Abstract
Lipopolysaccharide (LPS), a common bacteria-derived product, has long been recognized as a key factor implicated in periodontal bone loss. However, the precise cellular and molecular mechanisms by which LPS induces bone loss still remains controversial. Here, we show that LPS inhibited osteoclastogenesis from freshly isolated osteoclast precursors but stimulated osteoclast formation from those pretreated with RANKL in vitro in tissue culture dishes, bone slices, and a co-culture system containing osteoblasts, indicating that RANKL-mediated lineage commitment is a prerequisite for LPS-induced osteoclastogenesis. Moreover, the RANKL-mediated lineage commitment is long term, irreversible, and TLR4-dependent. LPS exerts the dual function primarily by modulating the expression of NFATc1, a master regulator of osteoclastogenesis, in that it abolished RANKL-induced NFATc1 expression in freshly isolated osteoclast precursors but stimulated its expression in RANKL-pretreated cells. In addition, LPS prolonged osteoclast survival by activating the Akt, NF-κB, and ERK pathways. Our current work has not only unambiguously defined the role of LPS in osteoclastogenesis but also has elucidated the molecular mechanism underlying its complex functions in osteoclast formation and survival, thus laying a foundation for future delineation of the precise mechanism of periodontal bone loss.
LPS,2 a common bacteria-derived product, has long been recognized as a key factor implicated in the development of chronic periodontitis. LPS plays an important role in periodontitis by initiating a local host response in gingival tissues that involves recruitment of inflammatory cells, production of prostanoids and cytokines, elaboration of lytic enzymes and activation of osteoclast formation and function to induce bone loss (1-3).
Osteoclasts, the body's sole bone-resorbing cells, are multinucleated giant cells that differentiate from cells of hematopoietic lineage upon stimulation by two critical factors: the macrophage/monocyte colony-forming factor (M-CSF) and the receptor activator of NF-κB ligand (RANKL) (4-6). RANKL exerts its effects on osteoclast formation and function by binding to its receptor, RANK (receptor activator of NF-κB) expressed on osteoclast precursors and mature osteoclasts (7-9). RANKL also has a decoy receptor, osteoprotegerin, which inhibits RANKL action by competing with RANK for binding RANKL (10, 11).
RANK is a member of the tumor necrosis factor receptor (TNFR) family (12). Members of the TNFR family lack intrinsic enzymatic activity, and hence they transduce intracellular signals by recruiting various adaptor proteins including TNF receptor-associated factors (TRAFs) through specific motifs in the cytoplasmic domain (13, 14). It has been established that RANK contains three functional TRAF-binding sites (369PFQEP373, 559PVQEET564, and 604PVQEQG609) that, redundantly, play a role in osteoclast formation and function (15, 16). Collectively, through these functional TRAF-binding motifs, RANK activates six major signaling pathways, NF-κB, JNK, ERK, p38, NFATc1, and Akt, which play important roles in osteoclast formation, function, and/or survival (15, 17-19). In particular, NFATc1 has been established as a master regulator of osteoclast differentiation (20-22).
The involvement of osteoclasts in the pathogenesis of periodontal bone loss is supported by observations that osteoclasts are physically present and functionally involved in bone resorption in periodontal tissues (23-27). RANKL and RANK knockout mice develop osteopetrosis and show failure in tooth eruption due to a lack of osteoclasts (24, 25, 28). Moreover, op/op mice, in which a mutation in the coding region of the M-CSF gene generates a stop codon that leads to premature termination of translation of M-CSF mRNA, also show osteopetrosis and failure in tooth eruption due to a defect in osteoclast development (26, 27).
Whereas the role of osteoclasts in periodontal disease associated alveolar bone destruction has been well established, the precise role of LPS in osteoclastogenesis still remains controversial. The vast majority of the previous studies demonstrated that LPS stimulates osteoclastogenesis. This is consistent with the role that LPS, a well recognized pathogenic factor in periodontitis, presumably plays in periodontal bone loss (29-33). However, two previous studies demonstrated, surprisingly, that LPS plays bifunctional roles in osteoclastogenesis in that although it inhibits osteoclast formation from normal osteoclast precursors, it reverses to promote osteoclastogenesis from osteoclast precursors pretreated with RANKL (34, 35). Given that this finding is inconsistent with the presumed role of LPS as a pathogenic factor in periodontal bone loss and lacks careful and further validation, the prevalent view is still that LPS stimulates osteoclastogenesis (1-3). Importantly, if LPS indeed has a dual function in osteoclastogenesis, the molecular mechanism by which LPS exerts a dual effect on osteoclastogenesis need to be further elucidated.
In the present work, using various in vitro assays, we have demonstrated independently that LPS inhibits osteoclastogenesis from normal osteoclast precursors but promotes the development of osteoclasts from RANKL-pretreated cells in tissue culture dishes and bone slices in single-cell and co-culture settings, confirming the two previous observations that LPS play a bifunctional role in osteoclastogenesis (34, 35). Moreover, we have further shown that the RANKL-mediated lineage commitment is long term and irreversible in LPS-mediated osteoclastogenesis. More importantly, we have revealed that LPS inhibits osteoclastogenesis by suppressing NFATc1 expression and JNK activation while it prolongs osteoclast survival by activating the Akt, NF-κB, and ERK pathways. These studies have not only unambiguously and precisely defined the role of LPS in osteoclastogenesis but, more importantly, may also lead to a paradigm shift in future investigation of the molecular mechanism of periodontal bone loss.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents—Chemicals were purchased from Sigma unless indicated otherwise. Ultra-pure LPS and standard LPS were purchased from InvivoGen (San Diego, CA). The following antibodies were purchased from Cell Signaling Technology, Inc. (Beverly, MA): antibodies against IκBα (catalog No. 9242), phospho-IκBα (2859s), p44/42ERK (9102), phosphop44/42ERK (9101s), JNK (9252), phospho-JNK (9251s), p38 (9212), phospho-p38 (9211s), Akt (9272), and phospho-Akt (9275s). Antibodies against NFATc1 (sc-7294) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Alexa Fluor-488 phalloidin (A12379) and Hoechst-33258 (H1398) were purchased from Invitrogen.
In Vitro Mouse Osteoclastogenesis Assays—Male C57BL/6 mice at different ages, as indicated in individual experiments, were purchased from Harlan Industries (Indianapolis, IN). TLR4-/- breeding pairs were obtained under a materials transfer agreement from Dr. Shikuo Akira (Osaka University, Osaka, Japan). Bone marrow macrophages (BMMs) were isolated from long bones of mice as described previously (36) and cultured in α-MEM containing 10% heat-inactivated fetal bovine serum in the presence of M-CSF. To generate osteoclasts from BMMs with M-CSF and RANKL treatment, 5 × 104 cells were plated per well in 24-well plates and cultured in the presence of 44 ng/ml M-CSF and various concentrations of purified glutathione S-transferase-RANKL as indicated in individual experiments (37). Osteoclasts were stained for TRAP expression with a leukocyte acid phosphatase kit (387-A) from Sigma. To generate osteoclasts in vitro using the co-culture systems, BMMs were co-cultured in α-MEM containing 10% heat-inactivated fetal bovine serum with osteoblasts isolated from newborn mice calvarias in 24-well tissue culture plates (5 × 104 BMMs and 5 × 103 osteoblasts/well). The co-cultures are treated with 20 nm 1,25-dihydroxyvitamin D3 and 100 nm dexamethasone. Under these conditions, osteoclasts began to form at days 6 to 7. The cultures were stained for TRAP activity at days 8-10 with a leukocyte acid phosphatase kit (387-A) from Sigma.
In Vitro Bone Resorption Assays—5 × 104 BMMs were plated on bovine cortical bone slices in 24-well plates, and the cultures were treated with M-CSF (44 ng/ml) and RANKL (100 ng/ml) for 4 days to stimulate osteoclast formation. The cultures were then continued for 3 more days to allow osteoclasts to resorb bone. In bone resorption assays involving co-cultures, BMMs were co-cultured with osteoblasts in α-MEM containing 10% heat-inactivated fetal bovine serum on bone slices in 24-well tissue culture plates (5 × 104 BMMs and 5 × 103 osteoblasts/well) in the presence of 20 nm 1,25-dihydroxyvitamin D3 and 100 nm dexamethasone for 11 days. Then, bone slices were harvested and cells removed from the bone slices with 0.25 m ammonium hydroxide and mechanical agitation. Bone slices were then subjected to scanning electron microscopy (SEM) using a Philips 515 SEM (Materials Engineering Department, University of Alabama at Birmingham). The data were quantified by measuring the percentage of the pits area in four random resorption sites. The percentage of the pits area was determined using ImageJ analysis software obtained from National Institutes of Health.
Immunofluorescence Assays—5 × 104 BMMs were plated on bovine cortical bone slices in 24-well plates, and the cultures were treated with M-CSF (44 ng/ml) and RANKL (100 ng/ml) for 5 days to stimulate osteoclast formation. Bone slices were then fixed with 3.7% formaldehyde solution in PBS for 10 min at room temperature, and each bone slice was placed in 0.1% Triton X-100 in PBS for 8 min and then stained with Alexa-488 phalloidin and Hoechst-33258 for 15 min for actin ring and nucleic staining, respectively. Bone slices were then subjected to confocal imaging using a Leica DMIRBE inverted UV SP1 confocal microscope system with Leica confocal software at the University of Alabama at Birmingham imaging facility.
