Abstract
Ischemia/reperfusion injury (IRI) induces an innate immune response, leading to an inflammatory reaction and tissue damage that have been attributed to engagement of the Toll-like receptor (TLR) 2 and 4. However, the respective roles of TLR2 and/or TLR4 in mediating downstream activation of mitogen-activated protein kinase (MAPK) pathways during IRI have not been fully elucidated. Here we show that extracellular signal-regulated kinase (ERK)1/2 is activated in both intact kidneys and cultured renal tubule epithelial cells (RTECs) from wildtype and Tlr4 knockout mice, but not those from Tlr2 knockout mice subjected to transient ischemia. Geldanamycin (GA), an inhibitor of heat shock protein 90 and reticulum endoplasmic-resident gp96, and gp96 mRNA silencing (siRNA), did not affect ERK1/2 activation in either post-hypoxic wild-type or Tlr4-deficient RTECs, but did restore its activation in post-hypoxic Tlr2-deficient RTECs. Immunoprecipitation studies revealed that gp96 co-immunoprecipitates with the serine-threonine protein phosphatase 5 (PP5), identified as a negative modulator of the mitogen extracellular kinase (MEK)-ERK pathway, in unstressed wild-type and post-hypoxic Tlr2-deficient RTECs. In contrast, PP5 co-immunoprecipitation with gp96 was strikingly reduced in post-hypoxic wild-type RTECs, suggesting that the inactivation of PP5 resulting from the dissociation of PP5 from gp96 allows the activation of ERK1/2 to occur. Inhibition of PP5 by okadaic acid, and Pp5 siRNA also restored TLR2-mediated phosphorylation of ERK1/2, and apoptosis signal-regulating kinase 1 (ASK1)/c-Jun N-terminal kinase (JNK)-mediated apoptosis in post-hypoxic Tlr2-deficient RTECs. These findings indicate that gp96 interacts with PP5 and controls TLR2-mediated induction of ERK1/2 in post-hypoxic renal tubule cells.
Ischemia/reperfusion injury (IRI)3 leads to the activation of an innate immune response that can cause tissue damage (1). The initial sterile injury activates many immune signaling pathways mediated by pattern-recognition receptors. Toll-like receptors (TLRs), which recognize molecular motifs shared by microorganisms sense a variety of endogenous damaged-associated molecular pattern molecules released from damaged/ischemic cells (2-4). Among them, TLR2 and TLR4 expressed both in bone marrow-derived cells and also in renal tubule epithelial cells (5), play key roles in mediating inflammatory responses and apoptosis after renal IRI (6, 7). Ischemic-reperfused kidney and renal tubule epithelial cells (RTECs) from TLR2- and TLR4-deficient mice were shown to be better protected against IRI and exhibit less apoptotic tubule cells than their counterpart wild-type mice (6, 7). However, the mechanisms of TLR-mediated downstream signaling pathways activated during IRI remain unclear.
The oxidative stress injury caused by IRI has been attributed to the activation of mitogen-activated protein kinase (MAPK) pathways including extracellular signal-regulated protein kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 (8, 9). Activation of JNK/p38, via the activation of the Ser/Thr MAP kinase kinase kinase apoptosis signal-regulating kinase 1 (ASK1) plays a key role in cytokine- and stress-induced apoptosis (10, 11). The activation of ERK, which can be associated with drug-induced apoptosis (12-14), has also been shown to be implicated in cell survival following oxidant injury or induction of endoplasmic reticulum (ER) stress-induced cell death signaling (15-17). The protein phosphatase 5 (PP5), a member of the PPP family of serine/threonine phosphatases, ubiquitously expressed in mammalian cells (18), has been shown to act as a physiological negative regulator of the ASK1-JNK/p38 pathway that facilitates apoptosis in hypoxic and H2O2-stressed cell (19, 20). Rapamycin, an inhibitor of the serine/threonine target of rapamycin (mTOR) was also shown to activate ASK1 signaling by suppressing protein phosphatase 5 activity (21). PP5 has been identified as an inactivator of the MEK-ERK pathway through its interaction with the kinase Raf-1 initiating this pathway (22). PP5 was also shown to physically interact with the heat shock protein (Hsp) 90, which is complexed with the transcription factor heat shock factor 1 (HSF1), a key regulatory protein responsible in Hsp synthesis (23). This complex present in unstressed cells dissociates in stressed cells. Inhibition of complexed Hsp90 or dissociation of the complex under the condition of stress leads to an activation of HSF1 (24-27). Moreover, PP5 was shown to act as a negative regulator of HSF1 (28). Therefore, the question arises whether a similar mechanism of activation/deactivation of PP5 might also account in the control of TLR-mediated activation of ERK1/2 in ischemic cells.
In the present study, we analyzed the regulatory mechanisms that govern TLR-mediated ERK pathway in an experimental murine model of IRI and in primary cultures of renal tubule epithelial cells (RTECs) subjected to transient hypoxia. TLR2 and TLR4 both activate JNK, but the activation of ERK1/2 in post-ischemic kidneys appears selectively mediated by TLR2. We show that PP5 co-immunoprecipitates with gp96, the ER-resident homologue to cytosolic Hsp90, shown to play a key role in TLR-mediated innate immunity (29, 30), in non-hypoxic wild-type RTECs. PP5 co-immunoprecipitation with gp96 was strikingly reduced in post-hypoxic wild-type mice, but not in the protected post-hypoxic TLR2 knockout RTECs, suggesting that dissociation of the gp96-PP5 interaction leads to the inactivation of PP5. The benzoquinone ansamycin, geldanamycin (GA), an inhibitor of Hsp90 and gp96 (31, 32), and silencing RNA (siRNA) gp96 mRNA expression, which had no effect in non-hypoxic and post-hypoxic wild-type RTECs, restored the activation of ERK1/2, but not of JNK, in TLR2-deficient post-hypoxic RTECs. In addition, extinction of Pp5 mRNA expression by siRNA restored both the activation of ERK1/2, ASK1, and JNK, and subsequent cell apoptosis in TLR2 knockout RTECs. Taken together, these findings have provided strong evidence that gp96/PP5 interactions play a key role in the control of the selective TLR2-mediated activation of the ERK pathway in ischemic renal tubule cells.
EXPERIMENTAL PROCEDURES
Mice—Experiments were carried out on pathogen-free 10-12-week-old female wild-type C57BL/6J mice obtained from the Jackson Laboratory (Bar Harbor, ME), and Tlr2-/-, Tlr4-/-, and double Tlr2,4-/-(DKO) knockout mice originally obtained from S. Akira (Osaka University, Osaka, Japan). All the mice were further backcrossed at least eight times into C57BL/6 mice to ensure similar genetic backgrounds. All experiments were conducted in accordance with the guidelines of the French Agricultural Office, and in compliance with the legislation governing animal studies.
Renal I/R Injury in Mice—Renal I/R injury was induced as described with slight modifications (7). Mice were anesthetized by the intraperitoneal injection of 2,2,2-tribromoethanol (5 mg/mouse) (Sigma-Aldrich). The kidneys were exposed by bilateral lumbar incisions, and the renal pedicles were clamped with non-traumatic microvascular clamps for 30 min. The clamps were then removed, and the lumbar incisions were sutured after checking that the blood flow had been restored. Sham-operated mice (referred to as controls) underwent the same procedure, except that the renal vessels were not clamped. Surgery was performed on a homeothermic table to maintain a body temperature between 36.5 and 38 °C. All mice received 200 μl of sterile 0.9% NaCl dripped onto the lumbar incisions to keep the tissue moist. Mice (6-10 mice per group) were sacrificed 1, 2, and 7 days after surgery. Before sacrifice, a blood sample was collected, and the level of serum creatinine was measured using an Olympus AU400 auto-analyzer (Olympus). Mice were sacrificed 1, 2, and 7 days after the transient ischemia, and the kidneys were removed, and fixed or stored at -80 °C until use.
