Abstract
The neuropeptide galanin has been shown to play a role in psychiatric disorders as well as in other biological processes including regulation of pain threshold through interactions with three G-protein coupled receptors, GalR1 - 3. While most of the pharmacological studies on galanin in stress-related disorders have been done with rats, the continuous development of genetically engineered mice involving galanin or its receptor subtype(s) validates the importance of mouse pharmacological studies. The present study on mice examined the homeostatic, endocrinological and neuroanatomical effects of the galanin, injected intracerebroventricularly (icv), in regulation of stress responses after restraint stress. Furthermore, the roles of galanin receptor subtype 1 (GalR1) on these effects were studied using GalR1 knockout (KO) mice. The core body temperature and the locomotor activity were monitored with radio telemetry devices. Galanin (icv) decreased locomotor activity and exerted a bidirectional effect on the restraint stress-induced hyperthermia; a high dose of galanin significantly attenuated the stress-induced hyperthermic response, while a low dose of galanin moderately enhanced this response. The bidirectional effect of galanin was correlated with changes in stress hormone levels (adrenocorticotropic hormone and corticosterone). To neuroanatomically localize the effects of galanin on stress response, cFos immunoreactivity was assessed in galanin receptor rich areas; paraventricular nucleus (PVN) of the hypothalamus and the locus coeruleus (LC), respectively. A high dose of galanin significantly induced cFos activity in the LC but not in the PVN. In GalR1KO mice, a high dose of galanin failed to induce any of the above effects, suggesting the pivotal role of GalR1 in decreased locomotor activity and stress-resistant effects caused by galanin icv injection studied here.
Keywords: anxiety, hyperthermia, restraint stress, temperature, GPCR, neuropeptide
Augmented stress responses have been implicated in the etiology of many somatic as well as psychiatric disorders including anxiety and depression. Stress exposure is known to increase body temperature rapidly, which is referred to as stress-induced hyperthermia (SIH). SIH is time-dependent with a rapid rise and relatively long return phase; approximately 60 min to return to the basal temperature (Van der Heyden et al., 1997). The SIH response is correlated with, among others, the elevation of two stress hormones, adrenocorticotropic hormone (ACTH) and corticosterone (Groenink et al., 1994, Groenink et al., 1995, Spooren et al., 2002), that are the key players in the hypothalamic-pituitary-adrenal (HPA) axis (Tsigos and Chrousos, 2002). SIH phenomenon commonly occurs across a large variety of species (Adriaan Bouwknecht et al., 2007) and is mediated by activation of the autonomic nervous system. Persistent autonomic hyperactivity is regarded as one of the diagnostic markers of generalized anxiety by the Diagnostic and Statistical Manual (DSM-IV) produced by the American Psychiatric Association, and indeed the SIH paradigm is a valid and well-known tool to identify drug candidates for the treatment of anxiety (Spooren et al., 2002). Since current pharmacological treatments for anxiety and depression are not totally effective in a significant proportion of patients, a great deal of effort has been therefore directed to understand the molecular basis of these disorders in the hope to find a more efficacious treatment with fewer side-effects.
Galanin, a 29 amino-acid (30 in humans) neuropeptide (Tatemoto et al., 1983), has been indicated to play a role in stress-related disorders (Weiss et al., 1998, Holmes and Picciotto, 2006, Karlsson and Holmes, 2006, Wrenn and Holmes, 2006, Lu et al., 2007, Kuteeva et al., 2008a, Kuteeva et al., 2008b, Mitsukawa et al., 2008) as well as in numerous other biological and physiological functions such as learning and memory, seizure, pain and feeding (Wrenn and Crawley, 2001, Abramov et al., 2004, Walton et al., 2006, Counts et al., 2008, Crawley, 2008, Lerner et al., 2008, Xu et al., 2008), through actions at three G-protein coupled receptors, GalR1 – 3 (Branchek et al., 2000). Galanin and galanin receptor subtypes are abundantly expressed in the brain regions that are critical for the stress responses including the PVN and the LC (Skofitsch and Jacobowitz, 1985, O'Donnell et al., 1999, Jungnickel and Gundlach, 2005). In the rodent brain, over 80 % of the norepinephrine neurons in the LC and 60 % of serotonergic neurons in the dorsal raphe nucleus (Melander et al., 1986, Holets et al., 1988, Xu and Hokfelt, 1997, Xu et al., 1998, Lang et al., 2007, Sharkey et al., 2008) have galanin-like immunoreactivity. To date, most of the pharmacological studies on the role of galanin in stress-related behaviors (Karlsson and Holmes, 2006, Wrenn and Holmes, 2006, Kuteeva et al., 2008b) have been conducted on rats. However, due to the lack of galanin receptor subtype selective pharmacological tools except recently introduced GalR3 selective antagonists (Swanson et al., 2005, Barr et al., 2006), transgenic mice carrying gene mutation of galanin or its receptor subtype(s) have been developed to gain a better understanding of the galaninergic system and it is critical to confirm the pharmacological effects of galanin in mice. Therefore despite many studies on the role of galanin in stress-related behaviors, the role of galanin and the galanin receptor subtypes involved in stress responses have not been entirely clear. Recently GalR2 agonists were suggested as putative antidepressant agents (Lu et al., 2005a, Kuteeva et al., 2008b) and GalR3 has been suggested as a putative target for the development of antidepressants and anxiolytics by use of novel GalR3 antagonists (Swanson et al., 2005, Barr et al., 2006).
In the present study, the effects of galanin on stress responses are evaluated at different levels. We monitored the effects of galanin (icv) on restraint stress-induced hyperthermia and measured ACTH and corticosterone levels. In addition, to neuroanatomicaly investigate the effects of galanin on restraint stress, cFos immunoreactivity as a neuronal activity marker was assessed in the PVN and the LC. Moreover, the use of GalR1KO mice enabled us to delineate the role of this galanin receptor subtype in the effects of galanin on restraint stress.
Materials and Methods
Animals
GalR1KO mice were generated as described previously (Jacoby et al., 2002, Blakeman et al., 2003, Fetissov et al., 2003, Grass et al., 2003, Holmes et al., 2003, Zorrilla et al., 2007). Age-matched, adult (11 - 16 weeks old of age) male GalR1KO mice backcrossed for at least 7 generations onto a C57BL/6J background, their wild-type (WT) littermates and C57BL/6J mice (The Jackson Laboratory) were given ad libitum access to food and water. The mice were housed separately and maintained on a 12 h light/dark cycle (lights on at 6:00). All procedures adhered to the National Institutes of Health Guide for the Care and Use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of the Scripps Research Institute.
