Abstract
This study examined the role of dorsal hippocampal NMDA receptors and PKA activation in 17β-estradiol (E2)-induced enhancement of object memory consolidation. Mice explored two identical objects during training, after which they immediately received intraperitoneal injections of 0.2 mg/kg E2, and bilateral dorsal hippocampal infusions of Vehicle, the NMDA receptor antagonist APV (2.5 μg/side), or the cAMP inhibitor Rp-cAMPS (18.0 μg/side). Retention was tested 48 hours later. The enhanced object memory and increased ERK phosphorylation observed with E2 alone was reduced by APV and Rp-cAMPS, suggesting that estrogenic enhancement of object memory involves NMDA receptors and PKA activation within the dorsal hippocampus.
Keywords: object recognition, estrogen, ERK, PKA, glutamate
Estradiol improves several different types of hippocampal-dependent memory in young female rodents, including spatial reference memory in the Morris water maze (El-Bakri et al., 2004; Gresack & Frick, 2006; Harburger, Bennett, & Frick, 2007; Packard & Teather, 1997), spatial working memory in the radial-arm maze and T maze (Bohacek & Daniel, 2007; Gibbs, 2002; Gresack & Frick, 2004), contextual fear (Jasnow, Schulkin, & Pfaff, 2006), and object recognition (Gresack & Frick, 2006; Luine, Jacome, & Maclusky, 2003). Further, estradiol promotes structural changes and modifications in synaptic strength within the hippocampus that may underlie the formation of lasting memories. For example, estrogen increases CA1 dendritic spine synapse density (Frick, Fernandez, Bennett, Prange-Kiel, MacLusky, & Leranth, 2004; Woolley & McEwen, 1992), and enhances long-term potentiation (LTP) of hippocampal cells in vitro (Smith & McMahon, 2006). However, these alterations have yet to be directly linked with improvements in hippocampal-dependent memory. Thus, the molecular mechanisms underlying estrogen—induced improvements in memory remain unknown.
Estradiol-induced increases in hippocampal spines and LTP are dependent on the activation of NMDA receptors (Smith & Mc-Mahon, 2006; Woolley & McEwen, 1994). The activation of NMDA receptors leads to an increase in intracellular calcium and activates calcium-sensitive second messengers, including protein kinase A (PKA). Although PKA can have a wide-array of effects on hippocampal-dependent memory and physiology (Nguyen & Woo, 2003), information about the role of PKA in mediating estradiol’s effects on hippocampal cellular function is limited. Shingo and Kito (2005), demonstrated that 17β-estradiol (E2) conjugated to bovine serum albumin (BSA), a molecule that cannot penetrate the cell membrane, activated PKA 45 seconds after bath application, in vitro. A role for PKA in E2-induced differentiation in dopaminergic neurons, and in cerebellar granule cells, has also been reported (Belcher, Le, Spurling, & Wong, 2005; Beyer & Karolczak, 2000). To date, no published studies have examined the involvement of PKA in the effects of E2 on hippocampal-dependent memory.
PKA phosphorylation can lead to the activation of extracellular signal-regulated kinase (ERK), which is a proposed link between PKA and the transcription factor cyclic-AMP response element binding protein (CREB). The importance of ERK to the mediation of estrogen action within the hippocampus has been demonstrated previously in rats. For example, Kuroki, Fukushima, Kanda, Mizuno, and Watanabe (2002), reported a threefold increase in levels of phosphorylated ERK (pERK) 5 minutes after intrahippocampal infusion of BSA-conjugated E2 within the dorsal hippocampus. In addition, our lab has demonstrated that the enhancement of object memory induced in female mice by a single posttraining intraperitoneal injection of E2 is dependent on ERK activation in the dorsal hippocampus (Fernandez et al., submitted). Given the importance suggested by these data of ERK in mediating estrogenic enhancement of object memory, we hypothesized that this enhancement might also depend on NMDA and PKA-induced activation of ERK within dorsal hippocampus. The present data suggest that NMDA and PKA activation are necessary for E2 to enhance ERK activation and object memory consolidation.