TUNEL—The number of apoptotic cells analyzed in kidney tissue sections fixed in Dubosc-Brazil solution was determined using the terminal deoxyribonucleotide transferase (TdT)-mediated dUTP nick end labeling (TUNEL) method using the Apop Tag kit (Chemicon Int.) according to the manufacturer's instructions. The percentage of apoptotic cells was determined on 2 or 3 enlarged fields (×200) from 2-3 different tissue sections from 3-5 kidneys in each condition tested.
Cultured Cells—Experiments were carried out on primary cultures of RTECs isolated from the kidneys of naive wild-type mice or Tlr2-/- mice. Briefly, the kidneys were rapidly removed under sterile conditions, and primary cultures of RTECs were prepared using previously described methods (8, 33). Kidneys were injected using fine needles with modified defined medium (DM) as described (33) supplemented with 0.1% collagenase (Roche Diagnostics GmBH). Thin kidney slices were then minced and incubated in the same collagenase-supplemented DM for 45 min at 37 °C. Kidney slices were gently dispersed using sterile needles, and then poured through 100- and 40-μm nylon gauzes, as described (33). The resulting cell suspension was then seeded on chambered coverglass slides or collagen-coated 48-well trays (∼ 2 × 104 cells per well), and grown at 37 °C in a 5% CO2-95% air atmosphere in the same modified defined DM medium. The medium was changed every 2 days. Two weeks after seeding, cultured RTECs formed confluent layers of highly enriched epithelial renal cells expressing cytokeratins8-18 and the tight junction-associated protein ZO-1 (not shown). Hypoxia was induced in cultured RTECs by immersing cell layers in mineral oil as described (7). After replacing with fresh medium, 1 ml of sterile mineral oil (Sigma-Aldrich) was added for 30 min at 37 °C. The mineral oil was then removed by rinsing twice with fresh medium, and the cells were then incubated at 37 °C for a further 24 h before being analyzed. All reagents, media, and buffers used in the assays were checked by using the Limulus amebocyte gelation activity test (BioWhittaker Inc) to ensure that they had not been contaminated by endotoxin. Mouse renal proximal tubule PKSV-PR cells (34) were also used for co-immunoprecipitation studies (see below).
Immunohistochemical Studies—Cultured RTECs were labeled with an active caspase-3 antibody (Promega) to estimate the percentage of apoptotic cells determined on 2 or 3 enlarged fields (×200) from at least five separate cultures derived from 2 to 3 separated wild-type and Tlr2-/- mice kidneys for each condition tested.
Small Interfering RNA (siRNA) Transfection—Experiments were performed using predesigned HP GenomeWide (Qiagen) siRNAs for the murine gp96 gene (5′-ATGAATGATATCAAACCAATA-3′; sense: GAAUGAUAUCAAACCAAUAdTdT; antisense UAUUGGUUUGAUAUCAUUCdAdT), or Pp5 gene (5′-CAGCAAGATTGTGAAGCAGAA-3′; sense: GCAAGAUUGUGAAGCAGAAdTdT; antisense UUCUGCUUCACAAUCUUGCdTdG). A universal negative control siRNA (target DNA sequence: AATTCTCCGAACGTGTCACGT; sense: UUCUCCGAACGUGUCACGUdTdT; antisense: ACGUGACACGUUCGGAGAAdTdT) was also used. Single strand sense and antisense RNA nucleotides were annealed (90 °C for 1 min, and then 37 °C for 1 h) to generate a RNA duplex according to the manufacturer's instructions. Subconfluent cultures of RTECs (between day 6 and day 8 after seeding) grown in 48-well plates were incubated with a mixture of 0.6 pmol of siRNA duplex and 2 μl of INTERFERin™ prepared according to the manufacturer's instructions (Polyplus-Transfection Inc) for 48 h at 37 °C before use.
Immunoblotting—Frozen kidney samples and cultured RTECs grown in 48-well plates were lysed in 50 μl of ice-cold lysis buffer containing 62.5 mm Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 50 mm dithiothreitol, and then sonicated for 15 s at 4 °C. For each condition tested, cell lysates from 8 separate wells were pooled. Samples (25-50 μg/lane) were electrophoresed using 10% SDS-PAGE, and analyzed by immunoblot (35). Antibodies against p-ASK1, p-JNK, p-ERK1/2, p-p38, p38, p-AKT, AKT (Cell Signaling Technology), ASK1, JNK, ERK1/2 (Santa Cruz Biotechnology), β-actin (Sigma Aldrich), and gp96 (Stressgen) were used to detect the corresponding antigens.
Immunoprecipitation (IP)—IPs were performed on cell extracts from untreated confluent cultures of PKSV-PR cells and primary cultures of RTECs subjected or not to transient hypoxia as described above. For each condition tested, RTECs grown in individual 48-well plates were pooled to obtain sufficient amount of material. Cells were lysed in a lysis buffer, sonicated, centrifuged, and precleared with 75 μl of protein G-Sepharose overnight at 4 °C using the Immunoprecipitation kit (protein G) (GE Healthcare). Precleared supernatants from untreated PKSV-PR cells and wild-type RTECs or post-hypoxic RTECS were then sequentially incubated overnight at 4 °C with an antibody raised against gp96 (12 μg/ml, Stressgen) for 2 h at 4 °C, and then with 50 μl of protein G-Sepharose overnight at 4 °C. Bound proteins were then rinsed twice in the Immunoprecipitation kit lysis buffers (Roche Diagnostics) according to the manufacturer's instructions. Sepharose-bound proteins were separated on 8-15% SDS acrylamide gels. After being transferred onto nitrocellulose, the filters were incubated with antibodies directed against gp96 (1:2000), or PP5 (1:1000), and then with the ECL anti-rabbit IgG, horseradish peroxidase-linked species-specific whole antibody (GE Healthcare) or the Rabbit IgG TrueBlot™ (eBioscience). Labeled bands were detected using the ECL+ Western blotting Detection System (GE Healthcare).
Data Analysis—All data are representative of a minimum of three independent experiments. Values are expressed as means ± S.E. Statistical differences between groups were analyzed using one-way analysis of variance with the Bonferroni test. A p value < 0.05 was considered significant.
RESULTS
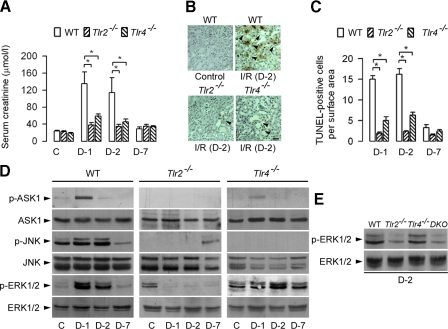
Differential TLR2- and TLR4-mediated Activation of ERK1/2 in Ischemic-reperfused Kidneys—Renal IRI induces transient renal insufficiency in wild-type mice, assessed by the rapid increase of serum creatinine which peaked during the 2 days following surgery, and returned to almost normal values 7 days after the 30-min bilateral clamping of the renal pedicles. In accordance with previous studies (7, 8), the rise in serum creatinine was significantly less marked in Tlr2-/- and to a lesser extent in Tlr4-/- than in wild-type operated mice (Fig. 1A). The number of apoptotic cells that were TUNEL-positive was also significantly lower in post-ischemic Tlr2-/- and Tlr4-/- than wild-type post-ischemic kidneys (Fig. 1, B and C), suggesting that TRL2 and TLR4 are both implicated in renal insufficiency and IRI-induced apoptosis (7, 8). We next analyzed the consequences of IRI on TLR2- and TLR4-mediated activation of MAPKs. IRI induced transient increase in the expression of phosphorylated (p) ASK1 in day-1 post-ischemic wild-type mice kidneys (Fig. 1D). IRI also stimulated the expression of p-JNK and p-ERK1/2 in day-1 and day-2 post-ischemic wildtype mice kidneys (Fig. 1D). In any case, IRI affected the total amounts of ASK1, ERK½, and JNK. In sharp contrast, the invalidation of Tlr2, but not of Tlr4, impaired the activation of p-ERK1/2 in post-ischemic kidneys (Fig. 1D). No activation of p-ERK1/2 was also detected in the day-2 post-ischemic Tlr2,4-/- double knockout (DKO) mice kidneys (Fig. 1E). In contrast, IRI did not significantly affect much the expression of the p-p38 MAPK in post-ischemic wild-type, Tlr2-/- and Tlr4-/- kidneys (not shown). These results indicate that both TLR2 and TLR4 mediate the activation of JNK in post-ischemic kidneys, and that the IRI-induced activation of ERK1/2 is controlled by TLR2.
