Abstract
Background:
Adenosine tri-phosphate (ATP), a potent vascular regulator in the cerebral circulation, initiates conducted vasomotor responses which may be impaired after pathological insults. We analyzed the mechanism of ATP-induced local vasomotor responses and their effect on conducted vasomotor responses in rat cerebral penetrating arterioles.
Methods:
Arterioles were cannulated and their internal diameter monitored. Vasomotor responses to ATP were observed in the absence/presence of inhibitors or after endothelial impairment. Smooth muscle membrane potentials were measured in some vessels.
Results:
Microapplication of ATP produced a biphasic response (constriction followed by dilation), which resulted in conducted dilation preceded by a membrane hyperpolarization. α,β-metATP or PPADS blunted the ATP-mediated constriction and enhanced local and conducted dilation. Nω -monomethyl-L-arginine, endothelial impairment, and MS-PPOH reduced the local dilation to ATP. The conducted dilation was attenuated by MS-PPOH and endothelial impairment, but not Nω -monomethyl-L-arginine or indomethacin.
Conclusion:
ATP-induced conducted dilation is preceded by a membrane hyperpolarization. Local ATP induces initial local constriction via smooth muscle P2X1 and subsequent dilation via endothelial P2Y receptors. Nitric oxide, cytochrome P450 metabolites, intermediate and large conductance KCa channels mediate dilation to ATP. ATP-induced conducted dilation is dependent upon both the endothelium and cytochrome P450 metabolites.
Keywords: Adenosine 5′- triphosphate, cerebrovascular circulation, microcirculation, cytochrome P450, P2 receptors, potassium channels
Introduction
Cerebral penetrating arterioles are precapillary micro-vessels regulating the distribution of blood into the capillary cortical bed and may contribute up to 23% of total cerebrovascular resistance [1]. The penetrating arteriole running in the Virchow-Robin space may serve as a means by which neural activity and synaptic transmission can be metabolically coupled with local cerebral blood flow [2]. Neuronal cerebral stimulation releases adenosine tri-phosphate (ATP), a potent vasoactive agonist in the cerebral circulation [3-6]. Such ATP as well as ATP released from other cerebral sources including perivascular nerves, parenchymal tissue, red blood cells, and platelets (see [4] could cause local vasomotor responses. Since communication of this local agonist-induced vasoactivity to the proximal supply vessels is required to appropriately adjust local blood flow, the local response must initiate a response which is spread along the vessel, i.e., the response must be conducted. [7].
In rat penetrating arterioles, locally applied ATP elicits a biphasic response consisting of an initial transient constriction followed by a dilation which is subsequently conducted along the vessel wall [8-10]. In hamster retractor muscle, extra-luminal micro-applied ATP caused conducted arteriolar vasoconstriction while intra-luminal ATP caused conducted vasodilation [11]. In hamster cheek pouch arterioles, Duza and Sarelius observed that micro-applied ATP caused a local vasoconstriction followed by a conducted vasodilation, a response similar to that observed in cerebral vessels. They determined that the local transient constriction was mediated by P2x receptors with the subsequent dilation via P1 receptors with their agonist the ATP hydrolysis product, adenosine [12]. In rat penetrating arterioles we found that extraluminal ATP stimulates smooth muscle P2X1 and endothelial P2Y1 and P2Y2 receptors [13]. The contribution of P2 receptors initiating conducted vasomotor responses in penetrating arterioles has not been described.
Inhibition of nitric oxide synthase resulted in diminished conducted dilation to intraluminal ATP and enhanced conducted constriction in hamster retractor muscle arterioles indicating a possible contribution of NO to the conducted vasomotor responses in these arterioles [11]. Although the contribution of EDHF to ATP-induced conduction has not been studied, Hoepfl did report that in hamster cremaster arterioles, the response to locally applied ACh was mediated via EDHF and not by NO. [14]. In rat penetrating arterioles P2Y receptor stimulation releases NO and possibly EDHF but not prostanoids [13]. The contribution of NO or EDHF to the conducted response has not been studied in penetrating arterioles.
The underlying mechanism of conducted vasomotor responses is the spread of a local membrane potential change along the vessel via electro-mechanical coupling. Dora et al found in hamster cheek pouch arterioles that local acetylcholine caused a hyperpolarization with subsequent dilation followed by conducted hyperpolarization preceding a conducted dilation [15]. A role for a similar conducted membrane potential change in the ATP-induced conduction has not been studied.
The goal of this study was to examine if electromechanical coupling underlies the observed conducted vasomotor responses to ATP in rat cerebral penetrating arterioles. Further, we wanted to determine the contribution of nitric oxide synthase, cyclooxygenase and cytochrome P450 inhibition on local ATP-receptor mediated smooth-muscle and endothelial mechanisms involved in conducted vasomotor responses to topical micro-application of ATP in cerebral penetrating arterioles in vitro. Since cytochrome P450 produced epoxyeicosatrienoic acids (EETs) induce vasodilation via stimulation of calcium activated potassium channels (KCa) [16], in additional experiments we also examined the possible contribution of KCa inhibition on the response to extraluminal ATP stimulation. We also examined the possible contribution of ecto-ATPases to extraluminal ATP-induced vasomotor responses.
Materials and Methods
Isolation and cannulation of penetrating arterioles
All procedures were approved by the Washington University Advisory Committee for Animal Resources. Male Sprague-Dawley rats (350-450 g, Harlan, Indianapolis, IN) were anesthetized with pentobarbital sodium (65 mg/kg intraperitoneally) and sacrificed. The cerebral penetrating arterioles were excised from the middle cerebral artery. Arterioles were transferred to an organ bath (2.5 ml volume) mounted on the stage of an inverted video microscope (Diaphot, Nikon, Melville, NY) and cannulated with glass micropipettes. No intraluminal flow was applied and the transmural pressure was set at 60 mmHg and continuously monitored. We observed the internal diameter of the vessels using a computerized diameter tracking system (Diamtrak, Montech Pty Ltd., Australia).
The arterioles were superfused continuously with a physiological saline solution (37.5°C; pH 7.3) of the following composition (in mmol/L): 144 NaCl, 3 KCl, 2.5 CaCl2, 1.4 MgSO4, 2.0 pyruvate, 5.0 glucose, 0.02 ethylenediaminetetraacetic acid (EDTA), and 2.0 3-(N-morpholino) propanesulfonic acid (MOPS), 1.21 NaH2PO4. After equilibration, the vessels developed spontaneous tone and we confirmed their viability by changing the extraluminal pH from 7.3 to 6.8 and from 7.3 to 7.65. Vessels with poor tone (less than 20% decrease from the maximum diameter) or poor response to pH (less than 15% change in diameter after pH change) were excluded.