Method
Subjects
Female C57BL/6 mice (N = 18–19/group) were obtained from Taconic (Germantown, NY) at 12 weeks of age. The mice were group housed, until surgical procedures, in the Department of Psychology’s animal colony at Yale University and maintained on a 12:12 light/dark cycle. Mice acclimated to our colony for 1 week and were handled daily (5 min/day). All behavioral procedures were performed during the light cycle. Food and water were available ad libitum. All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Yale University Institutional Animal Care and Use Committee.
Surgical Procedures
Mice were ovariectomized 1 week before the start of treatment as described previously (Fernandez & Frick, 2004). Mice were first anesthetized with isoflurane (5% for induction, 2% for maintenance) in 100% oxygen and then were placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA) for subsequent cannula implantation (see below). The ovaries, oviducts, and tips of the uterine horn were bilaterally removed via two dorsal incisions just above the tips of the pelvis. Immediately after ovariectomy, mice were implanted with stainless steel guide cannulae (Plastics One, Roanoke, VA) aimed at the dorsal hippocampus. The scalp was incised and retracted to expose the skull, and Bregma and Lambda were aligned in the same horizontal plane. Small holes (1 mm in diameter) were drilled bilaterally for placement of dorsal hippocampal guide cannulae (C232GC, 22 gauge, Plastics One). Each guide cannula with inserted dummy cannula (C232DC; Plastics One) was directed toward the dorsal hippocampus (−1.7 mm posterior to bregma, ±1.5 mm lateral to midline, and −2.3 mm (injection site) ventral to the skull surface), based on Paxinos and Franklin (1997). Each cannula was fixed to the skull with dental cement that also served to close the wound. Mice were allowed to recover for at least 5 days before behavioral testing.
Drugs and Infusions
17β-Estradiol (2-hydroxypropyl-β-Cyclodextrin (HBC)- encapsulated 17β-estradiol; 0.2 mg/kg), APV (D-2-Amino-5-phosphonovaleric acid; NMDA receptor antagonist; 5.0 mg/ml), Rp-cAMPS (Rp-Cyclic 3′,5′-hydrogen phosphorothioate adenosine triethylammonium salt; inhibitor of cAMP; 36 mg/ml), and HBC Vehicle were obtained from Sigma Chem. Co. (St. Louis, MO). For intraperitoneal injections, HBC dissolved in saline was used as the Vehicle. For intrahippocampal infusions, physiological saline was used as the Vehicle and all drugs were dissolved in saline. It is important to note that this form of E2 is metabolized within 24 hours (Pitha & Pitha, 1985). Therefore, it is not in circulation during either phase of testing, which ensures that nonmnemonic effects of E2 (e.g., on motivation or anxiety) cannot influence performance in this task. Further, previous studies have demonstrated that posttraining E2 administration must occur within 2 hours of training to observe enhancement in both the Morris water maze and object recognition tasks (Luine et al., 2003; Packard & Teather, 1997), thus warranting the immediate posttraining administration of E2.
Immediately after the sample phase, mice were restrained and dummy cannulae were replaced with injection cannulae (22 gauge; extending 0.8 mm beyond the tip of the 1.5 mm guide) attached to polyethylene tubing (PE50; Plastics One). The PE50 tubing was connected to a 10 μl Hamilton syringe that was controlled by a microinfusion pump (KDS 100, KD Scientific; New Hope, PA). Estradiol or saline were administered intraperitoneally (ip). The 0.2 mg/kg dose of E2 enhanced object memory in our previous studies (Gresack & Frick, 2004, 2006). Saline, APV, or Rp-cAMPS were infused into the hippocampus at 0.5 μl/min at a volume of 0.5 μl/side, resulting in doses of 2.5 and 18.0 μg/side of APV or Rp-cAMP, respectively. A previous study demonstrated that this infusion protocol results in approximately 1 mm3 of drug diffusion (Lewis & Gould, 2007) and, given the site of infusion within the dorsal hippocampus, suggests that the effects of APV or Rp-cAMPS were likely restricted to the dorsal hippocampus. Intraamygdala doses of these drugs have been shown to disrupt cued and contextual fear conditioning (Lee & Kim, 1998; Schafe, Nadel, Sullivan, Harris, & LeDoux, 1999).
