Abstract
Matrix metalloproteinase- (MMP-9) is involved in processes that occur during cutaneous wound healing such as inflammation, matrix remodeling, and epithelialization, To investigate its role in healing, full thickness skin wounds were made in the dorsal region of MMP-9-null and control mice and harvested up to 14 days post wounding. Gross examination and histological and immunohistochemical analysis indicated delayed healing in MMP-9-null mice. Specifically, MMP-9-null wounds displayed compromised reepithelialization and reduced clearance of fibrin clots. In addition, they exhibited abnormal matrix deposition, as evidenced by the irregular alignment of immature collagen fibers. Despite the presence of matrix abnormalities, MMP-9-null wounds displayed normal tensile strength. Ultrastructural analysis of wounds revealed the presence of large collagen fibrils, some with irregular shape. Keratinocyte proliferation, inflammation, and angiogenesis were found to be normal in MMP-9-null wounds. In addition, VEGF levels were similar in control and MMP-9-null wound extracts. To investigate the importance of MMP-9 in wound reepithelialization we tested human and murine keratinocytes in a wound migration assay and found that antibody-based blockade of MMP-9 function or MMP-9 deficiency retarded migration. Collectively, our observations reveal defective healing in MMP-9-null mice and suggest that MMP-9 is required for normal progression of wound closure.
Keywords: matrix metalloproteinase, wound healing, collagen, keratinocytes
Introduction
Matrix metalloproteinases (MMP) comprise a family of zinc-dependent endopeptidases with matrix-degradative properties (Page-McCaw et al., 2007; Parks et al., 2004). MMP-2 and MMP-9 are considered a subclass of the MMPs due to their gelatinolytic activity and have been shown to participate in the wound healing response. In general, injury to tissues elicits a series of responses that include inflammation, extracellular matrix remodeling, angiogenesis, and epithelial regeneration (Eming et al., 2007; Tonnesen et al., 2000). The activity of MMP-9 has been shown to be critical for several of these processes in the context of pathological conditions or trauma to tissues such as cornea and brain (Page-McCaw et al., 2007).
Injury to the skin elicits responses that include hemostasis, edema formation and inflammation, eschar formation, cell proliferation and migration, production and remodeling of the ECM, angiogenesis, and reepithelialization (Singer and Clark, 1999). As a result, these processes regress as the wound matures, leading to stabilization of the repaired tissue. However, due to the lack of regenerative capacity, healed skin tissue in adults lacks specialized structures such as hair follicles and sweat glands and is frequently subject to excessive scar formation (Eming et al., 2007; Gurtner et al., 2008; Singer and Clark, 1999). Therefore, various strategies to improve wound healing have been pursued with limited success. We assume that a critical understanding of the cellular and molecular processes that dictate the course of healing would facilitate the development of interventional strategies that could lead to improved healing. Likewise, the identification of molecules whose function is critical for the various processes could lead to the development of specific therapeutic strategies.
In cutaneous wound healing, these tissue responses have been shown to occur in an overlapping manner and to influence each other (Singer and Clark, 1999; Tonnesen et al., 2000). For example, secretory products of inflammatory cells such as vascular endothelium growth factor (VEGF) and fibroblast growth factor can induce angiogenesis. It is also appreciated that, in turn, angiogenic cells modify the extracellular matrix by expressing matrix-degrading enzymes. Consistent with an overall positive role for MMPs in wound healing, it has been previously shown that wounds treated with MMP inhibitors heal at a delayed rate (Agren et al., 2001; Mirastschijski et al., 2004). However, delayed wound healing has also been observed when MMPs, such as MMP-1, are overexpressed indicating that normal wound healing requires balanced MMP activity.
Studies in MMP-9-null mice have implicated MMP-9 as a critical factor in the response to injury. Specifically, it was shown that the expression of MMP-9 is upregulated following brain injury and that MMP-9-null mice developed smaller lesions and acquired less motor deficits (Wang et al., 2000). In addition, MMP-9-null mice displayed enhanced healing of injured corneas due to increased cell proliferation and inflammation (Mohan et al., 2002). Furthermore, in a model of myocardial infarction, these mice displayed increased angiogenesis (Lindsey et al., 2006). The latter finding was inconsistent with the observation of reduced neovascularization of polymeric scaffolds and delayed angiogenesis in the developing bones of MMP-9-null mice (Sung et al., 2005; Vu et al., 1998). Therefore, the role of MMP-9 in neovascularization appears to be complex.
To address some of the issues regarding the function of MMP-9 in post-injury processes, we compared the wound healing response in MMP-9-null and littermate control mice. Here we report that MMP-9-null mice heal wounds at a decelerated rate and display defects in keratinocyte migration and collagen fibrillogenesis. Our findings suggest that these effects lead to delayed reepithelialization and irregular matrix remodeling, respectively.
Materials and Methods
Animal Model
The generation of MMP-9-null mice has been described (Vu et al., 1998). In this study, unless stated otherwise, three-month-old and sex-matched MMP-9-null and littermate control mice were used. A total of fifty mice per genotype were used. All experiments were approved by the Institutional Animal Care and Use Committee at the University of Washington and Yale University.
Wounds
Full-thickness excisional wounds were made in the dorsal region of mice anesthetized with Avertin, as described previously (Kyriakides et al., 1999). Each mouse received two 6-mm wounds with the aid of a biopsy punch (Acuderm, Ft Lauderdale, FL), giving a total of 16 wounds per time point. All wounds were excised with a 3 mm rim of unwounded tissue, and 10 wounds were processed for histology and 6 for protein extraction. Wound reepithelialization was estimated in a blind fashion from H&E-stained sections and judged to be complete when the entire wound surface was covered with a new epithelial layer. Reepithelialization was quantified by measuring both the migration of epithelium from the wound edges and the gap between migrating ends. Percent re-epithelialization was determined by adding all three values (left and right migrating ends plus the gap) to obtain the total length and expressed as the percent of the total occupied by the migrating ends. 10 sections per wound, and 5 wounds per time point per genotype were examined. For the generation of wound extracts, a 3-mm biopsy punch was used to collect wound tissue from the center of each wound. Six d 7 and d 14 wounds per genotype were processed for analysis by transmission electron microscopy. For tensile strength analysis, two full-thickness longitudinal incisions, each 1.0 cm in length and separated by 1.5 cm, were made on the dorsal skin of each mouse with the aid of a standard #10 surgical blade. The first wound was administered on d 0 and the second on d 7. A total of 8 wounds per time point per genotype were made. All d 7 and d 14 wounds were collected on the same day and analyzed by tensiometry.
