induction of autophagy, cells were typically grown to 75% confluency and washed twice and incubated in amino acid-free DMEM for 1 h (or complete medium as a control) unless indicated. Rapamycin at 500 nm was included in complete growth medium where indicated.
Transfection and Transduction—293T cells and U2OS cells were transfected with Lipofectamine2000 (Invitrogen) according to the manufacturer's recommendations and typically analyzed 24-h post-transfection. MEF cells were stably transduced using a Moloney murine leukemia retrovirus-based pBabe system with 293T cells used as the packaging cell line.
Tandem Affinity Purification of ULK1-binding Partners—40 15×-cm plates of MEF cells, stably expressing low levels of FLAG and S-tagged ULK1 or control cells expressing vector alone were placed on ice, washed twice, in cold PBS, and lysed in IP buffer (50 mm HEPES, pH 7.4, 150 mm NaCl, 1 mm EDTA, 0.5 mm DTT, 10% glycerol, 0.5% Triton X-100) plus protease inhibitors. Lysates were then centrifuged for 10 min at 5,000 × g, 4 °C, followed by an additional spin at 100,000 × g for 1 h at 4 °C. Clarified lysates were then incubated with anti-FLAG-agarose beads (Sigma) overnight with rotation at 4 °C. Following washes, bound proteins were eluted in IP buffer containing 200 μg/ml 3× FLAG peptide (Sigma) for 30 min on ice. Eluates were then incubated with anti-S tag beads (Novagen) for 4 h at 4 °C with rotation. Following four washes in IP buffer and one wash in PBS, samples were eluted in SDS-sample buffer and proteins identified by mass spectrometry.
RNA Interference—The shRNA constructs against mouse FIP200 and ATG13 were generated using pSuperRetro vector according to the manufacturer's procedure (OligoEngine). The FIP200 DNA sequences used in the RNAi constructs were: GAGAGAACTTGTTGAGAAA and ACATGAAGGCTCAGAGAAA. The ULK1 sequences: GAGCAAGAGCACACGGAAA and AGACTCCTGTGACACAGAT. The ATG13 sequences: GAGAAGAATGTCCGAGAAT and ACAGGAAGGACTTGGACAA. Following transfection and puromycin selection, multiple stable clones were chosen and showed no significant variation in phenotype with respect to protein knock-down.
RT-PCR—Control and ATG13 knock-down cells grown in a 6×-cm plate were harvested using the Total RNA Mini kit (Bio-Rad). Total RNA was measured, and 0.5 μg of each sample was used for first strand cDNA synthesis (iScript cDNA Synthesis kit, Bio-Rad). One-tenth of the total cDNA reaction served as the template for the PCR reaction. Primers for both ATG13 and GAPDH were designed to amplify roughly 500 bp of the 3′-region of both genes. PCR was carried out using the Taq Polymerase per routine protocol.
Immunoprecipitations—Following induction of autophagy, cells grown in 10×-cm plates were placed on ice and washed twice in ice-cold PBS followed by lysis in IP buffer plus protease inhibitors. Lysates were then centrifuged at maximum speed in a benchtop microcentrifuge at 4 °C for 10 min. Clarified lysates were then incubated with mouse anti-GFP for 1 h with rotation at room temperature, followed by addition of protein G-Sepharose beads (GE Healthcare) prewashed in IP buffer for an additional 30 min. Beads were then washed three times in IP buffer, followed by one wash in PBS before elution in SDS-sample buffer and analysis by SDS-PAGE and immunoblot.
Binding Assays—Purified, recombinant protein at 100 nm was incubated in binding buffer (25 mm Tris, pH 7.5, 100 mm NaCl, 0.5 mm DTT, 0.5 mg/ml bovine serum albumin, 0.1% Triton X-100) for 1 h at room temperature with rotation. For ULK1 pull-down assays, reactions were incubated with rabbit anti-ULK1 for 30 min, followed by protein A-Sepharose beads, prewashed in binding buffer, for an additional 30 min. Beads were then washed four times in binding buffer followed by elution in sample buffer and analysis by SDS-PAGE and immunoblot. For ATG13 pull-down assays, samples were incubated with prewashed T7-agarose beads (Novagen) for 1 h before washing in binding buffer and analysis as for ULK1 pull-down.
Gel Filtration—The indicated recombinant proteins, at 100 nm, were preincubated in gel filtration buffer (25 mm Tris, pH 7.5, 100 mm NaCl, 1 mm DTT) and 0.5 mg/ml bovine serum albumin for 1 h before loading 50 μl onto a SMART-FPLC system Superose 6 column, pre-equlibrated in gel filtration buffer. 100-μl fractions were collected and analyzed by SDS-PAGE and immunoblot.
Kinase Assays—Typically, 10 nm purified recombinant ULK1 and the indicated amount of FIP200 or ATG13 was incubated in kinase buffer (25 mm HEPES, pH 7.4, 50 mm NaCl, 5 mm MgCl2, 1 mm DTT, 0.5 mg/ml bovine serum albumin) containing 0.3 mg/ml myelin basic protein, 30 μm cold ATP, and 0.5 μCi of [γ32-P]ATP for 10 min at 30 °C. Reactions were stopped by the addition of sample buffer, followed by SDS-PAGE, transfer to nitrocellulose, and analysis by phosphorImager.
Immunofluorescence—Cells were processed for immunofluorescence essentially as described (29). Slides were visualized on A Nikon Eclipse TE2000-U microscope and images processed using Adobe Photoshop.