Object Recognition
This task, described previously in Frick and Gresack, (2003), assesses hippocampal-dependent (Baker & Kim, 2002; Clark, Zola, & Squire, 2000) object memory and consists of habituation, sample, and choice phases. Mice were first habituated to an empty white box by allowing them to freely explore for 5 minutes. Twenty-four hours later, mice were placed in the empty box for 1 minute of additional habituation. Mice were removed while two identical objects were placed in the northeast and northwest corners of the box, approximately 5 cm from the walls. The objects (approximately 5 cm × 7 cm in height and width) used were a triangular plastic prism with colored edges or a half inch threaded brass faucet with a cast-iron handle. Mice were then placed back in the box facing the middle of the south wall and allowed to investigate the objects until they accumulated a total of 30 seconds exploring the objects, at which point the sample phase was terminated. Object exploration was scored when the mouse’s nose or front paws touched the object. Estradiol and all drugs were administered immediately after the completion of the sample phase and then mice were returned to their home cages. The use of 30 seconds of total exploration time rather than a fixed trial duration minimizes the effects of group differences in activity (Frick & Gresack, 2003). Forty-eight hours later, mice were tested in the choice phase. This timing is an important aspect of our protocol; typically, control animals do not show a preference for the novel object at this delay, but do show a preference when tested after 24 hours (Gresack, Kerr, & Frick, 2007). Thus, testing mice at a time point after which OVX controls have forgotten the familiar object allows for detection of object memory enhancement by estradiol. The choice phase was identical to the sample phase, except that a novel object was substituted for one of the objects used in the sample phase. The location of the novel object (northeast or northwest corner) was counterbalanced. Both time spent with the novel object and elapsed time (time needed to accumulate 30 seconds of object exploration) were recorded. The observer scoring exploration was blind to the treatment groups. For both phases, objects and the box were cleaned with 70% EtOH between mice.
Western Blotting
A separate set of behaviorally naïve mice underwent similar handling and surgery, and was used for Western blotting analysis of pERK levels (N = 8/group). Behaviorally naïve mice were used to specifically examine the effects of E2 on ERK. Previous studies have demonstrated that exposure to objects and/or context can increase ERK activation (Kelly, Laroche, & Davis, 2003). Control mice in our paradigm typically do not demonstrate object memory after 48 hours and, thus, any ERK activation that occurs after object and/or context exposure is insufficient to support memory and would simply occlude the effects of E2 on ERK. As such, behaviorally naïve mice were treated with Vehicle, E2, E2+ APV, or E2+ Rp-cAMPS as described above. One hour after infusion, the dorsal hippocampus was immediately bilaterally dissected on ice. Western blotting was conducted according to a protocol based on Schafe, Atkins, Swank, Bauer, Sweatt, and LeDoux (2000). On the day of assay, tissue samples were resuspended 1:50 wt/vol in lysis buffer and homogenized with a probe sonicator. The total protein content of the samples was measured using a Bio-Rad (Bio-Rad Laboratories, Hercules, CA) protein assay (Bradford, 1976). Sample buffer was added to the homogenates, and the samples were boiled for 4 minutes. Samples were then electrophoresed on 10% SDS-polyacrylamide gels and transferred to a PVDF membrane. Blots were blocked and then incubated with either antiphospho-p44/42 ERK (Thr202/Tyr204) (1:1000; Cell Signaling Technology, Danvers, MA) or antitotal p44/42 ERK antibody (1:2000; Cell Signaling Technology) overnight. Blots were then incubated with antirabbit IgG HRP-linked secondary antibody (1:20,000; Cell Signaling Technology) for 1 hour, and developed using enhanced chemiluminescence (Pierce Biotechnology, Inc, Rockford, IL). Signal was detected using a Kodak Image Station 440CF. Densitometry was conducted using accompanying Kodak 1D 3.6 software. To accurately assess differences in p42/p44 ERK activation, phospho-ERK levels were normalized to total p42/p44 ERK levels. Data are presented as percent increase in immunoreactivity relative to Vehicle controls.
