Abstract
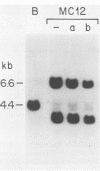
T lymphocytes from a patient with Shigella flexneri dysentery and postdysenteric reactive arthritis were cloned by limiting dilution with recombinant interleukin-2 and a strain of S. flexneri different from that which had infected her. Five of eight clones produced proliferated in response to the shigellae used to generate the clones. The response required irradiated syngeneic blood mononuclear cells as antigen-presenting cells. One such clone, MC12, proliferated in response to both the shigellae used to generate the clones and the infecting shigellae but not to other shigellae, Salmonella heidelberg, or control Escherichia coli. MC12 was CD3+, CD4+, CD8-, and human histocompatibility leukocyte antigen (HLA)-DR+. The proliferative response to the shigellae was blocked by antibody to HLA-DR but not by antibody to HLA-A,B,C. The response required antigen-presenting cells that shared HLA-DR antigens with the clone and appeared to be restricted by HLA-DR2. The epitope recognized by MC12 was associated with the bacterial membranes. Thus, T-lymphocyte clones that proliferate in response to some shigellae can be isolated from patients with shigellosis.
Full text
PDF
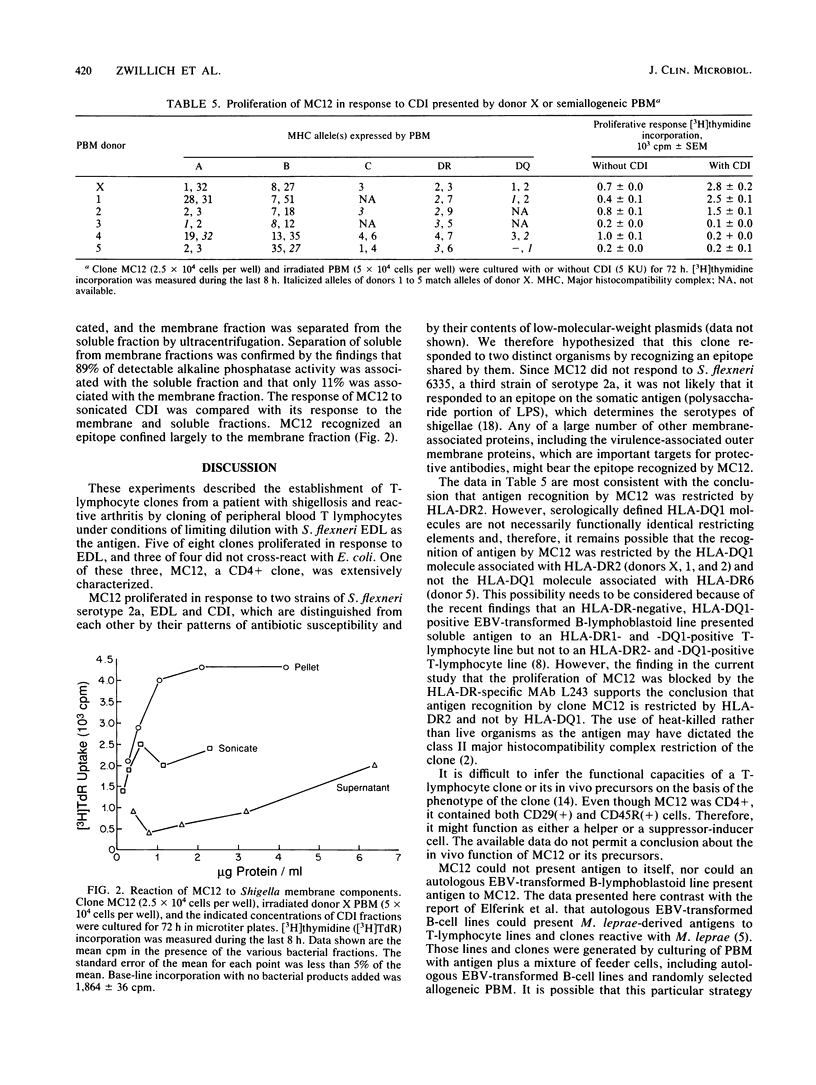

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baskin D. H., Lax J. D., Barenberg D. Shigella bacteremia in patients with the acquired immune deficiency syndrome. Am J Gastroenterol. 1987 Apr;82(4):338–341. [PubMed] [Google Scholar]
- Braciale T. J., Morrison L. A., Sweetser M. T., Sambrook J., Gething M. J., Braciale V. L. Antigen presentation pathways to class I and class II MHC-restricted T lymphocytes. Immunol Rev. 1987 Aug;98:95–114. doi: 10.1111/j.1600-065x.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Carl M., Robbins F. M., Hartzman R. J., Dasch G. A. Lysis of cells infected with typhus group rickettsiae by a human cytotoxic T cell clone. J Immunol. 1987 Dec 15;139(12):4203–4207. [PubMed] [Google Scholar]
- Duby A. D., Klein K. A., Murre C., Seidman J. G. A novel mechanism of somatic rearrangement predicted by a human T-cell antigen receptor beta-chain complementary DNA. Science. 1985 Jun 7;228(4704):1204–1206. doi: 10.1126/science.3839095. [DOI] [PubMed] [Google Scholar]