Western Blot—BMMs were cultured in serum-free α-MEM in the absence of M-CSF for 16 h before treatment with RANKL and/or LPS for various times as indicated in the individual experiments. For assays involving osteoclasts, BMMs were treated with M-CSF (44 ng/ml) and RANKL (100 ng/ml) for 4 days to stimulate osteoclast formation. After osteoclasts were formed, the cultures were then treated with PBS or RANKL for 4 h. BMMs or mature osteoclasts were washed twice with ice-cold PBS and then lysed in lysis buffer from Cell Signaling Technology (catalog No. 9803), 1× protease inhibitor mixture 1 (Sigma, P-2850), and 1× protease inhibitor mixture 2 (Sigma, P-5726). 25 μg of cell lysates was boiled in the presence of SDS sample buffer (0.5 m Tris-HCl, pH 6.8, 10% (w/v) SDS, 10% glycerol, 0.05% (w/v) bromphenol blue) for 5 min and loaded for electrophoresis on 10% SDS-PAGE. Proteins were transferred to nitrocellulose membranes (catalog No. 162-0147) from Bio-Rad using a semidry blotter (Bio-Rad). Membranes were blocked in blocking solution (5% nonfat dry milk in TBS containing 0.1% Tween 20) for 1 h to prevent nonspecific binding and then washed three times with TBS-T (TBS containing 0.1% Tween 20). Membranes were incubated primary antibodies in TBS-T containing 5% bovine serum albumin (Sigma, A-7030) overnight at 4 °C. Next day, membranes were then washed three times with TBS-T and incubated with secondary antibody in TBS-T containing 5% nonfat dry milk for 1 h. Membranes were washed extensively, and an enhanced chemiluminescence (ECL) detection assay was performed using the SuperSignal West Dura kit from Pierce.
RESULTS
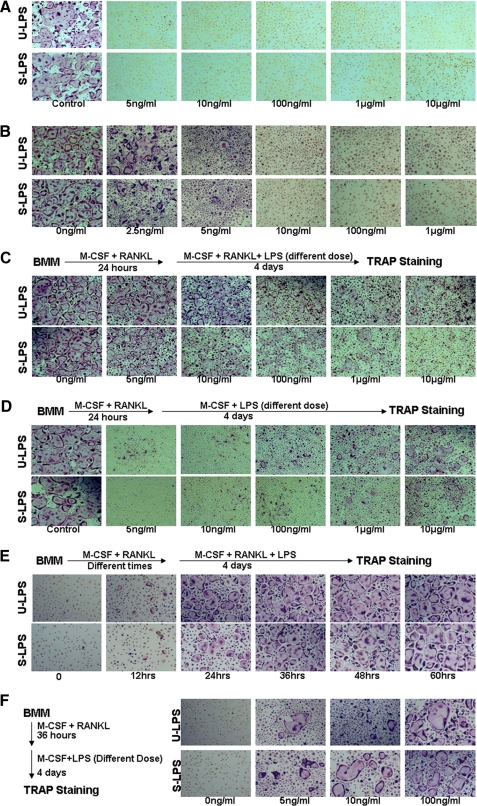
LPS Plays a Bifunctional Role in Osteoclastogenesis—The role of LPS in osteoclastogenesis still remains controversial. To further address the issue, we independently and carefully carried out numerous assays to examine the role of LPS in osteoclastogenesis. First, we determined whether LPS could promote osteoclast formation in the presence of M-CSF. To this end, primary BMMs (namely primary osteoclast precursors) from 6-week-old male C57BL/6 mice were treated with M-CSF and different doses of LPS (5 ng/ml-10 μg/ml) for 4 days (Fig. 1A). The assays were performed with both standard LPS and ultrapure LPS from InvivoGen. Standard LPS contains other bacterial components such as lipopeptides, thus targeting both TLR4 and TLR2. Ultra-pure LPS is further purified using a protocol developed by Hirschfeld et al. (38) and therefore activates only TLR4. As control, BMMs treated with M-CSF and RANKL formed numerous typical osteoclasts (Fig. 1A). However, cells treated with M-CSF and LPS at concentrations as high as 10 μg/ml failed to form any osteoclasts (Fig. 1A), indicating that LPS cannot replace RANKL in osteoclastogenesis. This finding is consistent with the current notion that osteoclastogenesis requires two essential factors, M-CSF and RANKL (24-27). We then determined whether LPS could additively or synergistically promote osteoclastogenesis with RANKL. Hence, we treated BMMs with M-CSF, RANKL, and different doses of LPS for 4 days (Fig. 1B). The control cultures containing no LPS formed numerous osteoclasts, but LPS at concentrations as low as 10 ng/ml totally abolished osteoclast formation (Fig. 1B), revealing that LPS inhibits RANKL-mediated osteoclastogenesis from freshly isolated BMMs rather than additively or synergistically stimulating osteoclastogenesis with RANKL. This finding is in agreement with two previously documented observations (34, 35).
FIGURE 1.
LPS plays a bifunctional role in osteoclastogenesis in a tissue culture dish. A, LPS is incapable of promoting osteoclastogenesis in the presence of M-CSF. BMMs were treated with 44 ng/ml M-CSF and increasing concentrations of LPS (5 ng/ml, 10 ng/ml, 100 ng/ml, 1 μg/ml, and 10 μg/ml) for 4 days. As control, BMMs were treated with 44 ng/ml M-CSF and 100 ng/ml RANKL for 4 days. The cultures were then stained for TRAP activity. Each condition had three replicates (wells). A representative area of the cultures from each condition is shown. U-LPS, ultra-pure LPS; S-LPS, standard LPS. B, LPS inhibits RANKL-mediated osteoclastogenesis from freshly isolated BMMs. BMMs were treated with 44 ng/ml M-CSF and 100 ng/ml RANKL in the absence of LPS as control or in the presence of increasing concentrations of LPS (2.5 ng/ml, 5 ng/ml, 10 ng/ml, 100 ng/ml, and 1 μg/ml) for 4 days. The cultures were then stained for TRAP activity. Each condition had three replicates (wells). A representative area of the cultures from each condition is shown. C, LPS stimulates osteoclastogenesis from BMMs that are pretreated with RANKL. BMMs were pretreated with 44 ng/ml M-CSF and 100 ng/ml RANKL for 24 h. Different doses of LPS (0, 5 ng/ml, 10 ng/ml, 100 ng/ml, 1 μg/ml, and 10 μg/ml) were then added to the cultures, and the cultures were continued for 4 more days. The cultures were stained for TRAP activity. Each condition had three replicates (wells). A representative area of the cultures from each condition is shown. D, LPS and M-CSF can promote osteoclastogenesis from RANKL-pretreated BMMs. BMMs were pretreated with 44 ng/ml M-CSF and 100 ng/ml RANKL for 24 h. The cultures were then continued with 44 ng/ml M-CSF and different doses of LPS (5 ng/ml, 10 ng/ml, 100 ng/ml, 1 μg/ml and 10 μg/ml) for 4 more days. As control, the cultures were continued with 44 ng/ml M-CSF and 100 ng/ml RANKL for 4 more days. The cultures were stained for TRAP activity. Each condition had three replicates (wells). A representative area of the cultures from each condition is shown. E, pretreatment of BMM by RANKL for as short as 36 h fully commits BMMs into the osteoclast lineage. BMMs were pretreated with 44 ng/ml M-CSF and 100 ng/ml RANKL for different times (0, 12, 24, 36, 48, or 60 h). LPS (10 μg/ml) was then added to the cultures, which were continued for 4 more days. The cultures were stained for TRAP activity. Each condition had three replicates (wells). A representative area of the cultures from each condition is shown. F, RANKL-mediated full commitment of BMMs into the osteoclast lineage is a prerequisite for subsequent differentiation into osteoclast in response to LPS stimulation. BMMs were pretreated with 44 ng/ml M-CSF and 100 ng/ml RANKL for 36 h. The cultures were then continued with 44 ng/ml M-CSF and different doses of LPS (0, 5, 10 or 100 ng/ml) for 4 more days. The cultures were stained for TRAP activity. Each condition had three replicates (wells). A representative area of the cultures from each condition is shown.
Next, we examined the effect of LPS on osteoclastogenesis from BMMs that were pretreated with RANKL. BMMs were treated with M-CSF and RANKL for 24 h, and then different doses of LPS were added to the cultures to complete the process (Fig. 1C). Our finding is consistent with previous reports that once osteoclast precursors are pretreated with RANKL, the inhibitory effect of LPS on RANKL-mediated osteoclastogenesis is significantly reduced (Fig. 1C) (34, 35). We then took one more step to investigate whether LPS and M-CSF could promote osteoclastogenesis from RANKL-pretreated BMMs in the absence of RANKL. Significantly, our assays demonstrated that LPS and M-CSF, without RANKL, are sufficient to promote osteoclastogenesis from RANKL-pretreated BMMs in a dose-dependent manner (Fig. 1D).
As shown in Fig. 1C, high doses of LPS (100 ng/ml or higher), especially standard LPS, can still exert a significant inhibitory effect on osteoclastogenesis from RANKL-pretreated BMMs. It has been postulated previously that RANKL pretreatment commit BMMs to the osteoclast lineage (39, 40). It is likely that 24-h RANKL pretreatment is not long enough to fully commit BMMs and/or to commit all of the BMMs in the cultures, and therefore this treatment would fail to completely abolish the inhibitory effect. We addressed the possibility by pretreating BMMs with M-CSF and RANKL for various times followed by addition of high dose LPS (10 μg/ml) (Fig. 1E). The data revealed that a longer RANKL pretreatment (36 h or longer) fully abrogated the inhibitory effect of a high dose of LPS (10 μg/ml) on osteoclastogenesis, supporting the prospect that 36-h pretreatment of BMM by RANKL at 100 ng/ml fully commits all BMMs in the cultures into the osteoclast lineage. Moreover, it was also noted that only high doses of LPS (100 ng/ml or higher) promoted osteoclastogenesis from RANKL-pretreated BMMs in the presence of M-CSF (Fig. 1D). This is once again likely the result of incomplete commitment of BMMs by the 24-h RANKL treatment. To test this possibility, BMMs pretreated with RANKL for 36 h, as shown in Fig. 1E, were cultured in the presence of M-CSF and LPS to promote osteoclastogenesis. Our results demonstrate that BMMs pretreated with RANKL for a longer time (36 h) became capable of differentiating into osteoclasts in response to LPS at concentrations as low as 5 ng/ml (Fig. 1F), indicating that the RANKL-mediated full commitment of BMMs into the osteoclast lineage is critical for subsequent differentiation into osteoclast in response to LPS stimulation. Taken together, these data support the finding that LPS plays a bifunctional role in osteoclastogenesis in tissue culture dish in vitro.