Tissue Processing and Immunohistochemistry
Excised wounds were fixed in 10 % zinc-buffered formalin (Z-fix, Anatech, Battle Creek, MI) and embedded in paraffin. 5 μm-thick sections were generated and stained with anti-PECAM-1 to visualize blood vessels and Mac3 to detect macrophages, as described previously (Kyriakides et al., 1999). Proliferating cells were detected with the Ki-67 antibody (DAKO, Carpinteria, CA; 1:10). Fibrin(ogen) was detected with anti-fibrinogen antibody (Accurate Chemical and Scientific Corp. Westbury, NY; 1:200). A Nikon Eclipse 800 microscope was used for all examinations. For TEM, samples were dehydrated in a graded series of ethanol, washed, and embedded in Epon. 100-nm sections were examined by a Technai Bio Twin EM microscope, equiped with a Morada camera.
Histomorphometry
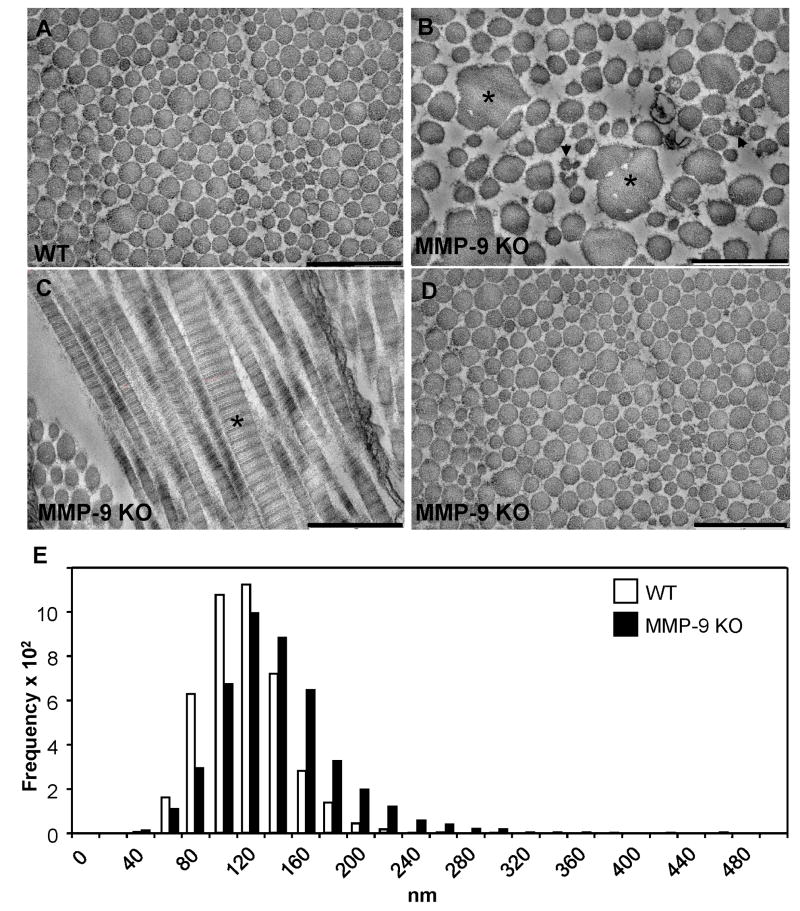
For quantification of angiogenesis, high power digital images from PECAM-1-stained sections were collected and analyzed with the aid of imaging software (Metamorph, Universal Imaging Co., Downingtown, PA), as described previously (Kyriakides et al., 2001). Briefly, the background immunoreaction levels in control slides were used to threshold images and PECAM-1-positive vessels were identified and quantified by the software. For quantification of macrophages, images from Mac-3-stained sections were analyzed as described previously (Agah et al. 2002). For determination of fibril diameters, collagen bundles in the wound area were imaged at 430,000x. Fibril diameters were analyzed quantitatively using Metamorph image analysis software (n=1198 WT fibrils, n=1337 KO fibrils).
Analysis of wound extracts
Protein extracts from d 0, 5, 7, 10, and 14 wounds were prepared in extraction buffer (phosphate-buffered saline containing 2 mM phenylmethyl sulfonyl fluoride and 1 mg/ml each of aprotinin, leupeptin, and pepstatin (Sigma Chemical Co., St Louis, MO) as described previously (Agah et al., 2002). The protein content of each sample was determined by the BCA assay (Bio-Rad, Hercules, CA). Zymograms were performed as described previously (Krady et al., 2008). A total of three samples per time point per genotype were analyzed and the results were repeated in triplicate. Soluble VEGF was prepared by gentle extraction of wound tissues in extraction buffer at 4°C for 30 min. Western blot analysis was performed on pooled samples with anti-VEGF antibody at 0.2 μg/ml (Abcam, ab9953).
Tensile Strength Analysis
Intact and wounded skin was subjected to tensile strength analysis. For intact skin, a dumbbell shaped pattern 1.5 cm long and 0.5 cm wide in the middle was used to excise a segment of skin. A total of 6 samples per genotype were analyzed. Wounds, two per animal, were generated by a full-thickness longitudinal incision, 0.5 cm in length with the aid of a standard #10 surgical blade. The first wound was produced on d 0 and the second on d 7. Wounds were allowed to heal naturally for 7 or 14 days without any stitches or staples. Wounds were removed and samples were cut using a No. 10 scalpel blade and placed in moist gauze (1x PBS). A total of 8 wounds per time point per genotype were analyzed. All wounds were analyzed with the aid of an Instron 5848 MicroTester (Instron, Canton MA), as described previously (Wu et al., 2003).
