Abstract
The purpose of this study was to determine whether in vivo images of oral mucosa obtained with a fiber optic confocal reflectance microscope could be used to differentiate normal and neoplastic tissues. We imaged 20 oral sites in 8 patients undergoing surgery for squamous cell carcinoma. Normal and abnormal areas within the oral cavity were identified clinically, and real-time videos of each site were obtained in vivo using a fiber optic confocal reflectance microscope. Following imaging, each site was biopsied and submitted for histopathologic examination. We identified distinct features, such as nuclear irregularity and spacing, which can be used to qualitatively differentiate between normal and abnormal tissue. Representative confocal images of normal, pre-neoplastic, and neoplastic oral tissue are presented. Previous work using much larger microscopes has demonstrated the ability of confocal reflectance microscopy to image cellular and tissue architecture in situ. New advances in technology have enabled miniaturization of imaging systems for in vivo use.
Keywords: Confocal microscopy, squamous cell carcinoma, dysplasia detection, oral cavity
INTRODUCTION
Early detection and accurate diagnosis of neoplastic changes could significantly reduce the morbidity and mortality associated with epithelial malignancies. Current techniques rely heavily on the ability of clinicians to recognize sometimes subtle changes in gross morphology and tissue architecture followed by surgical removal of tissue and pathologic assessment. However, premalignant or early malignant lesions can be clinically indistinguishable from more common benign lesions. Diagnostic delays often result because patients and clinicians are reluctant to perform the invasive biopsy currently needed to identify neoplastic changes. A technology that can quickly provide this pathologic information without surgical removal of tissue could improve the management of a number of epithelial malignancies, including cancers of the colon, bladder, skin, esophagus and oral cavity. Confocal reflectance microscopy currently can provide real-time, non-invasive imaging of intact tissue and cellular structure in vitro. The purpose of this study is to evaluate whether confocal reflectance microscopy could be used in vivo to aid diagnosis of one representative epithelial malignancy, oral carcinoma. Oral cancer is the fifth most common malignancy worldwide. Roughly 34 000 new cases and over 7 500 deaths from this disease occur each year in the United States.1
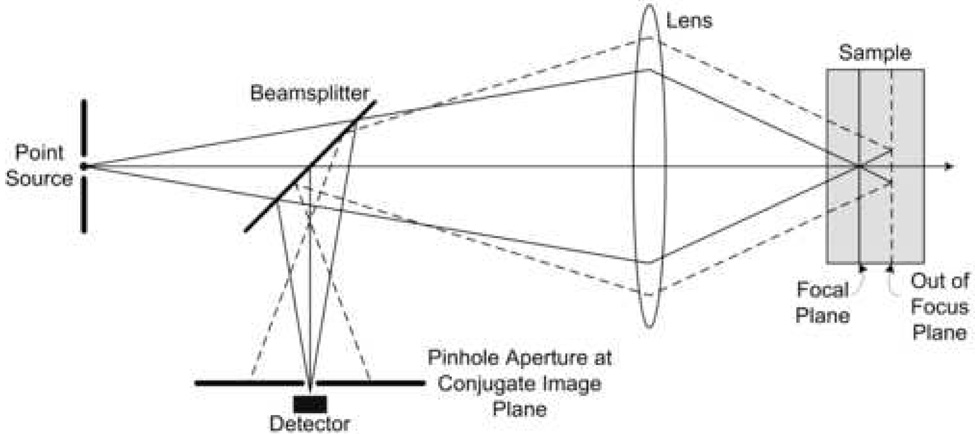
Confocal microscopy (Figure 1) allows high resolution imaging of intact tissue by selectively imaging light reflected from a specific depth within the tissue and rejecting light originating from tissue layers above and below that plane.2 Confocal microscopy is a well established biological imaging technique for ex vivo specimens that has recently been applied to human tissue in vivo. In vivo confocal reflectance microscopy can provide images of tissue architecture and cellular morphology with resolution comparable to histology in real time, without tissue removal, sectioning, and staining. This is based on the principal that backscattering of light from cellular and tissue structures provides signal, and image contrast is generated by differences in refractive indexes of cellular components.3–5 Resolution as low as 1 µm, field of view greater than 400 µm, and penetration depth as great as 500 µm have been achieved in confocal imaging of epithelial tissue.6–12 Taking advantage of optical fibers,13, 14 miniaturization of optics,15–17 and improved beam scanning mechanisms,12, 18 confocal microscopes are becoming more clinically feasible. So far, examples of confocal images have been successful obtained in vivo from the skin,3, 19–21 eye,22, 23 cervix,24, 25 and oral cavity.7, 9, 26
Fig. 1.