Local micro-stimulation by ATP
For topical micro-application, ATP (10 mmol/L in distilled water) was back-filled into borosilicate glass micropipettes (World Precision Instruments, Sarasota, FL) pulled (Model P-87, Sutter Instrument, Novato, CA) to a tip with 1.5-2.0 μm inner diameter. The micropipette was positioned near the arteriolar wall (within 20 μm) and ATP was applied by pressure ejection (Picospritzer II, General Valve, Fairfield, NJ; 50 to 450 msec in 100 msec steps at 20 psi using 100% nitrogen gas) resulting in ejected volumes of 50 to 500 pL onto the abluminal surface of the vessel. These stimulation parameters provided a vasomotor response similar to that observed previously for ATP applied globally into the tissue bath with a 450 msec pulse of ATP equivalent to a global concentration of 100 μmol/L ATP [8;9]. Initially, responses were observed at the site of stimulation (local). The micropipette was then positioned at the opposite end (remote site) of the vessel and the same stimulation used enabling observation of conducted responses. The ejected agonist did not spread further than 70 μm from the tip at the highest pulse duration, consistent with previous observations. Ejections of the control solution had no impact on vessel diameter. [8]
Measurement of membrane potential
Membrane potential measurements of vascular smooth muscle were obtained as described earlier [17]. Intracellular electrodes were back filled with 4 mol/L potassium acetate and attached to a direct current (DC) amplifier (WPI, input impedance of 300 MΩ) with an Ag-AgCl reference electrode. The amplifier output and vessel diameter were recorded simultaneously. Smooth-muscle cells were identified according to their location (the cells next to the clearly visible endothelium). To facilitate electrode penetration we used a piezo stepper (Eppendorf, Model 5172) and penetration was considered successful when we observed a sudden change of membrane potential to at least -25 mV with removal of the electrode inducing a return of the membrane potential to baseline.
Pharmacological analysis of vasomotor responses to local ATP stimulation
The vessel was stimulated locally and remotely in the presence/absence of globally applied inhibitors or after endothelial impairment. All drugs were obtained from Sigma Chemical (St. Louis, MO). We used the following inhibitors to analyze the ATP-induced local and conducted responses: One μmol/L α,β-methylene-ATP (α,β-MetATP; a P2X1 receptor antagonist) and 3 μmol/L pyridoxal phosphate-6-azophenyl-2',4'-disulphonic acid (PPADS; a P2X1 antagonist at this concentration) [4;18]; 100 μmol/L PPADS (a P2X1 and P2Y1 receptor antagonist at this concentration) [4;18]; 10 μmol/L Nω -monomethyl-L-arginine (L-NMMA; a NO synthase inhibitor), 10 μmol/L indomethacin (cyclo-oxygenase inhibitor), one μmol/L N-methylsulfonyl-6-(2-propargyloxyphenyl) hexanamide (MS-PPOH, inhibitor to specifically inhibit the production of epoxyeicosatrienoic acids (EETs))[19]. Air emboli applied at 60 mmHg intraluminal pressure were used to functionally impair the endothelium without smooth muscle damage [4].
Calcium sensitive potassium channels and extraluminally applied ATP
Calcium sensitive potassium channels are activated by EETs [16] which may be released in response to ATP. To examine the contribution of KCa, we observed vasomotor responses to extraluminally applied ATP in the absence/presence of the following potassium channel inhibitors: 1-[(2-Chlorophenyl)dipenylmethyl]-1H-pyrazole (TRAM-34; 0.1 μmol/L) applied intraluminally to inhibit endothelial intermediate conductance calcium activated potassium channels (IKCa) [20]. Extraluminally, we applied apamin (0.1 μmol/L) to specifically inhibit small conductance potassium channels (SKCa)[21] and iberiotoxin (0.1 μmol/L) to specifically inhibit large conductance potassium channels (BKCa) [20].
Contribution of ATP hydrolysis to extraluminally applied ATP
Since ecto-ATPases can hydrolyze ATP to adenosine which could stimulate P1 receptors [12] and since adenosine receptors are present on the smooth muscle cells of rat penetrating arterioles [22;23], we used 1 μmol/L suramin to inhibit ecto-ATPases thus reducing the production of adenosine [24].
Statistics
In this study, only one vessel was used from each animal. All data are presented as mean ± SEM, with n representing the number of observations. Statistics were conducted on absolute vessel diameters. Differences were considered significant at p < 0.05 and determined by repeated-measures analysis of variance RANOVA with Student-Newman-Keuls test as post test or paired Student's t-test where appropriate. The data are presented as relative diameter change (% relative vessel Diameter = DATP/DTone*100) where DTone is the baseline diameter of the vessel before the stimulation with ATP, and DATP is the diameter of the vessel after the stimulation. If a treatment modified the tone diameter (e.g. air embolism or NOS inhibition) data are presented as percent maximal diameter to correct for the changed control diameter and was calculated by the following formula: % maximum dilation = [(DATP − Dtone) / (Dmax − Dtone)]* 100, where Dmax is the maximum diameter of the vessel at 60 mm Hg before the development of spontaneous tone [3;13].