Keratinocyte culture and wound migration assay
Human foreskin keratinocytes were isolated and cultured on tissue culture grade polystyrene as described previously, and used at passage four (Fleckman et al., 1987; Hitomi et al., 2003). Mouse skin keratinocytes were isolated from newborn WT and MMP-9-null pups and cultured on tissue culture grade polystyrene coated with collagen type IV as described previously (Pirrone et al., 2005). To evaluate wound migration in vitro, confluent keratinocytes cultures were scraped with the aid of a pipette tip in order to create a wound 0.3 mm in width. Migration of keratinocytes into the wound area was evaluated by photographic images collected at 12, 24, and 48 h post wounding. Specifically, migration was determined by measuring the area of the original wound occupied by migrating cells and expressed as percent closure. To control for the possible contribution of cell proliferation, the cultures were treated with mitomycin (10 μg/ml) for 3 h prior to wounding. Equal amounts (50 μg) of WT and MMP-9-null keratinocyte conditioned media were analyzed by zymography. Human keratinocyte migration was evaluated in the presence of 2.5 μg/ml function-blocking anti-human MMP-9 mouse antibody (Chemicon International; catalog number MAB13415) or 2.5 μg/ml isotype control mouse IgG. The function-blocking activity of the anti-MMP-9 antibody was demonstrated previously (Visscher et al., 1994).
Statistical Analysis
All data are expressed as means ± SEM. Statistical differences were determined by either Student’s t test or one or two-way analysis of variance followed by Bonferroni post hoc test. A value of p < 0.05 was considered significant.
Results
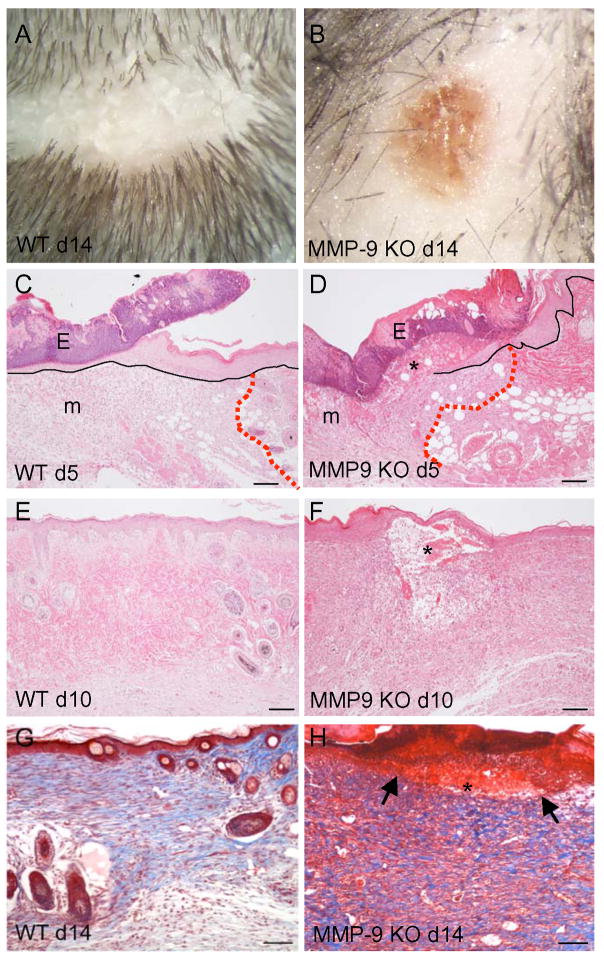
Gross examination of healing excisional wounds indicated that wound closure was delayed in MMP-9-null mice. Specifically, MMP-9-null wounds remained open longer and resolved at a rate slower than WT wounds, as evidenced by the persistence of eschar at d 14 (Fig 1; Supplemental Fig. 1). Histological analysis of d 5–14 H&E-stained sections revealed insufficient reepithelialization and the prolonged presence of provisional matrix in MMP-9-null wounds (Fig. 1). Overall, the wound epithelial layer appeared thicker and displayed reduced migration over time. Specifically, reepithelialization, measured as percent wound coverage, was retarded in MMP-9-null wounds (Table 1). In addition, the presence of wound provisional matrix, containing fibrin clot and inflammatory cells, was evident in the neodermis. Detection of fibrin(ogen) in d 10 wounds by immunohistochemistry was suggestive of reduced fibrinolysis in MMP-9-null wounds (Supplemental Fig. 2). In contrast, d 10 WT wounds were fully reepithelialized and lacked provisional matrix. To confirm changes in matrix remodeling, d 14 wound sections were stained with Masson’s trichrome to visualize mature collagen fibers. MMP-9-null wounds were found to contain irregularly organized fibers (Fig. 1G, H), suggesting a defect in developing a network of mature collagen. However, it should be noted that MMP-9-null wounds appear to contain comparable amounts of collagen.
Figure 1. Delayed wound healing in MMP-9-null mice.
Two weeks after the creation of full-thickness excisional wounds, in contrast to WT (A), MMP-9-null mice retained a substantial part of the original eschar, which is associated with persistent redness (B). Representative images of H&E-stained sections from d 5 (C, D) and d 10 (E, F) wounds from WT (C, E) and MMP-9-null (D, F) mice are shown. MMP-9-null mice display persistence of a fibrin clot (*) and delayed reepithelialization; black lines indicate the epithelium and red dotted lines indicate the wound edge. Eschar (E) and provisional matrix (m) can be observed in both groups. Representative images of Masson’s trichrome-stained sections of d14 wounds from WT (G) and MMP-9-null (H) mice are shown. Irregular mature matrix deposition (blue color), persistence of the fibrin clot (*), and incomplete reepithelialization are evident in the MMP-9-null wound (arrows in H denote the edges of migrating epithelium). Bar = 100 μm (c-f), 50 μm (G, H).
Table 1.
Delayed wound re-epithelialization in MMP-9-null mice.
| WOUND CLOSURE AND PERCENT REEPITHELIALIZATION |
||
|---|---|---|
| DAY | WT | MMP-9-null |
| 5 | 2/5 (69 ± 40) | 0/5 (19 ± 11)* |
| 7 | 4/5 (87 ± 21) | 1/5 (47 ± 19)* |
| 10 | 5/5 (100 ± 0) | 2/5 (62 ± 17)* |
| 14 | 5/5 (100 ± 0) | 4/5 (90 ± 22) |
WT, wild type. Measurements were obtained from H&E-stained sections and expressed as percent re-epithelialization. The results are presented as average ± SEM.
≤ 0.05, n = 5 wounds.