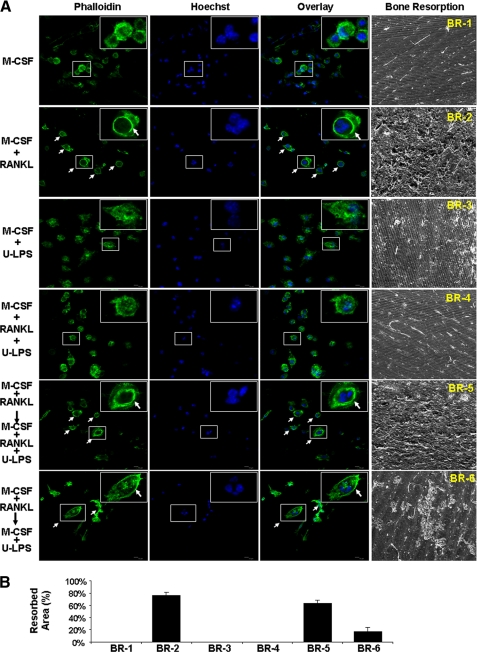
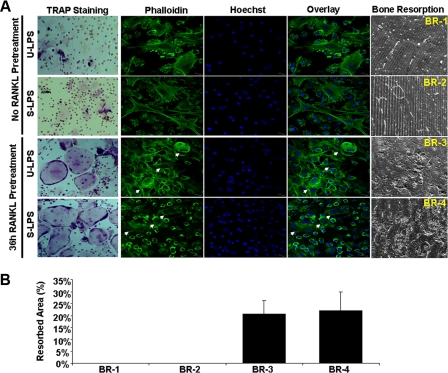
Thus far, all of the osteoclastogenesis assays undertaken to investigate the role of LPS in osteoclastogenesis, including ours shown above and those done previously by others (34, 35), have been performed to assess the effect of LPS on osteoclastogenesis in tissue culture dishes. To obtain more physiologically relevant data, we further asked whether we could recapitulate the dual role of LPS in osteoclastogenesis on bone slices. Toward this end, we repeated the key osteoclastogenesis assays on bone slices (Fig. 2). As control, BMMs grown on bone slices in the presence of M-CSF alone were mononuclear and did not form an actin ring (Fig. 2A, top row, first three panels from left). More importantly, no bone resorption was detected (Fig. 2A, right panel). M-CSF and RANKL were sufficient to promote osteoclast formation on bone slices from fresh BMMs (Fig. 2A, second row from top), indicated by the presence of two important structural features of osteoclasts, actin ring formation visualized by phalloidin staining (leftmost panel) and multinucleation revealed by Hoechst staining (second panel from left), in the majority of cells attached on bone slices. The overlay of the two different staining images further highlights the multinucleated cells with typical actin ring (Fig. 2A, third panel from left). The formation of osteoclasts on the bone slices were eventually confirmed by revelation of bone resorption activities on bone slices (Fig. 2A, rightmost panel). In contrast, M-CSF and LPS (10 μg/ml) failed to promote osteoclastogenesis on bone slices from fresh BMMs, as indicated by the absence of multinucleated cells with actin rings on the bone slices and lack of bone resorption activity (Fig. 2A, third row from top). Moreover, 5 ng/ml LPS completely blocked RANKL-mediated osteoclastogenesis on bone slices from fresh BMMs (Fig. 2A, fourth row from top). Importantly, 10 μg/ml LPS not only was unable to inhibit RANKL-mediated osteoclastogenesis from RANKL-pretreated BMMs on bone slices (Fig. 2A, fifth row from top) but also was capable of stimulating osteoclastogenesis from RANKL-pretreated BMMs on bone slices in the presence of M-CSF (Fig. 2A, bottom row). The bone resorption assays are quantified in Fig. 2B. These data further substantiate that LPS plays a bifunctional role in osteoclastogenesis.
FIGURE 2.
LPS plays a bifunctional role in osteoclastogenesis on bone slices. A, BMMs were cultured on bone slices in 24-well tissue culture plates with M-CSF (44 ng/ml) alone, M-CSF (44 ng/ml) and RANKL (100 ng/ml), M-CSF (44 ng/ml), and ultra-pure LPS (10 μg/ml) or M-CSF (44 ng/ml) and RANKL (100 ng/ml) plus ultrapure LPS (5 ng/ml) for 5 days to promote osteoclastogenesis. At day 5, a portion of bone slices were stained for actin ring formation (alexa-488-phalloidin) and multinucleation (Hoechst-33258), two important features of osteoclasts. The remaining cultures were continued for 3 additional days, and bone slices were then harvested for SEM scanning to visualize bone resorption cavities. In addition, BMMs were cultured on bone slices with M-CSF (44 ng/ml) plus RANKL (100 ng/ml) for 36 h, and the cultures were then continued with M-CSF (44 ng/ml) and RANKL (100 ng/ml) plus ultra-pure LPS (10 μg/ml) or with M-CSF (44 ng/ml) plus ultra-pure LPS (10 μg/ml) for an additional 5 days. Similarly, at day 5, a portion of bone slices were stained for actin ring formation and multinucleation. The remaining cultures were continued for 3 additional days, and bone slices were then harvested for SEM. Each condition had three replicates (wells); a representative area of the phalloidin, Hoechst staining, and the SEM image is shown. A representative cell is shown at higher magnification in inserts (top right corners). Osteoclasts are indicated by arrows. U-LPS, ultra-pure LPS. B, quantification of the bone resorption assays. Four areas in bone slices from each bone resorption assay (BR-1, BR-2, BR-3, BR-4, BR-5, and BR-6) shown in A were chosen randomly, and the percentage of resorbed area was determined as described under “Experimental Procedures.” Bars show averages ± S.D.
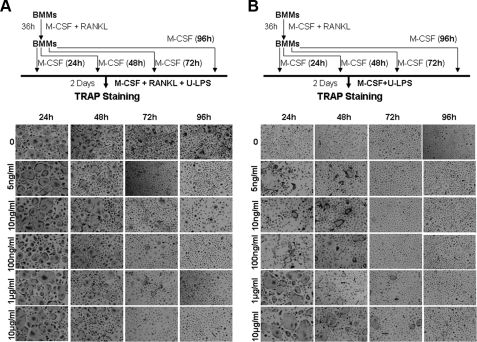
RANKL-mediated Osteoclast Lineage Commitment is Long Term—Having demonstrated that LPS promotes osteoclastogenesis from BMMs precommitted by RANKL, we next determined whether the RANKL-mediated lineage commitment was transient or long term. We treated BMMs with M-CSF and RANKL for 36 h. RANKL was then removed, and the cultures were continued with M-CSF alone (serving as a proliferating and surviving factor) on an untreated culture dish for 24, 48, 72, or 96 h (Fig. 3). BMMs were lifted and replated in 24-well treated tissue culture plates and cultured with M-CSF and RANKL plus different doses of ultra-pure LPS for 4 days (Fig. 3A) or with M-CSF and different doses of ultra-pure LPS for 4 days (Fig. 3B). In Fig. 1E, we show that, immediately following pretreatment of BMMs with RANKL for 36 h, LPS was no longer able to inhibit RANKL-mediated osteoclastogenesis. Fig. 3A further demonstrates that as long as 48 h after the 36-h RANKL pretreatment, LPS was still unable to completely block RANKL-mediated osteoclastogenesis, indicating that the RANKL-mediated lineage commitment can persist for at least 48 h. Moreover, as shown in Fig. 3B, LPS was still capable of promoting osteoclastogenesis from the RANKL-pretreated BMMs 48 h after the completion of the 36-h RANKL pretreatment, further supporting the idea that the RANKL-mediated lineage commitment is durable. Similar results were obtained with standard LPS (data not shown).
FIGURE 3.
The RANKL-mediated osteoclast lineage commitment is long term. A, 48 h after the 36-h RANKL pretreatment, BMMs can still form osteoclasts in response to M-CSF, RANKL, and LPS. BMMs were treated with 44 ng/ml M-CSF and 100 ng/ml RANKL for 36 h. RANKL was then removed, and the cultures were continued with 44 ng/ml M-CSF alone for 24, 48, 72, or 96 h. BMMs were treated with 44 ng/ml M-CSF, 100 ng/ml RANKL plus different doses of ultra-pure LPS for 2 days (0, 5 ng/ml, 10 ng/ml, 100 ng/ml, 1 μg/ml, or 10 μg/ml). The cultures were stained for TRAP. Each condition had three replicates (wells), and a representative area is shown. U-LPS, ultra-pure LPS. B, 48 h after the 36-h RANKL pretreatment, BMMs can still form osteoclasts in response to M-CSF and LPS. BMMs pretreated with 44 ng/ml M-CSF and 100 ng/ml RANKL for 36 h were continued with 44 ng/ml M-CSF alone for 24, 48, 72, or 96 h. BMMs were then treated with 44 ng/ml M-CSF and different doses of ultra-pure LPS for 2 days (0, 5 ng/ml, 10 ng/ml, 100 ng/ml, 1 μg/ml, or 10 μg/ml). The cultures were stained for TRAP. Each condition had three replicates (wells), and a representative area is shown.