Results
Diameter and membrane potential response to local ATP application
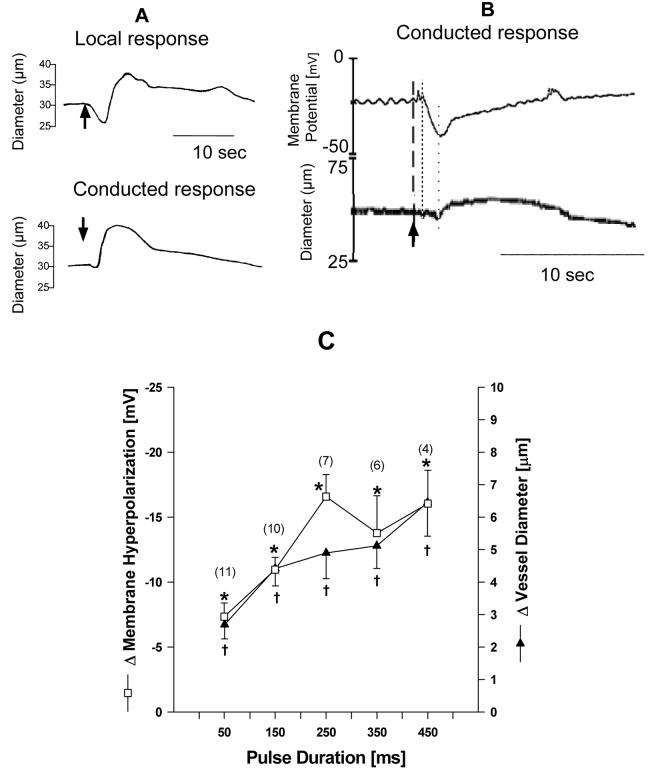
The vessels used for membrane potential measurements had an average length of 1240±230 μm, a maximum diameter of 59.3±1.3 μm and a tone diameter of 44.8±1.1 μm with a resting membrane potential of – 36.4±3.0 mV (n = 6). Micro-application of ATP resulted in a brief local vasoconstriction followed by a vasodilation (Figure 1A). The conducted response was consistently a vasodilation (Figure 1A). This conducted vasodilation was preceded by a membrane hyperpolarization with stimulus dependent increases in conducted dilation and membrane hyperpolarization as shown in Figure 1B and 1C. For 450 msec pulses, the average maximum hyperpolarization was -16 mV (= 72.5%) resulting in a significant 6.5 μm (= 10%) dilation at the remote site (Figure 1C). The remote site hyperpolarization was initiated 0.6±0.1 seconds after pulse application, preceding vessel dilation which occurred at 1.7±0.1 seconds (p < 0.05) with both responses independent of pulse duration (data not shown). Occasionally we observed a small yet insignificant depolarization with a subsequent small conducted vasoconstriction prior to the conducted hyperpolarization and dilation (data not shown).
Figure 1.
Panel A shows diameter traces of the local and conducted vasomotor response to micro-applied ATP (arrow) with a transient local constriction (downstroke) followed by a dilation with only a small conducted initial constriction but a strong conducted dilation (upstroke). Panel B shows the simultaneous measurement of conducted vasomotor responses and membrane potential to micro-applied ATP (arrow) with the hyperpolarization (fine dotted line) preceding the dilation (coarse dotted line). Panel C shows that stimulus-dependent increase between conducted dilation and membrane hyperpolarization to increased ATP pulses. * and †denote p < 0.05 from pre-stimulation control (ANOVA).
Evaluation of the ATP-induced vasomotor responses to purinoceptor inhibition
The vessels used for pharmacological evaluation of local and conducted vasomotor mechanisms had a tone diameter of 36.6±1.9 μm and a length of 1072±47 μm (n = 31). Micro-applied ATP induced significant local transient constrictions and subsequent dilations. We consistently observed a conducted dilation which was sometimes preceded by a small vasoconstriction.
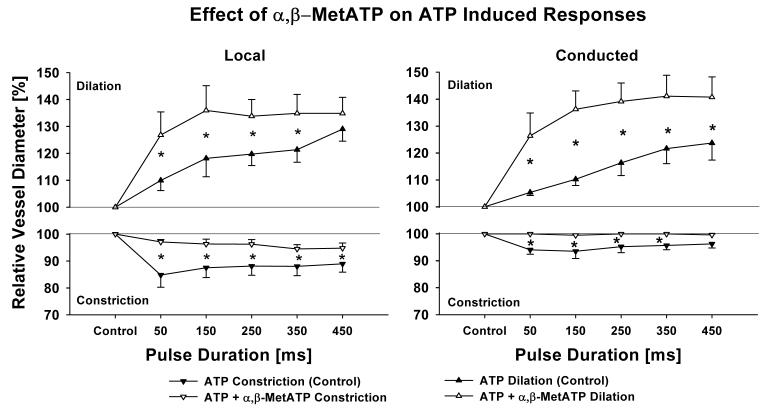
Vessels treated with α,β-MetATP had a maximum diameter of 53.8±5.8 μm and a tone diameter of 35.1±5.7 μm (n = 5). One μmol/L α,β-MetATP applied extraluminally transiently constricted the vessels via smooth muscle P2X1 receptors with the vessels subsequently returning to their basal diameter due to P2x1 receptor desensitization [4]. After this desensitization, subsequent ATP-induced local and conducted constrictions were significantly attenuated in the presence of α,β-MetATP, while local and conducted dilations were greatly enhanced (Figure 2).
Figure 2.
Desensitizing P2X1 receptor with α,β-MetATP (1 μmol/L) significantly reduces local (left panel) and conducted (right panel) initial vasoconstriction and enhances local and conducted dilation. This indicates that P2X1-receptors cause the initial transient vasoconstriction which attenuates P2Y-receptor induced dilation. n = 5; * denotes p < 0.05 RANOVA between control and after desensitization with α,β-MetATP.
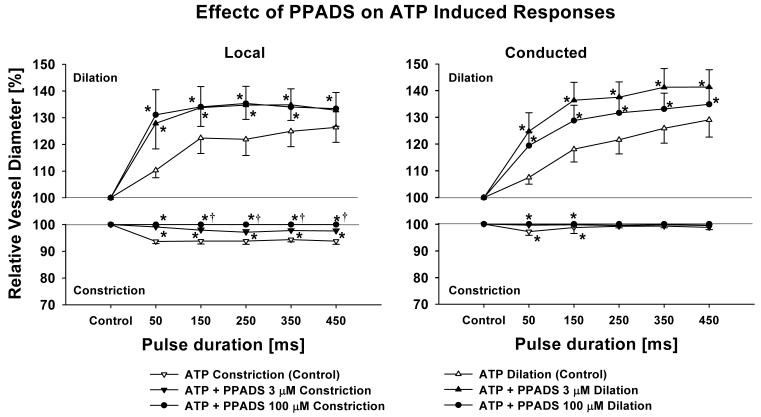
Vessels treated with PPADS had a maximum diameter of 45.9±4.0 μm with a tone diameter of 35.8 μm (n = 7). PPADS (3 and 100 μmol/L) had no effect on the control diameter (data not shown). Three μmol/L PPADS (P2X1 receptor antagonist at this concentration) significantly blocked local transient constrictions to ATP similar to the effect of α,β-MetATP (Figure 3). The ATP-induced local and conducted dilation was potentiated by 3 μmol/L PPADS (Figure 3). Addition of 100 μmol/L PPADS (P2X1 and P2Y1 receptor antagonists at this concentration) [18] further inhibited the local transient constriction but had no additional effect on the conducted dilation (Figure 3).