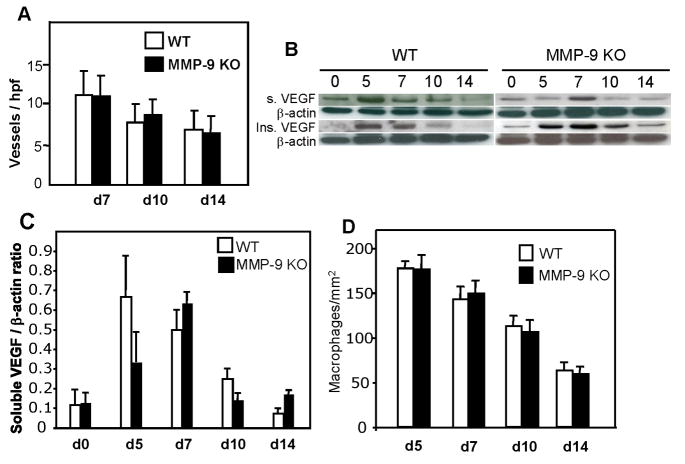
Histomorphometric analysis of wound neovascularization was performed following the immunohistochemical detection of endothelial cells with PECAM-1 antibody and showed no differences in blood vessel density between MMP-9-null and WT wounds (Fig. 2). Analysis of soluble and insoluble VEGF levels in wound extracts revealed overall similar levels between MMP-9-null and WT samples, except that the level of soluble VEGF peaked earlier in the latter. Specifically, the levels of soluble VEGF in WT wounds were high in early wounds (d 5–7) and decreased over time (Fig. 2). A similar pattern was observed in MMP-9-null wounds, except that levels peaked at d 7 instead of d 5. Densitometric analysis of western blots verified a trend for reduced soluble VEGF at d 5, however the difference was not significant (Fig. 2C). Insoluble VEGF levels were similar between MMP-9-null and WT wounds. Thus, the overall levels were similar between the two groups and matched the vascular density, which decreased progressively from d 7 to d 14. It should be noted however, that the somewhat reduced levels of VEGF in d 5 wounds might influence the delay in migration of keratinocytes in MMP-9-null wounds. Moreover, zymographic analysis of d 7 and d 10 wounds indicated comparable levels of MMP-2 between WT and MMP-9-null wounds suggesting the absence of compensatory MMP2 upregulation (Supplemental Figure 3).
Figure 2. Normal angiogenesis, VEGF levels, and macrophage content in MMP-9-null wounds.
Sections of wounds were stained with anti-PECAM1 antibodies and visualized with the peroxidase reaction. A. Results of vessel quantification for d 7, 10, and 14 wounds. A total of 50 sections per time point per genotype were analyzed. B. Representative Western blots of soluble (top panel) and insoluble VEGF (lower panel) in pooled samples from d 0, 5, 7, 10, and 14 wounds are shown. Immunodetection of β-actin is shown as loading control. The blots were repeated in triplicate with separate extracts and produced similar results. C. Densitometric analysis of western blots showing the ratio of soluble VEGF to β-actin. n = 3. D. Wounds from WT and MMP-9-null mice were stained with the Mac3 antibody and the number of macrophages was quantified from digital images obtained from d 5, 7, 10, and 14 wounds. A total of 50 images from five wounds per time point per genotype were analyzed.
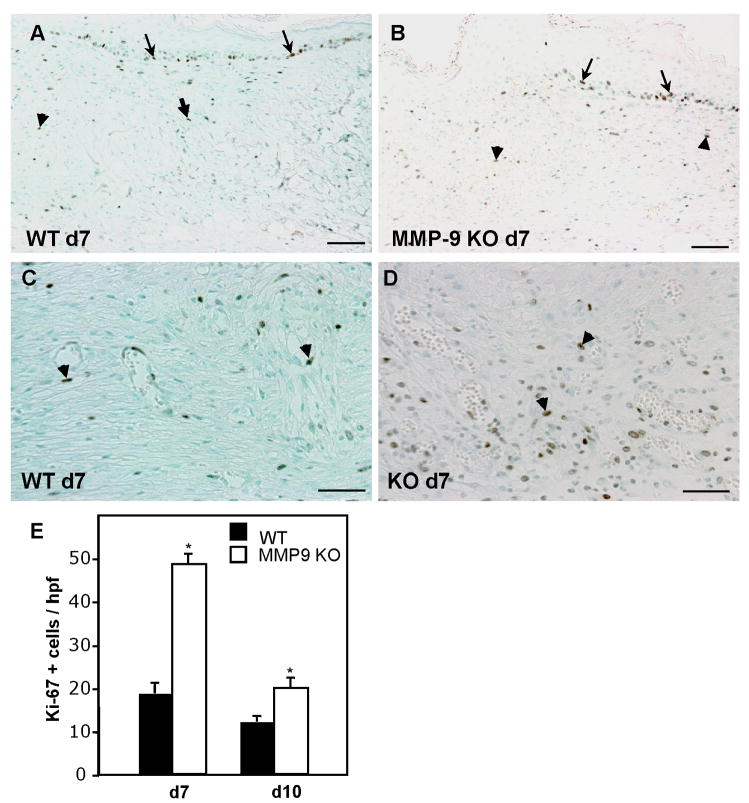
Similar to our capillary density findings, we detected normal macrophage infiltration and resolution of inflammation in both groups (Fig. 2D). It is possible that changes in the number of immature macrophages, negative for Mac-3, or other inflammatory cell types such as neutrophils, might be present. Nevertheless, our findings do not indicate an abnormal inflammatory response in MMP-9-null wounds. Previously, in a model of corneal epithelial regeneration, it was shown that MMP-9 deficiency was associated with increased cell replication (Mohan et al., 2002). Analysis of d 7 and d 10 wounds with the Ki-67 antibody, which is specific for proliferating cells, indicated normal keratinocyte proliferation in MMP-9-null wounds (Fig. 3). The majority of the Ki-67 positive cells were located at the trailing edge of the migrating epithelium. During examination of the stained sections we noticed the frequent presence of proliferating cells in the wound bed, especially in MMP-9-null wounds. Therefore, we enumerated the number of Ki-67 positive cells per high power field and found that it was elevated in MMP-9-null mice, especially at d 7, suggesting that MMP-9 limits the proliferation of cells during repair (Fig. 3E). Based on the morphology of the cells and association of some with vessels, we conclude that endothelial cells and fibroblasts comprised the majority of positive cells. Overall, the number of proliferating cells decreased from d 7 to d 10 in both WT and MMP-9-null wounds, indicating progression of the healing process.
Figure 3. Increased cell proliferation in MMP-9-null wounds.