Stereotaxic operations and telemetry device implantation
The mice were anesthetized with isoflorane (induction, 3–5%; maintenance, 0.9–1.5%) and placed in a stereotaxic frame. A stainless steel guide cannula (24 gauge, 7 mm long; Plastics One, Roanoke, VA, USA) was implanted into the right lateral ventricle (0.3 mm posterior to bregma, 1 mm lateral to the mid-sagittal line and 2.3 mm ventral to the surface of the skull) and anchored to the skull with a 1.6 mm stainless steel screw (Plastics One, Roanoke, VA, USA) and dental cement. A 31 gauge dummy cannula (Plastics One, Roanoke, VA, USA) was kept inside the guide cannula when not in use. The mice used in restraint stress-induced hyperthermia test were implanted with radio telemetry devices (TA10TA-F20; Data Sciences, Inc., St. Paul, MN, USA) into the peritoneal cavity just before the intracerebroventricular (icv) cannulation surgery.
Measurement of core body temperature and locomotor activity and injection
Mice were individually housed and allowed to recover for at least one week from the surgery. Mice were subjected to daily handling and mock injection for 3 days before the start of stress paradigm and the injection of galanin or its vehicle. Temperature and locomotor activity, transmitted via digital telemetry devices, were recorded with the RPC-1 receivers (Data Sciences, Inc., St. Paul, MN, USA) and forwarded to the Dataquest A.R.T.™ (Data Sciences, Inc., St. Paul, MN, USA). Temperature and locomotor activity from the radio telemetry devices were sampled at a 5 min interval. Galanin (Biopeptide CO., LLC, San Diego, CA, USA) or the vehicle (artificial cerebrospinal fluid: ACSF) was injected i.c.v via cannula, at a volume of 3 μl, over 4 min.
Restraint stress and blood sample collection for basal and stress-induced conditions
In order to investigate the effects of galanin on stress-induced conditions, mice were placed in an adjustable restraining device one hour after the injection of either galanin or its vehicle (ACSF). Then, after 40 min period of restraint stress exposure, mice were deeply and quickly anesthetized and the blood was collected through the right atrium very quickly in an adjacent room between 13:00 and 15:00.
Transcardial perfusion and Tissue slice preparation for immunohistochemistry
After the mice were deeply anesthetized and blood samples were collected, they were transcardially perfused with 0.1 M phosphate buffer (PB) followed by freshly prepared 4 % paraformaldehyde (PFA) in 0.1 M PB. Brains were post-fixed in 4 % PFA overnight and subsequently cryoprotected with 30 % sucrose in 0.1 M PB for 24 - 48 hours at 4 °C. Serial 35 μm coronal sections were cut from each brain with a cryostat (Leica Microsystems, Bannockburn, IL, USA)
Hormone measurement
Plasma corticosterone and ACTH concentrations were measured using commercially available radioimmunoassay kits (MP Biomedicals, Costa Mesa, CA, USA). The inter- and intra-assay coefficients of variability for ACTH and corticosterone were similar to the manufacturer's reported values.
Immunohistochemistry
Sections were pre-incubated with 0.1 M phosphate-buffered saline (PBS) containing 1 % bovine serum albumin (BSA) and 0.2 % Triton X-100 for 30 min, followed by the wash with 0.1 M PBS containing 0.5 % BSA. Then slices were incubated overnight with primary antibody (rabbit anti-cFos, 1: 5000; Santa Cruz, Santa Cruz, CA, USA) in 0.1 M PBS containing 0.5 % BSA and 0.05 % sodium azide. After rinsing with 0.1 M PBS, slices were incubated in the secondary antibody (biotinylated anti-rabbit, made in goat, 1:500; Vector laboratories, Burlingame, CA, USA) in 0.1 M PBS containing 0.5 % BSA for an hour. Then after the wash in 0.1 M PBS, they were placed in avidin-biotin-peroxidase complex (ABC kit; Vector laboratories, Burlingame, CA, USA) for one hour, followed by the diaminobenzine-nickel (DAB-Ni) reaction (0.25 mg/ml DAB, 0.009 % H2O2, 0.04 % NiCl2 in 0.1 M PB) for 5 min. All sections were mounted in 0.1 M PB on slide glasses and after the dehydration they were examined under the light microscope. For each animal, the counts of cFos positive neurons per brain regions were determined bilaterally. Brain regions were identified by using the atlas of Franklin & Paxinos (1997): PVN, bregma −0.58 to −1.22; LC, −5.34 to −5.80.
Statistical analysis
Data are reported as means ± S.E.M. Values of galanin-injected groups were compared with each vehicle control group by using the factorial analyses of variance (ANOVAs). When appropriate, Tukey's test for one-way ANOVA and Bonferroni's test for two-way ANOVA were used for post hoc analysis. Comparisons involving only two groups were evaluated by Student's t-test. The level of significance was evaluated at p < 0.05.
Results
Effects of galanin (icv) in C57BL/6J mice: SIH and stress hormone responses
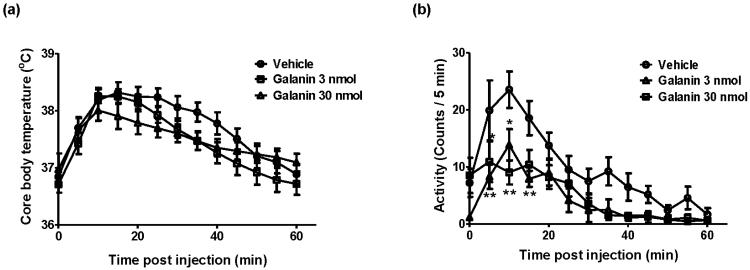
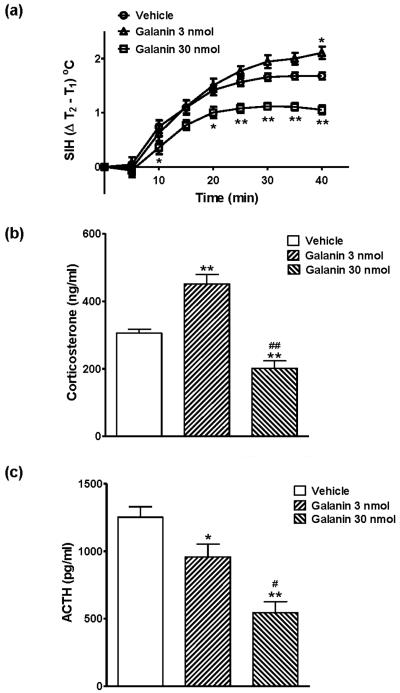
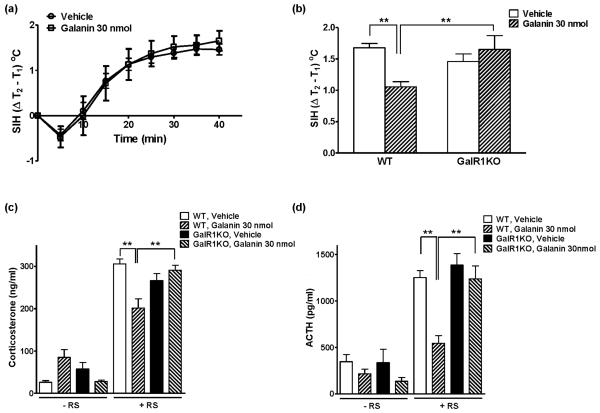