Histology
Behaviorally tested mice were checked for cannula placements. Five percent methylene blue in 0.9% saline was infused through the injection cannula to visualize the injection sites. Brains were removed and stored in 10% formalin solution (Fisher Scientific, Pittsburgh, PA) until sectioning. Coronal sections (60 μm thick collected proximal to cannula tracts) were cut on a cryostat (−18 °C) and mounted on PLUS slides (Fisher Scientific). After drying, the slides were stained with Cresyl violet and cover slipped. Injection sites were verified under a light microscope and only those mice with correct cannula placements were included in the data analysis. No animals were excluded because of incorrect cannula placement.
Data Analyses
Time spent with the novel object during the choice phase and total time to complete both the sample and choice phases were analyzed using a one-way analysis of variance with treatment as the independent variable, followed by Tukey post hoc comparisons. For Western blots, independent samples t tests were performed based on a priori hypotheses that E2 administration would increase p42 ERK, and that this increase would be attenuated by coadministration of either APV or Rp-cAMPS (Keppel & Zedeck, 1989).
Results
Object Recognition
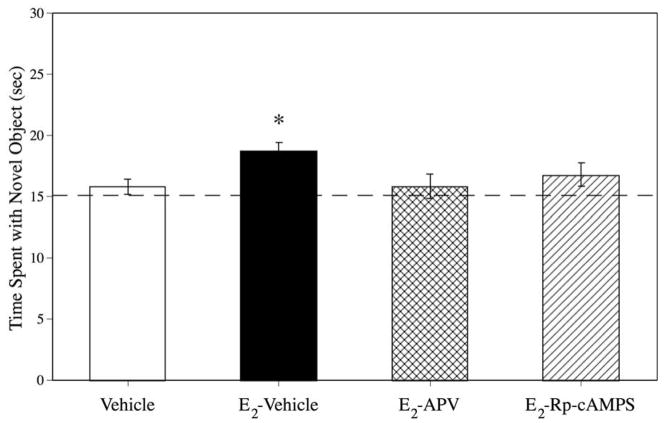
Time spent with the novel objects in the choice phase is presented in Figure 1. The main effect of treatment was significant, F(3, 70) = 3.1, p < .05. Post hoc analysis revealed that the group injected with ip E2 plus intrahippocampal Vehicle (E2 –Vehicle) spent significantly more time with the novel object during the choice phase than Vehicle controls ( p < .05). Mice receiving ip E2 and either intrahippocampal APV or Rp-cAMPS were not significantly different from Vehicle controls ( ps > .05 for both comparisons), demonstrating that coadministration of either APV or Rp-cAMPS reduced the memory enhancing effect of E2. Further, APV-treated mice spent significantly less time with the novel object than E2-treated mice (p < .05), and a trend was apparent for Rp-cAMPS treated mice relative to E2-treated mice (p = .086). There was no significant main effect of treatment on elapsed time during the sample phase (F(3, 70) = 0.63, p > .05; Vehicle: 307.3 ± 32.3; E2 –Vehicle: 371.3 ± 58.2; E2-APV: 362.2 ± 38.7; E2-Rp-cAMPS: 309.1 ± 37.4) or the choice phase (F(3, 70) = 0.23, p > .05; Vehicle: 331.7 ± 39.0; E2 –Vehicle: 353.8 ± 50.2; E2-APV: 330.8 ± 41.3; E2-Rp-cAMPS: 385.0 ± 74.3), suggesting no effect of E2 or the inhibitors on the total time taken to accumulate 30 seconds of exploration.