RESULTS
ULK1 Forms a Triple Complex with ATG13 and FIP200—To understand the precise role of ULK1 in autophagy initiation, we screened for ULK1-interacting partners using a tandem affinity tag immunoprecipitation (IP) approach. We generated a mouse embryonic fibroblast (MEF) cell line stably expressing a ULK1 construct with an N-terminal FLAG and S tag (F/S-ULK1). Following sequential IP with anti-FLAG and anti-S tag antibodies, ULK1-associated proteins were identified by mass spectrometry. Two proteins identified were FIP200 and hypothetical protein LOC51897. During the course of this study, two reports were published describing FIP200 as a ULK-interacting protein (25) and a human ULK-interacting protein distantly related to yeast Atg13 (30), which had been previously identified in a bioinformatics analysis as a potential equivalent to yeast Atg13 (31). This latter protein turns out to be the homolog of the mouse LOC51897 discovered here. Further, although sequence similarity between LOC51897 and yeast Atg13 is very limited, we found significant sequence homology between LOC51897 and Caenorhabditis elegans ATG13 (data not shown). Based on these observations and the functional analysis described below, we now refer to LOC51897 as ATG13.
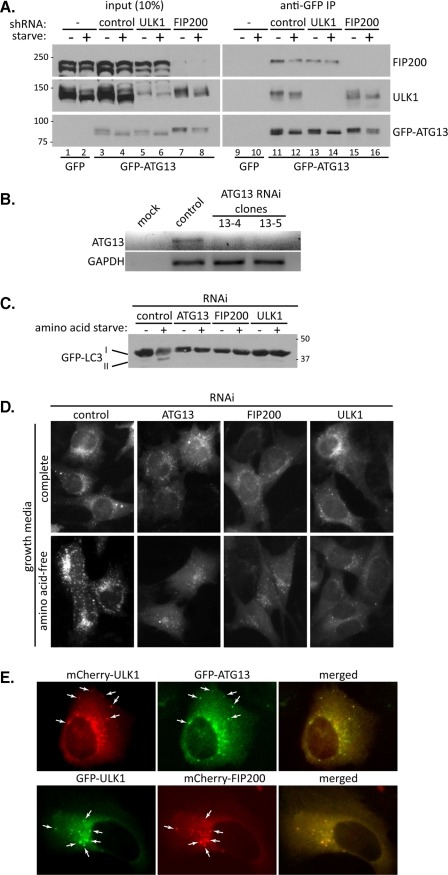
Although FIP200 and ATG13 have been identified as binding proteins of ULK1, how they regulate ULK1 is not clear. Therefore in this study we seek to understand the effect of FIP200 and ATG13 on ULK1 function. We hypothesize that ULK1 may form a complex with both proteins, analogous to the yeast Atg1·Atg13·Atg17 complex. Using MEF cells expressing GFP-ATG13, we confirmed the interaction between ULK1 and ATG13. Importantly, IP of GFP-ATG13 with anti-GFP led to not only the co-IP of endogenous ULK1 but also endogenous FIP200, whereas GFP alone failed to co-IP ULK1 or FIP200 (Fig. 1A). Interestingly, we found that both ULK1 and GFP-ATG13 run as multiple bands on SDS-PAGE and that upon starvation there is a downshift of both proteins to a faster migrating form (Fig. 1A, compare lane 3 with 4). This is indicative of a starvation-induced dephosphorylation event and indeed, treatment of cell lysate with phosphatase results in a similar downshift of ULK1 and ATG13 (see below and Fig. 5). In addition, ULK1 has previously been shown to be phosphorylated in cells (25, 30). Induction of autophagy by amino acid starvation failed to significantly alter the amount of ULK1 or FIP200 associating with GFP-ATG13 (Fig. 1A, compare lane 11 with 12), implying that ATG13 is interacting with ULK1 and FIP200 prior to autophagy induction.
FIGURE 1.
ATG13 interacts with both ULK1 and FIP200 in vivo. A, GFP-ATG13 immunoprecipitations. MEF cells, depleted of the indicated proteins by RNAi, were transduced with retrovirus expressing GFP-ATG13. Following a 1-h incubation in complete growth medium or amino acid-free medium (starve), cells were lysed, and GFP-ATG13 immunoprecipitated using mouse anti-GFP. Co-immunoprecipitated proteins were analyzed by immunoblotting as indicated. B, RNAi depletion of ATG13. Agarose gel depicting RT-PCR of ATG13 and GAPDH mRNA isolated from control-depleted and two ATG13-depleted cell clones (13-4 and 13-5) is shown. C, loss of ULK1 complex proteins inhibits autophagy. MEF cells, depleted of the indicated proteins and expressing GFP-LC3, were incubated in complete medium or amino acid-free medium for 1 h, followed by lysis, SDS-PAGE, and immunoblot with anti-GFP to detect GFP-LC3. D, same cells described in C were grown on glass coverslips and following incubation in complete medium or amino acid-free medium for 1 h, were fixed and GFP-LC3 visualized under the fluorescence microscope. E, ULK1, ATG13, and FIP200 colocalize upon autophagy stimulation. U2OS cells, grown on glass coverslips, were transfected with mCherry-ULK1 and GFP-ATG13 (top panels) or GFP-ULK1 and mCherry-FIP200 (bottom panels). 24-h post-transfection, cells were washed and incubated in amino acid-free medium for 1 h, followed by fixing and visualization under the fluorescence microscope. Arrows indicate examples of co-localization between mCherry-ULK1 and GFP-ATG13 (top panels) or GFP-ULK1 and mCherry-FIP200 (bottom panels).
FIGURE 5.