Core body temperature (Figure 1a) and locomotor activity (Figure 1b) were continuously monitored by radio telemetry after the injection of either vehicle or galanin in adult male mice. As shown in Figure 1a, core body temperature increased immediately following the icv injection in both vehicle- and galanin-treated groups, peaked at 10 - 15 min and recovered by 60 min after the injection (F[12, 32] = 48.86, P < 0.0001). There were no significant differences of core body temperature between vehicle- and galanin-treated mice. During the same period of time, locomotor activity was significantly decreased by injection of galanin (Figure 1b, F[2, 32] = 5.71, P = 0.0076). Figure 2 illustrates the effects of galanin icv injection on restraint stress. Mice were injected with either galanin or vehicle (icv), and were subjected to restraint stress one hour later. Figure 2a shows the difference in core body temperature (T2 − T1: T1 is the pre-restraint stress basal body temperature whereas T2 is the post-restraint stress body temperature measured at the indicated time point) that is considered to reflect the SIH. Restraint stress markedly increased body temperature that reached the plateau in 30 min both in vehicle- and in galanin-treated groups (F[8, 200] = 277.3, P < 0.0001). Interestingly, the effects of galanin on the restrain stress-induced hyperthermic response was bidirectional (F[2, 200] = 11.37, P < 0.0001); a low dose (3 nmol) of galanin showed significantly (P < 0.05) increased SIH response by 25 %, while a high dose (30 nmol) of galanin dramatically (P < 0.0001) attenuated by 37 %, compared to the vehicle-injected group (Figure 2a). The basal body temperature (T1), which is shown at 60 min post injection in Figure 1a, was not different between the groups. These effects of galanin in the restraint stress-induced hyperthermia were correlated with changes in serum stress hormone levels. As indicated in Figure 2b, icv administration of a low dose (3 nmol) of galanin showed the significantly increased corsticosterone level (451.3 ± 28.03 ng/ml) under the restraint stress while a high dose (30 nmol) of galanin elicited markedly lower level of plasma corticosterone (201.6 ± 21.95 ng/ml) compared to the vehicle (305.6 ± 11.88 ng/ml), which is consistent with the SIH response in Figure 2a, indicating the bidirectional effect of acute treatment with galanin on the restraint stress. At the same time points (13:00 – 15:00), however, both 3 and 30 nmol galanin icv injection produced a decline, in a dose-dependent manner, in the ACTH level after the 40 min duration of restraint stress (Figure 2c).
Figure 1.
The effects of galanin injection (icv) on the core body temperature and the locomotor activity. Mice were injected vehicle or galanin (3 or 30 nmol, icv) and kept in individual home cages for 60 min while being measured the core body temperature (a) and the locomotor activity (b) with the implanted radio telemetry devices. The injection-related stress increased body temperature in vehicle- and galanin-treated mice and the injection of galanin decreased locomotor activity (* p < 0.05, ** p < 0.01; vs. Vehicle). The data represents means ± SEM (n ≥ 6).
Figure 2.
The effects of galanin injection (icv) on the restraint stress-induced hyperthermia test (a) and on its associated plasma corticosterone (b) and ACTH (c) levels. Mice were injected vehicle or galanin (3 or 30 nmol, icv), kept in individual home cages for 60 min and then given the restraint stress for 40min, followed by the blood sample collection (13:00 - 15:00). Both vehicle- and galanin-injected mice showed the increased core body temperature in response to restraint stress. A low dose of galanin (3 nmol, icv) showed the significantly increased core body temperature while a high dose of galanin (30 nmol, icv) showed the markedly decreased core body temperature, compared to the vehicle injection (a; * p < 0.05, ** p < 0.01; vs. Vehicle). These changes were correlated with corticosterone and ACTH levels (b, c; * p < 0.05, ** p < 0.01; vs. Vehicle, # p < 0.05, ## p < 0.01; vs. Galanin 3 nmol). The data represents means ± SEM (n ≥ 7).
Effects of galanin on cFos induction in C57BL/6J mice
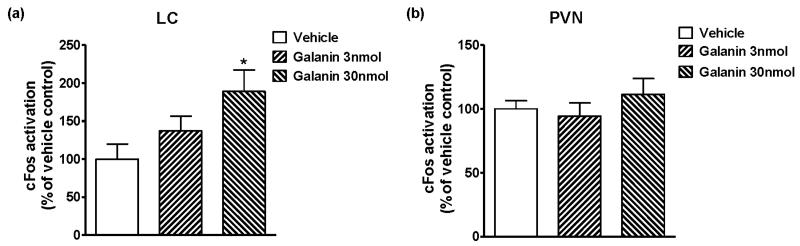
We investigated the effects of galanin (icv) on restraint stress-induced neuronal activity in the LC and in the PVN; two areas with rich galanin receptor expression (O'Donnell et al., 1999, Jungnickel and Gundlach, 2005), by using cFos immunoreactivity as a neuronal activity marker. As shown in Figure 3a, in the LC, the higher dose of galanin (30 nmol, icv) injection significantly induced cFos immunoreactivity to almost twice the level observed in the vehicle-treated group (189.11 ± 28.20 % of vehicle control; P < 0.05 vs. Vehicle), while a lower dose of galanin (3 nmol, icv) did not cause significant difference compared to vehicle control (Figure 3a). In the PVN (Figure 3b), there were no significant differences in cFos staining between vehicle- and galanin-injected groups at either a lower (3 nmol) or a higher (30 nmol) dose of galanin (icv).
Figure 3.
The effects of galanin injection (icv) on cFos immunoreactivity in the LC (a) and the PVN (b) of restraint-stressed mice. Mice were injected vehicle or galanin (3 or 30 nmol, icv), kept in individual home cages for 60 min and then given the restraint stress for 40 min, followed by the perfusion of brain samples which were later immunostained for cFos. In the LC, galanin 30 nmol injection significantly increased cFos immunoreactivity (* p < 0.05 vs. Vehicle). There were no significant differences of cFos activation in the PVN between vehicle- and galanin-injected groups. The data represents means ± SEM (n ≥ 6). The levels of cFos activation are shown relative to each vehicle-treated group that is set as 100 %.