Sections of wounds from d 7 (A–D) from WT (A, C) and MMP-9-null (B, D) mice were stained with the Ki-67 antibody and visualized with the peroxidase reaction. Representative low (A, B) and high (C, D) power imaged are shown. Arrows and arrowheads indicate proliferating keratinocytes and cells in the wound bed, respectively. E. Results of enumeration of Ki-67 positive cells for d 7 and d 10 wounds. A total of 50 sections per time point per genotype were analyzed. Error bars represent SEM. n = 6; * p value ≤ 0.01. Bar = 100 μm (c–f), 50 μm (g, h).
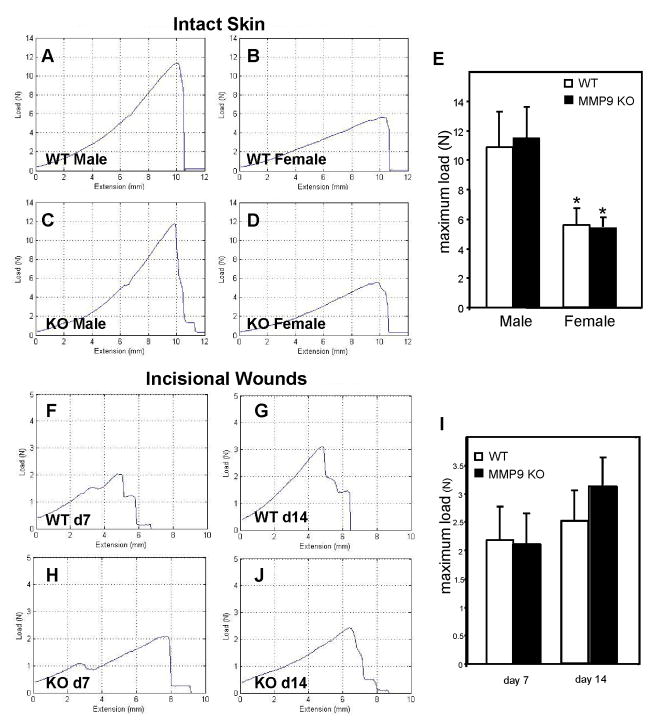
The delay in wound closure and collagen matrix assembly prompted us to examine the mechanical integrity of the wounds. Therefore, we performed tensiometric analysis of d7 and d14 incisional wounds. In addition, we examined the tensile strength of uninjured skin in order to obtain baseline values. Analysis of normal skin revealed no differences between WT and MMP-9-null mice (Fig. 4, A–D). However, differences in the strength of the uninjured skin between male (Fig. 4A, C) and female (Fig. 4B, D) mice were detected in both genotypes and confirmed by statistical analysis (Fig. 4E). Based on this observation, we opted to analyze the strength of healing wounds from male mice. Consistent with previous wound healing studies, we observed a decrease in breaking strength of both d 7 (Fig. F, H) and d 14 (G, H) wild type and MMP-9-null wounds in comparison to uninjured skin. In addition, both groups displayed an increase in breaking strength between d 7 and d 14, which is indicative of healing (Fig. 4I).
Figure 4. MMP-9-null mice recover normal tensile strength following wounding.
Samples of WT and MMP-9-null normal skin from male and female 3 month-old mice were assessed for tensile strength with an Instron tensiometer. Representative load-extension curves of normal skin from WT (A, male; B, female) and MMP-9-null (C, male; D, female) mice are shown. Strength of healing incisional wounds was determined in male mice. Representative curves from d 7 (F, H) and d 14 (G, J) wounds from WT (F, G) and MMP-9-null (H, J) mice are shown. Quantification of breaking strength of uninjured skin (E) and healing wounds (I) is shown. No differences between MMP-9-null and WT mice were detected. Error bars represent SEM (n = 10).
Based on our histological analysis of healing MMP-9-null wounds, we found the lack of abnormalities in tensile strength surprising. To probe this finding in greater depth, we performed ultrastructural analysis of d 14 wounds by TEM. Consistent with our histological findings, we detected extensive areas in MMP-9-null wounds that contained abnormal collagen fibrils. Specifically, we observed many abnormally large fibrils as well as numerous fibrils with irregular contours (Fig. 5B, C). In addition, MMP-9-null fibrils possessed irregular edges evidenced by the presence of fine material surrounding each fibril. Furthermore, we observed the presence of abnormally large fibrils in areas of apparently normal collagen fibrillogenesis (Fig. 5C). No abnormalities were observed in the areas of skin surrounding wounds in MMP-9-null mice (Fig. 5D). Analysis of the distribution of fibril diameters revealed a shift towards larger diameter in the MMP-9-null wounds, with the majority of the fibrils being in the 100–200 nm range (median 122.32 ± 46 nm). In contrast, the fibril diameter distribution in WT wounds was less spread and predominantly smaller, in the 80–140 nm range (median 103.38 ± 30.1 nm). In addition, fibrils in MMP-9 wounds reached diameters over 450 nm, whereas fibrils in WT wounds reached maximum diameters of 325 nm (Fig. 5E).
Figure 5. Abnormal collagen fibrillogenesis during wound healing in MMP-9 KO mice.
Electron micrographs of collagen fibrils in d 14 wounded dermis from WT (A) and MMP-9-null (B) mice. The presence of larger (*) and normal size (arrowheads) fibrils with irregular contours in the wound from the mutant animal, in comparison with the more uniformly-sized circular fibrils in the control tissue, is evident. Bar, 500 nm (A–D). C. Longitudinal section of collagen fibrils from a MMP-9 KO wound displaying extremely thick fibrils (*). D. Normal fibrils in non-injured MMP-9-null skin. E. Distribution of collagen fibril diameters, as measured from electron micrographs of skin wounds from WT (white bars) and MMP-9-null (black bars) mice. The distribution is skewed in the direction of larger fibrils in the mutant tissue. n = 1000.