ULK1 and ATG13 are subjected to mTOR-mediated phosphorylation. A, ULK1 remains partially phosphorylated following autophagy induction. MEF cells were incubated in amino acid-free medium (starve) for 1 h followed by lysis. Lysates were then treated with phosphatase, or mock-treated, followed by immunoblot to analyze the mobility of ULK1. B, ATG13 is dephosphorylated upon autophagy induction. Lysates derived from MEF cells stably expressing GFP-ATG13 were either treated or mock-treated with phosphatase then subject to SDS-PAGE and immunoblotting anti-GFP. C, ULK1 and ATG13 are dephosphorylated upon autophagy induction. MEF cells expressing GFP-ATG13 were incubated in complete medium (control), medium containing 500 nm rapamycin (rapamycin) or amino acid-free medium (starve), followed by lysis and immunoblot with anti-ULK1 (top panel) or anti-GFP (bottom panel). Samples were loaded in duplicate. D, mTOR overexpression increases ATG13 phosphorylation. 293T cells were transfected with Myc-tagged ATG13, either alone or in combination with Myc-tagged wild type (WT) or kinase-dead (dead) mTOR as indicated. 24-h post-transfection cells were incubated in complete medium, medium containing 500 nm rapamycin, or amino acid-free medium (starve) for 1.5 h. Cells were then lysed, loaded in duplicate, and subjected to SDS-PAGE and immunoblot with anti-Myc.
The co-IP of both ULK1 and FIP200 with GFP-ATG13 suggests that either ATG13 interacts with both proteins simultaneously or that there are two pools of ATG13: one that interacts with ULK1 exclusively and one that interacts with FIP200. In yeast, Atg1 is thought to primarily interact with Atg17 through Atg13 (17). To determine if the interaction of GFP-ATG13 with ULK1 is mediated by FIP200 we carried out the co-IP of ULK1 in FIP200-depleted cells. We found that similar amount of ULK1 was co-precipitated with GFP-ATG13 in cells stably depleted of FIP200 by shRNA compared with control cells (Fig. 1A, compare lanes 11 and 12 with lanes 15 and 16). Conversely, the interaction between GFP-ATG13 and FIP200 was unaffected by depletion of ULK1 (Fig. 1A, compare lanes 11 and 12 with lanes 13 and 14), suggesting that ATG13 can interact independently with both ULK1 and FIP200 (though in the case of ULK1-depletion we cannot rule out ULK2 acting as a replacement). It could be argued that even though shRNA-mediated depletion of ULK1 or FIP200 is efficient (with ∼90% of the respective protein gone) there is still trace amounts of FIP200 (or ULK1) sufficient to mediate the interaction of ULK1 (or FIP200) with GFP-ATG13. We think this is unlikely as the depletion is sufficient to disrupt starvation-induced autophagy in these cells, as measured by immunofluorescence and Western blot of GFP-LC3 (Fig. 1, C and D).
To confirm that all three proteins are required for starvation-mediated autophagy, we expressed GFP-LC3 in cells depleted of ATG13, FIP200, and ULK1. Due to a lack of available ATG13 antibodies, we used mRNA levels and RT-PCR to monitor ATG13 depletion. As is depicted in Fig. 1B, ATG13 mRNA levels were almost undetectable in two stable MEF clones (unless stated, clone 13-5 was used in the experiments described below. All other RNAi experiments were also performed using two independent stable cell clones with the same outcome), indicating efficient depletion. Stable RNAi-depletion of ULK1 and FIP200 was monitored by Western blot (see Figs. 1A and 4C and supplemental Fig. S2A). Conjugation of cytosolic LC3 (form I) to the lipid phosphatidylethanolamine (form II) on the autophagosomal membrane, coupled with its subsequent degradation as the autophagosomes fuse with lysosomes is a hallmark of autophagy. When GFP-LC3 levels were monitored in control-depleted cells, there was clear conversion of the unconjugated form I to the lipidated form II and an overall reduction in GFP-LC3 levels upon amino acid withdrawal, indicating autophagy induction (Fig. 1C). However, in ATG13, FIP200 and ULK1-depleted cells, the conversion of LC3 to the lipidated form or the reduction in levels was inhibited, indicating a block of autophagy in response to amino acid deprivation (Fig. 1C). This result is further supported by GFP-LC3 immunofluorescence in cells (Fig. 1D): in control cells there is an increase in GFP-LC3 punctae upon starvation, indicating a large increase in autophagosome formation and correlating well with the Western blot data in Fig. 1C; In contrast, no significant increase in GFP-LC3 punctae was observed in the ATG13, FIP200, or ULK1-depleted cells, confirming the autophagy block in these cells.
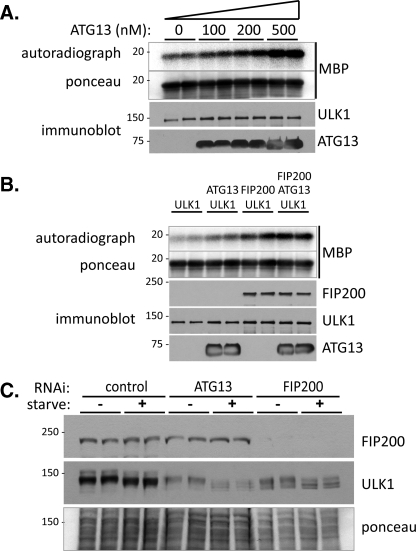
FIGURE 4.