Effect of galanin on restraint stress in GalR1KO mice: SIH and stress hormone responses
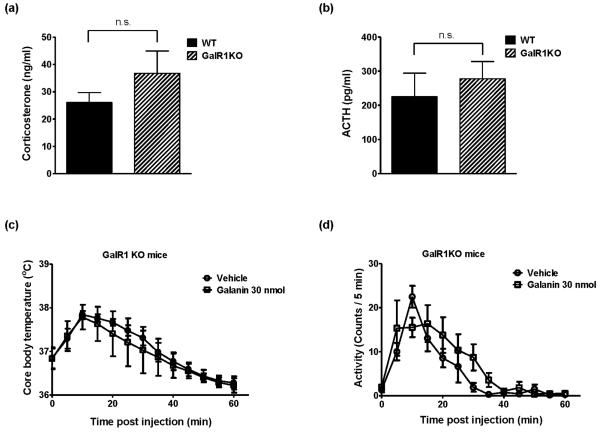
To determine the galanin receptor subtype(s) involved in SIH and stress hormone responses, the same experimental procedure as in control C57BL/6J mice was carried out using GalR1KO mice and WT mice. There were no significant differences of basal plasma corticosterone (Figure 4a) and ACTH (Figure 4b) levels between GalR1KO and WT mice. Figure 4c shows no difference in core body temperature between vehicle- and galanin-injected groups of GalR1KO mice during the 60 min observation after the injection, although injection-related stress significantly increased core body temperature in both groups (F[12, 120] = 16.94, P < 0.0001). Locomotor activity was not significantly different between the two groups during the same time period (Figure 4d). One hour after the injection of either vehicle or galanin, GalR1KO mice were exposed to restraint stress for 40 min. As shown in Figure 5a, restraint stress increased core body temperature in both vehicle- and galanin-treated GalR1KO mice (F[8, 80] = 71.79, P < 0.0001), similarly to WT mice. However, injection of 30 nmol galanin had no effect, compared to the vehicle injection, on the SIH response in GalR1KO mice (Figure 5a) and the difference observed between vehicle- and galanin-treated group in WT mice disappeared in GalR1KO mice at 40 min after the restraint stress exposure (Figure 5b). Consistently, plasma corticosterone (Figure 5c) and ACTH (Figure 5d) levels in both vehicle- and galanin-treated GalR1KO mice were dramatically increased in response to restraint stress and they were not significantly different between these two groups. In WT mice, galanin 30 nmol treatment (icv) showed significantly decreased levels of corticosterone (Figure 5c) and ACTH (Figure 5d) in response to restraint stress. These results indicate the important roles of GalR1 in a high dose of galanin injection–induced decrease of locomotor activity, SIH and stress hormone responses, respectively.
Figure 4.
The basal plasma corticosterone (a) and ACTH (b) levels in WT mice and in GalR1KO mice, and the effects of galanin injection (icv) on the core body temperature (c) and the locomotor activity (d) in GalR1KO mice. Mice treatments are as described in Figure 1. There were no significant differences in basal corticosterone and ACTH levels between WT and GalR1KO mice. In GalR1KO mice, there were no significant differences in core body temperature and in locomotor activity between vehicle- and galanin-injected groups. The data represents means ± SEM (n ≥ 6). n.s.; not significant
Figure 5.
The effects of galanin injection (icv) on the restraint stress-induced hyperthermia test in GalR1KO mice (a) and its comparison between WT and GalR1KO mice at 40 min after the restraint stress exposure (b). Plasma corticosterone (c) and ACTH (d) levels were measured with or without restraint stress in WT and GalR1KO mice. Mice treatments are as described in Figure 2. There was no effect of 30 nmol galanin on restraint stress-induced hyperthermia in GalR1KO mice and the lower core body temperature observed in WT mice at 40 min after galanin 30 nmol injection, compared to vehicle injection, was not observed in GalR1KO mice. These results were well correlated with corticosterone and ACTH levels. The data represents means ± SEM (n ≥ 6). ** p < 0.01
Effects of galanin on cFos induction in GalR1KO mice
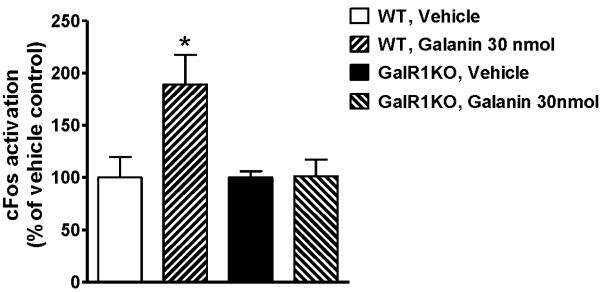
We investigated whether the effect of galanin on neuronal activation in the LC as measured by cFos staining shown in Figure 3a is mediated via GalR1 activation. Although in WT control mice, the number of cFos positive cells in the LC of galanin-injected group was almost twice (189.11 ± 28.20 %) as many compared with the vehicle-injected group (Figure 6, P < 0.05), this effect of galanin was not observed in GalR1KO mice (101.12 ± 16.06 % for galanin-injected group; Figure 6), indicating that increased neuronal activity in the LC by galanin icv injection under restraint stress is likely to be mediated by GalR1 activation.
Figure 6.
The effects of galanin injection (icv) on cFos immunoreactivity in the LC of restraint-stressed WT and GalR1KO mice. The injection of 30 nmol galanin increased cFos immunoreactivity in WT mice (* p < 0.05, vs. Vehicle), however this effect of galanin was not observed in GalR1KO mice. The data represents means ± SEM (n ≥ 5).
Discussion
The present study demonstrates that acute injection of a high dose of galanin (30 nmol, icv) reduces the SIH response, suppresses the associated increase in stress hormone release (corticosterone and ACTH), and causes increased neuronal activation in the LC as measured by cFos staining. In contrast, mice with a targeted deletion of the gene for GalR1 failed to show any of these effects of galanin icv injection, indicating the critical role of GalR1 for the effects of a high dose of galanin on stress responses; the reduction in SIH, the correlated decrease in stress hormone levels and increased cFos immunoreactivity in the LC. The present study also shows that a low dose of galanin (3 nmol, icv) induces a moderate increase in SIH with the associated changes in stress hormone levels. Furthermore, we also show the decreased locomotor activity after galanin injection, as previously observed (Fone and Dixon, 1991, Ericson and Ahlenius, 1999, Weiss et al., 2005). We attribute, for the first time, this effect to be mediated through GalR1 by using GalR1KO mice.