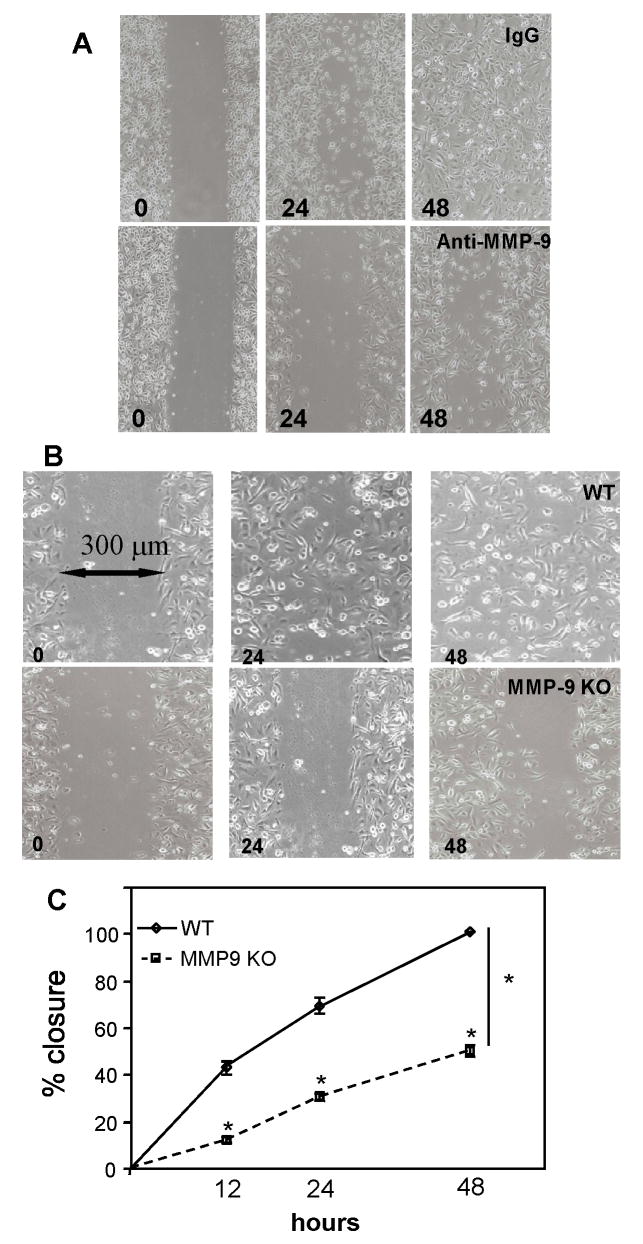
A previous study of corneal wound healing in MMP-9-null mice identified a role for MMP-9 in delaying wound closure by controlling the replication of epithelial cells (Mohan et al., 2002). In order to expand on this study and to investigate the delayed wound closure phenotype in the MMP-9-null skin wounds, we utilized an established wound migration in vitro assay. To probe for participation of MMP-9 in the process of migration, we performed our studies in the presence of mitomycin, an inhibitor of proliferation. First, we examined the ability of human keratinocytes to migrate in response to a wound in the presence of a function-blocking MMP-9 antibody or isotype control. Evaluation of wound closure at 24, and 48 h indicated that it was significantly delayed in the presence of the anti-MMP-9 antibody (Fig. 6A). Determination of the wound area, measured as percent area covered by cells, at 24 and 48 hr revealed a significant delay in the anti-MMP-9 treatment group (24 hr: 65 ± 12 % for IgG and 28 ± 7 % for anti-MMP-9; 48hr: 100 ± 1 % and 47 ± 6 % for anti-MMP-9; p value ≤ 0.01). Second, to examine the possibility that MMP-9-null keratinocytes have an intrinsic migration defect, we isolated neonatal MMP-9-null and WT skin keratinocytes and subjected them to the same assay. Consistent with our finding with human cells, we detected a profound wound migration defect in MMP-9-null keratinocytes (Fig. 6B). Determination of the wound area, measured as percent area covered by cells, at 12, 24, and 48 h indicated reduced migration of MMP-9-null keratinocytes (Fig. 6C). Analysis of conditioned media from WT and MMP-9-null keratinocytes revealed comparable levels of MMP-2 (Supplemental Figure 3). Thus, we conclude that in addition to changes in fibrin clot resolution, wound maturation, and collagen network formation, a defect in keratinocyte migration contributes to the delayed healing of MMP-9-null wounds.
Figure 6. MMP-9 is critical for keratinocyte wound migration in vitro.
A. Human keratinocyte monolayer cultures were wounded and allowed to heal in the presence of a function-blocking anti-MMP-9 antibody. Representative images from cultures treated with isotype control antibody (top row) or anti-MMP-9 antibody (bottom row) at 24 and 48 hr show the retarded healing in the latter. B. Representative images of mouse keratinocytes from WT (top row) and MMP-9 KO (bottom row) mice subjected to the same scratch assay at 0, 24, and 48 hr are shown. C. Measurement of migration, expressed as percent closure, indicated a defect in MMP-9 KO keratinocytes. Triplicate wells were used and the experiment was repeated three times. n = 6; * p value ≤ 0.01.
Discussion
We have performed a detailed analysis of the skin wound healing response in MMP-9-null mice with a focus on inflammation, matrix remodeling, angiogenesis, and reepithelialization. Gross examination of healing wounds indicated that healing was impaired in MMP-9-null mice due to a delay in wound closure. Despite the well-established role of MMP-9 in monocyte recruitment, we did not observe a reduction in the accumulation of macrophages in the MMP-9-null wounds. In addition, we did not detect changes in vascular density despite somewhat reduced levels of soluble VEGF in d-5 MMP-9-null wound extracts. Previously, MMP2 and/or MMP-9 were shown to release matrix-bound VEGF (Belotti et al., 2003; Bergers et al., 2000; Lee et al., 2005). Conceivably, other MMPs might be able to compensate for the loss of MMP-9 in this model. Consistent with this hypothesis, we have previously shown high levels of MMP2 in healing wounds (Agah et al., 2004). Therefore, we believe that conditions exist within the MMP-9-null wounds that allow for the proper progression of the inflammatory and angiogenic responses.
To identify processes that might be compromised in MMP-9-null mice, we investigated ECM deposition and remodeling, and reepithelialization. Based on our histological and TEM analyses we conclude that MMP-9 is critical for the deposition and remodeling of wound ECM. One of the major defects of MMP-9-null wounds is the prolonged presence of fibrin clots and provisional matrix. This observation is consistent with the findings in the healing corneas of MMP-9-null mice, where it was suggested that MMP-9 can influence clot removal directly by cleaving fibrinogen, or indirectly, by activating plasminogen activator and inactivating inhibitors of fibrinolysis such as PAI-1 (Mohan et al., 2002). In addition to the persistent fibrin clots, MMP-9-null wounds displayed abnormalities in collagen fiber assembly, and ultrastructural analysis revealed the presence of numerous extremely large collagen fibrils as well as some that were irregularly-shaped.