Stimulation of ULK1 kinase activity by ATG13 and ULK1. A, ATG13 stimulates ULK1 kinase activity. 10 nm recombinant ULK1 was incubated with the indicated amounts of recombinant FLAG-ATG13 in the presence of 0.3 mg/ml MBP and [γ-32P]ATP as described under “Experimental Procedures.” Reactions were stopped by the addition of SDS sample buffer followed by SDS-PAGE and transfer to nitrocellulose. The upper panel shows the autoradiograph of 32P-labeled MBP with the total amount of MBP shown by Ponceau-S staining of the membrane below. The quantities of ULK1 and FLAG-ATG13 in each reaction are indicated by immunoblot in the lower panels. B, ATG13 and FIP200 act additively to stimulate ULK1 kinase activity. 10 nm ULK1, 200 nm FLAG-ATG13, or 200 nm hisFIP200 were incubated alone or in combination, as indicated, along with MBP and [γ-32P]ATP and processed as in A. C, ULK1 kinase activity is impaired in ATG13- and FIP200-depleted cells. MEF cells, either control-, ATG13-, or FIP200-depleted were incubated in complete medium or amino acid-free medium for 1 h, followed by lysis and immunoblotting of FIP200 (top panel) and ULK1 (bottom panel). Protein loading of the duplicate samples is shown by Ponceau-S staining in the lower panel.
If ULK1, ATG13, and FIP200 interact in cells, then it is reasonable to assume that they will colocalize together by immunofluorescence microscopy. ULK1 and FIP200 have been shown to be primarily cytosolic in resting cells but localize with the isolation membrane marker ATG5, upon stimulation of autophagy (see Fig. 3A and Ref. 25). In the recent report detailing the discovery of human ATG13, a FLAG-tagged version showed a partial co-localization with GFP-LC3, which marks both the isolation membrane and mature autophagosome, upon starvation (30). To determine whether indeed ATG13 is localizing specifically to the isolation membrane, we transduced F/S-ULK1 stable MEFs with GFP-ATG13 retrovirus. Both ULK1 and GFP-ATG13 displayed a cytosolic staining pattern under normal growth conditions. However, they extensively co-localized to punctate structures upon starvation (supplemental Fig. S1). To confirm the co-localization of these three proteins, we transiently transfected U2OS cells with mCherry-ULK1 and GFP-ATG13 or GFP-ULK1 and mCherry-FIP200, under amino acid-starved conditions. As in Fig. 1E, the majority of mCherry-ULK1 punctae and GFP-ATG13 punctae colocalize with each other (top panels), and so do the majority of GFP-ULK1 punctae and mCherry-FIP200 punctae (bottom panels), while in normal culture medium these proteins displayed a diffusive, cytosolic staining pattern (data not shown). Therefore a significant proportion of ULK1, ATG13, and FIP200 colocalize at the isolation membrane upon amino acid starvation, supporting the hypothesis that all three proteins interact in a complex.
FIGURE 3.
Localization of ULK1 to the isolation membrane requires both ATG13 and FIP200. A, ULK1 localizes to isolation membranes upon autophagy stimulation. MEF cells stably expressing GFP-ATG5 alone (top panels) or GFP-ATG5 plus FLAG-S-tagged ULK1 (bottom panels) were grown on glass coverslips and following incubation in amino acid-free medium for 1 h, were fixed and processed for immunofluorescence. Costaining for endogenous ULK1 (red) is shown in the top panels along with GFP fluorescence (green), while staining with anti-S tag (for FLAG-S-ULK1) along with GFP fluorescence is shown in the bottom panels. B, localization of ULK1 is disrupted in ATG13- and FIP200-depleted cells. Left panels show 1-h amino acid-starved MEF cells, with the indicated protein depleted by RNAi, stained with anti-ULK1 (green) and DAPI (blue, to mark the nuclei). Right panels show the same depleted MEF cells expressing FLAG-S-tagged ULK1, treated as in the left panels, but stained with anti-S tag (red) to detect the tagged ULK1 as well as DAPI (blue).
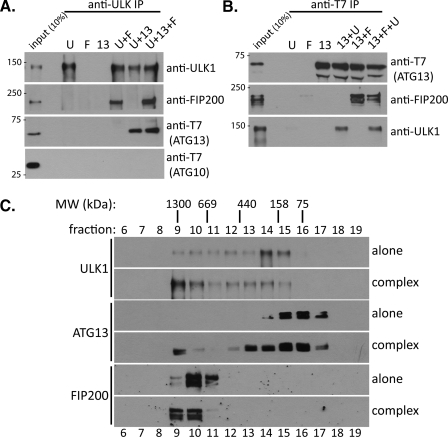
To gain definitive evidence that ULK1, ATG13, and FIP200 can interact directly and form a complex, we carried out in vitro binding assays using purified recombinant proteins produced in Sf9 cells (Fig. 2). We found that in ULK1 pull-down assays, ULK1 interacted directly with FIP200 and ATG13 (T7-tagged), both independently and in combination (Fig. 2A). Importantly, addition of FIP200 to the binding reaction did not alter the amount of ATG13 interacting with ULK1 and vice versa; ATG13 did not alter the interaction of FIP200 with ULK1 either, indicating that ULK1 interacts with FIP200 and ATG13 at distinct sites. As a control, T7-tagged ATG10 was included in all binding reactions and failed to interact with ULK1 (Fig. 2A, bottom panel). Similarly, in ATG13 pull-down assays, ATG13 also interacted with FIP200 and ULK1, both independently and in combination (Fig. 2B). To further examine whether ULK1, ATG13, and FIP200 form a triple complex, we conducted a gel filtration analysis using the purified recombinant proteins (Fig. 2C). ULK1 alone eluted at around fractions 14 and 15, correlating well with the observed size of ULK1 in SDS-PAGE of 150 kDa, indicating ULK1 is primarily a monomer in the absence of other proteins. ATG13 alone eluted in a peak corresponding to fractions 15 and 16, indicating a monomer, or possibly a homodimer in the absence of other proteins. FIP200 alone elutes at around fraction 10. Given the apparent molecular weight of FIP200 is ∼200 kDa, FIP200 is likely a homooligomer in the absence of other proteins. Importantly, when all three proteins are incubated together and resolved by gel filtration, a large macromolecular complex containing all three proteins was formed and eluted in fractions 9 and 10. Based on the gel filtration analysis, the apparent molecular mass of this protein complex is probably over 1 million daltons (Fig. 2C, top panels). However, since gel filtration separations are based on molecular shape rather than mass directly, additional experiments are needed in the future for accurate measurement of the molecular mass of this complex. It is interesting that when incubated together, a portion of both ULK1 and ATG13 were shifted to fractions 13 and 14, possibly reflecting ULK1·ATG13 complex formation.