A multitude of behavioral studies using stress-related animal models have been done to examine the role of galanin and its receptors in anxiety and affective disorders (Karlsson and Holmes, 2006, Bailey et al., 2007, Rajarao et al., 2007, Kuteeva et al., 2008a, Kuteeva et al., 2008b, Lu et al., 2008). There are many conflicting reports of the role of galanin in stress-related behavioral phenotypes, such as anxiogenic-like (Moller et al., 1999, Khoshbouei et al., 2002b, Echevarria et al., 2005, Swanson et al., 2005), anxiolytic-like (Bing et al., 1993, Holmes et al., 2002, Khoshbouei et al., 2002a, Barrera et al., 2006) or no effects (Karlsson et al., 2005, Kuteeva et al., 2005). The role of galanin in stress-related behaviors seems to be complex depending on the animal species, route and site of drug administration and behavioral paradigms employed (Lu et al., 2005b, Karlsson and Holmes, 2006, Rajarao et al., 2007), reflecting the complex galaninergic innervation in the CNS and the discrete yet partially overlapping patterns of the three galanin receptor subtypes (GalR1 – 3). The present series of experiments demonstrate for the first time that galanin (icv) has bidirectional effects on stress responses depending on its doses, in mice with C57BL/6J genetic background under the restraint stress condition. Previous data showed that 3 nmol galanin (icv) reduced anxiety-like behavior in the Vogel conflict test in rats (Bing et al., 1993), while Karlsson et al. (2005) have shown in C57BL/6J mice that centrally administered galanin have no anxiety-related effects in two commonly used tests, the elevated plus maze test or in the light-dark exploration test, suggesting the results are species and paradigm dependent. It should also be noted that the doses of galanin used in the previous studies were lower than those of the current study. There may be differences between rats and mice in the affinity and pharmacokinetics for galanin and higher doses of galanin may be required to detect the end-point readouts of stress-related tests in mice. Indeed, a lower dose of galanin used here only had a moderate effect in the SIH paradigm. Consistent with a moderate increase of SIH response, increased level of corticosterone was observed at 3 nmol galanin (icv), compared to the vehicle control. At the same time point, however, the ACTH level was lower than the vehicle control. This may reflect the negative feedback system of the HPA axis since corticosterone is known to be able to suppress the ACTH secretion (Sapolsky et al., 2000, Tsigos and Chrousos, 2002). A comprehensive temporal analysis of these stress hormone levels may be required to fully investigate the difference between the effects of galanin injection on corticosterone and ACTH level at a low dose.
We also examined whether galanin affects the restraint stress-induced neuronal activity, using cFos immunoreactivity, in the hypothalamic PVN and in the LC. We chose these two brain areas because they are the well-studied central control stations of the stress system in the brain (Tsigos and Chrousos, 2002) and areas where galanin and galanin receptors are predominantly expressed in the brain (O'Donnell et al., 1999, Jungnickel and Gundlach, 2005, Mitsukawa et al., 2008). The LC is a primary source of norepinephrine and the LC-norepinephrine system plays an important role together with HPA axis during the stress exposure, underlying the fight or flight response and serving as an alarm system (Carrasco and Van de Kar, 2003, Valentino and Van Bockstaele, 2008). The PVN and the LC are the regions where stressors including anxiogenic stimuli and anxiolytic agents including benzodiazepines (Beck and Fibiger, 1995, Compaan et al., 1996, Salminen et al., 1996, Kovacs, 1998) induce cFos expression. In the present study, 30 nmol galanin icv injection significantly increased restraint stress-induced cFos immunoreactivity at the LC. On the other hand, however, we could not detect galanin induced-significant difference of the cFos-positive immunoreactivity in the PVN under the restraint stress, although HPA axis was less activated as a result of 30 nmol galanin icv injection as it showed the decreased levels of both corticosterone and ACTH. Consistent with our data, intraventricular galanin infusion prior to challenge with fresh cage exposure did not change cFos expression level in the PVN compared to the vehicle infusion (Stone et al., 2007). Our finding is also consistent with results obtained using a group II metabotropic glutamate receptor (mGluR) agonist LY354740 which has shown anxiolytic-related effects in preclinical models (Monn et al., 1997, Helton et al., 1998, Ferris et al., 2001, Spooren et al., 2002) and in clinical findings (Grillon et al., 2003, Schoepp et al., 2003), having no effects on cFos in the PVN but increased level of cFos in the LC in mice that were exposed to the elevated plus maze as a stressor (Linden et al., 2004). Both galanin and group II mGluR agonists inhibit glutamate release (Zini et al., 1993, Kinney et al., 1998, Muly et al., 2007) therefore the decreased level of glutamate may be the common possible mechanism of their effects on cFos expression. We report that cFos activation following galanin adminstration observed in this study is similar to that of Group II mGluR agonists that are presently in clinical trials suggesting that galanin may be a novel therapeutic agent for anxiety.
GalR1KO mice were used to investigate which receptor subtype(s) is involved in the effect of galanin on SIH, stress hormone levels and cFos immunoreactivity in the LC. Among the three galanin receptor subtypes, GalR1 is the most abundant and predominantly expressed receptor throughout the brain including the PVN and the LC (Hawes and Picciotto, 2004, Mitsukawa et al., 2008). The icv injection of a low dose of galanin (3 nmol) showed only a moderate effect on the SIH in control C57BL/6J mice, therefore only a high dose of galanin was tested in GalR1KO mice. GalR1 KO mice failed to show the decrease of the locomotor activity, the decrease of the SIH and its associated stress hormone levels (both ACTH and corticosterone), or the increase of cFos immunoreactivity in the LC, all of which were shown in control WT mice, indicating that GalR1 plays a critical role in the anxiolytic-like effects of galanin under the stressful conditions. A potentially interesting experiment one can perform in the future to demonstrate a somewhat pure GalR1 effect is to investigate the anxiety related effect of galanin in GalR2 and GalR3 double KO mice. Given that GalR3 antagonists have anxiolytic-like effects (Swanson et al., 2005) and that GalR2 KO mice have a weak, if any, anxiety-like phenotype (Gottsch et al., 2005, Bailey et al., 2007, Lu et al., 2008), we predict that a stronger anxiolytic-like effect of galanin may be seen in mice expressing GalR1 subtype alone (i.e. GalR2 and GalR3 double KO mice) than in WT mice. In addition, we did not observe differences in locomotor activity upon galanin injection in GalR1KO mice, suggesting the important role of GalR1 in the effect of galanin on locomotor activity. However it can not be ruled out that the results observed here may be secondary to alterations in other neuronal circuits, not yet identified, which are influenced in a compensatory manner by the null mutation of GalR1. Holmes et al. (2003) have shown that GalR1KO mice exhibit increased anxiety-like behavior specific to the elevated plus maze test, suggesting the anxiolytic-like actions of galanin exerted via GalR1, under conditions of relatively high stress. This is supported by the present data that showed no differences of basal stress hormone levels between GalR1KO mice and WT mice. However, restraint stress, considered as relatively severe stress condition, did not evoke any temperature or endocrine differences in the vehicle-injected groups between GalR1KO mice and WT mice (Figure 5). The reason for this is currently unclear, but it is possible that ceiling response was observed in this study.