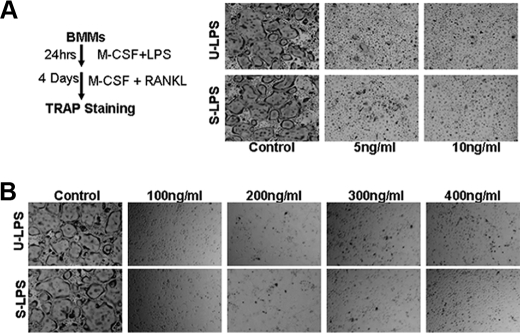
The Inhibitory Effect of LPS on Osteoclastogenesis Is Irreversible and Dominant over That of RANKL—As shown above, LPS is not only unable to stimulate osteoclastogenesis from fresh BMMs in the presence of M-CSF (Fig. 1A), but LPS actually inhibits RANKL-mediated osteoclastogenesis when LPS and RANKL were simultaneously added to the cultures (Fig. 1B). These findings raise another question: can RANKL mediate osteoclastogenesis from BMMs pre-exposed to LPS? To address this issue, we pretreated BMMs with different doses of LPS in the presence of M-CSF for 24 h. Then, RANKL (100 ng/ml) was added into the cultures. As shown in Fig. 4A, pretreatment of BMMs with LPS as low as 5 ng/ml ultra-pure LPS or standard LPS blocked osteoclastogenesis in response to subsequent RANKL stimulation, suggesting that LPS pretreatment permanently prevents BMMs from differentiating into osteoclasts. To further investigate this issue, we treated BMMs with M-CSF, LPS (10 ng/ml), and increasing concentrations of RANKL. As shown in Fig. 4B, as high a concentration as 400 ng/ml RANKL still could not promote osteoclast formation in the presence of 10 ng/ml LPS, indicating that the inhibitory effect of LPS on osteoclastogenesis overrides that of RANKL.
FIGURE 4.
The inhibitory effect of LPS on osteoclastogenesis is irreversible and dominant over that of RANKL. A, BMMs pre-exposed to LPS cannot form osteoclasts in response to RANKL treatment. As shown in the left panel, BMMs were pretreated with 44 ng/ml M-CSF and different doses of LPS (5 ng/ml or 10 ng/ml) for 24 h. LPS was then removed, and the cultures were continued with 44 ng/ml M-CSF and 100 ng/ml RANKL for 4 days. As control, BMMs without LPS pretreatment were treated with 44 ng/ml M-CSF and 100 ng/ml RANKL for 4 days. The cultures were stained for TRAP. Each condition had three replicates (wells), and a representative area is shown. U-LPS, ultra-pure LPS; S-LPS, standard LPS. B, high RANKL concentrations cannot reverse the inhibitory effect of LPS in osteoclastogenesis. BMMs were treated with 44 ng/ml M-CSF, 10 ng/ml LPS, and increasing concentrations of RANKL (100, 200, 300, and 400 ng/ml) for 4 days. The cultures were stained for TRAP. Each condition had three replicates (wells), and a representative area is shown.
LPS Also Plays a Bifunctional Role in Osteoclastogenesis in the Co-culture System—Up to the present time, all our experiments have been performed in single cell-type cultures that contain only BMMs. It has been shown that osteoblasts are indirectly involved in LPS-mediated osteoclast formation by producing numerous osteoclastogenic factors including RANKL in response to LPS stimulation (1, 41). Thus, we further examined the role of LPS in osteoclastogenesis in co-culture systems containing osteoblasts as described previously (36). In our co-culture experiments, we used BMMs pretreated with RANKL (100 ng/ml) for 36 h or fresh BMMs (Fig. 5A). The co-culture assays were treated with ultra-pure or standard LPS (10 ng/ml). These co-culture assays show that although BMMs failed to form osteoclasts in the co-culture systems containing fresh BMMs in response to LPS treatment, the co-culture assays containing RANKL-pretreated BMMs formed osteoclasts in response to LPS (Fig. 5A, first column from left), indicating that LPS plays a bifunctional role in osteoclastogenesis in the co-culture systems. We next determined whether LPS played a bifunctional role in osteoclastogenesis on bone slices in the co-culture systems by repeating the same set of the assays on bone slices. The osteoclast formation on the bone slices was assessed by visualization of actin ring formation and multinucleation (Fig. 5A, columns 2-4 from left) and bone resorption activity (Fig. 5A, rightmost column). Our data demonstrate that whereas no multinucleated cells with actin ring and bone resorption activity were seen on bone slices from the co-culture systems containing fresh BMMs in response to LPS treatment (Fig. 5A, top two rows), the co-culture assays containing RANKL-pretreated BMMs had formed multinucleated cells with an actin ring and exhibited bone resorption activity in response to LPS (Fig. 5A, bottom two rows). The bone resorption assays in Fig. 5A are quantified in Fig. 5B. Taken together, these co-culture assays demonstrate that the dual effect of LPS on osteoclastogenesis seen in the tissue culture plates can be recapitulated on bone slices.
FIGURE 5.
LPS also plays a bifunctional role in osteoclastogenesis in the co-culture systems. A, BMMs were treated with M-CSF (44 ng/ml) alone for 36 h (No RANKL Pretreatment) or with M-CSF (44 ng/ml) and RANKL (100 ng/ml) for 36 h (36h RANKL Pretreatment). These cells were then co-cultured with osteoblasts as described under “Experimental Procedures” in the presence of standard (S-LPS) or ultra-pure LPS (U-LPS) for 10 days before staining for TRAP activity (left column). In addition, the untreated BMMs and RANKL-pretreated BMMs were used to perform co-culture assays on bone slices. At day 7, a portion of bone slices were stained for actin ring formation and multinucleation. The remaining cultures were continued for three additional days, and bone slices were then harvested for SEM. A representative area of the staining or SEM image from each condition is shown. Osteoclasts are indicated by arrows. B, bone resorption assays (BR-1, BR-2, BR-3, and BR-4) shown in A were quantified as described in the legend for Fig. 2.
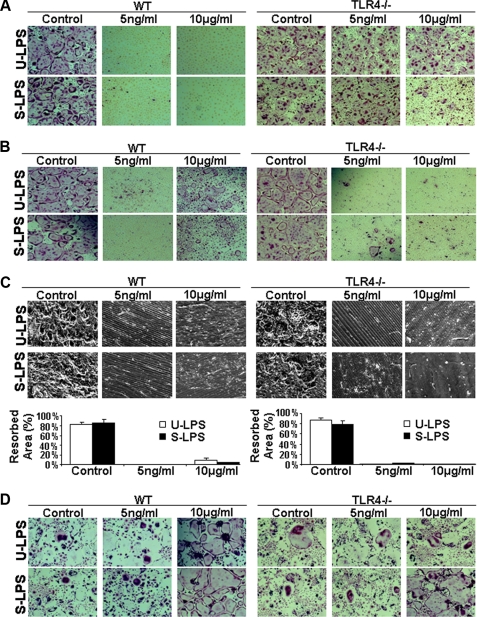
TLR4 Primarily Mediates the Complex Roles of LPS in Osteoclast Formation and Survival—Both TLR2 and TLR4 are expressed on BMMs (34). Although TLR4 is a primary receptor for LPS, a few studies suggest that TLR2 is also potentially involved in LPS signaling (42, 43). To determine whether TLR4 is solely responsible for the role of LPS in osteoclast formation and survival, we performed the following experiments with BMMs from wild-type (WT) or TLR4 knock-out mice (TLR4-/-). As shown in Fig. 6A, we treated freshly isolated WT or TLR4-/- BMMs with M-CSF, RANKL, and LPS to promote osteoclastogenesis. Although WT BMMs failed to form osteoclasts, TLR4-/- BMMs were fully capable of forming osteoclasts in response to M-CSF and RANKL in the presence of ultra-pure LPS, indicating that the LPS-activated inhibitory effect in fresh BMMs is mediated by TLR4. Moreover, standard LPS at a high concentration can still inhibit osteoclastogenesis in TLR4-/- BMMs, indicating that although TLR4 is a predominant receptor for LPS, TLR2 can also mediate the effect. Next, we repeated the assay using WT and TLR4-/- BMMs pretreated with RANKL. The data again indicate that LPS-activated stimulatory effect in RANKL-pretreated BMMs is also mediated primarily via TLR4 (Fig. 6B). Next, we repeated the assay shown in Fig. 6B on bone slices to investigate whether TLR4 is predominantly involved in mediating the LPS-induced osteoclastogenesis on bone slices from RANKL-pretreated BMMs. Our data indicate that TLR4 plays a major role in mediating the stimulatory effect of LPS on osteoclastogenesis from RANKL-pretreated BMMs on the physiologic substratum (Fig. 6C). Finally, we performed the osteoclast survival assays with WT and TLR4-/- BMMs. The results from the survival assays confirmed that TLR4 is mainly responsible for mediating LPS-stimulated surviving signaling pathway(s) in mature osteoclasts (Fig. 6D).
FIGURE 6.