FIGURE 2.
ULK1, ATG13, and FIP200 form a large protein complex. A, ULK1 interacts with ATG13 and FIP200. Equimolar amounts of the indicated recombinant proteins (U, ULK1; F, FIP200; 13, ATG13) were incubated together at room temperature for 1 h before precipitation of ULK1 with anti-ULK1 IgG. Following washes, bound proteins were subjected to immunoblotting with the indicated antibodies. B, ATG13 interacts with ULK1 and FIP200. Binding assay was carried out as in A, but T7-tagged ATG13 was precipitated using anti-T7 antibody followed by washing and analysis of bound proteins with immunoblotting. C, ULK1, ATG13, and FIP200 form a large protein complex. The indicated proteins were incubated alone or in combination followed by gel filtration over a Superose 6 column. The indicated fractions were subjected to immunoblotting with the relevant antibodies. The gel filtration position of the molecular weight standards (MW) is shown at the top.
ULK1 Function Requires both ATG13 and FIP200—As all three proteins can interact together in a complex, we investigated whether both FIP200 and ATG13 were required for proper ULK1 function. We first looked at the localization of ULK1 upon autophagy stimulation. ULK1 has previously been shown to localize to the isolation membrane upon amino acid starvation (25). We confirm this here, by showing both endogenous ULK1 and F/S-tagged ULK1 shift from a cytosolic staining pattern to a punctate pattern upon amino acid starvation, mirroring, almost exactly, GFP-ATG5 localization (Fig. 3A), while both ULK1 and ATG5 displayed a diffusive, cytosolic staining pattern under normal growth conditions (data not shown). It is possible that only one of the two binding partners of ULK1 is required for its cellular translocation upon starvation. However, we find that depletion of either ATG13 or FIP200 blocks the localization of endogenous ULK1 or F/S-ULK1 to the isolation membrane in MEF cells (Fig. 3B, compare middle and bottom panels with top panels). Therefore, a complete ULK1 complex, containing both FIP200 and ATG13, is required for ULK1 localization to isolation membrane upon sensing autophagic signals.
In addition to regulation of ULK1 localization, FIP200 and ATG13 might also stimulate the kinase activity of ULK1. Because we have generated purified recombinant proteins for ULK1, ATG13, and FIP200, for the first time regulation of Atg1/ULK1 enzymatic activity can be unambiguously assessed in vitro. As with yeast Atg1, the kinase substrates of ULK1 are unknown so we utilized MBP as the kinase substrate. In the absence of ULK1, ATG13 or FIP200 could not phosphorylate MBP (data not shown) while ULK1 alone caused modest MBP phosphorylation (Fig. 4A). Importantly, addition of recombinant ATG13 stimulated ULK1 kinase activity toward MBP in the absence of FIP200 and in an ATG13 dose-dependent manner. Similarly, FIP200 alone is also able to stimulate the kinase activity of ULK1 in the absence of ATG13. The presence of both ATG13 and FIP200 resulted in an additive stimulation of ULK1 activity (Fig. 4B). Therefore, maximal ULK1 kinase activity requires both ATG13 and FIP200 in vitro.
We also obtained cellular evidence that supported the role of ATG13 and FIP200 in enhancing the kinase activity of ULK1. ULK1 autophosphorylation has been reported previously (25, 30, 32), and this is confirmed by our experiments in 293T cells expressing ULK1 with a mutation in either the kinase active site (K46R) or the ATP-binding pocket (M92A). Both mutants showed faster migration in SDS-PAGE than wild-type ULK1, and phosphatase treatment converts the wild-type ULK1 to the faster migrating band (see supplemental Fig. S2B and data not shown). Using this as the readout, we assessed the cellular autophosphorylation activity of ULK1 in both ATG13- and FIP200-depleted MEF cells and reproducibly found that ULK1 migrates faster in ATG13- or FIP200-depleted cells than that in control-depleted cells (Fig. 4C). This faster migration is irrespective of autophagy-stimulating conditions, but is especially evident in amino acid-starved cells (Fig. 4C, compare lanes 3 and 4 with 7 and 8 and lanes 11 and 12). It therefore appears that ULK1 kinase activity is impaired in ATG13- and FIP200-depleted cells, supporting the in vitro data that both proteins are required for ULK1 maximal kinase activity. It is interesting to note that ULK1 levels in ATG13- and FIP200-deleted cells are reduced relative to those in control-depleted cells (Fig. 4C, middle panel). It has previously been shown that ULK1 is destabilized in FIP200-depleted cells (25), and now it appears that ATG13 is also required, supporting our hypothesis that ULK1 is present in a complex with both ATG13 and FIP200, and that the proper complex formation is required for ULK1 stability.