In summary, we showed the bidirectional (anxiolytic and anxiogenic) actions of galanin in stress-related paradigm and that GalR1-mediated signaling participates, at least in part, in the anxiolytic actions of galanin. It is interesting to note that galanin has been also shown to exert bidirectional, dose-dependent effects on pain responses; a low dose of galanin increases neuropathic pain and a high dose of galanin has predominantly inhibitory effects on neuropathic pain (Wiesenfeld-Hallin et al., 1988, 1989, Liu and Hökfelt, 2002). This is consistent with our present data as pain is known as an important stressor. These results underlie the importance of developing GalR1 agonists that may act as putative anxiolytic agents.
Acknowledgement
We would like to thank Dr. Manuel Sanchez-Alavez for his critical and insightful suggestions and discussions. We also would like to thank Viktor Zhukov for his excellent technical assistance. This work has been supported by National Institutes of Health grant MH063080 (TB), MH074055 (TB), Swiss National Science Foundation (KM), Novartis Stiftung, vormals Ciba-Geigy-Jubiläums-Stiftung (KM) and JSPS Postdoctoral Fellowship for Research Abroad (KM).
Abbreviations
- ACTH
adrenocorticotropic hormone
- GalR1
galanin receptor subtype 1
- icv
intracerebroventricularly
- KO
knockout
- LC
locus coeruleus
- PVN
paraventricular nucleus
- SIH
stress-induced hyperthermia
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramov U, Floren A, Echevarria DJ, Brewer A, Manuzon H, Robinson JK, Bartfai T, Vasar E, Langel U. Regulation of feeding by galnon. Neuropeptides. 2004;38:55–61. doi: 10.1016/j.npep.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Adriaan Bouwknecht J, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. doi: 10.1016/j.neubiorev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN. Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacol Biochem Behav. 2007;86:8–20. doi: 10.1016/j.pbb.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Kinney JW, Hill MN, Lu X, Biros S, Rebek J, Jr., Bartfai T. A novel, systemically active, selective galanin receptor type-3 ligand exhibits antidepressant-like activity in preclinical tests. Neurosci Lett. 2006;405:111–115. doi: 10.1016/j.neulet.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Barrera G, Hernandez A, Poulin JF, Laforest S, Drolet G, Morilak DA. Galanin-mediated anxiolytic effect in rat central amygdala is not a result of corelease from noradrenergic terminals. Synapse. 2006;59:27–40. doi: 10.1002/syn.20208. [DOI] [PubMed] [Google Scholar]
- Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing O, Moller C, Engel JA, Soderpalm B, Heilig M. Anxiolytic-like action of centrally administered galanin. Neurosci Lett. 1993;164:17–20. doi: 10.1016/0304-3940(93)90846-d. [DOI] [PubMed] [Google Scholar]
- Blakeman KH, Hao JX, Xu XJ, Jacoby AS, Shine J, Crawley JN, Iismaa T, Wiesenfeld-Hallin Z. Hyperalgesia and increased neuropathic pain-like response in mice lacking galanin receptor 1 receptors. Neuroscience. 2003;117:221–227. doi: 10.1016/s0306-4522(02)00779-0. [DOI] [PubMed] [Google Scholar]
- Branchek TA, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends Pharmacol Sci. 2000;21:109–117. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Compaan JC, Groenink L, van der Gugten J, Maes RA, Olivier B. 5-HT1A receptor agonist flesinoxan enhances Fos immunoreactivity in rat central amygdala, bed nucleus of the stria terminalis and hypothalamus. Eur J Neurosci. 1996;8:2340–2347. doi: 10.1111/j.1460-9568.1996.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Counts SE, Perez SE, Mufson EJ. Galanin in Alzheimer's disease: neuroinhibitory or neuroprotective? Cell Mol Life Sci. 2008;65:1842–1853. doi: 10.1007/s00018-008-8159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Galanin impairs cognitive abilities in rodents: relevance to Alzheimer's disease. Cell Mol Life Sci. 2008;65:1836–1841. doi: 10.1007/s00018-008-8158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarria DJ, Hernandez A, Diogenes A, Morilak DA. Administration of the galanin antagonist M40 into lateral septum attenuates shock probe defensive burying behavior in rats. Neuropeptides. 2005;39:445–451. doi: 10.1016/j.npep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ericson E, Ahlenius S. Suggestive evidence for inhibitory effects of galanin on mesolimbic dopaminergic neurotransmission. Brain Res. 1999;822:200–209. doi: 10.1016/s0006-8993(99)01144-0. [DOI] [PubMed] [Google Scholar]
- Ferris P, Seward E, Dawson GR. Interactions between LY354740, a group II metabotropic agonist and the GABA(A)-benzodiazepine receptor complex in the rat elevated plus-maze. J Psychopharmacol. 2001;15:76–82. doi: 10.1177/026988110101500203. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Jacoby AS, Brumovsky PR, Shine J, Iismaa TP, Hökfelt T. Altered hippocampal expression of neuropeptides in seizure-prone GALR1 knockout mice. Epilepsia. 2003;44:1022–1033. doi: 10.1046/j.1528-1157.2003.51402.x. [DOI] [PubMed] [Google Scholar]
- Fone KC, Dixon DM. Acute and chronic effects of intrathecal galanin on behavioural and biochemical markers of spinal motor function in adult rats. Brain Res. 1991;544:118–125. doi: 10.1016/0006-8993(91)90892-y. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Zeng H, Hohmann JG, Weinshenker D, Clifton DK, Steiner RA. Phenotypic analysis of mice deficient in the type 2 galanin receptor (GALR2) Mol Cell Biol. 2005;25:4804–4811. doi: 10.1128/MCB.25.11.4804-4811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass S, Jacoby AS, Iismaa TP, Crawley JN, Xu XJ, Wiesenfeld-Hallin Z. Flexor reflex excitability in mice lacking galanin receptor galanin-R1. Neurosci Lett. 2003;345:153–156. doi: 10.1016/s0304-3940(03)00516-0. [DOI] [PubMed] [Google Scholar]
- Grillon C, Cordova J, Levine LR, Morgan CA., 3rd Anxiolytic effects of a novel group II metabotropic glutamate receptor agonist ( LY354740) in the fear-potentiated startle paradigm in humans. Psychopharmacology (Berl) 2003;168:446–454. doi: 10.1007/s00213-003-1444-8. [DOI] [PubMed] [Google Scholar]
- Groenink L, Compaan J, van der Gugten J, Zethof T, van der Heyden J, Olivier B. Stress-induced hyperthermia in mice. Pharmacological and endocrinological aspects. Ann N Y Acad Sci. 1995;771:252–256. doi: 10.1111/j.1749-6632.1995.tb44686.x. [DOI] [PubMed] [Google Scholar]