TLR4 primarily mediates the complex role of LPS in osteoclast formation and survival. A, TLR4 is predominantly involved in the LPS-mediated inhibition of osteoclastogenesis from fresh BMMs. BMMs from WT or TLR4 knock-out (TLR4-/-) mice were treated with M-CSF (44 ng/ml) and RANKL (100 ng/ml) plus LPS (5 ng/ml or 10 μg/ml) for 4 days. The cultures were then stained for TRAP activity. Each condition had three replicates (wells). A representative area of the cultures from each condition is shown. B, TLR4 is also primarily involved in LPS-mediated osteoclastogenesis from RANKL-pretreated BMMs. BMMs from WT or TLR4-/- mice were treated with M-CSF (44 ng/ml) and RANKL (100 ng/ml) for 36 h, and then the cultures were continued with M-CSF (44 ng/ml) and LPS (5 ng/ml or 10 μg/ml) for 4 days. The cultures were then stained for TRAP activity. Each condition had three replicates (wells). A representative area of the cultures from each condition is shown. C, TLR4, for the most part, mediates LPS-dependent osteoclastogenesis from RANKL-pretreated BMMs on bone slices. The assays described in B were repeated on bone slices, and bone resorption cavities were examined by SEM. A representative area of the SEM image from each condition is shown. The bone resorption assays, quantified as described in the legend for Fig. 2, are shown in graph form below the images. D, TLR4 is mainly responsible for mediating the LPS-stimulated signaling pathway(s) in mature osteoclasts. BMMs from WT or TLR4-/- mice were treated with M-CSF (44 ng/ml) and RANKL (100 ng/ml) for 4 days and then continued with M-CSF (44 ng/ml) and LPS (5 ng/ml or 10 μg/ml) for 2 days. The cultures were then stained for TRAP activity. Each condition had three replicates (wells). A representative area of the cultures from each condition is shown. U-LPS, ultra-pure LPS; S-LPS, standard LPS.
LPS Inhibits Osteoclastogenesis from Fresh BMMs by Suppressing RANKL-mediated JNK Activation and NFATc1 Expression—We next set out to delineate the molecular mechanism by which LPS exerts a dual effect on osteoclastogenesis. RANKL has been shown to promote osteoclastogenesis by activating various intracellular signaling pathways in BMMs including NF-κB (7, 44), JNK (7, 45), ERK (7, 46), and p38 (46-48). In addition, RANKL also stimulates the expression of NFATc1 in BMMs, which is an essential factor for osteoclastogenesis (20-22). Thus, we investigated whether LPS regulates osteoclastogenesis via interference with these signaling pathways and/or the expression of the critical transcription factor.
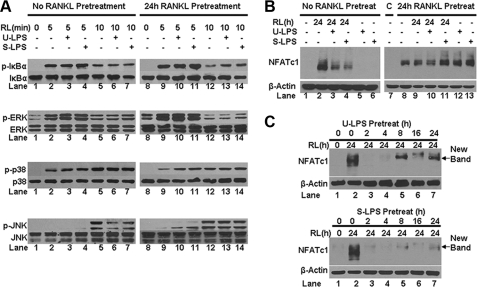
To this end, we treated freshly isolated BMMs with RANKL for 0, 5, or 10 min in the absence or presence of LPS, and the activation of the NF-κB, ERK JNK, and p38 pathways was determined by Western analysis (Fig. 7A, left panel). This set of assays was also repeated with BMMs pretreated with RANKL for 24 h (Fig. 7A, right panel). LPS had no effect on RANKL-mediated activation of the NF-κB or ERK pathways in fresh BMMs (Fig. 7A, lane 2 versus lanes 3 and 4; or lane 5 versus lanes 6 and 7). Additionally, LPS did not have any significant effect on the RANKL-mediated ERK activation in RANKL-pretreated BMMs as well (Fig. 7A, lane 9 versus lanes 10 and 11; or lane 12 versus lanes 13 and 14). Interestingly, LPS enhanced RANKL-dependent NF-κB activation in RANKL-pretreated BMMs (Fig. 7A, lane 12 versus lanes 13 and 14). These data indicate that LPS does not block osteoclastogenesis through suppression of RANKL-mediated activation of the NF-κB or ERK pathway. Moreover, LPS was able to transiently enhance RANKL-dependent activation of the p38 pathway in both fresh BMMs (Fig. 7A, lane 2 versus lanes 3 and 4) and RANKL-pretreated BMMs (lane 9 versus lanes 10 and 11), ruling out the possibility that LPS inhibits osteoclastogenesis by blocking RANKL-mediated p38 activation. Significantly, we found that LPS suppresses RANKL-mediated activation of the JNK pathway in fresh BMMs (Fig. 7A, lane 5 versus lanes 6 and 7) but not in RANKL-pretreated BMMs (lane 12 versus lanes 13 and 14), supporting the idea that LPS inhibits osteoclastogenesis in part by suppressing RANKL-mediated activation of the JNK pathway.
FIGURE 7.
LPS inhibits osteoclastogenesis from fresh BMMs by suppressing RANKL-mediated JNK activation and NFATc1 expression. A, LPS exerts an inhibitory effect on RANKL-mediated activation of the JNK pathway. Fresh BMMs or BMM pretreated with RANKL for 24 h were cultured with serum-free media for 16 h prior to treatment with RANKL for 0, 5, or 10 min in the absence or presence of ultra-pure LPS (U-LPS) or standard LPS (S-LPS). Activation of the NF-κB, ERK, JNK, and p38 pathways was assessed as phosphorylation of IκB, ERK, JNK, and p38 using Western analysis with antibodies against phospho-IκB, phospho-ERK, phospho-JNK, and phospho-p38. The same volume of lysates was run and then probed with antibodies against IκB, ERK, JNK, and p38 as loading control. RL, RANKL. B, LPS inhibits RANKL-mediated NFAFc1 expression in fresh BMMs but stimulates NFATc1 expression in RANKL-pretreated BMMs. Fresh BMMs or BMM pretreated with RANKL for 24 h were treated with RANKL for 24 h in the absence or presence of ultrapure LPS or standard LPS. C, lane 7, BMMs were cultured with M-CSF (44 ng/ml) alone for 48 h. Lane 8, BMMs were pretreated with 44 ng/ml M-CSF and RANKL for 24 h and then with 44 ng/ml alone for an additional 24 h. Both served as important controls. NFATc1 expression was assessed by using Western analysis with an antibody against NFATc1. Blots were stripped and then reprobed with β-actin antibody as loading control. Pretreat, pretreatment. C, LPS pretreatment of BMMs blocks RANKL-mediated NFATc1 expression. BMMs were pretreated with 10 ng/ml ultra-pure LPS or standard LPS for various times and then treated with RANKL (100 ng/ml) for 24 h. NFATc1 expression was determined as described in B.
We next examined the impact of LPS treatment on RANKL-induced expression of NFATc1. We found that LPS significantly suppresses RANKL-induced NFATc1 expression in fresh BMMs (Fig. 7B, lane 2 versus lanes 3 and 4). More importantly, the suppression was not seen in assays with RANKL-pretreated BMMs (Fig. 7B, lane 9 versus lanes 10 and 11). These data support the idea that LPS inhibits osteoclastogenesis from fresh BMMs in part by suppressing RANKL-induced NFATc1 expression. Once BMMs are pretreated with RANKL, LPS is no longer able to exert an inhibitory effect on RANKL-induced NFATc1 expression and therefore cannot block osteoclastogenesis from RANKL-pretreated BMMs. Moreover, we also revealed that although LPS is incapable of inducing NFATc1 expression in fresh BMMs (Fig. 7B, lanes 5 and 6), they are as potent as RANKL in up-regulating NFATc1 expression in RANKL-pretreated BMMs (lane 8 versus lanes 12 and 13). This finding is consistent with the observation that LPS can promote osteoclastogenesis from RANKL-pretreated BMMs in the presence of M-CSF (Fig. 1, D and F).
As shown in Fig. 4A, LPS-pretreated BMMs failed to form osteoclasts in response to RANKL, raising the possibility that the failure of RANKL in promoting osteoclastogenesis from LPS-pretreated BMMs results from its inability to induce NFATc1 expression in these cells. To address this possibility, we examined the ability of RANKL to induce NFATc1 expression in BMMs that are pretreated with LPS for various times (Fig. 7C). Our data demonstrate that not only RANKL is unable to induce NFATc1 expression in LPS-pretreated BMMs but also that LPS pretreatment for as little as 2 h completely BMMs renders unresponsive to RANKL in inducing NFATc1 expression (Fig. 7C, top and bottom panels, lane 2 versus lane 3). More interestingly, longer pretreatment of BMMs with LPS induces the expression of proteins with slower mobility (Fig. 7C, top and bottom panels, lanes 5 and 6, New Bands), the identities of which are unknown, but they represent either LPS-induced new proteins or LPS-induced modification of NFATc1. Future studies aimed at elucidation and characterization of the proteins/modifications may lead to a better understanding of the molecular mechanism underlying the dual function of LPS in osteoclastogenesis. Nonetheless, collectively, the data from these current studies support the idea that LPS blocks osteoclastogenesis from fresh BMMs by suppressing RANKL-induced JNK activation and NFATc1 expression.