Figure 3.
Inhibiting P2X1 with PPADS (3 μmol/L) significantly reduces local (left panel) and conducted (right panel) initial vasoconstriction and greatly enhances local and conducted dilation similarly to α,β-MetATP (Fig. 2). Inhibiting all P2-receptors except P2Y2 with 100 μmol/L PPADS did further reduce local transient constriction but has no further effect on local or conducted dilation indicating that ATP stimulates P2Y2-receptors to cause dilation. n = 7; * denotes p < 0.05 RANOVA between ATP control and 3 μmol/L PPADS, †denotes p < 0.05 between 3 μmol/L and 100 μmol/L PPADS (RANOVA).
Since ecto-ATPases on vascular cells can hydrolyze ATP to adenosine which activates P1 receptors causing vasodilation [12], we used suramin which has been shown to reduce ecto-ATPase activity at micromolar concentrations [24]. Vessels used to test the effect of suramin had a maximum diameter of 68.3±5.6 μm and a tone diameter of 48.0±3.8 μm (n = 8). When we compared vessel dilations to extraluminal 100 μmol/L ATP before and after suramin (1 μmol/L) we found no difference in either the dilatory or constrictor responses. Higher concentrations of suramin needed to inhibit P2Y2 receptors [24]} induced complete vessel dilation within the incubation period of 20 minutes and could not be used. Similarly the ecto-ATPase inhibitor Reactive Blue 2 [24] used on seven additional vessels again dilated the vessels at concentrations higher than 1 μmol/L (data not shown).
Contribution of endothelial impairment and endothelial factors (NO, prostanoids, EETs) to local and conducted vasomotor responses
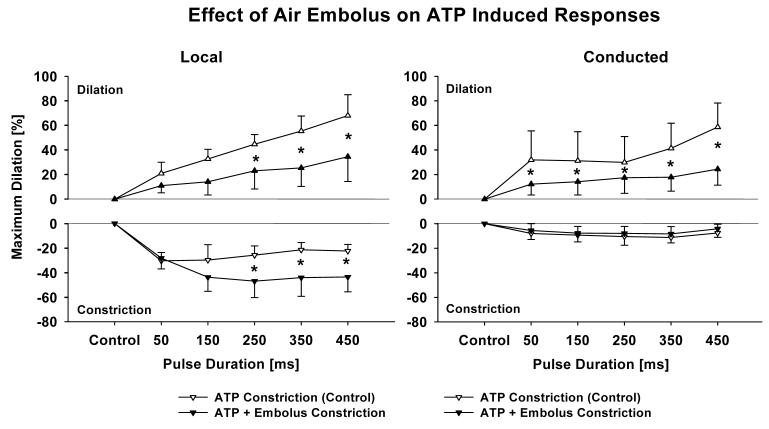
Vessels used with air embolization had a maximum diameter of 66.8±7.8 μm and a tone diameter of 46.4±8.8 μm (n = 4). Air embolization significantly reduced the control diameter indicating successful endothelial damage (46.4±8.8 versus 40.4±9.7 μm, p < 0.05). ATP-induced local constriction was potentiated by the endothelial impairment and dilation was attenuated (Figure 4). The conducted dilation was also reduced after air embolization but the conducted constriction was unchanged (Figure 4).
Figure 4.
Air Embolization significantly enhances local initial constriction and reduces local dilation at high pulse durations (left panel) and reduces conducted dilation but not conducted constriction (right panel). These results indicate that the P2X1 receptors causing transient constriction reside on smooth muscle cells and that the P2Y2 receptors causing dilation are present on the endothelium. Data are presented as % maximum diameter to compensate for control diameter change induced by the embolism. n = 4; * denotes p < 0.05 RANOVA between control and after embolization.
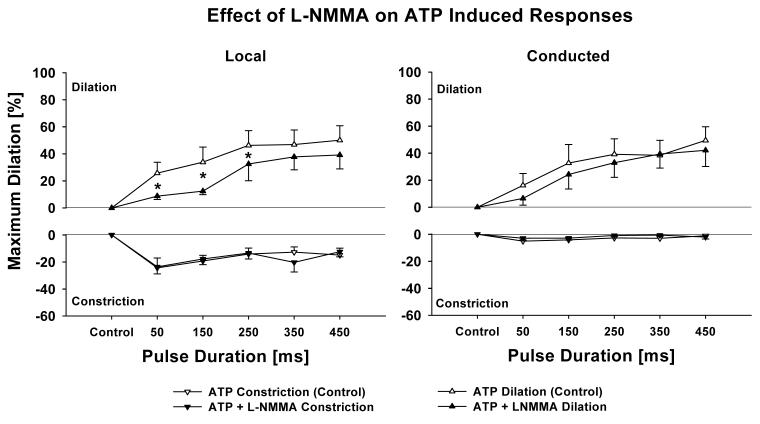
Vessels exposed to L-NMMA had a maximum diameter of 48.2±6.8 μm with a tone diameter of 30.7±3.5 μm (n = 5). Inhibition of nitric oxide synthase with L-NMMA (10 μmol/L) constricted the vessels (39.9±5.7 versus 33.5±6.0 μm, p < 0.05) and partially affected the local dilation at low pulse durations (Figure 5). However, L-NMMA had no effect on the conduction (Figure 5).
Figure 5.
Inhibition of nitric oxide synthase with L-NMMA (10 μmol/L) significantly reduces local dilation at low pulse durations (left panel) but not conducted (right panel) responses. This indicates that NO as an endothelial dilator is released at low ATP pulses but that NO is not involved in the conducted vasomotor responses. Data are presented as % maximum diameter to compensate for control diameter change induced after NOS inhibition. n = 5; * denotes p < 0.05 RANOVA between control and after L-NMMA.
Cyclooxygenase inhibition with indomethacin (10 μmol/L) influenced neither local nor conducted vasomotor responses (maximum vessel diameter = 51.6±2.9 μm, tone = 30.9±4.6 μm, n = 4, data not shown).
