Abstract
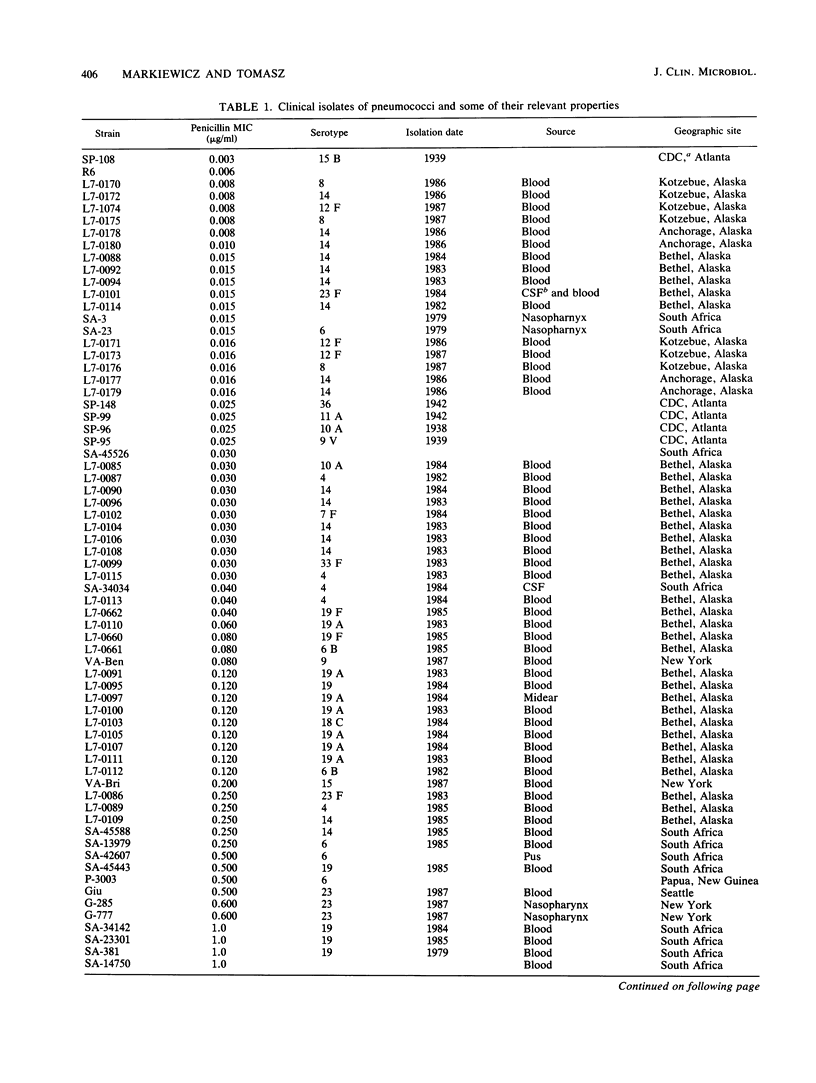
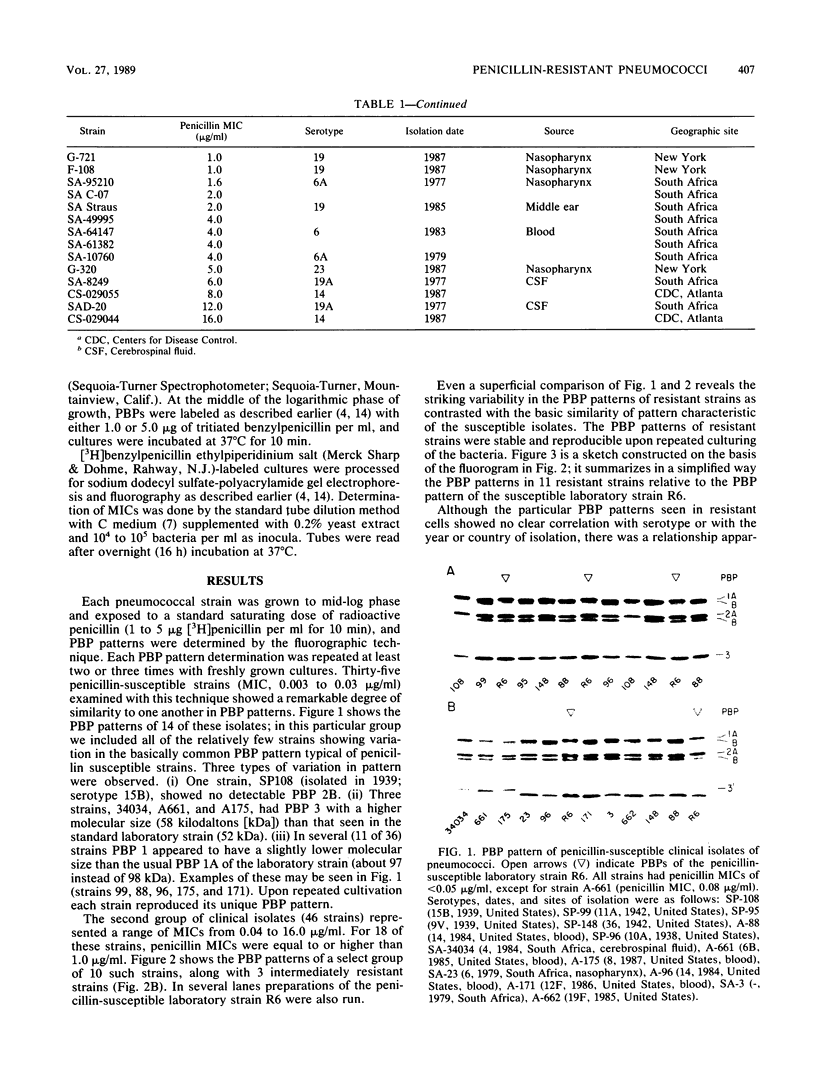
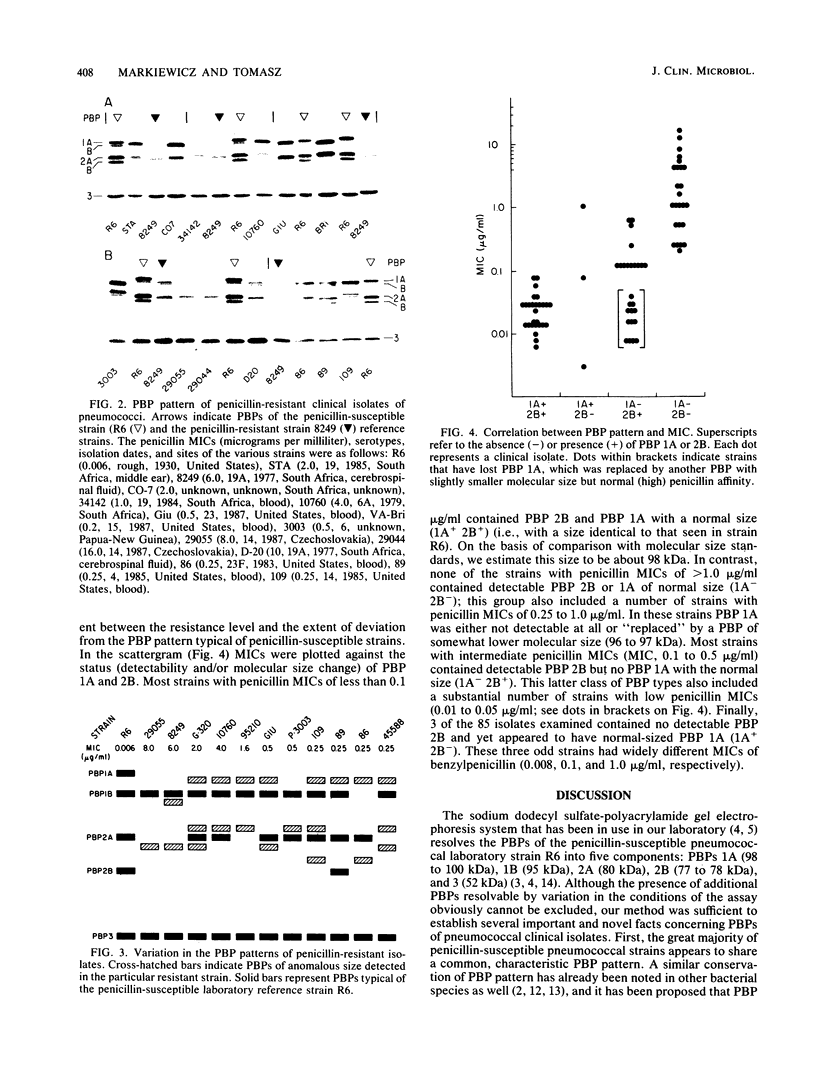
A large number of pneumococcal isolates (over 80 strains) from a variety of geographic locales and representing a spectrum of resistance levels from a penicillin MIC of 0.003 microgram/ml up to an MIC of 16 micrograms/ml were analyzed for their penicillin-binding protein (PBP) patterns. With a few exceptions, the great majority of strains with penicillin MICs up to about 0.05 microgram/ml contained the same set of five PBPs with molecular sizes typical of those of susceptible pneumococci. In strains with penicillin MICs of about 0.1 microgram/ml and up, virtually all isolates showed two common features: (i) all isolates showed loss of PBP 1A (98 kilodaltons) with or without a parallel appearance of a "new" PBP that ranged in molecular size between 96 and 97 kilodaltons; and (ii) in strains with penicillin MICs of 0.5 microgram/ml or more, PBP 2B could not be detected on the fluorograms even with very high concentrations of radioactive penicillin. Beyond these two common features, resistant strains with similar penicillin MICs showed a surprising variety of PBP profiles (i.e., in the number and molecular sizes of PBPs), each characteristic of a given isolate. We suggest that in pneumococci remodeling of critical PBPs in more than one way may result in comparable levels of penicillin resistance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Garcia-Bustos J. F., Chait B. T., Tomasz A. Structure of the peptide network of pneumococcal peptidoglycan. J Biol Chem. 1987 Nov 15;262(32):15400–15405. [PubMed] [Google Scholar]