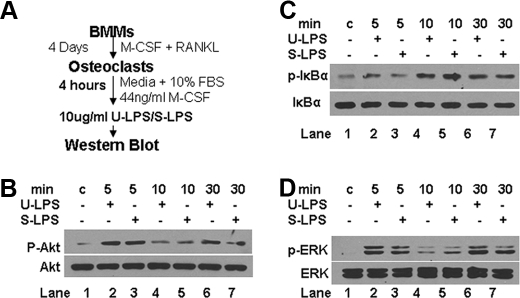
LPS Promotes Osteoclast Survival by Activating the Akt, NF-κB, and ERK Pathways—To delineate the molecular mechanism by which LPS stimulates osteoclast survival, we investigated whether LPS activates three known pathways (Akt, NF-κB, and ERK) in osteoclasts for two reasons: 1) these pathways have been shown previously to play a role in regulating osteoclast survival (16, 44, 49, 50); and 2) TLR4 has been implicated in the activation of the NF-κB, Akt, and MAPK pathways (JNK, ERK, and p38) (23, 51-54). As depicted in Fig. 8A, we prepared osteoclasts by treating BMMs with M-CSF and RANKL for 4 days. Mature osteoclasts were then cultured with medium containing M-CSF alone for 4 h prior to LPS treatment. The activation of the Akt, NF-κB, and ERK pathways by LPS was determined by Western analysis. These assays demonstrate that LPS significantly activates the Akt pathway (Fig. 8B, lane 1 versus lanes 2 and 3), the NF-κB pathway (Fig. 8C, lane 1 versus lanes 4 and 5), and the ERK pathway (Fig. 8D, lane 1 versus lanes 2 and 3) in osteoclasts, indicating that LPS prolongs osteoclast survival by activating these three signaling pathways.
FIGURE 8.
LPS promotes osteoclast survival by activating the Akt, NF-κB and ERK pathways. A, experimental procedures for assessing activation of the Akt, NF-κB, and ERK pathways in mature osteoclasts. BMMs were treated with M-CSF (44 ng/ml) and RANKL (100 ng/ml) for 4 days to promote osteoclasts. Osteoclasts were then cultured in media containing 44 ng/ml M-CSF for 4 h prior to treatment with 1 μg/ml ultra-pure LPS (U-LPS) or standard LPS (S-LPS) for 5, 10, or 30 min. Cells were then lysed for Western blot analysis. B, LPS activates the Akt pathway in mature osteoclasts. The cell lysates from A were used to assess Akt activation as described in the legend for Fig. 7A. C, LPS stimulates the NF-κB pathway in mature osteoclasts. The cell lysates from A were used to assess NF-κB activation as described in the legend for Fig. 7A. D, LPS activates the ERK pathway in mature osteoclasts. The cell lysates from A were used to assess ERK activation as described in the legend for Fig. 7A.
DISCUSSION
It has been well established that periodontitis is initiated by specific oral pathogens that colonize and invade the oral tissues (1, 2, 55). Over 600 different bacteria are able to colonize the human oral cavity, and on average most individuals typically harbor 15-200 different types of bacteria (57). Among these, about 15-20 are believed to play a pathological role in the initiation of periodontitis (58-60). The main complication of periodontitis is progressive loss of bone, which may in turn result in tooth loss if left untreated (61, 62). However, the development of periodontal bone loss cannot be explained by the mere existence of bacteria, and it is now widely believed that the host response to the microbial antigens is a major determinant of disease progression (3, 62, 63).
As a common bacteria-derived product, LPS has been widely recognized as a key factor implicated in the development of chronic periodontitis (1-3). Supporting this notion, the vast majority of previous studies showed that LPS stimulates osteoclastogenesis and/or bone resorption in in vitro assays and in animal models (29-33, 64-70). However, we have long been puzzled by this widely held view on the role of LPS in osteoclastogenesis. For instance, in our laboratory, to generate good osteoclasts in vitro, we have always taken extra precautions to prevent bacteria or LPS contamination, which has detrimental effects on in vitro osteoclast generation. We keep a separate set of glassware, such as medium bottles specifically reserved for osteoclast work, to prevent LPS contamination.
Interestingly, two papers published in 2002 reported that LPS potently inhibits osteoclast formation (34, 35). Moreover, one of these groups further shows that LPS-mediated inhibitory effect on osteoclastogenesis is abolished in osteoclast precursors pretreated with RANKL (35). However, this intriguing finding has not thus far received enough attention, as almost every recent review paper on periodontal bone loss still emphasizes that LPS simply plays a stimulatory role in osteoclast formation (3, 29-31). The failure in further validation and prompt recognition of this interesting finding is likely to result from the fact that a stimulatory effect of LPS on osteoclast formation is contradictory to the well established stimulatory role of LPS in development of periodontal bone loss. Nonetheless, the reported inhibitory effect of LPS on osteoclastogenesis supports our routine laboratory observation that LPS contamination inevitably leads to a disastrous consequence in in vitro osteoclast generation. We feel that the newly revealed dual effect of LPS on osteoclastogenesis may truly represent the complex actions of LPS in osteoclastogenesis but, more significantly, may also have the potential to lead to a paradigm shift in the understanding of the mechanism underlying development of periodontal bone loss. Specifically, the dual effect of LPS on osteoclastogenesis may help explain why the mere existence of bacteria does not always lead to periodontal bone loss and how the host response to microbial antigens dictates disease progression.
To establish the paradigm shift, it is first very critical to further and independently validate the previous finding. To this end, we carried out various assays to more precisely and convincingly investigate the role of LPS in osteoclastogenesis. Our first set of assays was carefully planned and performed to examine the effect of LPS treatment on osteoclastogenesis in tissue culture dishes (Fig. 1). Our data recapitulated the key findings reported previously by two other groups that LPS inhibits osteoclastogenesis from fresh osteoclast precursors but the inhibition is attenuated once osteoclast precursors are pretreated with RANKL (34, 35). Importantly, we also demonstrated that RANKL pretreatment of osteoclast precursors for as little as 36 h renders them fully capable of differentiating into osteoclasts in response to LPS and M-CSF. Moreover, we examined the effect of LPS stimulation on osteoclastogenesis on bone slices (Fig. 2). Our assays indicate that LPS exerts the same effects on osteoclastogenesis on the physiologic substratum, further confirming that LPS plays a biphasic role in osteoclastogenesis. More importantly, we further revealed that LPS is still able to mediate osteoclastogenesis from RANKL-pretreated BMMs 48 h after the completion of RANKL pretreatment (Fig. 3), supporting the idea that the RANKL-mediated lineage commitment is durable. Finally, we also showed that the pretreatment of osteoclast precursors with LPS totally abolishes the stimulatory effect of RANKL on osteoclastogenesis (Fig. 4A) and that the inhibitory effect of LPS on osteoclastogenesis cannot be reversed by high concentration of RANKL (Fig. 4B). Taken together, these studies support the proposal that BMMs have two fates: they function as phagocytes in response to agents such as LPS, or alternatively they differentiate into osteoclasts upon RANKL stimulation. Once RANKL commits BMMs to the osteoclast lineage, they will inevitably choose the destination. Conversely, the exposure of uncommitted BMMs to LPS keeps the cells as phagocytes. Importantly, once a fate is chosen, it is no longer reversible. More importantly, the effect of LPS on cell fate determination appears to supersede that of RANKL, because the simultaneous treatment of BMMs with both RANKL and LPS leads to inhibition of osteoclastogenesis.
Although our current studies, in combination with previous studies from other groups, have unambiguously established the role of LPS in osteoclastogenesis, the molecular mechanism underlying the dual function of LPS has not been fully and convincingly elucidated. Upon discovery of the dual function of LPS in osteoclastogenesis, Zou and Bar-Shavit (35) further investigated the potential molecular mechanism underlying the unexpected and intriguing effects of LPS on osteoclastogenesis and showed that LPS inhibits RANKL-mediated osteoclastogenesis by down-regulating the expression of c-fms and RANK, the receptors for M-CSF and RANKL, respectively. Although LPS appears to exert an inhibitory effect on c-fms and RANK expression, the kinetics and magnitude of the inhibition is not sufficient to explain the striking inhibitory effect of LPS on osteoclastogenesis. For instance, LPS treatment progressively suppresses the expression of RANK and c-fms genes with a kinetics of 20-30% reduction for every 24-h treatment (35). In our assays, pretreatment of BMMs with LPS for 24 h completely blocked osteoclastogenesis (Fig. 4A). It is unlikely that a 20-30% decrease in RANK and c-fms levels can give rise to such dramatic inhibition of osteoclastogenesis. Moreover, we showed that once BMMs were pretreated with RANKL for 36 h, LPS was no longer able to inhibit osteoclastogenesis (Fig. 1E). It is noteworthy to mention that LPS was present in the entire culture period (4 days) after the pretreatment (Fig. 1E) and therefore LPS should exert the same kinetics and magnitude of the inhibition on RANK and c-fms expression as in the osteoclastogenic cultures involving fresh BMMs. Based on these observations, we reasoned that LPS exerts a potent inhibitory effect on osteoclastogenesis by targeting the intracellular signaling pathways. Thus, we investigated the effect of LPS on the known signaling pathways activated by RANKL to promote osteoclastogenesis: NF-κB (7, 44), JNK (7, 45), ERK (7, 46), and p38 (46-48). Our data indicate that LPS strongly inhibits RANKL-induced expression of NFATc1 while slightly suppressing RANKL-mediated JNK activation in BMMs (Fig. 7A). Because JNK activation is only slightly down-regulated by LPS, the suppression of JNK activation contributes to the LPS-mediated inhibition of osteoclastogenesis but does not play a decisive role in the process. In contrast, given that LPS significantly suppresses RANKL-induced NFATc1 expression and that NFATc1 has been well established as a master regulator of osteoclastogenesis (20-22), we conclude that LPS inhibits osteoclastogenesis from fresh BMMs primarily by suppressing RANKL-mediated NFATc1 expression. Consistent with the functional assays, we found that the LPS-mediated suppression of NFATc1 expression is abolished in RANKL-pretreated BMMs (Fig. 7B) and that prior treatment of BMMs with LPS totally abrogates the RANKL-induced expression of NFATc1 (Fig. 7C), further supporting a critical role of NFATc1 in LPS-mediated inhibition of osteoclastogenesis.