Illustration of optical sectioning by confocal microscopy.
Our preliminary studies carried out in vitro demonstrate the capability of confocal reflectance microscopy to identify neoplastic changes, based on morphologic features such as changes in nuclear size and density in oral cavity tissue biopsies.27 While a few studies have reported in vivo images of the oral cavity of normal volunteers,9, 28 these previous studies of in vivo confocal reflectance imaging of the oral cavity have generally used large, bench-type microscope objectives. For confocal imaging, the objective lens must be in close proximity to the imaging site. Consequently, imaging has been limited to the most accessible areas of the mouth, such as the lip and tongue and is impractical for even pilot clinical trials. We have recently developed a fiber optic confocal reflectance microscope with a miniaturized objective lens, allowing access to mucosal surfaces like the deeper parts of the oral cavity, in a clinical setting.11 The goals of this study are to obtain confocal reflectance images of normal and neoplastic sites throughout the oral cavity in vivo and to determine if these images can be used to identify neoplastic changes.
MATERIALS AND METHODS
In Vivo Confocal Imaging System
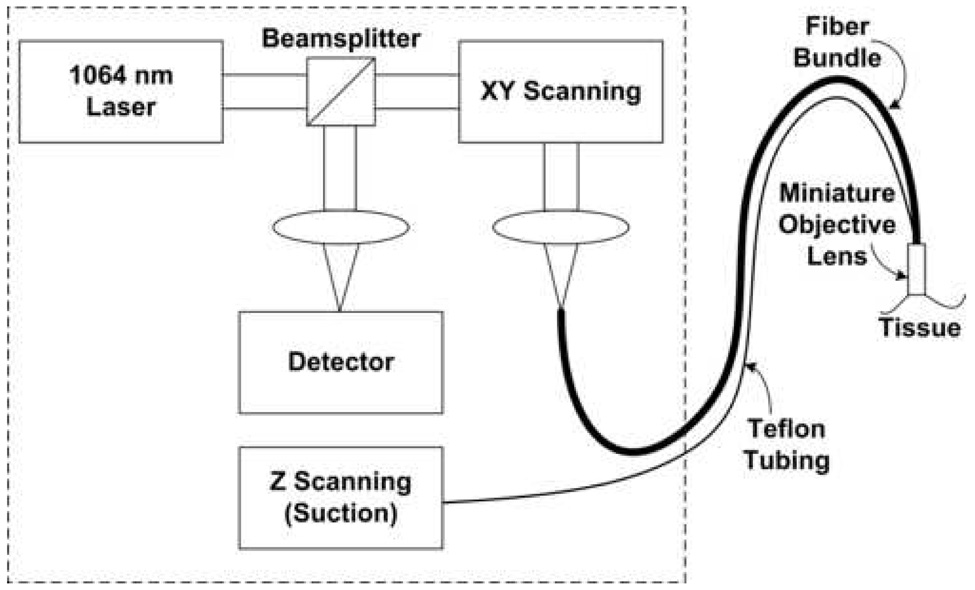
Reflectance confocal images were obtained in vivo from mucosal sites within the oral cavity using a non-invasive, real-time, fiber optic confocal microscope.15 A schematic diagram of the imaging system is shown in Figure 2, and photographs demonstrating how confocal images are acquired from a subject’s tongue lesion with the system are shown in Figure 3. Light at 1064 nm wavelength is directed to the mucosal surface through a 30 000 optical fiber image guide and a complex, water immersion miniature objective lens, with an outer diameter of 7 mm.15 The miniature objective lens has a magnification of 3.3 and a numerical aperture of 1.0. Raster scanning the laser beam across the fiber bundle allows point scanning in the focal plane in the tissue sample. In this configuration, each fiber acts as a confocal pinhole, and reflectance signal is produced by refractive index differences in the tissue. This microscope generates en face reflectance confocal images at 15 frames/second. The measured axial resolution, or image thickness, is less than 10 µm. The lateral resolution is approximately 2 µm, and the imaging field of view is approximately 230 × 230 µm. The entire epithelial depth is imaged by sequentially moving the tissue through the focal plane of the microscope using hydraulic suction controlled by a syringe pump. Although the imaging depth in the tissue is not quantitatively known, high quality images can be obtained at depths starting at the tissue surface and extending to the basement membrane. Determination of these two positions in the tissue permits visualization of the nuclear density in superficial, intermediate, and basal layers. All of the large optics and electronics are housed on a portable cart that can be wheeled into the operating room or examination room. The miniature objective lens, which is connected to the optical system through the fiber bundle and to the axial scanning system through Teflon tubing, is placed on the tissue for image acquisition. The probe housing which protects the objective lens measures 1 cm in diameter.