- Groenink L, van der Gugten J, Zethof T, van der Heyden J, Olivier B. Stress-induced hyperthermia in mice: hormonal correlates. Physiol Behav. 1994;56:747–749. doi: 10.1016/0031-9384(94)90237-2. [DOI] [PubMed] [Google Scholar]
- Hawes JJ, Picciotto MR. Characterization of GalR1, GalR2, and GalR3 immunoreactivity in catecholaminergic nuclei of the mouse brain. J Comp Neurol. 2004;479:410–423. doi: 10.1002/cne.20329. [DOI] [PubMed] [Google Scholar]
- Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ. Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. J Pharmacol Exp Ther. 1998;284:651–660. [PubMed] [Google Scholar]
- Holets VR, Hökfelt T, Rokaeus A, Terenius L, Goldstein M. Locus coeruleus neurons in the rat containing neuropeptide Y, tyrosine hydroxylase or galanin and their efferent projections to the spinal cord, cerebral cortex and hypothalamus. Neuroscience. 1988;24:893–906. doi: 10.1016/0306-4522(88)90076-0. [DOI] [PubMed] [Google Scholar]
- Holmes A, Kinney JW, Wrenn CC, Li Q, Yang RJ, Ma L, Vishwanath J, Saavedra MC, Innerfield CE, Jacoby AS, Shine J, Iismaa TP, Crawley JN. Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology. 2003;28:1031–1044. doi: 10.1038/sj.npp.1300164. [DOI] [PubMed] [Google Scholar]
- Holmes A, Picciotto MR. Galanin: a novel therapeutic target for depression, anxiety disorders and drug addiction? CNS Neurol Disord Drug Targets. 2006;5:225–232. doi: 10.2174/187152706776359600. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Crawley JN. Evaluation of an anxiety-related phenotype in galanin overexpressing transgenic mice. J Mol Neurosci. 2002;18:151–165. doi: 10.1385/JMN:18:1-2:151. [DOI] [PubMed] [Google Scholar]
- Holmes PV, Koprivica V, Chough E, Crawley JN. Intraventricular administration of galanin does not affect behaviors associated with locus coeruleus activation in rats. Peptides. 1994;15:1303–1308. doi: 10.1016/0196-9781(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Jacoby AS, Hort YJ, Constantinescu G, Shine J, Iismaa TP. Critical role for GALR1 galanin receptor in galanin regulation of neuroendocrine function and seizure activity. Brain Res Mol Brain Res. 2002;107:195–200. doi: 10.1016/s0169-328x(02)00451-5. [DOI] [PubMed] [Google Scholar]
- Jungnickel SR, Gundlach AL. [125I]-Galanin binding in brain of wildtype, and galanin- and GalR1-knockout mice: strain and species differences in GalR1 density and distribution. Neuroscience. 2005;131:407–421. doi: 10.1016/j.neuroscience.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Holmes A. Galanin as a modulator of anxiety and depression and a therapeutic target for affective disease. Amino Acids. 2006;31:231–239. doi: 10.1007/s00726-006-0336-8. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Holmes A, Heilig M, Crawley JN. Anxiolytic-like actions of centrally-administered neuropeptide Y, but not galanin, in C57BL/6J mice. Pharmacol Biochem Behav. 2005;80:427–436. doi: 10.1016/j.pbb.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Dove S, Javors M, Morilak DA. Behavioral reactivity to stress: amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacol Biochem Behav. 2002a;71:407–417. doi: 10.1016/s0091-3057(01)00683-9. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Morilak DA. Modulatory effects of galanin in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuropsychopharmacology. 2002b;27:25–34. doi: 10.1016/S0893-133X(01)00424-9. [DOI] [PubMed] [Google Scholar]
- Kinney GA, Emmerson PJ, Miller RJ. Galanin receptor-mediated inhibition of glutamate release in the arcuate nucleus of the hypothalamus. J Neurosci. 1998;18:3489–3500. doi: 10.1523/JNEUROSCI.18-10-03489.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Kuteeva E, Hökfelt T, Ogren SO. Behavioural characterisation of transgenic mice overexpressing galanin under the PDGF-B promoter. Neuropeptides. 2005;39:299–304. doi: 10.1016/j.npep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Kuteeva E, Hökfelt T, Wardi T, Ogren SO. Galanin, galanin receptor subtypes and depression-like behaviour. Cell Mol Life Sci. 2008a;65:1854–1863. doi: 10.1007/s00018-008-8160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuteeva E, Wardi T, Lundstrom L, Sollenberg U, Langel U, Hökfelt T, Ogren SO. Differential Role of Galanin Receptors in the Regulation of Depression-Like Behavior and Monoamine/Stress-Related Genes at the Cell Body Level. Neuropsychopharmacology. 2008b doi: 10.1038/sj.npp.1301660. [DOI] [PubMed] [Google Scholar]
- Lang R, Gundlach AL, Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther. 2007;115:177–207. doi: 10.1016/j.pharmthera.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Lerner JT, Sankar R, Mazarati AM. Galanin and epilepsy. Cell Mol Life Sci. 2008;65:1864–1871. doi: 10.1007/s00018-008-8161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden AM, Greene SJ, Bergeron M, Schoepp DD. Anxiolytic activity of the MGLU2/3 receptor agonist LY354740 on the elevated plus maze is associated with the suppression of stress-induced c-Fos in the hippocampus and increases in c-Fos induction in several other stress-sensitive brain regions. Neuropsychopharmacology. 2004;29:502–513. doi: 10.1038/sj.npp.1300321. [DOI] [PubMed] [Google Scholar]
- Liu HX, Hökfelt T. The participation of galanin in pain processing at the spinal level. Trends Pharmacol Sci. 2002;23:468–474. doi: 10.1016/s0165-6147(02)02074-6. [DOI] [PubMed] [Google Scholar]
- Lu X, Barr AM, Kinney JW, Sanna P, Conti B, Behrens MM, Bartfai T. A role for galanin in antidepressant actions with a focus on the dorsal raphe nucleus. Proc Natl Acad Sci U S A. 2005a;102:874–879. doi: 10.1073/pnas.0408891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Lundstrom L, Langel U, Bartfai T. Galanin receptor ligands. Neuropeptides. 2005b;39:143–146. doi: 10.1016/j.npep.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Lu X, Ross B, Sanchez-Alavez M, Zorrilla EP, Bartfai T. Phenotypic analysis of GalR2 knockout mice in anxiety- and depression-related behavioral tests. Neuropeptides. 2008;42:387–397. doi: 10.1016/j.npep.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Sharkey L, Bartfai T. The brain galanin receptors: targets for novel antidepressant drugs. CNS Neurol Disord Drug Targets. 2007;6:183–192. doi: 10.2174/187152707780619335. [DOI] [PubMed] [Google Scholar]