To reconcile the inhibitory effect of LPS on osteoclastogenesis in in vitro assays with its well established role in the development of periodontal bone loss, it was argued that the inhibitory effect of LPS on osteoclastogenesis observed in in vitro studies may not recapitulate the in vivo role of LPS in osteoclastogenesis because the in vivo action of LPS may involve cells other than osteoclast precursors, including osteoblasts, fibroblasts, and T-cells (34, 35). In particular, osteoblasts, fibroblasts (gingival fibroblasts and periodontal ligament fibroblasts), and T-cells have been implicated in periodontal bone loss by serving as a source of M-CSF and also by producing RANKL in response to LPS (3, 56). It is possible that osteoblasts, fibroblasts, and/or T-cells may indirectly regulate osteoclastogenesis by increasing the local concentration of RANKL and/or M-CSF, therefore altering the outcome. To test this possibility, we repeated our assays in co-culture systems containing osteoclast precursors and osteoblasts. Our data show that LPS inhibits osteoclastogenesis from fresh osteoclast precursors but stimulates osteoclastogenesis from those pretreated with RANKL in the co-culture assays (Fig. 5), indicating that the involvement of other cell types may not be sufficient to explain the discrepancy. This finding is consistent with our data showing that high doses of RANKL failed to reverse the LPS-mediated inhibitory effect on osteoclastogenesis (Fig. 4B).
Based on these findings, we propose the following model to explain the discrepancy between the inhibitory effect of LPS on osteoclastogenesis observed from in vitro assays and its established role in periodontal bone loss as well as why the mere existence of bacteria does not always lead to periodontal bone loss. The commitment status of osteoclast precursors determines the role of LPS in osteoclastogenesis and subsequently its role in the development of periodontal bone loss. More specifically, in healthy individuals, osteoclast precursors are not abnormally or prematurely committed to the osteoclast lineage. Thus, even though these individuals may harbor bacteria in their oral cavities, LPS derived from the microbial pathogens are unable to promote osteoclastogenesis, and as a matter of fact, LPS inhibits osteoclastogenesis. However, when these individuals develop certain pathological conditions in which osteoclast precursors are committed to osteoclast lineage by RANKL before they migrate into periodontal tissues, LPS stimulates osteoclast formation leading to periodontal bone loss. Thus, it is the factor that is intrinsic to the host (namely, the commitment status of osteoclast precursors) that determines the outcome of LPS action in vivo in relation to osteoclastogenesis.
In summary, our present work has more convincingly and precisely defined the role of LPS in osteoclast biology and provided new insights into the mechanisms underlying the dual action of LPS in osteoclastogenesis. More significantly, based on these studies, we propose a new model that may explain the discrepancy between the inhibitory effect of LPS on osteoclastogenesis and its established role in the development of periodontal bone loss. Future investigation aimed at establishing this new model will enable us to fully understand the molecular mechanism underlying the pathogenesis of periodontal bone loss and will also elucidate novel diagnostic methods and therapeutic targets.
This work was supported, in whole or in part, by National Institutes of Health Grant AR47830 from NIAMS.
Footnotes
The abbreviations used are: LPS, lipopolysaccharide; M-CSF, monocyte/macrophage colony-stimulating factor; TLR, Toll-like receptor; BMM, bone marrow macrophage; RANK, receptor activator of NF-κB; RANKL, RANK ligand; TNF, tumor necrosis factor; TNFR, tumor necrosis factor receptor; TRAF, TNF receptor-associated factor; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; NF-κB, nuclear factor κB; NFATc1, nuclear factor of activated T cells c1; TRAP, tartrate-resistant acid phosphatase; SEM, scanning electron microscope (microscopy); PBS, phosphate-buffered saline; TBS, Tris-buffered saline; WT, wild type; α-MEM, minimal essential medium.
References
- 1.Henderson, B., and Nair, S. P. (2003) Trends Microbiol. 11 570-577 [DOI] [PubMed] [Google Scholar]
- 2.Nair, S. P., Meghji, S., Wilson, M., Reddi, K., White, P., and Henderson, B. (1996) Infect. Immun. 64 2371-2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagasawa, T., Kiji, M., Yashiro, R., Hormdee, D., Lu, H., Kunze, M., Suda, T., Koshy, G., Kobayashi, H., Oda, S., Nitta, H., and Ishikawa, I. (2007) Periodontol. 2000 43 65-84 [DOI] [PubMed] [Google Scholar]
- 4.Suda, T., Takahashi, N., and Martin, T. J. (1992) Endocr. Rev. 13 66-80 [DOI] [PubMed] [Google Scholar]
- 5.Teitelbaum, S. L., Tondravi, M. M., and Ross, F. P. (1997) J. Leukocyte Biol. 61 381-388 [DOI] [PubMed] [Google Scholar]
- 6.Ross, F. P., and Teitelbaum, S. L. (2001) in Osteoporosis (Marcus, R., Feldman, D., and Kelsey, J., eds) pp. 73-105, Academic Press, San Diego
- 7.Hsu, H., Lacey, D. L., Dunstan, C. R., Solovyev, I., Colombero, A., Timms, E., Tan, H.-L., Elliott, G., Kelley, M. J., Sarosi, I., Wang, L., Xia, X. Z., Elliott, R., Chiu, L., Black, T., Scully, S., Capparelli, C., Morony, S., Shimamoto, G., Bass, M. B., and Boyle, W. J. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 3540-3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacey, D. L., Timms, E., Tan, H. L., Kelley, M. J., Dunstan, C. R., Burgess, T., Elliott, R., Colombero, A., Elliott, G., Scully, S., Hsu, H., Sullivan, J., Hawkins, N., Davy, E., Capparelli, C., Eli, A., Qian, Y. X., Kaufman, S., Sarosi, I., Shalhoub, V., Senaldi, G., Guo, J., Delaney, J., and Boyle, W. J. (1998) Cell 93 165-176 [DOI] [PubMed] [Google Scholar]
- 9.Burgess, T. L., Qian, Y., Kaufman, S., Ring, B. D., Van, G., Capparelli, C., Kelley, M., Hsu, H., Boyle, W. J., Dunstan, C. R., Hu, S., and Lacey, D. L. (1999) J. Cell Biol. 145 527-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suda, T., Takahashi, N., Udagawa, N., Jimi, E., Gillespie, M. T., and Martin, T. J. (1999) Endocr. Rev. 20 345-357 [DOI] [PubMed] [Google Scholar]
- 11.Teitelbaum, S. L. (2000) Science 289 1504-1508 [DOI] [PubMed] [Google Scholar]
- 12.Anderson, D. M., Maraskovsky, E., Billingsley, W. L., Dougall, W. C., Tometsko, M. E., Roux, E. R., Teepe, M. C., DuBose, R. F., Cosman, D., and Galibert, L. (1997) Nature 390 175-179 [DOI] [PubMed] [Google Scholar]
- 13.Locksley, R. M., Killeen, N., and Lenardo, M. J. (2001) Cell 104 487-501 [DOI] [PubMed] [Google Scholar]
- 14.Chung, J. Y., Park, Y. C., Ye, H., and Wu, H. (2002) J. Cell Sci. 115 679-688 [DOI] [PubMed] [Google Scholar]
- 15.Liu, W., Xu, D., Yang, H., Xu, H., Shi, Z., Cao, X., Takeshita, S., Liu, J., Teale, M., and Feng, X. (2004) J. Biol. Chem. 279 54759-54769 [DOI] [PubMed] [Google Scholar]
- 16.Liu, W., Wang, S., Wei, S., Sun, L., and Feng, X. (2005) J. Biol. Chem. 280 43064-43072 [DOI] [PubMed] [Google Scholar]
- 17.Boyle, W. J., Simonet, W. S., and Lacey, D. L. (2003) Nature 423 337-342 [DOI] [PubMed] [Google Scholar]
- 18.Takayanagi, H., Kim, S., Matsuo, K., Suzuki, H., Suzuki, T., Sato, K., Yokochi, T., Oda, H., Nakamura, K., Ida, N., Wagner, E. F., and Taniguchi, T. (2002) Nature 416 744-749 [DOI] [PubMed] [Google Scholar]
- 19.Feng, X. (2005) Gene 350 1-13 [DOI] [PubMed] [Google Scholar]
- 20.Takayanagi, H., Kim, S., Koga, T., Nishina, H., Isshiki, M., Yoshida, H., Saiura, A., Isobe, M., Yokochi, T., Inoue, J., Wagner, E. F., Mak, T. W., Kodama, T., and Taniguchi, T. (2002) Dev. Cell 3 889-901 [DOI] [PubMed] [Google Scholar]
- 21.Takayanagi, H. (2007) Nat. Rev. Immunol. 7 292-304 [DOI] [PubMed] [Google Scholar]