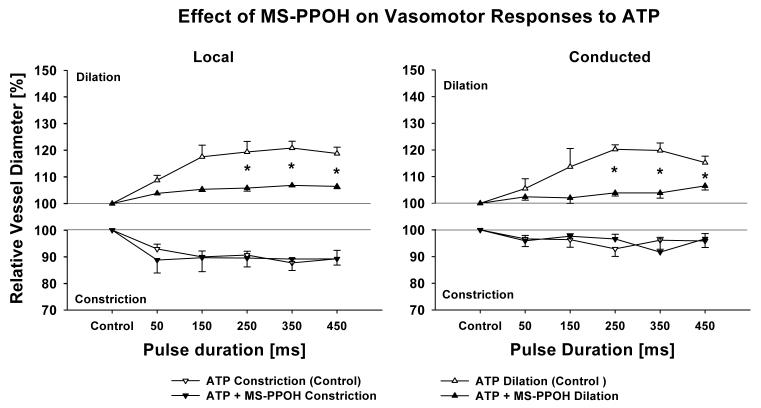
We used the specific cytochrome P450 inhibitor MS-PPOH to inhibit the de novo production of EETs [19]. The vessels used in these studies had a maximum diameter of 67.0±0.6 μm with a tone diameter of 52.0±2.5 μm. One μmol/L MS-PPOH did not affect the basal vessel diameter (54.3±3.8 μm) but did significantly attenuate both local and conducted dilation reducing the dilatory response for pulses from 250 to 450 ms (n = 4, Figure 6). In preliminary experiments we found that higher MS-PPOH concentrations used in in vivo studies dilated our vessel preparation.
Figure 6.
Inhibiting the production of EETs with MS-PPOH (1 μmol/L, cytochrome P450 inhibitor) significantly reduces local (left panel) and conducted (right panel) dilation. This indicates that a cytochrome P450 EET is released locally and that this product possibly acting as an EDHF contributes to the conducted vasomotor response. n = 4; * denotes p < 0.05 RANOVA between control and after MS-PPOH.
Calcium activated potassium channels and extraluminally applied ATP
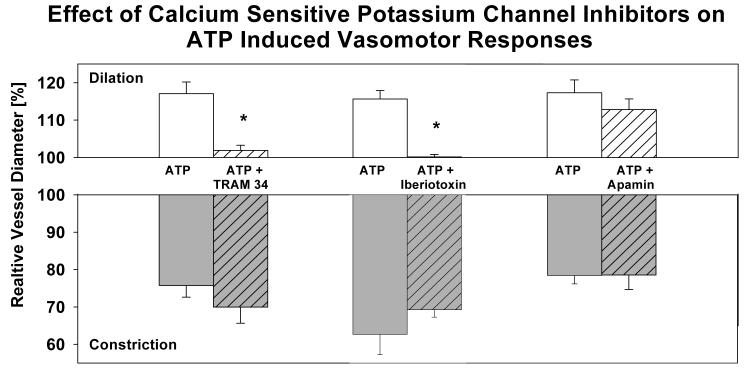
Since inhibition of cytochrome P450 resulted in reduced local and conducted vasomotor responses, EETs may contribute to ATP-induced dilation by activating calcium sensitive potassium channels (KCa)[25]. To elucidate the contribution of KCa to the ATP induced vasomotor responses, we applied ATP extraluminally in the absence or presence of specific KCa channel inhibitors. Vessels exposed to TRAM34 (specific inhibitor of intermediate conductance or IKCa channels) had a maximum diameter of 75.2±6.2 μm with a tone diameter of 53.2±7.8 μm (n = 4). Intraluminally applied TRAM-34 (10 μmol/L) did not affect basal tone diameter (53.3±3.4 μm) but inhibited dilation to ATP (Figure 7). Extraluminally applied TRAM-34 had no effect (data not shown). Vessels exposed to iberiotoxin (specific inhibitor of large conductance or BKCa channels) had a maximum diameter of 78.4±13.5 μm with a tone diameter of 47.0±4.5 μm (n = 4). Iberiotoxin did not affect the tone diameter (47.3±7.0 μm,) but inhibited dilation to ATP (Figure 7). Vessels treated with apamin had a maximum diameter of 79.8±5.7 μm with a tone diameter of 52.8±4.8 μm (n = 4). Inhibition of SKCa with 0.1 μmol/L apamin had no effect on the vessels' tone diameter (52.8±4.8 μm, n = 4) or ATP-induced vasomotor responses (Figure 7).
Figure 7.
Effect of calcium sensitive potassium channel blocker on vasomotor responses to extraluminally applied ATP (100 μmol/L). Inhibition of BKCa and IKCa but not SKCa inhibited the vessel dilation to ATP indicating that BKCa and IKCa activation dilates the penetrating arterioles.
Discussion
The major findings of this study were, first, that topically micro-applied ATP consistently initiated conducted dilation which was preceded by smooth muscle hyperpolarization at the remote site in a stimulus dependent manner. Second, endothelial impairment enhanced local but not conducted constriction and attenuated both local and conducted dilation. Third, micro-applied ATP stimulated smooth muscle P2X1-receptors causing a transient constriction. Inhibition of P2X1-receptors attenuated local and conducted constriction and enhanced local and conducted dilation. Local vessel dilation was mediated by endothelial P2Y-receptors resulting in conducted dilation. Fourth, NO contributes to local dilation at low stimulation pulses but does not contribute to conducted dilation. Cytochrome P450 metabolites contribute to local dilation and inhibition of cytochrome P450 greatly reduces both local and conducted dilation. Cyclooxygenase products are not involved in either local or conducted responses. Fifth, ATP induced dilation involves activation of IKCa and BKCa channels but not SKCa channels.
Locally induced ATP-induced vasomotor responses, conducted vasodilation and membrane potentials
Micro-application of ATP caused a biphasic response locally with an initial transient constriction followed by a dilation. This corresponds to previous observations by us and others [8;10;12] and is similar to globally applied ATP [4]. In our preparation, micro-applied adenosine tri-phosphate consistently resulted in conducted vasodilation (e.g 20 % at 450 ms pulses, Figure 2) while the conducted constriction was 5 % at a distance of 1200 μm. This result indicates that in our preparation dilation is more prominently conducted than constriction. Ngai and colleagues observed a 5% conducted dilation which was preceded by a 5% conducted constriction at a distance of 1000 μm [10]. In hamster cheek pouch arterioles Duza and Sarelius found that only dilation was conducted in response to local ATP [12]. In the present study, we found that at the remote site conducted vasodilation was preceded by a membrane hyperpolarization similar to local acetylcholine stimulation in hamster cheek pouch arterioles [15]. Dora et al. also observed that local acetylcholine-induce dilation was preceded by hyperpolarization indicating that a local membrane potential change is the generator for the observed vasomotor response and that it is this membrane potential change which is conducted resulting in the change in vessel diameter via electro-mechanical coupling [15]. We consistently observed smooth muscle hyperpolarization preceding the vessel dilation indicating that electro-mechanical coupling may also be responsible for the vessel diameter change in our preparation. The local membrane potential response to ATP has not yet been measured in penetrating arterioles. We observed that ATP induced an initial small depolarization with a similarly small constriction indicating that both membrane depolarization and vessel constriction can be conducted. However, these responses were significantly less pronounced compared with the hyperpolarization and subsequent dilation (Figure 1). The change in membrane hyperpolarization correlated well with vessel dilation. These findings indicate that in rat penetrating arterioles the dilation at the remote site is conducted electrically along the arteriolar wall and is caused by hyperpolarization similar to other vessel preparations [15].