- Hakenbeck R., Ellerbrok H., Briese T., Handwerger S., Tomasz A. Penicillin-binding proteins of penicillin-susceptible and -resistant pneumococci: immunological relatedness of altered proteins and changes in peptides carrying the beta-lactam binding site. Antimicrob Agents Chemother. 1986 Oct;30(4):553–558. doi: 10.1128/aac.30.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Tarpay M., Tomasz A. Multiple changes of penicillin-binding proteins in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):364–371. doi: 10.1128/aac.17.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerger S., Tomasz A. Alterations in penicillin-binding proteins of clinical and laboratory isolates of pathogenic Streptococcus pneumoniae with low levels of penicillin resistance. J Infect Dis. 1986 Jan;153(1):83–89. doi: 10.1093/infdis/153.1.83. [DOI] [PubMed] [Google Scholar]
- Kendall D. A., Bock S. C., Kaiser E. T. Idealization of the hydrophobic segment of the alkaline phosphatase signal peptide. Nature. 1986 Jun 12;321(6071):706–708. doi: 10.1038/321706a0. [DOI] [PubMed] [Google Scholar]
- Malouin F., Bryan L. E. Modification of penicillin-binding proteins as mechanisms of beta-lactam resistance. Antimicrob Agents Chemother. 1986 Jul;30(1):1–5. doi: 10.1128/aac.30.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Penicillin-binding proteins and the antibacterial effectiveness of beta-lactam antibiotics. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 3):S260–S278. doi: 10.1093/clinids/8.supplement_3.s260. [DOI] [PubMed] [Google Scholar]