Figure 1.
Time spent with the object during the choice phase. E2-Vehicle mice spent significantly more time with the novel object than Vehicle mice. Neither the E2-APV or E2-Rp-cAMPS groups spent significantly more time with the novel object than Vehicle controls, suggesting that dorsal hippocampal infusions of either APV or Rp-cAMPS reduced the E2-induced enhancement of object recognition to levels observed in Vehicle controls. The dotted line at 15 seconds represents chance performance. * = p < .05 relative to Vehicle. Error bars represent ± SEM.
Western Blotting
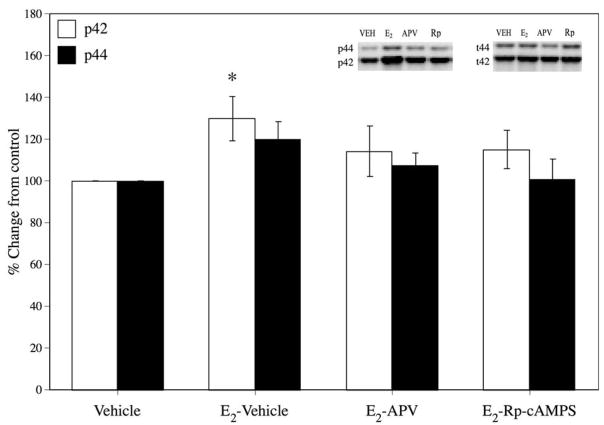
Western blotting results are presented in Figure 2. For p42, estradiol alone (E2-Vehicle) significantly increased p42 ERK, t(14) = 2.22, p < .05, relative to Vehicle controls. In contrast, p42 ERK was not increased relative to Vehicle controls in the E2-APV, t(13) = 1.00, p > .05, or Rp-cAMPS, t(13) = 1.26, p > .05 groups, suggesting that both APV and Rp-cAMP attenuated the increase in p42 ERK observed after E2 administration. Neither APV nor Rp-cAMP significantly decreased p42 ERK levels relative to E2-treated mice, suggesting an intermediate effect of both inhibitors. No treatment affected p44 ERK or total ERK protein.
Figure 2.
Effects of E2 on ERK activation. Estradiol alone significantly increased levels of phosphorylated p42 30% above Vehicle controls. Intrahippocampal infusion of the NMDA receptor antagonist APV or the cAMP inhibitor Rp-cAMPS attenuated this increase (14% over control). It should be noted that neither APV nor Rp-cAMP completely blocked the activation of p42. * = significant at p < .05. Error bars represent ± SEM. Inset: Representative Western blots for phosphorylated/total p44 and p42.
Discussion
The present study replicates our previous reports demonstrating that posttraining 0.2 mg/kg 17β-estradiol enhances object memory tested 48 hours after the sample phase (Gresack & Frick, 2004, 2006), and extends this work to show that this enhancement involves the activation of NMDA receptors and PKA within the dorsal hippocampus. To our knowledge, this is the first evidence that estradiol-induced enhancement of memory consolidation involves these pathways.
The precise mechanisms by which NMDA receptors influence the estrogenic mediation of object memory remain unclear. Previous research suggests that estradiol may indirectly influence NMDA receptor activity by increasing the phosphorylation of specific receptor subtypes. For example, Bi, Foy, Vouimba, Thompson, and Baudry (2001), found that elevated estrogen levels during the proestrus phase of the estrous cycle corresponded with increased phosphorylation of the NR2B subunit of the NMDA receptor and an increase in the magnitude of LTP. This increase in NR2B phosphorylation was mediated by tyrosine phosphorylation and the effect was, at least partially, mediated by ERK (Bi et al., 2001). This finding has recently been extended to show that the NR2B receptor is necessary for estrogen-induced facilitation of LTP (Smith & McMahon, 2006). Thus, a possible mechanism for the involvement of NMDA receptor activation in the effects of estradiol on memory may be mediated by tyrosine phosphorylation of NR2B subunits by ERK, although this possibility has yet to be tested directly.