FIGURE 1.
Differential expression of ERK1/2 in ischemic-reperfused wild-type, Tlr2-, and Tlr4-deficient mice. A, levels of serum creatinine in WT, Tlr2-/-, and Tlr4-/- sham-operated control (C) mice, and one (D-1), two (D-2), and seven (D-7) days after bilateral clamping of the renal pedicles. B, detection of TUNEL-positive tubule cells (arrowheads) in kidney sections from control (Control) and day-2 post-ischemic (I/R (D-2)) mice. Scale bar, 20 μm. C, quantification of the number of active caspase-3 positive cells per tissue section (200 μm2) in post-ischemic kidneys. Data are means ± S.E. (A, B: 6-9 kidneys). *, p < 0.05 between groups. D, immunoblot analysis of phospho (p-) and total ASK1-, JNK-, and ERK1/2-labeled bands detected in kidney homogenates from control (C), and D-1, D-2, and D-7 post-ischemic mice kidneys. E, immunoblot analysis of p- and total ERK1/2-labeled bands in D-2 post-ischemic WT, Tlr2-/-, Tlr4-/-, and Tlr2,4-/- DKO mice kidneys.
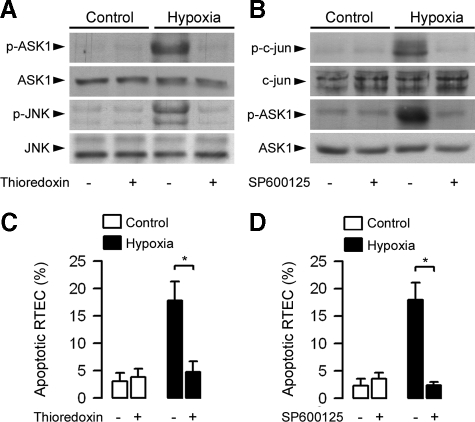
Inhibition of ASK1 and JNK Activation Impairs Hypoxia-induced Apoptosis in Wild-type RTECs—As in day-1 and day-2 post-ischemic wild-type kidneys, the expression of p-JNK and p-ASK1 increased in primary cultures of wild-type RTECs 24 h after the induction of transient hypoxia by immersing cell layers in mineral oil for 30 min when compared with untreated RTECs (Fig. 2A). Preincubating wild-type RTECs (30 min) with 200 ng/ml thioredoxin, a concentration used to inhibit ASK1 (36, 37) that does not affect cell viability (>96% viable cells as assessed by exclusion of the Trypan blue dye), impaired the activation of p-ASK1 and p-JNK in post-hypoxic wild-type RTECs without affecting total ASK1 and JNK (Fig. 2A). Similarly, preincubation of the cells with 2 μm of the selective JNK inhibitor SP600125, a concentration that does not affect the phosphorylation of other MAP kinases (35) and cell viability (>94% viable cells), did not affect total c-Jun, the substrate of JNK and ASK1, but in contrast, reduced both the activation of phosphorylated c-Jun and p-ASK1 caused by hypoxia (Fig. 2B). In addition, preincubation of the cells with SP600125 or thioredoxin both significantly reduced the percentage of apoptotic cells caused by hypoxia when compared with untreated post-hypoxic wild-type RTECs (Fig. 2, C and D). These results strongly suggest that the activation of the ASK1-JNK pro-apoptotic pathway mediated by TLR2/4 represents a key signal event responsible for cell apoptosis and renal damage, leading to increase in serum creatinine after ischemia/reperfusion injury.
FIGURE 2.
Inhibition of ASK1 and JNK activation impairs the induction of apoptosis in post-hypoxic wild-type renal tubule cells. A, immunoblot analysis of p- and total ASK1 and JNK in non-hypoxic (Control) and 24-h post-hypoxic (Hypoxia) wild-type RTECs preincubated without or with thioredoxin (200 ng/ml). B, immunoblot analysis of p- and total c-Jun and ASK1 in nonhypoxic and 24-h post-hypoxic wild-type RTECs preincubated without or with SP600125 (2 μm). C and D, percentage of apoptotic cells measured in non-hypoxic (Control) and 24 h post-hypoxic (Hypoxia) wild-type RTECs pre-incubated without or with thioredoxin (C) or SP600125 (D). Data are means ± S.E. from four different cell cultures from two different kidneys for each set of experimental conditions. *, p < 0.05 between groups.
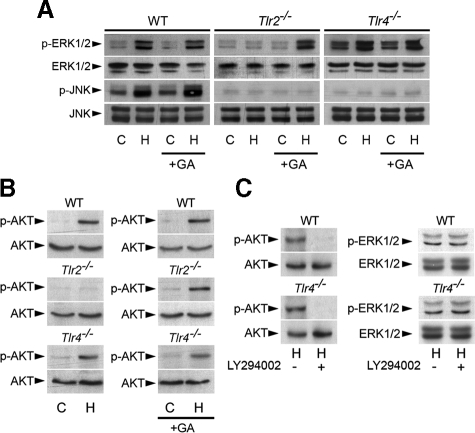
Geldanamycin Restores the Phosphorylation of AKT and ERK1/2 in Post-hypoxic Tlr2-/- RTECs—Hsp molecular chaperones play an essential role in the proper folding, maturation, and activity of client proteins (38). Hsp90 forms multimolecular complex with its client proteins, and controls the function of a number of signaling proteins, including serine/threonine Raf-1, which controls the MEK-ERK pathway (39). It has been shown that the benzoquinone ansamycin GA, which binds to Hsp90 and destabilizes kinase complexes (40), is able to disrupt signaling via the Raf-1-MEK-MAPK pathway in PMA-stimulated NIH 3T3 cells (41). We therefore investigated the effects of GA on the expression of ERK1/2 in control and post-hypoxic Tlr2-/- and Tlr4-/- RTECs. GA did not affect the phosphorylation status of p-ERK1/2 in control or post-hypoxic wildtype and Tlr4-/- RTECs, but did restore its phosphorylation in post-hypoxic Tlr2-/- RTECs (Fig. 3A). Interestingly, GA had no effect on activated p-JNK in post-hypoxic wild-type RTECs, and in contrast with its inducible effect on p-ERK1/2 in post-hypoxic Tlr2-/- RTECs, GA did not restore the activation of phosphorylated JNK post-hypoxic Tlr2-/- RTECs as well as in post-hypoxic Tlr4-/- RTECs. These results indicate that pharmacological inhibition of Hsps selectively restored the hypoxia-induced TLR2-mediated activation of ERK1/2.
FIGURE 3.