ATP-induced vasomotor responses and purinergic receptors
Desensitization of P2X1-receptors with 1 μmol/L α,β-MetATP or pretreatment with 3 μmol/L PPADS (P2X1-receptor antagonists at this concentration) diminished the local transient constriction while the subsequent dilation was potentiated. Endothelial denudation enhanced the ATP-induced vasoconstriction. These results indicate that ATP stimulates smooth muscle P2X1-receptors causing constriction [13]. Inhibition of smooth muscle P2X1-receptors enhanced conducted dilation, indicating that P2X1-receptor stimulation competes with the dilatory response both locally and during conduction. We did not measure the smooth muscle membrane potential at the local stimulation site due to strong vessel wall movement. However, we did observe small depolarizations and conducted constrictions at the distal site (Figure 1) indicating that membrane depolarization may have occurred locally and that this depolarization was conducted. P2X-receptor induced depolarization and contraction was previously observed in vas deferens [26]. The mechanism by which P2X1 stimulation causes depolarization in cerebral arterioles is not known.
Two endothelial P2 receptors, P2Y1 and P2Y2, are reported to cause dilation [27]. We recently reported that global ATP activated both endothelial P2Y1 and P2Y2-receptors. This conclusion was based on the observation that when the receptors were stimulated by receptor specific agonists [13], 100 μmol/L PPADS inhibited P2Y1-, but not P2Y2-receptors stimulation [4]. Inhibition of P2X1-receptors with 3 μmol/L PPADS enhanced local dilation indicating that endothelial P2Y-receptors are present [4]. However, we found no difference in ATP-induced local dilation in the presence 100 μmol/L PPADS an inhibitor of all P2Y-receptors except P2Y2 [27]. These findings suggest that micro-applied ATP produced dilation primarily via stimulation of the endothelial P2Y-receptor. We also observed no change in conducted vasodilation after the inhibition of P2Y1-receptors with 100 μmol/L PPADS indicating that local endothelial P2Y, possibly via P2Y2 stimulation, is the generator for the conducted vasodilation. Suramin and Reactive Blue 2 inhibit P2Y2 receptors at concentrations of 20 μmol/L and higher [24]. However, concentrations of these inhibitors greater than 1 μmol/L dilated our vessel preparation and could not be used. In hamster cheek pouch, Duza and Sarelius reported that conducted vasomotor responses to locally applied ATP and adenosine depend on endothelial P1 receptors with ATP being hydrolyzed to adenosine [12;24]. Rat penetrating arterioles have adenosine receptors present on the smooth muscle but not endothelium [22;23], strongly dilate to locally applied adenosine but did not show conduction [8]. We used a low concentration of suramin to reduce hydrolysis of ATP by ecto-ATPases [24] and found no change in vessel dilation to extraluminally applied ATP. This suggests that in rat penetrating arterioles, ATP hydrolysis with subsequent adenosine receptor stimulation was not causing the observed dilation. Due to the dilatory effect of the higher concentration of suramin (or Reactive Blue 2) we were unable to determine directly if P2Y2 receptor stimulation is responsible for the observed local ATP response. Future experiments with specific P2Y1 and P2Y2 agonists should help elucidate which receptor is involved in the local and conducted vasomotor responses to ATP [13].
Our results show that inhibiting the smooth muscle P2X1 receptor enhanced the local endothelial P2Y-receptor dependent dilation and the conducted dilation. This seems to indicate that the P2X1 receptor response counteracts the P2Y-induced hyperpolarization and dilation since the conducted dilation is also enhanced. Further only local responses which cause a membrane potential change contribute to conducted responses as indicated by others [15].
Contribution of NO, prostanoids and EDHF
Endothelial P2Y-activation releases NO, prostanoids, or endothelium-derived hyperpolarizing factor (EDHF), depending on species and P2Y-receptor type studied [27]. Inhibition of cyclooxygenase had no effect on ATP-induced local or conducted vasomotor responses in our preparation. The finding that prostanoids may not be involved is similar to our previous findings [13] and that of others in the cerebral circulation [28]. Inhibition of nitric oxide synthase (NOS) decreased dilation only at short pulse durations. This corresponds with our previous study where NOS inhibition only affected ATP induced dilation at low agonist concentrations [13] similar to larger cerebral vessels [28]. We previously found that specific P2Y1 receptor stimulation releases both NO (at low agonist concentrations) and an EDHF at higher concentration while P2Y2 stimulation released only an EDHF [13]. Inhibition of NOS had no effect on conducted vasomotor responses indicating that NO does not contribute to the generation of the conducted response or conduction itself possibly because NO does not affect the arteriolar membrane potential as an EDHF would do. The effect of NOS inhibition on the membrane potential has not been measured in our preparation. Hoepfl et al. observed that in hamster cremaster arterioles NO is not critical for acetylcholine induce conducted vasodilation [14]. However, Collins and Ellsworth reported that in hamster retractor muscle, inhibition of NOS did eliminate retrograde conducted vasodilation to intravenular ATP [29].
To test for the contribution of EDHFs we applied the specific cytochrome P450 inhibitor MS-PPOH which strongly attenuated both local and conducted ATP-induced dilation indicating that de novo synthesized EETs may be involved in the dilatory response [30]. These findings are similar to Hoepfl et al. who found that cytochrome P450 inhibition with 17-ODYA reduced local and conducted vasomotor responses to acetylcholine in hamster cremaster arterioles [14].