- Vlasuk G. P., Inouye S., Inouye M. Effects of replacing serine and threonine residues within the signal peptide on the secretion of the major outer membrane lipoprotein of Escherichia coli. J Biol Chem. 1984 May 25;259(10):6195–6200. [PubMed] [Google Scholar]
- Williamson R., Calderwood S. B., Moellering R. C., Jr, Tomasz A. Studies on the mechanism of intrinsic resistance to beta-lactam antibiotics in group D streptococci. J Gen Microbiol. 1983 Mar;129(3):813–822. doi: 10.1099/00221287-129-3-813. [DOI] [PubMed] [Google Scholar]
- Williamson R., Gutmann L., Horaud T., Delbos F., Acar J. F. Use of penicillin-binding proteins for the identification of enterococci. J Gen Microbiol. 1986 Jul;132(7):1929–1937. doi: 10.1099/00221287-132-7-1929. [DOI] [PubMed] [Google Scholar]
- Williamson R., Hakenbeck R., Tomasz A. In vivo interaction of beta-lactam antibiotics with the penicillin-binding proteins of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Oct;18(4):629–637. doi: 10.1128/aac.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zighelboim S., Tomasz A. Multiple antibiotic resistance in South African strains of Streptococcus pneumoniae: mechanism of resistance to beta-lactam antibiotics. Rev Infect Dis. 1981 Mar-Apr;3(2):267–276. doi: 10.1093/clinids/3.2.267. [DOI] [PubMed] [Google Scholar]
- Zighelboim S., Tomasz A. Penicillin-binding proteins of multiply antibiotic-resistant South African strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):434–442. doi: 10.1128/aac.17.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]