Fibril formation in wounds is an active process involving primarily types I and type III collagens, but the identity of regulatory molecules remains unclear (White et al., 2002). A role for MMP-9 in collagen synthesis has been suggested by studies in vitro with MMP-9-null smooth muscle cells, however there is no direct evidence for participation of MMP-9 in collagen fibril assembly (Sung et al., 2005). It is possible that loss of MMP-9 affects the ability of wound fibroblasts to organize the emerging collagen network. Supporting evidence for the participation of MMPs in fibrillogenesis can be found in the study of TSP2-null mice, in which increases in the levels of MMP-2 and MMP-9 are associated with aberrant collagen fibril formation (Bornstein et al., 2000). A common thread in these animal models is the presence of an imbalance in MMP levels leading to altered ECM assembly and remodeling. Ongoing studies with MMP-9-null fibroblasts should allow us to address this issue.
Previously, the deficiency in MMP-9 was shown to allow accelerated wound healing of injured corneas associated with the loss of MMP-9-mediated control of cell replication and inflammation (Mohan et al., 2002). Thus, both processes were enhanced in MMP-9-null mice via a delay in signal transduction through Smad2. Here, we show that MMP-9 deficiency is associated with increased proliferation of cells within the wound bed but not of keratinocytes. Our findings suggest that the participation of MMP-9 in wound healing of skin differs from that in cornea. Unlike injured corneas, skin wounds undergo significant contraction, an event that has profound effects on the healing process. Thus, MMP-9 deficiency may hinder repair in different tissue-specific ways. In the same study it was reported that skin wound closure was not compromised in these mice in the first two weeks following injury. We believe that the difference between this study and our findings is due to the fact that we measured reepithelialization, a more precise indicator of wound closure. In addition, we did not cover the wounds with dressing, which might have complicated the superficial examination of wound areas. We have found that determination of wound areas by gross measurements can be influenced by the presence of eschar and site-specific contraction.
Surprisingly, despite the presence of abnormally shaped collagen fibrils, the tensile strength of MMP-9-null wounds was not compromised. This finding could be due to the presence of large collagen fibrils that might provide mechanical compensation. However, there are conflicting reports regarding the relationship between fibril shape and diameter and tensile strength (Bradshaw et al., 2003; Christiansen et al., 2000; Lavagnino et al., 2005). In addition, the overall strength of healed wounds at 1 and 2 weeks is a small fraction of that of intact skin, and therefore it might be difficult to detect a minor defect. Alterations in ECM remodeling are also indicated by the prolonged presence of the wound fibrin clot and provisional matrix in MMP-9-null wounds. Fibrin is a substrate for MMP-9 and the deficiency of this protease could impair the removal and remodeling of the clot into provisional matrix (Sternlicht and Werb, 2001). Such a delay could influence keratinocyte migration and compromise reepithelialization. Wound keratinocyte migration is a coordinated process that involves alteration of keratinocyte phenotype and changes in the expression of adhesion receptors in order to facilitate interactions with the provisional matrix (Goldfinger et al., 1998; Kirfel and Herzog, 2004; Pilcher et al., 1997; Raja et al., 2007; Santoro and Gaudino, 2005). In addition, the activity of MMPs results in degradation and modulation of ECM proteins, leading to increased keratinocyte migration. In fact, integrin-mediated enhancement of MMP-9 secretion has been shown to increase migration of oral keratinocytes (Thomas et al., 2001). Moreover, the regulation of MMP-9 gene expression in human keratinocytes has been shown to be associated with keratinization (Kobayashi et al., 2001).
Previous studies have shown that wound keratinocytes can secrete VEGF and express its receptor, VEGF-R1, and that the latter plays a role in wound reepithelialization (Brown et al., 1992; Wilgus et al., 2005). The predominant effect of VEGF in this model was to influence keratinocyte proliferation. Based on the modest change in soluble VEGF levels in MMP-9-null wounds we conclude that it does not play a major role in influencing the phenotype. Furthermore, our in vitro migration assays were performed in the presence of mitomycin and thus, could not be influenced by changes in cell proliferation. The equal presence of Ki-67 positive keratinocytes in WT and MMP-9-null wounds provides additional support for the lack of a proliferation defect. In terms of matrix-related effects, the analysis of human keratinocytes was performed in the absence of exogenous matrix proteins. Mouse keratinocyte migration was evaluated on surfaces coated with collagen type IV. In both cases, we observed reduced migration when MMP-9 was blocked or absent. Moreover, we have found similar levels of MMP-2 in WT and MMP-9-null keratinocytes, suggesting that the migration defect cannot be compensated by MMP-2. Our findings are consistent with a recent report demonstrating impaired migration in human keratinocytes treated with MMP-9 siRNA (Xue and Jackson, 2008). Moreover, similar to the latter, we detected a very low secretion of MMP-2 in keratinocytes. Taken together, these observations suggest an inherent migration defect in MMP-9-null keratinocytes.
Our study suggests that MMP-9 plays a complex role in wound healing. This functional complexity is consistent with its ability to proteolytically cleave matrix components, growth factors and cytokines, and to contribute to the shedding of cell surface-associated proteins (Page-McCaw et al., 2007; Sternlicht and Werb, 2001). We propose that the MMP-9-null wound healing phenotype is predominantly influenced by the inability of these mice to remodel the provisional matrix and to properly assemble collagen fibers, and by a defect in keratinocyte migration. Subsequent studies should allow us to identify the spatiotemporal context in which these activities are critical and determine their relative contributions to healing.