Although critical for understanding how Atg1/ULK1 mediates autophagy response, their physiological substrates remain unknown. In this study we obtained evidence suggesting that FIP200 is a substrate of ULK1 (supplemental Fig. S2). In cells depleted of ULK1, endogenous FIP200 migrates as a faster form in SDS-PAGE when compared with control-depleted cells, indicative of a dephosphorylated form of FIP200 (supplemental Fig. S2A). In addition, overexpression of FLAG-tagged ULK1 in 293T cells in combination with Myc-tagged FIP200 results in a slower migrating form of FIP200 (supplemental Fig. S2B). This increase in mycFIP200 size is dependent on ULK1 kinase activity, as expression of the kinase impaired K64R or M92A ULK1 mutants fails to modify mycFIP200 (supplemental Fig. S2B). Further, treatment of cell lysates with phosphatase decreases mycFIP200 size back to the level observed in non-ULK1-overexpressing cells (supplemental Fig. S2C). Finally, recombinant ULK1 can phosphorylate FIP200, as well as ATG13, in vitro (supplemental Fig. S2D). It should be noted that upon autophagy treatment, we were not able to observe migration change of endogenous FIP200 in SDS-PAGE. Therefore the role of FIP200 phosphorylation by ULK1 in autophagy has not been established. On the other hand, it is possible that only a small population of FIP200 is subjected to ULK1 phosphorylation (and thus the migration shift undetectable), because FIP200 is apparently a multifunctional protein (33).
Regulation of the ULK1 Complex by mTOR-mediated Phosphorylation—We found that ULK1 exists in multiple phosphorylation states in the cell and induction of autophagy results in an increase in ULK1 mobility (Fig. 1A, compare lanes 1 with 2 and 3 with 4; Figs. 4C and 5C). As mentioned above, ULK1 mobility is increased in ATG13- and FIP200-depleted cells (loss of autophosphorylation), yet is further increased upon amino acid starvation (Fig. 4C). Importantly, the mobility of ULK1 from extracts of starved cells can also be further increased by phosphatase treatment, indicating ULK1 is regulated by multiple phosphorylation-dephosphorylation processes (Fig. 5A). In addition, Fig. 1A showed that ATG13 runs as a doublet in SDS-PAGE gels with the loss of the upper band upon autophagy stimulation. Indeed, this ATG13 upper band is a phosphorylated form as treatment of cell lysates with phosphatase causes its disappearance (Fig. 5B). In all eukaryotes analyzed so far, the TOR pathway acts as a negative regulator of autophagy and inhibition of TOR with rapamycin is sufficient to induce autophagy. In yeast it has been shown that treatment of cells with rapamycin results in dephosphorylation of Atg13 and this correlates well with autophagy induction. We observed a similar phenomenon in mammalian cells. Treatment of MEF cells stably expressing GFP-ATG13 with amino acid-free medium or rapamycin resulted in dephosphorylation of GFP-ATG13 and ULK1 (Fig. 5C). This suggests that mTOR is involved in the regulation of the mammalian ULK1·ATG13·FIP200 complex in a similar fashion to Tor regulation of the yeast Atg1·Atg13·Atg17 complex. Consistently, we found that overexpression of wild-type mTOR resulted in an increase of ATG13 phosphorylation (Fig. 5D; compare lanes 1 and 2 with 9 and 10). This increase in ATG13 phosphorylation was abolished upon amino acid starvation or treatment with rapamycin (Fig. 5D, lanes 3, 4 and 5, 6, respectively). As expected, this increase in ATG13 phosphorylation was dependent on mTOR kinase activity as overexpression of a kinase-dead form of mTOR resulted in loss of the ATG13 upper band (Fig. 5D, lanes 8 and 9). Likewise, preliminary data also suggest kinase-active mTOR overexpression can increase ULK1 phosphorylation (data not shown). Thus ATG13, and likely ULK1, are direct substrates of the mTOR kinase pathway.
DISCUSSION
We describe here identification and characterization of a large, multiprotein complex, essential for mammalian autophagy. This complex consists of at least three proteins: ULK1, ATG13, and FIP200. Various lines of in vitro and in vivo evidence presented here argue that all three proteins are present in a complex together. (A) All three proteins can interact independently with each other, indicating distinct, non-competing binding. (B) All three proteins elute from the same gel filtration fractions only when present together, indicating that they are part of the same macromolecular complex. (C) All three proteins co-localize at the isolation membrane upon autophagy stimulation. (D) The stability of ULK1 depends on the presence of both FIP200 and ATG13, as loss of either one result in reduced ULK1 levels at steady state. This phenomenon is often observed with proteins that form a tight and constitutive interaction together. We did not detect any significant loss of FIP200 in ULK1- or ATG13-depleted cells; however FIP200 is believed to function in multiple pathways (33), and it is possible only a small proportion of total cellular FIP200 interacts with ULK1. Finally, (E) maximal ULK1 kinase activity in vitro is only achieved when both ATG13 and FIP200 are present in combination, showing that functionally all three proteins are complexed together.