PKA appears to influence the estradiol-induced enhancement of object memory by activating ERK. Previous studies have established that PKA, acting through the PKA-Rap1-B-Raf-MEK pathway (Sweatt, 2001), is involved in several types of learning and memory (see Selcher, Weeber, Varga, Sweatt, & Swank, 2002 for review). Our data suggest that PKA-driven activation of ERK is involved in estradiol-induced modulation of object memory, as inhibition of PKA both reduced the enhancement of object memory and significantly decreased phosphorylated p42 ERK levels. Nevertheless, although ERK is activated following object recognition training (Kelly, Laroche, & Davis, 2003) the precise role of ERK in mediating the estrogenic enhancement of object memory has yet to be determined. As discussed above, one way could be via phosphorylation of NR2B subunits of NMDA receptors. Another may be through activation of CREB. Previous studies have demonstrated that CREB phosphorylation is necessary for the formation of stable memories, and that ERK is an integral component of CREB activation (Adams & Sweatt, 2002). As such, the enhancement of object memory observed here result from the activation of genes containing cAMP response elements and the subsequent production of proteins associated with long-term synaptic modifications.
One important aspect of ERK activation observed in the present study is that neither APV nor Rp-cAMPS completely blocked the estradiol—induced increase in ERK phosphorylation (see Figure 2), although both caused an approximately 50% reduction in activation of the protein. One potential reason for this result may be that the dose of the drugs used was insufficient to completely block protein activation. Another potential explanation for the residual ERK activation still observed after the administration of each inhibitor may be the fact that many other upstream factors can still actively contribute to ERK activation, for example, receptor tyrosine kinases and PKC (for review see Sweatt, 2004). Thus, although the present data suggest that both NMDA receptors and PKA activation are involved in the effects of estradiol on object memory, this modulation is likely because of complex interactions between these and numerous other proteins. However, the fact that object memory is completely disrupted after NMDA receptor antagonism and PKA inhibition suggests an important role for these proteins.
It is important to note that a previous study has demonstrated that ERK may be involved in object memory when tested after 24 hours. Kelly et al. (2003) demonstrated a significant increase in p44 ERK (ERK1) activation following the sample phase of an object recognition task. Although our Vehicle-treated mice do not demonstrate object memory when tested after 48 hours, the possibility remains that our infusions of APV or Rp-cAMP could have simply disrupted object memory formation while having little effect on the estradiol-induced enhancement of object memory. However, our data suggest that estradiol administration increased ERK2 (p42) rather than ERK1 (see Figure 2). Together, these findings suggest that object memory formation may involve the activation of ERK1, whereas estradiol-induced enhancement of object memory may involve the activation of ERK2, and thus, support the conclusion that the present findings are not due to disrupted object memory formation, per se.
The aforementioned mechanisms of estradiol action in the dorsal hippocampus assume that estradiol influences object memory and ERK via nongenomic mechanisms independent of the cloned genomic estrogen receptors alpha and beta. This assumption is based on recent evidence suggesting that estradiol may produce rapid intracellular effects by binding to estrogen receptors located at the cell membrane (Shingo & Kito, 2005). In support, recent studies suggest that estradiol can enhance long-term depression and spine density in the CA1 subregion of the hippocampus in an ERK-dependent fashion (Ogiue-Ikeda et al., 2007). Further, both ERα and ERβ agonists can rapidly modulate calcium dynamics in hippocampal neurons via ERK activation, an effect that is not blocked by the estrogen receptor antagonist ICI 182,780, suggesting a role for potential nongenomic effects of estradiol (Zhao & Brinton, 2007). Our data support this idea, as NMDA receptor antagonists or PKA inhibition would not be expected to interfere with the effects of estradiol on object memory if these effects were mediated solely by genomic mechanisms. We are currently investigating the downstream effectors that are critically involved in the effects of estradiol at the cell membrane.