- 22.Aliprantis, A. O., Ueki, Y., Sulyanto, R., Park, A., Sigrist, K. S., Sharma, S. M., Ostrowski, M. C., Olsen, B. R., and Glimcher, L. H. (2008) J. Clin. Investig. 118 3775-3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lombardo, E., Alvarez-Barrientos, A., Maroto, B., Bosca, L., and Knaus, U. G. (2007) J. Immunol. 178 3731-3739 [DOI] [PubMed] [Google Scholar]
- 24.Kong, Y. Y., Yoshida, H., Sarosi, I., Tan, H. L., Timms, E., Capparelli, C., Morony, S., Oliveira, d. S. A., Van, G., Itie, A., Khoo, W., Wakeham, A., Dunstan, C. R., Lacey, D. L., Mak, T. W., Boyle, W. J., and Penninger, J. M. (1999) Nature 397 315-323 [DOI] [PubMed] [Google Scholar]
- 25.Dougall, W. C., Glaccum, M., Charrier, K., Rohrbach, K., Brasel, K., De Smedt, T., Daro, E., Smith, J., Tometsko, M. E., Maliszewski, C. R., Armstrong, A., Shen, V., Bain, S., Cosman, D., Anderson, D., Morrissey, P. J., Peschon, J. J., and Schuh, J. (1999) Genes Dev. 13 2412-2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida, H., Hayashi, S.-I., Kunisada, T., Ogawa, M., Nishikawa, S., Okamura, H., Sudo, T., Shultz, L. D., and Nishikawa, S.-I. (1990) Nature 345 442-444 [DOI] [PubMed] [Google Scholar]
- 27.Kawata, T., Tokimasa, C., Fujita, T., Ozawa, S., Sugiyama, H., and Tanne, K. (1999) J. Craniofac. Genet. Dev. Biol. 19 221-225 [PubMed] [Google Scholar]
- 28.Li, J., Sarosi, I., Yan, X.-Q., Morony, S., Capparelli, C., Tan, H.-L., McCabe, S., Elliott, R., Scully, S., Van, G., Kaufman, S., Juan, S.-C., Sun, Y., Tarpley, J., Martin, L., Christensen, K., McCabe, J., Kostenuik, P., Hsu, H., Fletcher, F., Dunstan, C. R., Lacey, D. L., and Boyle, W. J. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 1566-1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaneko, H., Mehrotra, M., Alander, C., Lerner, U., Pilbeam, C., and Raisz, L. (2007) Prostaglandins Leukot. Essent. Fatty Acids 77 181-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Islam, S., Hassan, F., Tumurkhuu, G., Dagvadorj, J., Koide, N., Naiki, Y., Mori, I., Yoshida, T., and Yokochi, T. (2007) Biochem. Biophys. Res. Commun. 360 346-351 [DOI] [PubMed] [Google Scholar]
- 31.Hayashi, S., Tsuneto, M., Yamada, T., Nose, M., Yoshino, M., Shultz, L. D., and Yamazaki, H. (2004) Endocrinology 145 2721-2729 [DOI] [PubMed] [Google Scholar]
- 32.Jiang, Y., Mehta, C. K., Hsu, T. Y., and Alsulaimani, F. F. (2002) Infect. Immun. 70 3143-3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suda, K., Woo, J. T., Takami, M., Sexton, P. M., and Nagai, K. (2002) J. Cell Physiol. 190 101-108 [DOI] [PubMed] [Google Scholar]
- 34.Takami, M., Kim, N., Rho, J., and Choi, Y. (2002) J. Immunol. 169 1516-1523 [DOI] [PubMed] [Google Scholar]
- 35.Zou, W., and Bar-Shavit, Z. (2002) J. Bone Miner. Res. 17 1211-1218 [DOI] [PubMed] [Google Scholar]
- 36.Feng, X., Novack, D. V., Faccio, R., Ory, D. S., Aya, K., Boyer, M. I., McHugh, K. P., Ross, F. P., and Teitelbaum, S. L. (2001) J. Clin. Investig. 107 1137-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McHugh, K. P., Hodivala-Dilke, K., Zheng, M. H., Namba, N., Lam, J., Novack, D., Feng, X., Ross, F. P., Hynes, R. O., and Teitelbaum, S. L. (2000) J. Clin. Investig. 105 433-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirschfeld, M., Ma, Y., Weis, J. H., Vogel, S. N., and Weis, J. J. (2000) J. Immunol. 165 618-622 [DOI] [PubMed] [Google Scholar]
- 39.Lam, J., Takeshita, S., Barker, J. E., Kanagawa, O., Ross, F. P., and Teitelbaum, S. L. (2000) J. Clin. Investig. 106 1481-1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu, D., Wang, S., Liu, W., Liu, J., and Feng, X. (2006) J. Biol. Chem. 281 4678-4690 [DOI] [PubMed] [Google Scholar]
- 41.Kikuchi, T., Matsuguchi, T., Tsuboi, N., Mitani, A., Tanaka, S., Matsuoka, M., Yamamoto, G., Hishikawa, T., Noguchi, T., and Yoshikai, Y. (2001) J. Immunol. 166 3574-3579 [DOI] [PubMed] [Google Scholar]
- 42.Kawai, T., and Akira, S. (2007) Trends Mol. Med. 13 460-469 [DOI] [PubMed] [Google Scholar]
- 43.Akira, S., Uematsu, S., and Takeuchi, O. (2006) Cell 124 783-801 [DOI] [PubMed] [Google Scholar]
- 44.Wong, B. R., Besser, D., Kim, N., Arron, J. R., Vologodskaia, M., Hanafusa, H., and Choi, Y. (1999) Mol. Cell 4 1041-1049 [DOI] [PubMed] [Google Scholar]
- 45.Jimi, E., Akiyama, S., Tsurukai, T., Okahashi, N., Kobayashi, K., Udagawa, N., Nishihara, T., Takahashi, N., and Suda, T. (1999) J. Immunol. 163 434-442 [PubMed] [Google Scholar]
- 46.Wei, S., Wang, M. W., Teitelbaum, S. L., and Ross, F. P. (2002) J. Biol. Chem. 277 6622-6630 [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto, M., Sudo, T., Saito, T., Osada, H., and Tsujimoto, M. (2000) J. Biol. Chem. 275 31155-31161 [DOI] [PubMed] [Google Scholar]
- 48.Mansky, K. C., Sankar, U., Han, J., and Ostrowski, M. C. (2002) J. Biol. Chem. 277 11077-11083 [DOI] [PubMed] [Google Scholar]
- 49.Jimi, E., Nakamura, I., Ikebe, T., Akiyama, S., Takahashi, N., and Suda, T. (1998) J. Biol. Chem. 273 8799-8805 [DOI] [PubMed] [Google Scholar]
- 50.Lee, S. E., Chung, W. J., Kwak, H. B., Chung, C. H., Kwack, K. B., Lee, Z. H., and Kim, H. H. (2001) J. Biol. Chem. 276 49343-49349 [DOI] [PubMed] [Google Scholar]
- 51.Hung, L. C., Lin, C. C., Hung, S. K., Wu, B. C., Jan, M. D., Liou, S. H., and Fu, S. L. (2007) Biochem. Pharmacol. 73 1957-1970 [DOI] [PubMed] [Google Scholar]
- 52.Ojaniemi, M., Glumoff, V., Harju, K., Liljeroos, M., Vuori, K., and Hallman, M. (2003) Eur. J. Immunol. 33 597-605 [DOI] [PubMed] [Google Scholar]
- 53.Li, X., Tupper, J. C., Bannerman, D. D., Winn, R. K., Rhodes, C. J., and Harlan, J. M. (2003) Infect. Immun. 71 4414-4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones, B. W., Heldwein, K. A., Means, T. K., Saukkonen, J. J., and Fenton, M. J. (2001) Ann. Rheum. Dis. 60 Suppl. 3, iii6-iii12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Socransky, S. S., and Haffajee, A. D. (1992) J. Periodontol. 63 322-331 [DOI] [PubMed] [Google Scholar]
- 56.Teng, Y. T., Nguyen, H., Gao, X., Kong, Y. Y., Gorczynski, R. M., Singh, B., Ellen, R. P., and Penninger, J. M. (2000) J. Clin. Investig. 106 R59-R67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kazor, C. E., Mitchell, P. M., Lee, A. M., Stokes, L. N., Loesche, W. J., Dewhirst, F. E., and Paster, B. J. (2003) J. Clin. Microbiol. 41 558-563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zambon, J. J. (1996) Ann. Periodontol. 1 879-925 [DOI] [PubMed] [Google Scholar]
- 59.Socransky, S. S., Haffajee, A. D., Cugini, M. A., Smith, C., and Kent, R. L., Jr. (1998) J. Clin. Periodontol. 25 134-144 [DOI] [PubMed] [Google Scholar]
- 60.Socransky, S. S., and Haffajee, A. D. (2002) Periodontol. 2000 28 12-55 [DOI] [PubMed] [Google Scholar]
- 61.Schwartz, Z., Goultschin, J., Dean, D. D., and Boyan, B. D. (1997) Periodontol. 2000 14 158-172 [DOI] [PubMed] [Google Scholar]
- 62.Kirkwood, K. L., Cirelli, J. A., Rogers, J. E., and Giannobile, W. V. (2007) Periodontol. 2000 43 294-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ebersole, J. L. (2003) Periodontol. 2000 31 135-166 [DOI] [PubMed] [Google Scholar]
- 64.Rowe, D. J., and Hausmann, E. (1977) Calcif. Tissue Res. 23 283-289 [DOI] [PubMed] [Google Scholar]
- 65.Sveen, K., and Skaug, N. (1980) Scand. J. Dent. Res. 88 535-542 [DOI] [PubMed] [Google Scholar]
- 66.Shiina, Y., Yamaguchi, A., Yamana, H., Abe, E., Yoshiki, S., and Suda, T. (1986) Calcif. Tissue Int. 39 28-34 [DOI] [PubMed] [Google Scholar]
- 67.Sismey-Durrant, H. J., and Hopps, R. M. (1987) Arch. Oral Biol. 32 911-913 [DOI] [PubMed] [Google Scholar]
- 68.Umezu, A., Kaneko, N., Toyama, Y., Watanabe, Y., and Itoh, H. (1989) J. Periodontal Res. 24 378-383 [DOI] [PubMed] [Google Scholar]
- 69.Orcel, P., Feuga, M., Bielakoff, J., and de Vernejoul, M. C. (1993) Am. J. Physiol. 264 E391-E397 [DOI] [PubMed] [Google Scholar]
- 70.Abu-Amer, Y., Ross, F. P., Edwards, J., and Teitelbaum, S. L. (1997) J. Clin. Investig. 100 1557-1565 [DOI] [PMC free article] [PubMed] [Google Scholar]