Geldanamycin restores the phosphorylation of ERK1/2 in post-hypoxic Tlr2-/- renal tubule cells. A, immunoblot analyses of p- and total ERK1/2 and JNK in non-hypoxic (C) and 24-h post-hypoxic (H) WT, Tlr2-/-, Tlr4-/- RTECs preincubated without or with GA (+GA). B, immunoblot analyses of p- and total AKT in non-hypoxic (C) and day-1 post-hypoxic (H) WT, Tlr2-/-, Tlr4-/- RTECs preincubated without or with geldanamycin (+GA, 10 nm). C, immunoblot analyses of p- and total AKT and ERK1/2 in day-1 post-hypoxic (H) WT, and Tlr4-/- RTECs preincubated (+) or not (-) with LY294002 (20 μm).
Inhibition of Hsps function by GA was reported to transiently activate AKT and ERK in human breast cancer MCF7 cells (42). Inhibition of the phosphatidylinositol 3-kinase (PI3-K)-AKT pathway was also shown to prevent the activation of p38 and ERK1/2 in TLR2-stimulated neutrophils (43). Experiments were undertaken to test whether the PI3-K-AKT pathway might participate in the hypoxia-induced TLR2-mediated ERK1/2 activation. p-AKT, which was not detected in control cells, became activated in post-hypoxic wild-type and Tlr4-/- RTECs, but not in Tlr2-/- RTECs (Fig. 3B, left panels). GA did not affect the phosphorylation of AKT in either post-hypoxic wild-type or Tlr4-/- RTECs, but did restore its phosphorylation in Tlr2-/- RTECs (Fig. 3B, right panels). Incubating cells with the PI3-K inhibitor LY294002 (20 μm) completely inhibited p-AKT activation in post-hypoxic wild-type or Tlr4-/- RTECs (Fig. 3C). However, inhibition of PI3-K by LY294002 (20 μm) failed to inhibit the hypoxia-induced stimulation of p-ERK1/2 in wild-type or Tlr4-/- RTECs (Fig. 3C). These results suggest that the PI3-K-AKT pathway might not be the major pathway involved in the control of ERK1/2 activation in post-hypoxic renal cells. Furthermore, prevention of AKT activation by preincubating wild-type RTECs cells with LY294002 did not impair both the activation of p-JNK and p-ASK1 and the induction of apoptosis caused by hypoxia (supplemental Fig. S1).
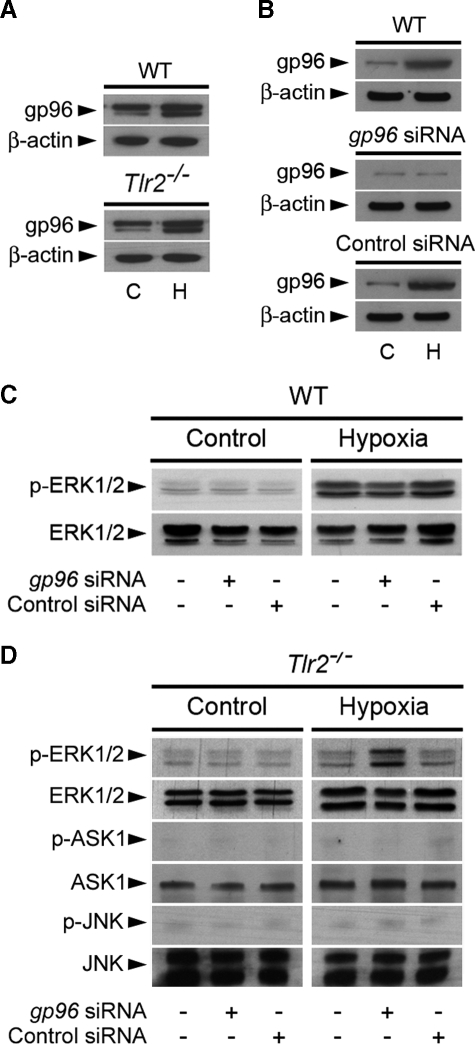
Inhibiting gp96 Restores the Activation of ERK1/2 in Post-hypoxic Tlr2-/- RTECs—gp96 (also known as grp94), one of the most abundant ER-residing Hsp proteins, which has been reported to be a master chaperone for most of the TLRs, including TLR2 and TLR4, in a pre B cell line and in macrophages (29, 30). Because GA was shown to inhibit both Hps90 and gp96 (31, 32), the question arises as to whether gp96 could interfere in TLR-mediated signaling in ischemic cells. The amount of gp96 increased significantly in both wild-type and Tlr2-/- RTECs subjected to hypoxia (Fig. 4A). Silencing the gp96 protein by gp96 siRNA (Fig. 4B) had no effect in non-hypoxic cells and did not impair the activation of p-ERK1/2 in post-hypoxic wildtype RTECs (Fig. 4C). Extinction of gp96 mRNA expression had also no effect in control Tlr2-/- RTECs, but, in sharp contrast, fully restored the activation of p-ERK1/2, but not of p-ASK1 and p-JNK, in post-hypoxic Tlr2-/- RTECs (Fig. 4D). These findings indicate that gp96 plays a key role in controlling TLR2-mediated ERK activation in post-hypoxic renal tubule cells.
FIGURE 4.
gp96 requirement for the induction of TLR2-mediated activation of ERK1/2. A, immunoblot analyses of gp96 and the correspondingβ-actin in untreated (C) and day-1 post-hypoxic (H) WT or Tlr2-/- RTECs. B and C, immunoblot analysis of gp96 and the corresponding β-actin (B), and phospho (p-) and total ERK1/2 (C) in non-hypoxic (C or Control) and day-1 post-hypoxic (H or Hypoxia) WT RTECs transfected or not with a specific gp96 siRNA or negative control siRNA. D, immunoblot analyses of p- and total ERK1/2, ASK1, and JNK in non-hypoxic and day-1 post-hypoxic Tlr2-/- RTECs transfected or not with gp96 siRNA or negative control siRNA.