Fig. 2.
Schematic of fiber optic confocal microscope.
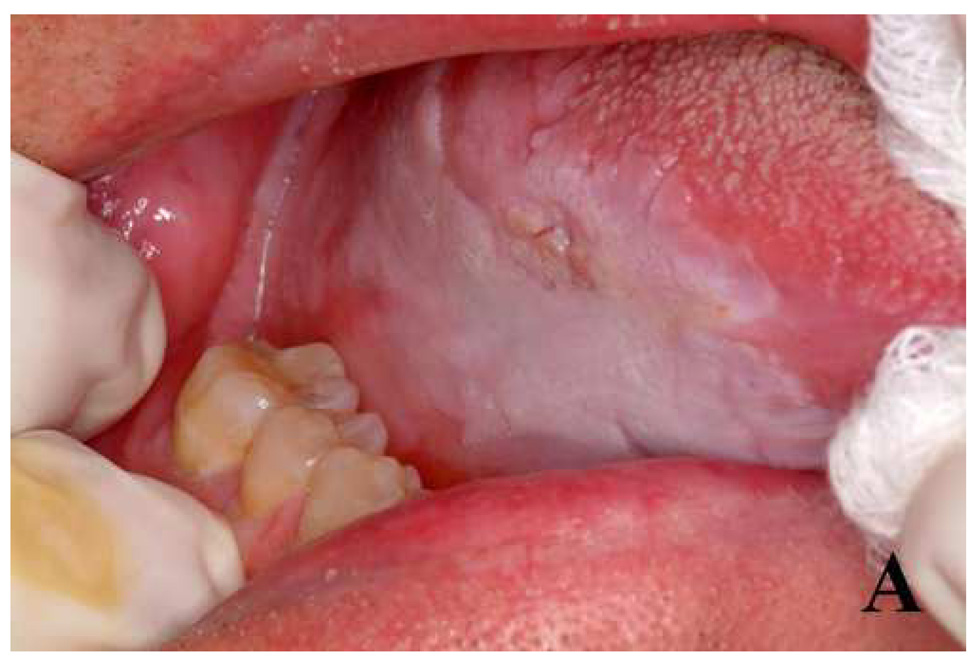
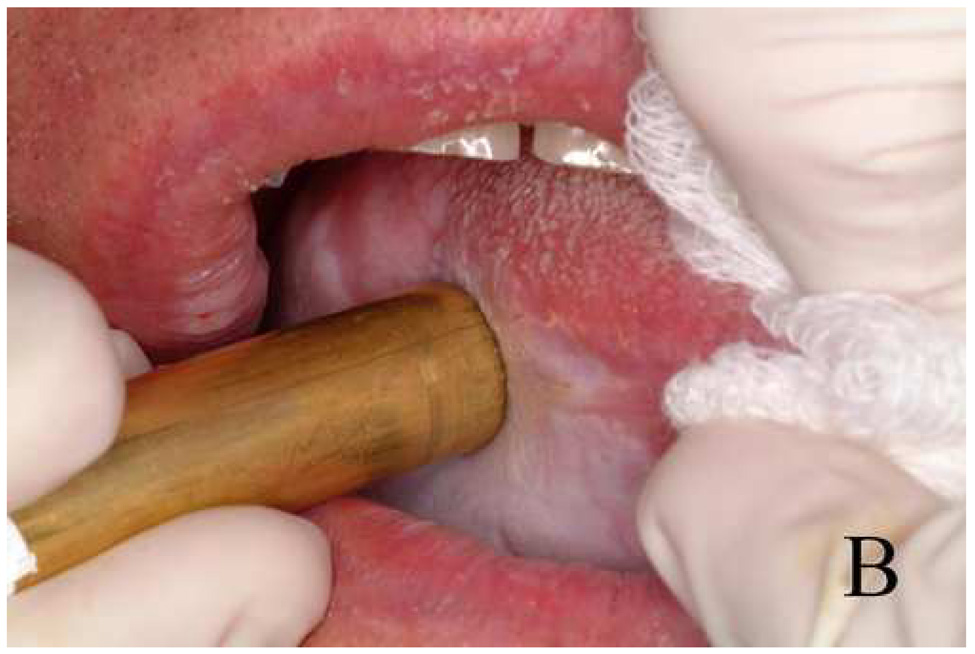
Fig. 3.