- Melander T, Hokfelt T, Rokaeus A, Cuello AC, Oertel WH, Verhofstad A, Goldstein M. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. J Neurosci. 1986;6:3640–3654. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsukawa K, Lu X, Bartfai T. Galanin, galanin receptors and drug targets. Cell Mol Life Sci. 2008;65:1796–1805. doi: 10.1007/s00018-008-8153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller C, Sommer W, Thorsell A, Heilig M. Anxiogenic-like action of galanin after intraamygdala administration in the rat. Neuropsychopharmacology. 1999;21:507–512. doi: 10.1016/S0893-133X(98)00102-X. [DOI] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Wright RA, Salhoff CR, Johnson BG, Howe T, Alt CA, Rhodes GA, Robey RL, Griffey KR, Tizzano JP, Kallman MJ, Helton DR, Schoepp DD. Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid ( LY354740): a potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J Med Chem. 1997;40:528–537. doi: 10.1021/jm9606756. [DOI] [PubMed] [Google Scholar]
- Muly EC, Mania I, Guo JD, Rainnie DG. Group II metabotropic glutamate receptors in anxiety circuitry: correspondence of physiological response and subcellular distribution. J Comp Neurol. 2007;505:682–700. doi: 10.1002/cne.21525. [DOI] [PubMed] [Google Scholar]
- O'Donnell D, Ahmad S, Wahlestedt C, Walker P. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. Journal of Comparative Neurology. 1999;409:469–481. [PubMed] [Google Scholar]
- Rajarao SJ, Platt B, Sukoff SJ, Lin Q, Bender CN, Nieuwenhuijsen BW, Ring RH, Schechter LE, Rosenzweig-Lipson S, Beyer CE. Anxiolytic-like activity of the non-selective galanin receptor agonist, galnon. Neuropeptides. 2007;41:307–320. doi: 10.1016/j.npep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Salminen O, Lahtinen S, Ahtee L. Expression of Fos protein in various rat brain areas following acute nicotine and diazepam. Pharmacol Biochem Behav. 1996;54:241–248. doi: 10.1016/0091-3057(95)02132-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ. LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress. 2003;6:189–197. doi: 10.1080/1025389031000146773. [DOI] [PubMed] [Google Scholar]
- Sharkey LM, Madamba SG, Siggins GR, Bartfai T. Galanin alters GABAergic neurotransmission in the dorsal raphe nucleus. Neurochem Res. 2008;33:285–291. doi: 10.1007/s11064-007-9524-5. [DOI] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985;6:509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Schoeffter P, Gasparini F, Kuhn R, Gentsch C. Pharmacological and endocrinological characterisation of stress-induced hyperthermia in singly housed mice using classical and candidate anxiolytics ( LY314582, MPEP and NKP608) Eur J Pharmacol. 2002;435:161–170. doi: 10.1016/s0014-2999(01)01562-x. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lehmann ML, Lin Y, Quartermain D. Reduced evoked fos expression in activity-related brain regions in animal models of behavioral depression. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1196–1207. doi: 10.1016/j.pnpbp.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Blackburn TP, Zhang X, Zheng K, Xu ZQ, Hökfelt T, Wolinsky TD, Konkel MJ, Chen H, Zhong H, Walker MW, Craig DA, Gerald CP, Branchek TA. Anxiolytic- and antidepressant-like profiles of the galanin-3 receptor (Gal3) antagonists SNAP 37889 and SNAP 398299. Proc Natl Acad Sci U S A. 2005;102:17489–17494. doi: 10.1073/pnas.0508970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K, Rokaeus A, Jornvall H, McDonald TJ, Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164:124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Heyden JA, Zethof TJ, Olivier B. Stress-induced hyperthermia in singly housed mice. Physiol Behav. 1997;62:463–470. doi: 10.1016/s0031-9384(97)00157-1. [DOI] [PubMed] [Google Scholar]
- Walton KM, Chin JE, Duplantier AJ, Mather RJ. Galanin function in the central nervous system. Curr Opin Drug Discov Devel. 2006;9:560–570. [PubMed] [Google Scholar]
- Weiss JM, Bonsall RW, Demetrikopoulos MK, Emery MS, West CH. Galanin: a significant role in depression? Ann N Y Acad Sci. 1998;863:364–382. doi: 10.1111/j.1749-6632.1998.tb10707.x. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Boss-Williams KA, Moore JP, Demetrikopoulos MK, Ritchie JC, West CH. Testing the hypothesis that locus coeruleus hyperactivity produces depression-related changes via galanin. Neuropeptides. 2005;39:281–287. doi: 10.1016/j.npep.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Villar MJ, Hökfelt T. Intrathecal galanin at low doses increases spinal reflex excitability in rats more to thermal than mechanical stimuli. Exp Brain Res. 1988;71:663–666. doi: 10.1007/BF00248760. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Villar MJ, Hökfelt T. The effects of intrathecal galanin and C-fiber stimulation on the flexor reflex in the rat. Brain Res. 1989;486:205–213. doi: 10.1016/0006-8993(89)90506-4. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Crawley JN. Pharmacological evidence supporting a role for galanin in cognition and affect. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:283–299. doi: 10.1016/s0278-5846(00)00156-1. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Holmes A. The role of galanin in modulating stress-related neural pathways. Drug News Perspect. 2006;19:461–467. doi: 10.1358/dnp.2006.19.8.1043963. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Hökfelt T, Wiesenfeld-Hallin Z. Galanin and spinal pain mechanisms: where do we stand in 2008? Cell Mol Life Sci. 2008;65:1813–1819. doi: 10.1007/s00018-008-8155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZQ, Hokfelt T. Expression of galanin and nitric oxide synthase in subpopulations of serotonin neurons of the rat dorsal raphe nucleus. J Chem Neuroanat. 1997;13:169–187. doi: 10.1016/s0891-0618(97)00043-4. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Shi TJ, Hökfelt T. Galanin/GMAP- and NPY-like immunoreactivities in locus coeruleus and noradrenergic nerve terminals in the hippocampal formation and cortex with notes on the galanin-R1 and -R2 receptors. J Comp Neurol. 1998;392:227–251. doi: 10.1002/(sici)1096-9861(19980309)392:2<227::aid-cne6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Zini S, Roisin MP, Langel U, Bartfai T, Ben-Ari Y. Galanin reduces release of endogenous excitatory amino acids in the rat hippocampus. Eur J Pharmacol. 1993;245:1–7. doi: 10.1016/0922-4106(93)90162-3. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Brennan M, Sabino V, Lu X, Bartfai T. Galanin type 1 receptor knockout mice show altered responses to high-fat diet and glucose challenge. Physiol Behav. 2007;91:479–485. doi: 10.1016/j.physbeh.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]