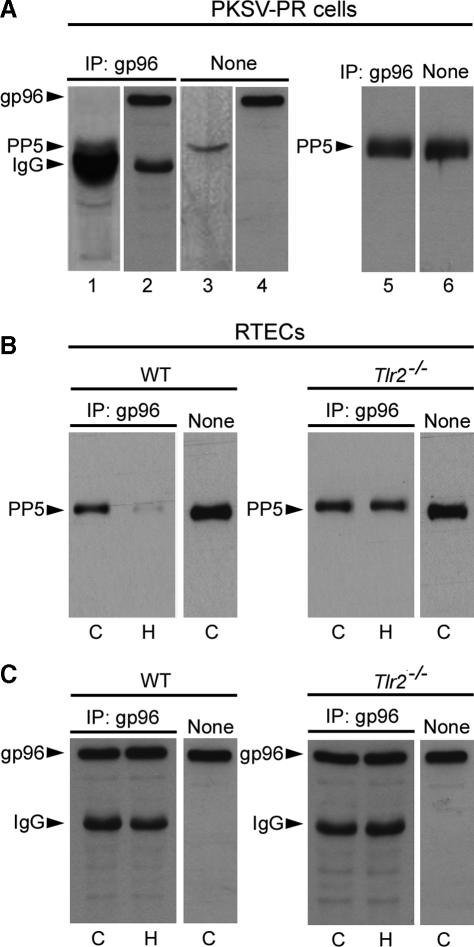
gp96 Interacts with PP5—The fact that the inhibition of gp96 restores the phosphorylation of ERK1/2 raised the possibility that a protein phosphatase-dependent dephosphorylation process might interfere with gp96-dependent p-ERK1/2 activation. Previous studies have shown that PP5 harboring a tetratricopeptide (TPR) domain selectively dephosphorylates and inhibits Raf-1 activity and downstream signaling of the MEK-ERK pathway (22). The crystal structure of PP5 revealed a mechanism of autoinhibition by its TPR domain inhibiting the C-terminal phosphatase domain, and activation by Hsp90 (44). These findings led us to investigate whether gp96 binds to PP5. Therefore, IP experiments using an antibody directed against gp96 were performed to test the interaction between PP5 and gp96 in non-hypoxic and post-hypoxic RTECs. Experiments were first performed on lysates of established murine PKSV-PR cells, which exhibit the main features of proximal tubule cells from which they were derived (34). Co-IP of PP5 using anti-gp96 antibody revealed that gp96 interact with PP5 in nonhypoxic PKSV-PR cells (Fig. 5A, lane 1). As controls, Western blot revealed the presence of gp96 in the same immunoprecipitated cell lysate (Fig. 5A, lane 2), as well as the presence of PP5 and gp96 in the non-immunoprecipitated cell lysates (Fig. 5A, lanes 3 and 4). Because the secondary ECL anti-rabbit IgG horseradish peroxidase antibody recognizes endogenous 55-kDa IgG heavy chains close to the 58-kDa PP5 protein (Fig. 5A), all subsequent IP experiments were performed using the Rabbit IgG TrueBlot™ (eBioscience), which does not detect the reduced, SDS-denatured form of IgG, to reveal PP5. Western blots of cell lysates from co-IP PP5 and non-immunoprecipitated cell lysates using the secondary IgG TrueBlot™ antibody confirmed that gp96 interact with PP5 in non-hypoxic PKSV-PR cells (Fig. 5A, lanes 5 and 6). Similar to PKSV-PR cells, PP5 also co-immunoprecipitated with gp96 in the nonhypoxic wild-type RTECs (Fig. 5B). We then checked the consequence of hypoxia on PP5-gp96 interactions in wild-type RTECs and Tlr2-/- RTECs. In contrast to non-hypoxic cells, PP5 co-immunoprecipitation with gp96 was dramatically reduced in the 24-h post-hypoxic wild-type RTECs (Fig. 5B). Consistent with the fact that Tlr2-/- post-hypoxic RTECs are better protected against hypoxia than their wild-type RTECs counterpart (7), co-IP experiments revealed that PP5 co-immunoprecipitated with gp96 in non-hypoxic Tlr2-/- RTECs as well as in post-hypoxic Tlr2-/- RTECs (Fig. 5B), suggesting that dissociation of the gp96-PP5 complex is highly dependent on TLR2 activation. As controls, Western blots revealed equal amounts of gp96 in the immunoprecipitated cell lysates from both non-hypoxic and post-hypoxic wild-type and Tlr2-/- RTECs (Fig. 5C). Altogether, these findings strongly suggest that hypoxia favors the dissociation of the gp96-PP5 complex in wild-type RTECs highly sensitive to the hypoxic stress.
FIGURE 5.
PP5 interacts with gp96. Lysates from non-hypoxic PKSV-PR cells (A) and wild-type or Tlr2-/- RTECs (B, C) were subjected to IP using an antibody against gp96. The IP material was then subjected to Western blot analysis, and proteins were detected with anti-PP5 or anti-gp96 antibodies, then revealed with the ECL anti-rabbit IgG, horseradish peroxidase-linked species-specific whole antibody (GE Healthcare) (A, lanes 1-4 and C) or the Rabbit IgG TrueBlot™ (eBioscience) (A, lanes 5 and 6, and B). A, untreated PKSV-PR cells lysates were immunoprecipitated (IP) with the anti-gp96 antibody, then blotted for PP5 (lane 1). As controls, immunoprecipitated cell lysates were blotted for gp96 (lane 2) and non-immunoprecipitated PKSV-PR cell lysates (None) were blotted for PP5 (lane 3) or gp96 (lane 4). Western blot identification of PP5 in non-hypoxic PKSV-PR cell lysates from co-IP (lane 5) and non-immunoprecipitated (None, lane 6) cell lysates using the secondary IgG TrueBlot™ antibody. B and C, untreated control (C) and post-hypoxic (H) WT or Tlr2-/- RTECs were immunoprecipitated with the anti-gp96 antibody, then blotted for PP5 (B) or gp96 (C). As controls, non-immunoprecipitated cell lysates were blotted either for PP5 (B) or gp96 (C).
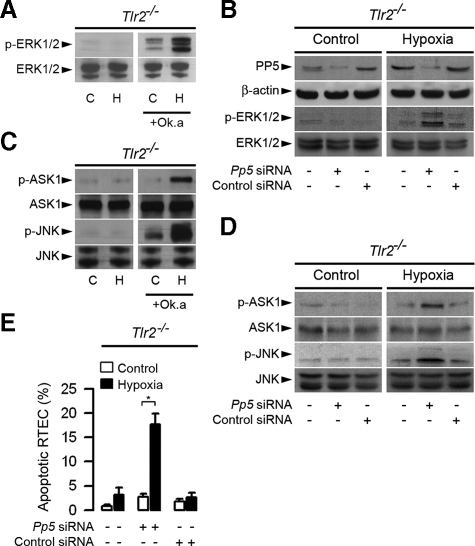
Inhibition of PP5 Restores the Phosphorylation of ERK1/2 and Apoptosis in Post-hypoxic Tlr2-/- RTECs—We next examined the consequence of inhibition of PP5 activity and PP5 siRNA silencing on the expression of ERK1/2 in Tlr2-/- RTECs subjected or not to transient hypoxia. Preincubating RTECs with 10 nm okadaic acid, a potent protein phosphatase inhibitor that inhibits the catalytic activity of PP5 (45), led to detectable activation of p-ERK1/2 in non-hypoxic control Tlr2-/- RTECs (Fig. 6A). In addition, the expression of p-ERK1/2 dramatically increased in okadaic acid-pretreated post-hypoxic Tlr2-/- RTECs (Fig. 6A). Pp5 mRNA extinction by silencing RNA led to decreased expression of the PP5 protein, but had no significant effect on p-ERK1/2 in non-hypoxic, control Tlr2-/- RTECs when compared with non-transfected Tlr2-/- RTECs or cells transfected with a control siRNA (Fig. 6B). In sharp contrast, and in accordance with the restoration of p-ERK1/2 activation in okadaic acid-treated post-hypoxic Tlr2-/- RTECs, extinction of PP5 by Pp5 siRNA caused the reactivation of ERK1/2 in post-hypoxic Tlr2-/- RTECs, which is not detected in nontransfected cells and cells transfected with the control siRNA (Fig. 6B). These results provide strong evidence that the selective TLR2-dependent activation of the ERK1/2 pathway caused by hypoxia appears highly dependent on PP5 activity in renal tubule cells.
FIGURE 6.
PP5 controls the phosphorylation of ERK1/2, ASK1, and JNK in post-hypoxic Tlr2-/- RTECs. A, immunoblot analyses of p- and total ERK1/2 in non-hypoxic (C) and 24-h post-hypoxic (H) Tlr2-/- RTECs preincubated or not with 5 nm okadaic acid (+Ok.a). B, immunoblot analyses of PP5 and corresponding β-actin, and p- and total ERK1/2 in non-hypoxic (Control) and day-1 post-hypoxic (Hypoxia) Tlr2-/- RTECs transfected or not with a Pp5 siRNA or negative control siRNA. C and D, immunoblot analyses of p- and total ASK1 and JNK in non-hypoxic (C) and day-1 post-hypoxic (H) Tlr2-/- RTECs preincubated or not with 10 nm okadaic acid (+Ok.a)(C), and non-hypoxic (Control) or day-1 post-hypoxic (Hypoxia) Tlr2-/- RTECs transfected or not with a Pp5 siRNA or negative control siRNA (D). E, percentage of apoptotic cells measured in non-hypoxic (Control) or day-1 post-hypoxic (Hypoxia) Tlr2-/- RTECs transfected or not with a Pp5 siRNA or negative control siRNA. Data are means ± S.E. from 4 different cell cultures from 3 different kidneys for each set of experimental conditions. *, p < 0.05 between groups.