Comparison between the Mammalian ULK1 Complex and the Yeast Atg1 Complex—Until now, it was questioned whether the mammalian system of autophagy closely paralleled the yeast system with respect to the Atg1/ULK1 kinase. In yeast, it is well established that inhibition of Tor kinase, through nutrient starvation or rapamycin treatment, results in dephosphorylation of Atg13 leading to the formation of a complex between Atg1, Atg13, and Atg17. Formation of this complex causes stimulation of Atg1 kinase activity, and this is thought to be the mechanism by which Tor signaling controls autophagy (19). In mammalian cells, ULK1 was discovered as the yeast counterpart to Atg1 (23) yet no homolog of Atg13 or Atg17 was readily discernable from the sequence databases, suggesting mammalian ULK1 might function differently in autophagy when compared with yeast Atg1. With the discovery of FIP200 as a ULK-interacting protein essential for autophagy (27), similarities were drawn to yeast Atg17 even though percentage sequence identity was very low (25, 27). We and others (30), have now identified mammalian ATG13. We further show here that this protein interacts with FIP200 as well as ULK1, providing compelling evidence that the mammalian ULK1·ATG13·FIP200 complex is the equivalent of the yeast Atg1·Atg13·Atg17 complex.
There are some significant differences between yeast Atg1 and mammalian ULK1 systems. Firstly, in yeast it is believed that the dephosphorylation of Atg13 triggers the formation of the Atg1·Atg13·Atg17 complex, while in mammalian cells the ULK1·ATG13·FIP200 complex is already formed, prior to autophagy induction. We could see no increase in ULK1·ATG13·FIP200 complex formation following induction of autophagy by amino acid starvation, despite seeing dephosphorylation of ATG13 (see Fig. 1A). Indeed, ULK1 can co-IP both phosphorylated and dephosphorylated forms of ATG13 to a similar extent (not shown). We also note that ULK1 is regulated by mTOR in a similar fashion to ATG13. Treatment with rapamycin, or starvation, results in dephosphorylation of ULK1 as well as ATG13. Additionally, overexpression of active mTOR decreases the mobility of endogenous ULK1 in 293T cells. Dephosphorylation of Atg1 has previously been observed in yeast upon stimulation of autophagy, though this was attributed to loss of autophosphorylation (16). We now show that this is dependent on the TOR pathway, at least in mammals, and potentially acts as another level of regulation in addition to ATG13 dephosphorylation. As both ULK1 and ATG13 become dephosphorylated upon amino acid starvation or rapamycin treatment, we propose that it is this event, rather than the formation of the complex that is the important trigger for autophagy in mammalian cells. It is important to note that this starvation/rapamycin-induced dephosphorylation of ULK1 and ATG13 occurs in cells depleted of their respective complex binding partners (Figs. 1A and 4C), suggesting that localization to the isolation membrane, which is inhibited in these cells, follows after dephosphorylation. Given the effect of rapamycin treatment on dephosphorylation of ULK1 and ATG13, we propose that like the yeast system, mTOR phosphorylates and inhibits the ULK1 kinase complex under normal growth conditions, thus providing the mechanism by which the mTOR pathway controls autophagy in higher eukaryotes. Secondly, it appears that no one protein bridges the interaction between the ULK1·ATG13·FIP200 complex. In yeast it has been proposed that the interaction between Atg1 and Atg13 is mediated by Atg17, as in atg17Δ cells Atg1 and Atg13 fail to interact by co-IP (17, 18). Here we find that in the absence of FIP200, ATG13 can still co-IP ULK1 in amounts similar to control cells, and likewise, ATG13 can still co-IP FIP200 in the absence of ULK1. More importantly, we demonstrated in vitro using purified, recombinant proteins that any two of ULK1, ATG13, and FIP200 can interact with each other independently of the third protein.
These results lead us to the exact function of the individual components of the ULK1 kinase complex, with respect to autophagy. One of the major functions of ATG13 and FIP200 is to regulate ULK1 kinase activity: either alone can do so in the absence of the other, however maximal stimulation of ULK1 activity requires both. On the other hand, both ATG13 and FIP200 are required for ULK1 localization to the isolation membrane, and absence of either of them prevents correct localization of ULK1. Therefore, it seems the proper formation of the ULK1·ATG13·FIP200 complex is essential for autophagy-induced ULK1 translocation. In addition, the proper complex formation appears to be important for ULK1 protein stability.
If a clear function of ATG13 and FIP200 is to stimulate ULK1 kinase activity, then the obvious, and long standing, questions are what are the physiological substrates of ULK1 and what are the consequences of their phosphorylation? The work presented here brings us closer to the answer to these questions. First, not only does it appear that ULK1 can phosphorylate both ATG13 and FIP200 in vitro but also that ULK1 can phosphorylate FIP200 in cells (supplemental Fig. S2). We are currently in the process of identifying the sites on FIP200 and ATG13 phosphorylated by ULK1 to determine their functional significance on autophagy. Second, to obtain maximal ULK1 kinase activity, both ATG13 and FIP200 need to be present, and these two proteins might be involved in recruitment of ULK1 substrates. Therefore any in vitro screen for ULK1 substrates need to utilize the complete ULK1·ATG13·FIP200 complex to increase the chances of finding physiologically relevant substrates.
Supplementary Material
Acknowledgments
We thank members of the X. J. laboratory for critical reading and discussion.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 CA113890 (to X. J.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: ULK, Unc-51-like kinase; DTT, dithiothreitol; PBS, phosphate-buffered saline; GFP, green fluorescent protein; IP, immunoprecipitation; MEF, mouse embryonic fibroblast; RNAi, RNA interference; MBP, myelin basic protein; mTOR, mammalian target of rapamycin.