Calcium sensitive potassium channels and ATP-induced vasomotor responses
EETs can cause dilation through activation of calcium sensitive potassium channels and subsequent membrane hyperpolarization [16]. Since it is not known if ATP activates calcium sensitive potassium channels in rat penetrating arterioles, we sought to elucidate the contribution of these channels to vasomotor responses to extraluminally applied ATP. We used specific inhibitors of these channels. We found that BkCa and IKCa but not SKCa channels contribute to ATP-induced dilation. Activation of KCa channels causes hyperpolarization contributing to the local dilation [31]. These results indicate that cytochrome P450 generated EETs may contribute to ATP-induced local dilation possibly by activating potassium channels [16].
Taken together, these results suggest that micro-applied ATP may locally release endothelial NO (at low stimulation pulses) and cytochrome P450 metabolites (P2Y-stimulation) causing dilation. Since EETs produced by cytochrome P450 can activate potassium channels [16] ATP may, in part, dilate penetrating arterioles via activating IKCa and BKCa channels to initiate the conducted response.
Local stimulation and extraluminal antagonists
In the present studies, we employed micro-application to cause a local vasomotor response and extraluminally applied inhibitors. Thus, after inhibition, changed vasomotor responses at the local site reflect the contribution of the mechanisms inhibited (e.g. NO or EDHF) on the local vessel response. Since we applied inhibitors globally, it is possible that the inhibitors not only affected the local vasomotor response but also the conduction along the vessel and/or the remote vasomotor response. Thus, future experiments with focal application of inhibitors (e.g. inhibitor only at the local or the remote site) have to be done to distinguish which mechanisms are involved in the local and/or remote vasomotor responses.
Pathology and Conducted Vasodilation in Cerebral Arterioles
In these studies, air-embolization-induced damage to the endothelium resulted in constriction of the penetrating arterioles, which effectively raises microvascular resistance. In addition, we observed that the local constriction was enhanced and the local and conducted vasodilation to ATP were attenuated. Air embolization of microvessels damages the endothelial function but does not remove the endothelial cells [4;32]. It is therefore possible that the residual endothelial cell layer still provided a conduit to allow conducted responses [4].
Conducted vasodilation is a prerequisite to allow an increase in micro-vascular flow in response to local metabolic demand [7]. Thus impairment of endothelial function and subsequent reduction in conducted vasodilation in penetrating arterioles may further decrease cerebral microvascular blood flow. The reduction in conducted dilation is similar to the effect of oxyhemoglobin [9].
Interplay of local endothelial and smooth muscle factors resulting in local and conducted vasomotor responses
Our results indicate that ATP locally stimulates smooth muscle P2X1 receptors causing local constriction and some conducted depolarization/constriction (Fig. 1C; 2 and 3). This indicates that P2X1 induced constriction may involve a depolarization locally. We found that ATP stimulates P2Y1 and P2Y2 receptors where P2Y1 stimulation appears to liberate NO and a non-NO and non-COX dependent factor, possibly an EDHF dependent on cytochrome P450 [13] and P2Y2 liberates only a non-NO and non-COX dependent factor i.e. EDHF [13]. As such, both NO and an EDHF may contribute to the local dilation. For a local response to lead to a conducted one, most studies indicate that a local membrane potential change has to occur. [15]. However, inhibition of NO did not result in a decreased conducted dilation. Thus it appears that only the EDHF component contributes to a local membrane potential change leading to conduction but NO causes local dilation without hyperpolarization. Therefore, while inhibition of NO decreased the local dilation, the EDHF component was unaltered and the conducted response unchanged. Inhibition of either NO or EDHF resulted in a decreased local dilatory response, but not enhanced constriction. It is possible that the presence of the non inhibited dilator (either NO or EDHF) was sufficient to prevent an increased constriction. This is supported by the observation that air embolus-induced endothelial damage caused increased smooth muscle constriction to ATP. Our data therefore show an intricate interplay among the various factors invoked by ATP stimulation on both local and conducted response.
In summary, in rat cerebral arterioles in vitro, micro-application of ATP produces a biphasic response resulting in a transient constriction via smooth muscle P2X1 receptors and subsequent dilation primarily due to endothelial P2Y-receptor activation. Conducted vasomotor responses travel along the vessel with a membrane hyperpolarization preceding vessel dilation. ATP produces NO and cytochrome P450 metabolites (EETs) which locally dilate the vessel but NO does not contribute to conduction. Activation of IKCa and BKCa but not SKCa channels may contribute to ATP-induced dilation.
Acknowledgment of Support
This work was supported by National Institutes of Health grants HL57540 (HHD), P01 NS32636 (HHD), NS30555 (RGD and HHD) and GM31278 (JRF).