In accordance with the fact that PP5 associates with and inactivates ASK1 and JNK under redox stress (19, 20), the expression of p-JNK, as well as p-ASK1 dramatically increased in okadaic acid-pretreated post-hypoxic Tlr2-/- RTECs (Fig. 6C). Silencing Pp5 mRNA expression had no effect on p-ASK1 and p-JNK in non-hypoxic, control Tlr2-/- RTECs (Fig. 6D), but did restore the activation of p-ASK1 and p-JNK in post-hypoxic Tlr2-/- RTECs (Fig. 6D). As a result, extinction of Pp5 mRNA expression also reactivated apoptosis in post-hypoxic Tlr2-/- RTECs (Fig. 6E). Because hypoxia failed to activate p-AKT, like ERK1/2, in Tlr2-/- RTECs the question arises to know as to whether PP5 controls the phosphorylation of AKT. Pp5 mRNA silencing did not restore the phosphorylation of AKT, suggesting thus that PP5 has no direct action on p-AKT (supplemental Fig. S2).
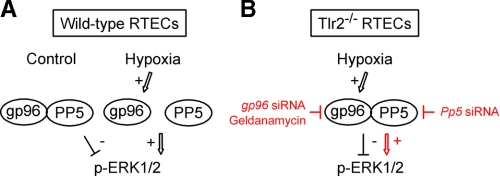
Fig. 7 provides a schematic representation of the proposed mechanism of TLR2-mediated, PP5-dependent activation/inactivation of ERK1/2 in non-hypoxic and post-hypoxic RTECs.
FIGURE 7.
Diagrammatic representation of the mechanism of TLR2-mediated ERK activation in post-hypoxic renal tubule cells. A, in non-hypoxic renal tubule cells, PP5 is associated with gp96, while transient hypoxia (Hypoxia) stimulates the expression of gp96, and induces the dissociation of gp96 bound to PP5, resulting in the inhibition of PP5 activity and downstream ERK1/2 phosphorylation. B, in Tlr2-/--deficient RTECs, hypoxia stimulates gp96, but does not trigger the dissociation of gp96 bound to PP5. In this case, PP5 can be expected to remain active, and inhibit raf-1 activity and downstream ERK1/2 phosphorylation. Inhibition of gp96 activity by GA, or mRNA extinction of gp96 induces the reactivation of ERK1/2, but not of JNK or ASK1 in Tlr2-/--deficient RTECs, and extinction of Pp5 mRNA expression also induces the reactivation of ERK1/2 phosphorylation and reactivation of p-JNK and ASK-1 (not shown in the diagram).
DISCUSSION
The present study shows that the selective TLR2-mediated activation of ERK1/2 is tightly dependent on gp96-PP5 interaction in post-ischemic cells. gp96, the ER-resident member of the Hsp family, has 50% homology with its cytosolic counterpart Hsp90, and its expression is up-regulated by a variety of stress conditions that perturb ER functions, such as glucose starvation, ER-calcium store depletion, glycosylation blockade, proteasome inhibition, or overexpression of misfolded proteins (reviewed in Ref. 46). Although mainly localized in the ER, gp96 is also found in other cell compartments, including plasma membrane, Golgi apparatus, or the nucleus (reviewed in Ref. 42). In contrast to the high number of client proteins associated with the Hsp90 (47), the number of proteins bound to gp96 is more limited. Several TLRs, including TLR2 and TLR4 have been shown to bind gp96 in dendritic cells and in a pre-B cell line (29, 48). Two studies using a mutant pre-B cell line that expressed truncated gp96, and conditionally gp96-deficient mice have highlighted the key role of gp96 in the maturation and proper folding of TLRs (29, 30). The absence of gp96 was also shown not to be essential in cellular survival of the pre-B cells, even under stress conditions (29). Here we provide lines of evidence that gp96 is essential in the control of hypoxia-induced activation of TLR2-mediated activation of ERK1/2. GA, which binds and alters the function of Hsp90 and gp96, did not impair the activation of AKT and ERK1/2 in wild-type RTECs, but restored their activation in post-hypoxic Tlr2-/- RTECs. These results are in accordance with previous reports, which showed that PI3-K and downstream AKT are involved in TLR2-induced activation of neutrophils (43). The control of ERK1/2 by gp96 seems to be highly dependent of TLR2, since GA did not induce reactivation of ERK1/2 in the post-hypoxic Tlr4-/- RTECs. Moreover, GA had no inducible action on the activation of p-JNK, which is lacking in both post-hypoxic Tlr2-/- and Tlr4-/- RTECs (Fig. 2B). These results suggest that the activation of p-JNK observed in ischemic-reperfused wild-type kidneys occurs by a mechanism different of the TLR2-mediated, gp96-dependent ERK activated pathway. The fact that the extinction of gp96 by siRNA has provided the same effects than GA suggested that disruption of gp96 complexed with client proteins would coincide with the activation of the ERK1/2.
Former studies have shown that the activity of client proteins can be regulated by Hsp molecular chaperone complexes. GA induces the rapid dissociation of Hsp90 to the double-stranded RNA-dependent kinase PKR, which resulted in an activation of PKR involved in ER stress (49). The complex mechanism of regulation of HSF1 activity was also shown to result from interactions with Hsp90 multichaperone complexes (24-27). Conde et al. (28) demonstrated that PP5 physically interacts with the HSF1-Hsp90 complex and functions as a negative regulator of HSF1. Protein phosphatase PP5 is implicated in a variety of cellular processes and associates with many proteins involved in cellular signaling, such as the glucocorticoid receptor-Hsp90 complex (45, 51), DNA-dependent protein kinase (52), Gα12/Gα13 subunits of the heteromeric G proteins (53), human blue-light photoreceptor cryptochrome 2 (54), Hsp90-dependent heme-regulated eukaryotic initiation factor 2α kinase (55), ASK1 (19, 21), or A-regulatory subunit of protein phosphatase 2A (21, 56). These studies have provided lines of evidence that PP5 plays important role in the regulation of the cellular responses to stress. PP5 was also shown to interact with Raf-1 and inactivate the activation of Raf-1 and downstream MEK-ERK signaling in COS-1 cells upon growth factor stimulation (22). PP5, which binds to the C-terminal domain of ASK1 (20), suppresses the hypoxia-induced phosphorylation of ASK1 and JNK, without affecting the stimulated phosphorylation of ERK1/2 and p38 MAPKs, suggesting that PP5 can play a role in cell survival by impairing the activation of the proapoptotic ASK1-JNK signaling pathway. Rapamycin, an inhibitor of mTOR, induces a cellular stress response characterized by a rapid and sustained activation of ASK1 and selective apoptosis in p53-mutant cells. Rapamycin does not affect either protein level of PP5 or association of PP5 with ASK1, but induces rapid dissociation of PP2A-B″ subunit (PR72) from PP5, that is associated with concomitant decreased phosphatase activity of PP5 and activating