References
- 1.Klionsky, D. J. (2005) J. Cell Sci. 118 7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine, B., and Klionsky, D. J. (2004) Dev. Cell 6 463-477 [DOI] [PubMed] [Google Scholar]
- 3.Mizushima, N. (2007) Genes Dev. 21 2861-2873 [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez, M. G., Master, S. S., Singh, S. B., Taylor, G. A., Colombo, M. I., and Deretic, V. (2004) Cell 119 753-766 [DOI] [PubMed] [Google Scholar]
- 5.Komatsu, M., Waguri, S., Chiba, T., Murata, S., Iwata, J., Tanida, I., Ueno, T., Koike, M., Uchiyama, Y., Kominami, E., and Tanaka, K. (2006) Nature 441 880-884 [DOI] [PubMed] [Google Scholar]
- 6.Pattingre, S., Tassa, A., Qu, X., Garuti, R., Liang, X. H., Mizushima, N., Packer, M., Schneider, M. D., and Levine, B. (2005) Cell 122 927-939 [DOI] [PubMed] [Google Scholar]
- 7.Singh, S. B., Davis, A. S., Taylor, G. A., and Deretic, V. (2006) Science 313 1438-1441 [DOI] [PubMed] [Google Scholar]
- 8.Hara, T., Nakamura, K., Matsui, M., Yamamoto, A., Nakahara, Y., Suzuki-Migishima, R., Yokoyama, M., Mishima, K., Saito, I., Okano, H., and Mizushima, N. (2006) Nature 441 885-889 [DOI] [PubMed] [Google Scholar]
- 9.De Duve, C., and Wattiaux, R. (1966) Annu. Rev. Physiol. 28 435-492 [DOI] [PubMed] [Google Scholar]
- 10.Thumm, M., Egner, R., Koch, B., Schlumpberger, M., Straub, M., Veenhuis, M., and Wolf, D. H. (1994) FEBS Lett. 349 275-280 [DOI] [PubMed] [Google Scholar]
- 11.Tsukada, M., and Ohsumi, Y. (1993) FEBS Lett. 333 169-174 [DOI] [PubMed] [Google Scholar]
- 12.Harding, T. M., Morano, K. A., Scott, S. V., and Klionsky, D. J. (1995) J. Cell Biol. 131 591-602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki, K., and Ohsumi, Y. (2007) FEBS Lett. 581 2156-2161 [DOI] [PubMed] [Google Scholar]
- 14.Klionsky, D. J., Cregg, J. M., Dunn, W. A., Jr., Emr, S. D., Sakai, Y., Sandoval, I. V., Sibirny, A., Subramani, S., Thumm, M., Veenhuis, M., and Ohsumi, Y. (2003) Dev. Cell 5 539-545 [DOI] [PubMed] [Google Scholar]
- 15.Yorimitsu, T., and Klionsky, D. J. (2005) Cell Death Differ 12, Suppl. 2, 1542-1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuura, A., Tsukada, M., Wada, Y., and Ohsumi, Y. (1997) Gene 192 245-250 [DOI] [PubMed] [Google Scholar]
- 17.Kabeya, Y., Kamada, Y., Baba, M., Takikawa, H., Sasaki, M., and Ohsumi, Y. (2005) Mol. Biol. Cell 16 2544-2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheong, H., Yorimitsu, T., Reggiori, F., Legakis, J. E., Wang, C. W., and Klionsky, D. J. (2005) Mol. Biol. Cell 16 3438-3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamada, Y., Funakoshi, T., Shintani, T., Nagano, K., Ohsumi, M., and Ohsumi, Y. (2000) J. Cell Biol. 150 1507-1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budovskaya, Y. V., Stephan, J. S., Reggiori, F., Klionsky, D. J., and Herman, P. K. (2004) J. Biol. Chem. 279 20663-20671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budovskaya, Y. V., Stephan, J. S., Deminoff, S. J., and Herman, P. K. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 13933-13938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrera, A. C. (2004) J. Cell Sci. 117 4615-4616 [DOI] [PubMed] [Google Scholar]
- 23.Chan, E. Y., Kir, S., and Tooze, S. A. (2007) J. Biol. Chem. 282 25464-25474 [DOI] [PubMed] [Google Scholar]
- 24.Young, A. R., Chan, E. Y., Hu, X. W., Kochl, R., Crawshaw, S. G., High, S., Hailey, D. W., Lippincott-Schwartz, J., and Tooze, S. A. (2006) J. Cell Sci. 119 3888-3900 [DOI] [PubMed] [Google Scholar]
- 25.Hara, T., Takamura, A., Kishi, C., Iemura, S., Natsume, T., Guan, J. L., and Mizushima, N. (2008) J. Cell Biol. 181 497-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kundu, M., Lindsten, T., Yang, C. Y., Wu, J., Zhao, F., Zhang, J., Selak, M. A., Ney, P. A., and Thompson, C. B. (2008) Blood 112 1493-1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hara, T., and Mizushima, N. (2009) Autophagy 5 85-87 [DOI] [PubMed] [Google Scholar]
- 28.Shao, Y., Gao, Z., Feldman, T., and Jiang, X. (2007) Autophagy 3 10-16 [DOI] [PubMed] [Google Scholar]
- 29.Ganley, I. G., Carroll, K., Bittova, L., and Pfeffer, S. (2004) Mol. Biol. Cell 15 5420-5430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan, E. Y., Longatti, A., McKnight, N. C., and Tooze, S. A. (2009) Mol. Cell. Biol. 29 157-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meijer, W. H., van der Klei, I. J., Veenhuis, M., and Kiel, J. A. (2007) Autophagy 3 106-116 [DOI] [PubMed] [Google Scholar]
- 32.Yan, J., Kuroyanagi, H., Kuroiwa, A., Matsuda, Y., Tokumitsu, H., Tomoda, T., Shirasawa, T., and Muramatsu, M. (1998) Biochem. Biophys. Res. Commun. 246 222-227 [DOI] [PubMed] [Google Scholar]
- 33.Gan, B., and Guan, J. L. (2008) Cell Signal 20 787-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.