Photographs of (A) a clinically suspicious imaging site on the ventral tongue, and (B) placement of probe on the tissue for confocal reflectance imaging.
In Vivo Imaging of Human Oral Tissue
All subjects included in this study gave informed consent. The protocol was reviewed and approved by the Institutional Review Boards of The University of Texas M. D. Anderson Cancer Center, The University of Texas at Austin, and Rice University. Eight subjects undergoing surgery for squamous cell carcinoma (SCC) of the oral cavity participated in the study. A head and neck surgeon identified clinically normal and abnormal areas for imaging and applied a sponge soaked in apple cider vinegar, in which acetic acid is the major constituent, to the tissue for approximately 1 minute. Low concentration acetic acid is often used for acetowhitening during cervical colposcopy and increases scattering from cell nuclei and improves demarcation of nuclei and cytoplasm.29, 30 The clinician then placed the confocal probe on at least one clinically suspicious tissue site and a contralateral clinically normal-appearing site. Figure 3 shows (A) an example of a suspicious lesion and (B) placement of the fiber optic confocal microscope probe on the tissue. After imaging, biopsies were acquired at each imaged site for histopathological assessment. Images of histology sections stained with hematoxylin and eosin (H&E) were acquired using a bright field microscope and a color charge-coupled device camera.
Image Processing
At each site, confocal video was acquired as the depth of imaging was varied. Individual confocal images from the superficial and intermediate layers of the epithelium were obtained from video files. In general, viewing of images in video format improves visualization of cellular and tissue structure. Raw fiber optic confocal videos can have image artifacts due to the non-linear scan of one of the two scanning mirrors, non-uniform specular reflection across the fiber bundle, fiber bundle “pixilation,” and patient or operator motion. Images have been resized in one axis to account for the non-linear optical beam scan,31 background subtracted, filtered using a median anisotropic diffusion filter to remove the fiber bundle pattern,11 and contrast enhanced to improve image viewing quality in print form. These image processing techniques could potentially be implemented in real-time during image acquisition.
RESULTS
In vivo reflectance confocal images were successfully obtained from 20 sites in 8 patients. Table 1 shows the site location, clinical and confocal appearance, and histopathological diagnosis of the biopsies taken from each of the 20 imaging sites. Of the 20 sites, 8 exhibited hyperkeratosis, 5 had hyperplasia, 4 had inflammation, 4 were diagnosed with dysplasia, and 5 with invasive SCC. Two clinically normal sites from one subject did not have a pathologic diagnosis because the patient requested that no biopsies be taken from normal-appearing areas. Confocal appearance is classified as “dispersed nuclei” for videos with regularly spaced low-density nuclei, “dense nuclei” for videos with regularly spaced but crowded nuclei, and “disordered tissue structure” for videos that show irregular reflective structures and may or may not include nuclei. Confocal videos from 2 sites showed even, regular tissue structure with few or no visible nuclei, and are therefore not classified into any of the 3 categories.
Table 1.
Imaging site location, clinical and confocal appearance, and histopathological diagnosis.