ASK1 (21). Conversely, overexpression of PP5, but not the PP2A catalytic subunit, protected cells from rapamycin-induced apoptosis (21). Here we show that gp96 co-immunoprecipitates with PP5 in unstressed wild-type and post-hypoxic Tlr2-/- RTECs, while PP5 tends to dissociate from gp96 in post-hypoxic wild-type RTECs. Disruption of the interaction between gp96 and PP5 coincides with the activation of the TLR2-mediated phosphorylation of ERK1/2. These results thus suggest that the deactivation/activation of PP5 activity closely depends on its association/dissociation from gp96. In contrast to the Ser/Thr proteins PP1 and PP2A, which activate the protein kinase Raf-1 upstream of MEK and ERK by dephosphorylating Ser-259 (50, 57), PP5 reduces Raf-1 kinase activity and downstream MEK-ERK signaling pathway via dephosphorylation of the Ser-388 residue located in the N-terminal region between the regulatory and kinase domains of Raf-1 (22). von Kriegsheim et al. (22) have shown that PP5 does not interfere with ERK phosphorylation using an activated MEK mutant. In contrast, PP5 was reported to directly dephosphorylate an essential phosphothreonine residue (Thr-845) within the kinase domain of ASK1 and thereby inactivate ASK1 activity (19). Consistent with the present findings, the stimulation of p-ASK1 caused by hypoxia has been also shown to be associated with an increase in JNK phosphorylation in human A549 lung carcinoma cells (20). Extinction of Pp5 mRNA expression by siRNA was also shown to be associated with an increase in the phosphorylation of MKK4, JNK, and c-Jun, suggesting that PP5 acts as a negative regulator of ASK1, which in turns leads to the activation of JNK. Altogether these findings indicate that PP5 directly dephosphorylates p-ASK1, leading to the activation of JNK, and through the dephosphorylation of Raf-1-MEK, indirectly dephosphorylates ERK1/2. The fact that okadaic acid and the extinction of Pp5 mRNA expression both restored hypoxia-induced p-ERK1/2 activation in Tlr2-/- RTECs also suggests that the TLR2-mediated signaling activated by hypoxic stress represses PP5-dependent dephosphorylation of Raf-1 by a mechanism that remains to be determined. PP5 also functions as a negative feedback inhibitor of the ASK1-JNK-p38 signaling in response to oxidative stress (19). Consequently, the inhibition of PP5 activity and the extinction of Pp5 mRNA expression in post-hypoxic Tlr2-/- RTECs both lead to the reinduction of p-ERK1/2 and p-ASK1 and the subsequent increase in the percentage of apoptotic cells. However, neither GA nor gp96 siRNA induced the reactivation of p-JNK in post-hypoxic Tlr2-/- RTECs where PP5 remains associated to gp96. These results suggest that the mechanisms of hypoxia-induced TLR-mediated ASK1-JNK signaling that promotes apoptosis do not necessarily require the dissociation of the gp96-PP5 complex. Because invalidation of TLR4, like TLR2, exerts protective effect on cell damages during IRI (7), experiments will be needed to determine whether TLR4 could play a role in the activation of the proapoptotic ASK1-JNK signaling pathway during IRI.
To conclude, this study has provided the first demonstration that the TLR2-dependent activation of p-ERK1/2 in post-ischemic kidney cells occurs via a dephosphorylating process due to the inhibition of PP5 activity resulting from the dissociation of gp96 bound to PP5 provoked by the hypoxic stress.
Acknowledgments
We thank S. Akira for permission to use Tlr2-/- and Tlr4-/- mice.
This work was supported by INSERM and in part by an Agence Nationale de la Recherche grant (ANR-08-MIE-030, to A. V.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: IRI, ischemia/reperfusion injury; ASK1, apoptosis signal-regulating kinase 1; DKO, double Tlr2,4 knockout mice; ER, endoplasmic reticulum; ERK, extracellular signal-regulated protein kinase; GA, geldanamycin; HSF1, heat shock factor 1; Hsp, heat shock protein; IP, immunoprecipitation; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MEK, mitogen extracellular kinase; PI3-K, phosphatidylinositol 3-kinase; PP5, serine/threonine protein phosphatase type 5; RTEC, renal tubule epithelial cell; siRNA, silencing RNA; TLR, Toll-like receptor; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling; WT, wild type.
References
- 1.Bonventre, J. V., and Weinberg, J. M. (2003) J. Am. Soc. Nephrol. 14 2199-2210 [DOI] [PubMed] [Google Scholar]
- 2.Beg, A. A. (2002) Trends Immunol. (2002) 23 509-512 [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., and Takeda, K. (2004) Nat. Rev. Immunol. 4 499-511 [DOI] [PubMed] [Google Scholar]
- 4.Marshak-Rothstein, A. (2006) Nat. Rev. Immunol. 6 823-835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuboi, N., Yoshikai, Y., Matsuo, S., Kikuchi, T., Iwami, K., Nagai, Y., Takeuchi, O., Akira, S., and Matsuguchi, T. (2002) J. Immunol. 169 2026-2033 [DOI] [PubMed] [Google Scholar]
- 6.Leemans, J. C., Stokman, G., Claessen, N., Rouschop, K. M., Teske, G. J., Kirschning, C. J., Akira, S., van der Poll, T., Weening, J. J., and Florquin, S. (2005) J. Clin. Investig. 115 2894-2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu, H., Chen, G., Wyburn, K. R., Yin, J., Bertolino, P., Eris, J. M., Alexander, S. I., Sharland, A. F., and Chadban, S. J. (2007) J. Clin. Investig. 117 2847-2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakano, H., Nakajima, A., Sakon-Komazawa, S., Piao, J. H., Xue, X., and Okumura, K. (2006) Cell Death Differ. 13 730-737 [DOI] [PubMed] [Google Scholar]
- 9.Matsuzawa, A., and Ichijo, H. (2005) Antioxid. Redox Signal. 7 472-481 [DOI] [PubMed] [Google Scholar]
- 10.Ichijo, H., Nishida, E., Irie, K., ten Dijke, P., Saitoh, M., Moriguchi, T., Takagi, M., Matsumot, K., Miyazono, K., and Gotoh, Y. (1997) Science 275 90-94 [DOI] [PubMed] [Google Scholar]
- 11.Tobiume, K., Matsuzawa, A., Takahashi, T., Nishitoh, H., Morita, K., Takeda, K., Minowa, O., Miyazono, K., Noda, T., and Ichijo, H. (2001) EMBO Rep. 2 222-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang, X., Martindale, J. L., and Holbrook, N. J. (2000) J. Biol. Chem. 275 39435-39443 [DOI] [PubMed] [Google Scholar]
- 13.Stefanelli, C., Tantini, B., Fattori, M., Stanic, I., Pignatti, C., Clo, C., Guarnieri, C., Caldarera, C. M., Mackintosh, C. A., Pegg, A. E., and Flamigni, F. (2002) FEBS Lett. 527 223-228 [DOI] [PubMed] [Google Scholar]
- 14.Sarró, E., Tornavaca, O., Plana, M., Meseguer, A., and Itarte, E. (2008) Kidney Int. 73 77-85 [DOI] [PubMed] [Google Scholar]