Acknowledgments
This work was supported by NIH Grant AG022525 to Karyn M. Frick.
References
- Adams JP, Sweatt JD. Molecular psychology: Roles for the ERK MAP kinase cascade in memory. Annual Review of Pharmacology and Toxicology. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learning and Memory. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal- regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein- and protein kinase A-dependent mechanism and intracellular activation of protein phosphatase 2A. Endocrinology. 2005;146:5397–5406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- Beyer C, Karolczak M. Estrogenic stimulation of neurite growth in midbrain dopaminergic neurons depends on cAMP/protein kinase A signaling. Journal of Neuroscience Research. 2000;59:107–116. [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proceedings of the National Academy of Science U S A. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Hormones and Behavior. 2007;52:237–243. doi: 10.1016/j.yhbeh.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annals of Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. Journal of Neuroscience. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, et al. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: Relationship to Morris water maze performance. Journal of Cellular and Molecular Medicine. 2004;8:537–544. doi: 10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behavioral Neuroscience. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Frick KM. Differential estrogen receptor mechanisms involved in memory consolidation. Program No. 462.5. 2006. Neuroscience Meeting Planner; Atlanta, GA: Society for Neuroscience; 2006. Online. [Google Scholar]
- Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. European Journal of Neuroscience. 2004;19:3026–3032. doi: 10.1111/j.1460-9568.2004.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behavioral Neuroscience. 2003;117:1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Hormones and Behavior. 2002;42:245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128:459–471. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacology, Biochemistry, and Behavior. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Kerr KM, Frick KM. Short-term environmental enrichment decreases the mnemonic response to estrogen in young, but not aged, female mice. Brain Research. 2007;1160:91–101. doi: 10.1016/j.brainres.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiology of Aging. 2007;28:602–610. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Hormones and Behavior. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. Journal of Neuroscience. 2003;12:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G, Zedeck S. Correction for multiple comparisons. In: Atkinson RC, editor. Data analysis for research designs. New York: Freeman; 1989. pp. 169–182. [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. European Journal of Pharmacology. 2002;400:205–209. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. Journal of Neuroscience. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MC, Gould TJ. Reversible inactivation of the entorhinal cortex disrupts the establishment and expression of latent inhibition of cued fear conditioning in C57BL/6 mice. Hippocampus. 2007;17:462–470. doi: 10.1002/hipo.20284. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Progress in Neurobiology. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Ogiue-Ikeda M, Tanabe N, Mukai H, Hojo Y, Murakami G, Tsurugizawa T, et al. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Research Reviews. 2007;57:363–375. doi: 10.1016/j.brainresrev.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: Cholinergic blockade and synergism. Neurobiology of Learning and Memory. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. San Diego, CA: Academic Press; 2003. [Google Scholar]
- Pitha J, Pitha J. Amorphous water-soluble derivatives of cyclodextrins: Nontoxic dissolution enhancing excipients. Journal of Pharmaceutical Sciences. 1985;74:987–990. doi: 10.1002/jps.2600740916. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. Journal of Neuroscience. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nadel N, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learning and Memory. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- Selcher JC, Weeber EJ, Varga AW, Sweatt JD, Swank M. Protein kinase signal transduction cascades in mammalian associative conditioning. Neuroscientist. 2002;8:122–131. doi: 10.1177/107385840200800208. [DOI] [PubMed] [Google Scholar]
- Shingo AS, Kito S. Estradiol induces PKA activation through the putative membrane receptor in the living hippocampal neuron. Journal of Neural Transmission. 2005;112:1469–1473. doi: 10.1007/s00702-005-0371-8. [DOI] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. Journal of Neuroscience. 2006;26:8517–8522. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: A biochemical signal integration system subserving synaptic plasticity and memory. Journal of Neurochemistry. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Current Opinion in Neurobiology. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. Journal of Neuroscience. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. Journal of Neuroscience. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Estrogen receptor α and β differentially regulate intracellular CA2+ dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Research. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]