Supplementary Material
Acknowledgments
We thank Drs. Michael Reidy and Joseph Madri for helpful discussions and Jianmin Zeng and Michael Michaud for technical assistance. This work was supported by National Institute of Health grant GM 072194-01 (to T.R.K.), HL47328 (M.R.S), AR45418 (to P.B), the Odland Endowed Research Fund (to P.F.), and the Alan A. and Edith L. Wolff Charitable Trust/Barnes-Jewish Hospital Foundation (to R.M.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161:831–839. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agah A, Kyriakides TR, Letrondo N, Bjorkblom B, Bornstein P. Thrombospondin 2 levels are increased in aged mice: consequences for cutaneous wound healing and angiogenesis. Matrix Biol. 2004;22:539–547. doi: 10.1016/j.matbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Agren MS, Mirastschijski U, Karlsmark T, Saarialho-Kere UK. Topical synthetic inhibitor of matrix metalloproteinases delays epidermal regeneration of human wounds. Exp Dermatol. 2001;10:337–348. doi: 10.1034/j.1600-0625.2001.100506.x. [DOI] [PubMed] [Google Scholar]
- Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224–5229. [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P, Kyriakides TR, Yang Z, Armstrong LC, Birk DE. Thrombospondin 2 modulates collagen fibrillogenesis and angiogenesis. J Investig Dermatol Symp Proc. 2000;5:61–66. doi: 10.1046/j.1087-0024.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Helene Sage E. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol. 2003;120:949–955. doi: 10.1046/j.1523-1747.2003.12241.x. [DOI] [PubMed] [Google Scholar]
- Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen DL, Huang EK, Silver FH. Assembly of type I collagen: fusion of fibril subunits and the influence of fibril diameter on mechanical properties. Matrix Biol. 2000;19:409–420. doi: 10.1016/s0945-053x(00)00089-5. [DOI] [PubMed] [Google Scholar]
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- Fleckman P, Holbrook KA, Dale BA, Sybert VP. Keratinocytes cultured from subjects with ichthyosis vulgaris are phenotypically abnormal. J Invest Dermatol. 1987;88:640–645. doi: 10.1111/1523-1747.ep12470251. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, Stack MS, Jones JC. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Hitomi K, Presland RB, Nakayama T, Fleckman P, Dale BA, Maki M. Analysis of epidermal-type transglutaminase (transglutaminase 3) in human stratified epithelia and cultured keratinocytes using monoclonal antibodies. Journal of dermatological science. 2003;32:95–103. doi: 10.1016/s0923-1811(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Kirfel G, Herzog V. Migration of epidermal keratinocytes: mechanisms, regulation, and biological significance. Protoplasma. 2004;223:67–78. doi: 10.1007/s00709-003-0031-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kishimoto J, Ge Y, Jin W, Hudson DL, Ouahes N, Ehama R, Shinkai H, Burgeson RE. A novel mechanism of matrix metalloproteinase-9 gene expression implies a role for keratinization. EMBO reports. 2001;2:604–608. doi: 10.1093/embo-reports/kve129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krady MM, Zeng J, Yu J, Maclauchlan S, Skokos EA, Tian W, Bornstein P, Sessa WC, Kyriakides TR. Thrombospondin-2 Modulates Extracellular Matrix Remodeling during Physiological Angiogenesis. Am J Pathol. 2008;173:879–891. doi: 10.2353/ajpath.2008.080128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides TR, Tam JW, Bornstein P. Accelerated wound healing in mice with a disruption of the thrombospondin 2 gene. J Invest Dermatol. 1999;113:782–787. doi: 10.1046/j.1523-1747.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Zhu YH, Yang Z, Huynh G, Bornstein P. Altered extracellular matrix remodeling and angiogenesis in sponge granulomas of thrombospondin 2-null mice. Am J Pathol. 2001;159:1255–1262. doi: 10.1016/S0002-9440(10)62512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavagnino M, Arnoczky SP, Frank K, Tian T. Collagen fibril diameter distribution does not reflect changes in the mechanical properties of in vitro stress-deprived tendons. Journal of biomechanics. 2005;38:69–75. doi: 10.1016/j.jbiomech.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM, Jr, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. American journal of physiology. 2006;290:H232–239. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- Mirastschijski U, Haaksma CJ, Tomasek JJ, Agren MS. Matrix metalloproteinase inhibitor GM 6001 attenuates keratinocyte migration, contraction and myofibroblast formation in skin wounds. Exp Cell Res. 2004;299:465–475. doi: 10.1016/j.yexcr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA, Lee Y, McCarthy BE, Wollenberg KR, Jester JV, Wang M, Welgus HG, Shipley JM, Senior RM, Fini ME. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem. 2002;277:2065–2072. doi: 10.1074/jbc.M107611200. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nature reviews. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol. 1997;137:1445–1457. doi: 10.1083/jcb.137.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrone A, Hager B, Fleckman P. Methods in molecular biology. Vol. 289. Clifton, N.J: 2005. Primary mouse keratinocyte culture; pp. 3–14. [DOI] [PubMed] [Google Scholar]
- Raja Sivamani K, Garcia MS, Isseroff RR. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front Biosci. 2007;12:2849–2868. doi: 10.2741/2277. [DOI] [PubMed] [Google Scholar]
- Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005;304:274–286. doi: 10.1016/j.yexcr.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung HJ, Johnson CE, Lessner SM, Magid R, Drury DN, Galis ZS. Matrix metalloproteinase 9 facilitates collagen remodeling and angiogenesis for vascular constructs. Tissue engineering. 2005;11:267–276. doi: 10.1089/ten.2005.11.267. [DOI] [PubMed] [Google Scholar]
- Thomas GJ, Poomsawat S, Lewis MP, Hart IR, Speight PM, Marshall JF. alpha v beta 6 Integrin upregulates matrix metalloproteinase 9 and promotes migration of normal oral keratinocytes. J Invest Dermatol. 2001;116:898–904. doi: 10.1046/j.1523-1747.2001.01352.x. [DOI] [PubMed] [Google Scholar]
- Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- Visscher DW, Hoyhtya M, Ottosen SK, Liang CM, Sarkar FH, Crissman JD, Fridman R. Enhanced expression of tissue inhibitor of metalloproteinase-2 (TIMP-2) in the stroma of breast carcinomas correlates with tumor recurrence. International journal of cancer. 1994;59:339–344. doi: 10.1002/ijc.2910590308. [DOI] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JF, Werkmeister JA, Darby IA, Bisucci T, Birk DE, Ramshaw JA. Collagen fibril formation in a wound healing model. Journal of structural biology. 2002;137:23–30. doi: 10.1006/jsbi.2002.4460. [DOI] [PubMed] [Google Scholar]
- Wilgus TA, Matthies AM, Radek KA, Dovi JV, Burns AL, Shankar R, DiPietro LA. Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes. Am J Pathol. 2005;167:1257–1266. doi: 10.1016/S0002-9440(10)61213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Jansen ED, Davidson JM. Comparison of mouse matrix metalloproteinase 13 expression in free-electron laser and scalpel incisions during wound healing. J Invest Dermatol. 2003;121:926–932. doi: 10.1046/j.1523-1747.2003.12497.x. [DOI] [PubMed] [Google Scholar]
- Xue M, Jackson CJ. Autocrine Actions of Matrix Metalloproteinase (MMP)-2 Counter the Effects of MMP-9 to Promote Survival and Prevent Terminal Differentiation of Cultured Human Keratinocytes. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.