- 15.Guyton, K. Z., Liu, Y., Gorospe, M., Xu, Q., and Holbrook, N. J. (1996) J. Biol. Chem. 271 4138-4142 [DOI] [PubMed] [Google Scholar]
- 16.Hung, C. C., Ichimura, T., Stevens, J. L., and Bonventre, J. V. (2003) J. Biol. Chem. 278 29317-29326 [DOI] [PubMed] [Google Scholar]
- 17.Hu, P., Han, Z., Couvillon, A. D., and Exton, J. H. (2004) J. Biol. Chem. 279 49420-49429 [DOI] [PubMed] [Google Scholar]
- 18.Cohen, P. T. (1997) Trends Biochem. Sci. 22 245-251 [DOI] [PubMed] [Google Scholar]
- 19.Morita, K., Saitoh, M., Tobiume, K., Matsuura, H., Enomoto, S., Nishitoh, H., and Ichijo, H. (2001) EMBO J. 20 6028-6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou, G., Golden, T., Aragon, I. V., and Honkanen, R. E. (2004) J. Biol. Chem. 279 46595-46605 [DOI] [PubMed] [Google Scholar]
- 21.Huang, S., Shu, L., Easton, J., Harwood, F. C., Germain, G. S., Ichijo, H., and Houghton, P. J. (2004) J. Biol. Chem. 279 36490-36496 [DOI] [PubMed] [Google Scholar]
- 22.von Kriegsheim, A., Pitt, A., Grindlay, G. J., Kolch, W., and Dhillon, A. S. (2006) Nat. Cell Biol. 8 1011-1016 [DOI] [PubMed] [Google Scholar]
- 23.Voellmy, E. R. (2004) Cell Stress Chaperones 9 122-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali, A., Bharadwaj, S., O'Carroll, R., and Ovsenek, N. (1998) Mol. Cell Biol. 18 4949-4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou, J., Guo Y., Guettouche, T., Smith, D. F., and Voellmy, R. (1998) Cell 94 471-480 [DOI] [PubMed] [Google Scholar]
- 26.Bharadwaj, S., Ali, A., and Ovsenek, N. (1999) Mol. Cell Biol. 19 8033-8041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo, Y., Guettouche, T., Fenna, M., Boellmann, F., Pratt, W. B., Toft, D. O., Smith, D. F., and Voellmy, R. (2001) J. Biol. Chem. 276 45791-45799 [DOI] [PubMed] [Google Scholar]
- 28.Conde, R., Xavier, J., McLoughlin, C., Chinkers, M., and Ovsenek, N. (2005) J. Biol. Chem. 280 28989-28996 [DOI] [PubMed] [Google Scholar]
- 29.Randow, F., and Seed, B. (2001) Nat. Cell Biol. 3 891-896 [DOI] [PubMed] [Google Scholar]
- 30.Yang, Y., Liu, B., Dai, J., Srivastava, P. K., Zammit, D. J., Lefrançois, L., and Li, Z. (2007) Immunity 26 215-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitesell, L., Mimnaugh, E. G., De Costa, B., Myers, C. E., and Neckers, L. M. (1994) Proc. Natl. Acad. Sci. U. S. A. 9 8324-8328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavany, C., Mimnaugh, E., Miller, P., Bitton, R., Nguyen, P., Trepel, J., Whitesell, L., Schnur, R., Moyer, J., and Neckers, L. (1996) J. Biol. Chem. 271 4974-4977 [DOI] [PubMed] [Google Scholar]
- 33.Vandewalle, A., Lelongt, B., Geniteau-Legendre, M., Baudouin, B., Antoine, M., Estrade, S., Chatelet, F., Verroust, P., Cassingena, R., and Ronco, P. (1989) J. Cell Physiol. 141 203-221 [DOI] [PubMed] [Google Scholar]
- 34.Lacave, R., Bens, M., Cartier, N., Vallet, V., Robine, S., Pringault, E., Kahn, A., and Vandewalle, A. (1993) J. Cell Sci. 104 705-712 [DOI] [PubMed] [Google Scholar]
- 35.Chassin, C., Goujon, J. M., Darche, S., du Merle, L., Bens, M., Cluzeaud, F., Werts, C., Ogier-Denis, E., Le Bouguénec, C., Buzoni-Gatel, D., and Vandewalle, A. (2006) J. Immunol. 177 4773-4784 [DOI] [PubMed] [Google Scholar]
- 36.Hsieh, C. C., and Papaconstantinou, J. (2006) FASEB J. 20 259-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, C., Zhang, M. X., Shen, Y. H., Burks, J. K., Zhang, Y., Wang, J., LeMaire, S. A., Yoshimura, K., Aoki, H., Coselli, J. S., and Wang, X. L. (2007) Arteriosclerosis, Thrombosis, Vasc. Biol. 27 1760-1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neckers, L. (2002) Trends Mol. Med. 8 S55-S61 [DOI] [PubMed] [Google Scholar]
- 39.Hindley, A., and Kolch, W. (2002) J. Cell Science 115 1575-1581 [DOI] [PubMed] [Google Scholar]
- 40.Schulte, T. W., Blagosklonny, M. V., Ingui, C., and Neckers, L. (1995) J. Biol. Chem. 270 24585-24588 [DOI] [PubMed] [Google Scholar]
- 41.Schulte, T. W., Blagosklonny, M. V., Romanova, L., Mushinski, J. F., Monia, B. P., Johnston, J. F., Nguyen, P., Trepel, J., and Neckers, L. M. (1996) Mol. Cell Biol. 16 5839-5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koga, F., Xu, W., Karpova, T. S., McNally, J. G., Baron, R., and Neckers, L. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 11318-11322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strassheim, D., Asehnoune, K., Park, J. S., Kim, J. Y., He, Q., Richter, D., Kuhn, K., Mitra, S., and Abraham, E. (2004) J. Immunol. 172 5727-5733 [DOI] [PubMed] [Google Scholar]
- 44.Yang, J., Roe, S. M., Cliff, M. J., Williams, M. A., Ladbury, J. E., Cohen, P. T., and Barford, D. (2005) EMBO J. 24 1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen, M. S., Silverstein, A. M., Pratt, W. B., and Chinkers, M. (1996) J. Biol. Chem. 271 32315-32320 [DOI] [PubMed] [Google Scholar]
- 46.Yang, Y., and Li, Z. (2005) Mol. Cells 20 173-182 [PubMed] [Google Scholar]
- 47.Zhao, R., Davey, M., Hsu, Y. C., Kaplanek, P., Tong, A., Parsons, A. B., Krogan, N., Cagney, G., Mai, D., Greenblatt, J., Boone, C., Emili, A., and Houry, W. A. (2005) Cell 120 715-727 [DOI] [PubMed] [Google Scholar]
- 48.Vabulas, R. M., Braedel, S., Hilf, N., Singh-Jasuja, H., Herter, S., Ahmad-Nejad, P., Kirschning, C. J., Da Costa, C., Rammensee, H. G., Wagner, H., and Schild, H. (2002) J. Biol. Chem. 277 20847-20853 [DOI] [PubMed] [Google Scholar]
- 49.Donzé, O., Abbas-Terki, T., and Picard, D. (2001) EMBO J. 20 3771-3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhillon, A. S., Meikle, S., Yazici, Z., Eulitz, M., and Kolch, W. (2002) EMBO J. 21 64-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silverstein, A. M., Galigniana, M. D., Chen, M. S., Owens-Grillo, J. K., Chinkers, M., and Pratt, W. B. (1997) J. Biol. Chem. 272 16224-16230 [DOI] [PubMed] [Google Scholar]
- 52.Wechsler, T., Chen, B. P., Harper, R., Morotomi-Yano, K., Huang, B. C., Meek, K., Cleaver, J. E., Chen, D. J., and Wabl, M. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 1247-1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaguchi, Y., Katoh, H., Mori, K., and Negishi, M. (2002) Curr. Biol. 12 1353-1358 [DOI] [PubMed] [Google Scholar]
- 54.Zhao, S., and Sancar, A. (1997) Photochem. Photobiol. 66 727-731 [DOI] [PubMed] [Google Scholar]
- 55.Shao, J., Hartson, S. D., and Matts, R. L. (2002) Biochemistry 41 6770-6779 [DOI] [PubMed] [Google Scholar]
- 56.Lubert, E. J., Hong, Y., and Sarge, K. D. (2001) J. Biol. Chem. 276 38582-38587 [DOI] [PubMed] [Google Scholar]
- 57.Jaumot, M., and Hancock, J. F. (2001) Oncogene 20 3949-3958 [DOI] [PubMed] [Google Scholar]