Reference List
- 1.Rosenblum WI, Kontos HA. The importance and relevance of studies of the pial microcirculation. Stroke. 1974;5:425–428. doi: 10.1161/01.str.5.4.425. [DOI] [PubMed] [Google Scholar]
- 2.Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004 Dec 11;95:e73–e81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- 3.You JP, Johnson TD, Marrelli SP, Mombouli JV, Bryan RM., Jr. P2u receptor-mediated release of endothelium-derived relaxing factor nitric oxide and endothelium-derived hyperpolarizing factor from cerebrovascular endothelium in rats. Stroke. 1999;30:1125–1132. doi: 10.1161/01.str.30.5.1125. [DOI] [PubMed] [Google Scholar]
- 4.Horiuchi T, Dietrich HH, Tsugane S, Dacey RG., Jr. Analysis of purine- and pyrimidine-induced vascular responses in the isolated rat cerebral arteriole. American Journal of Physiology - Heart & Circulatory Physiology. 2001;280:H767–H776. doi: 10.1152/ajpheart.2001.280.2.H767. [DOI] [PubMed] [Google Scholar]
- 5.You J, Golding EM, Bryan RM., Jr. Arachidonic acid metabolites, hydrogen peroxide, and EDHF in cerebral arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1077–H1083. doi: 10.1152/ajpheart.01046.2004. [DOI] [PubMed] [Google Scholar]
- 6.Saino T, Matsuura M, Satoh YI. Comparison of the effect of ATP on intracellular calcium ion dynamics between rat testicular and cerebral arteriole smooth muscle cells. Cell Calcium. 2002;32:153–163. doi: 10.1016/s0143-4160(02)00139-2. [DOI] [PubMed] [Google Scholar]
- 7.Kurjiaka DT, Segal SS. Conducted vasodilation elevates flow in arteriole networks of hamster striated muscle. Am J Physiol. 1995;269:H1723–H1728. doi: 10.1152/ajpheart.1995.269.5.H1723. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich HH, Kajita Y, Dacey RG., Jr. Local and conducted vasomotor responses in isolated rat cerebral arterioles. Am J Physiol Heart Circ Physiol. 1996;271:H1109–H1116. doi: 10.1152/ajpheart.1996.271.3.H1109. [DOI] [PubMed] [Google Scholar]
- 9.Kajita Y, Dietrich HH, Dacey RG., Jr. Effects of oxyhemoglobin on local and propagated vasodilatory responses induced by adenosine, adenosine diphosphate, and adenosine triphosphate in rat cerebral arterioles. J Neurosurg. 1996;85:908–916. doi: 10.3171/jns.1996.85.5.0908. [DOI] [PubMed] [Google Scholar]
- 10.Ngai AC, Nguyen TS, Meno JR, Britz GW. Postischemic augmentation of conducted dilation in cerebral arterioles. Stroke. 2007;38:124–130. doi: 10.1161/01.STR.0000252157.93998.47. [DOI] [PubMed] [Google Scholar]
- 11.McCullough WT, Collins DM, Ellsworth ML. Arteriolar responses to extracellular ATP in striated muscle. Am J Physiol. 1997;272:H1886–H1891. doi: 10.1152/ajpheart.1997.272.4.H1886. [DOI] [PubMed] [Google Scholar]
- 12.Duza T, Sarelius IH. Conducted dilations initiated by purines in arterioles are endothelium dependent and require endothelial Ca2+ Am J Physiol Heart Circ Physiol. 2003;285:H26–H37. doi: 10.1152/ajpheart.00788.2002. [DOI] [PubMed] [Google Scholar]
- 13.Horiuchi T, Dietrich HH, Hongo K, Dacey RG., Jr. Comparison of P2 receptor subtypes producing dilation in rat intracerebral arterioles. Stroke. 2003;34:1473–1478. doi: 10.1161/01.STR.0000071527.10129.65. [DOI] [PubMed] [Google Scholar]
- 14.Hoepfl B, Rodenwaldt B, Pohl U, de WC. EDHF, but not NO or prostaglandins, is critical to evoke a conducted dilation upon ACh in hamster arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H996–H1004. doi: 10.1152/ajpheart.01082.2001. [DOI] [PubMed] [Google Scholar]
- 15.Dora KA, Xia J, Duling BR. Endothelial cell signaling during conducted vasomotor responses. Am J Physiol Heart Circ Physiol. 2003;285:H119–H126. doi: 10.1152/ajpheart.00643.2002. [DOI] [PubMed] [Google Scholar]
- 16.Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27:971–979. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich HH, Dacey RG., Jr. Effects of extravascular acidification and extravascular alkalinization on constriction and depolarization in rat cerebral arterioles in vitro. J Neurosurg. 1994;81:437–442. doi: 10.3171/jns.1994.81.3.0437. [DOI] [PubMed] [Google Scholar]
- 18.Ralevic V, Burnstock G. Discrimination by PPADS between endothelial P2Y- and P2U- purinoceptors in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1996;118:428–434. doi: 10.1111/j.1476-5381.1996.tb15420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, Schwartzman ML. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. Journal of Pharmacology & Experimental Therapeutics. 1998;284:966–973. [PubMed] [Google Scholar]
- 20.Marrelli SP, Eckmann MS, Hunte MS. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. American Journal of Physiology - Heart & Circulatory Physiology. 2003;285:H1590–H1599. doi: 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- 21.Faraci FM, Lynch C, Lamping KG. Responses of cerebral arterioles to ADP: eNOS-dependent and eNOS-independent mechanisms. Am J Physiol Heart Circ Physiol. 2004;287:H2871–H2876. doi: 10.1152/ajpheart.00392.2004. [DOI] [PubMed] [Google Scholar]
- 22.Ngai AC, Coyne EF, Meno JR, West GA, Winn HR. Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. American Journal of Physiology - Heart & Circulatory Physiology. 2001;280:H2329–H2335. doi: 10.1152/ajpheart.2001.280.5.H2329. [DOI] [PubMed] [Google Scholar]
- 23.Ngai AC, Winn HR. Effects of adenosine and its analogues on isolated intracerebral arterioles: Extraluminal and intraluminal application. Circ Res. 1993;73:448–457. doi: 10.1161/01.res.73.3.448. [DOI] [PubMed] [Google Scholar]
- 24.Chen BC, Lee CM, Lin WW. Inhibition of ecto-ATPase by PPADS, suramin and reactive blue in endothelial cells, C6 glioma cells and RAW 264.7 macrophages. Br J Pharmacol. 1996;119:1628–1634. doi: 10.1111/j.1476-5381.1996.tb16082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alkayed NJ, Birks EK, Hudetz AG, Roman RJ, Henderson L, Harder DR. Inhibition of brain P-450 arachidonic acid epoxygenase decreases baseline cerebral blood flow. Am J Physiol Heart Circ Physiol. 1996;271:H1541–H1546. doi: 10.1152/ajpheart.1996.271.4.H1541. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy C. ATP as a cotransmitter in perivascular sympathetic nerves. J Auton Pharmacol. 1996;16:337–340. doi: 10.1111/j.1474-8673.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 27.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 28.You JP, Johnson TD, Childres WF, Bryan RM., Jr. Endothelial-mediated dilations of rat middle cerebral arteries by ATP and ADP. Am J Physiol Heart Circ Physiol. 1997;273:H1472–H1477. doi: 10.1152/ajpheart.1997.273.3.H1472. [DOI] [PubMed] [Google Scholar]
- 29.Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvasc Res. 1998;56:43–53. doi: 10.1006/mvre.1998.2076. [DOI] [PubMed] [Google Scholar]
- 30.Lovick TA, Brown LA, Key BJ. Neuronal activity-related coupling in cortical arterioles: involvement of astrocyte-derived factors. Exp Physiol. 2005;90:131–140. doi: 10.1113/expphysiol.2004.028811. [DOI] [PubMed] [Google Scholar]
- 31.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 32.Saito Y, Eraslan A, Lockard V, Hester RL. Role of venular endothelium in control of arteriolar diameter during functional hyperemia. Am J Physiol Heart Circ Physiol. 1994;267:H1227–H1231. doi: 10.1152/ajpheart.1994.267.3.H1227. [DOI] [PubMed] [Google Scholar]