- Elferink B. G., Ottenhoff T. H., de Vries R. R. Epstein-Barr virus-transformed B cell lines present M. leprae antigens to T cells. Scand J Immunol. 1985 Nov;22(5):585–589. doi: 10.1111/j.1365-3083.1985.tb01918.x. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Mellins E., Woelfel M., Pious D. Importance of HLA-DQ and -DP restriction elements in T-cell responses to soluble antigens: mutational analysis. Hum Immunol. 1987 Mar;18(3):211–223. doi: 10.1016/0198-8859(87)90086-3. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Mustafa A. S., Gill H. K., Nerland A., Britton W. J., Mehra V., Bloom B. R., Young R. A., Godal T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature. 1986 Jan 2;319(6048):63–66. doi: 10.1038/319063a0. [DOI] [PubMed] [Google Scholar]
- Oaks E. V., Hale T. L., Formal S. B. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986 Jul;53(1):57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenhoff T. H., Klatser P. R., Ivanyi J., Elferink D. G., de Wit M. Y., de Vries R. R. Mycobacterium leprae-specific protein antigens defined by cloned human helper T cells. Nature. 1986 Jan 2;319(6048):66–68. doi: 10.1038/319066a0. [DOI] [PubMed] [Google Scholar]
- Pawelec G., Schneider E. M., Wernet P. Acquisition of suppressive activity and natural killer-like cytotoxicity by human alloproliferative "helper" T cell clones. J Immunol. 1986 Jan;136(2):402–411. [PubMed] [Google Scholar]
- Qvigstad E., Digranes S., Thorsby E. Antigen-specific proliferative human T-lymphocyte clones with specificity for Chlamydia trachomatis. Scand J Immunol. 1983 Oct;18(4):291–297. doi: 10.1111/j.1365-3083.1983.tb01800.x. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B., Abbas A. K. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984 Oct 1;160(4):1102–1113. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Casadaban M. J., Shuman H. A., Beckwith J. R. Conversion of beta-galactosidase to a membrane-bound state by gene fusion. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3423–3427. doi: 10.1073/pnas.73.10.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Thompson P. A., Jelinek D. F., Lipsky P. E. Regulation of human B cell proliferation by prostaglandin E2. J Immunol. 1984 Nov;133(5):2446–2453. [PubMed] [Google Scholar]
- Toyonaga B., Yoshikai Y., Vadasz V., Chin B., Mak T. W. Organization and sequences of the diversity, joining, and constant region genes of the human T-cell receptor beta chain. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8624–8628. doi: 10.1073/pnas.82.24.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]