| Patient and site | Imaging site | Clinical appearance | Histopathological diagnosis | Confocal appearance | ||
|---|---|---|---|---|---|---|
| Dispersed nuclei | Dense nuclei | Disordered tissue structure | ||||
| Normal | ||||||
| 6D | Upper lip | Normal | No biopsy taken | × | ||
| 6E | Buccal mucosa | Normal | No biopsy taken | Nuclei not visible | ||
| 4A | Floor of mouth | Normal | Normal | × | ||
| 7A | Ventral tongue | Normal | Normal | × | ||
| 8A | Ventral tongue | Normal | Hyperplasia, hyperkeratosis | × | ||
| 1B | Ventral tongue | Normal | Hyperkeratosis, mild inflammation | × | ||
| 2A | Buccal mucosa | Normal | Hyperkeratosis | × | ||
| 3A | Ventral tongue | Normal | Hyperplasia, hyperkeratosis | × | ||
| 5A | Ventral tongue | Abnormal | Hyperplasia, hyperkeratosis | × | ||
| 6A | Upper lip | Abnormal | Hyperkeratosis, mild inflammation | Nuclei not visible | ||
| 6B | Buccal mucosa | Abnormal | Hyperplasia, hyperkeratosis, mild inflammation | × | ||
| 7B | Ventral tongue | Abnormal | Hyperplasia, hyperkeratosis | × | ||
| Dysplasia | ||||||
| 5B | Ventral tongue | Normal | Mild dysplasia | × | ||
| 4B | Floor of mouth | Abnormal | Moderate dysplasia, severe inflammation | × | ||
| 8B | Ventral posterior tongue | Abnormal | Dysplasia | × | ||
| SCC | ||||||
| 1A | Ventral tongue | Abnormal | Moderately differentiated, invasive SCC | × | ||
| 2B | Retromolar trigone | Abnormal | Moderate - severe dysplasia, invasive focal SCC | × | ||
| 3B | Anterior floor of mouth | Abnormal | Invasive SCC | × | ||
| 6C | Lower gum | Abnormal | Invasive SCC | × | ||
| 8C | Ventral anterior tongue | Abnormal | Invasive SCC | × | ||
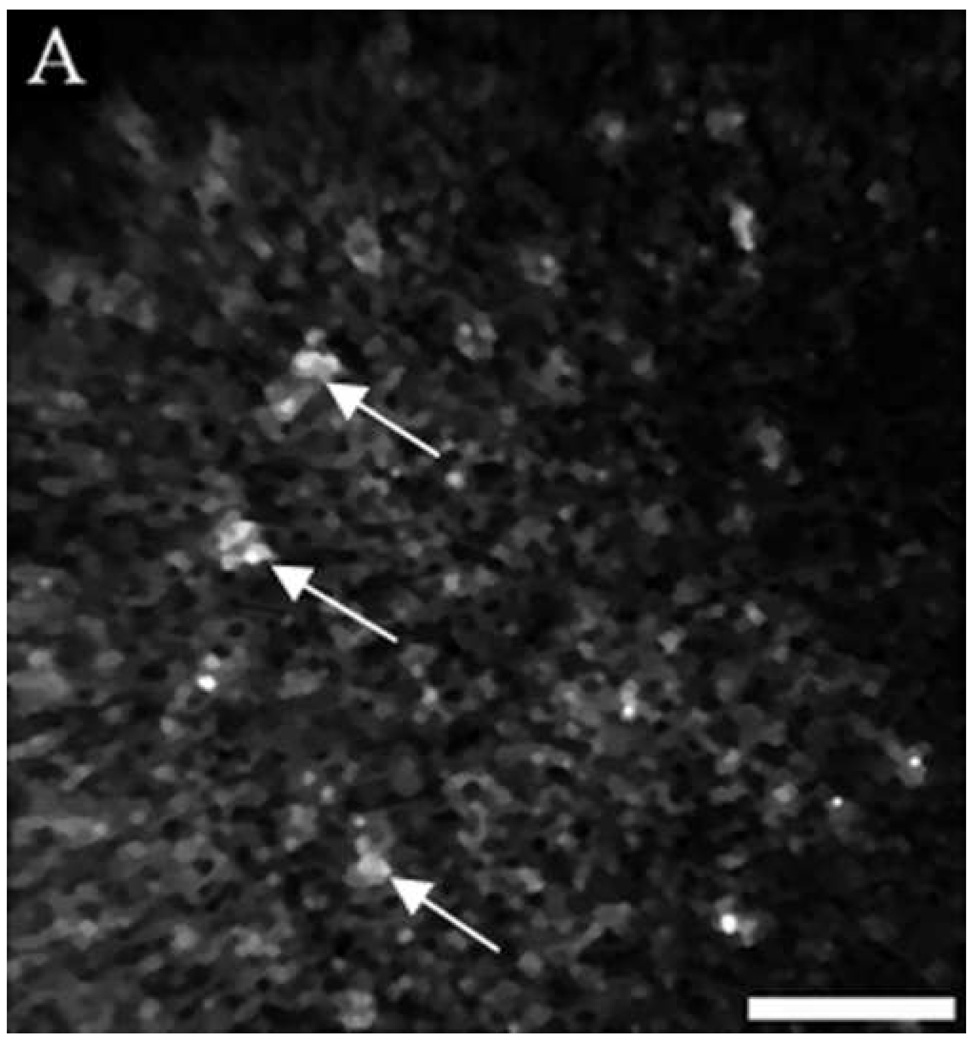
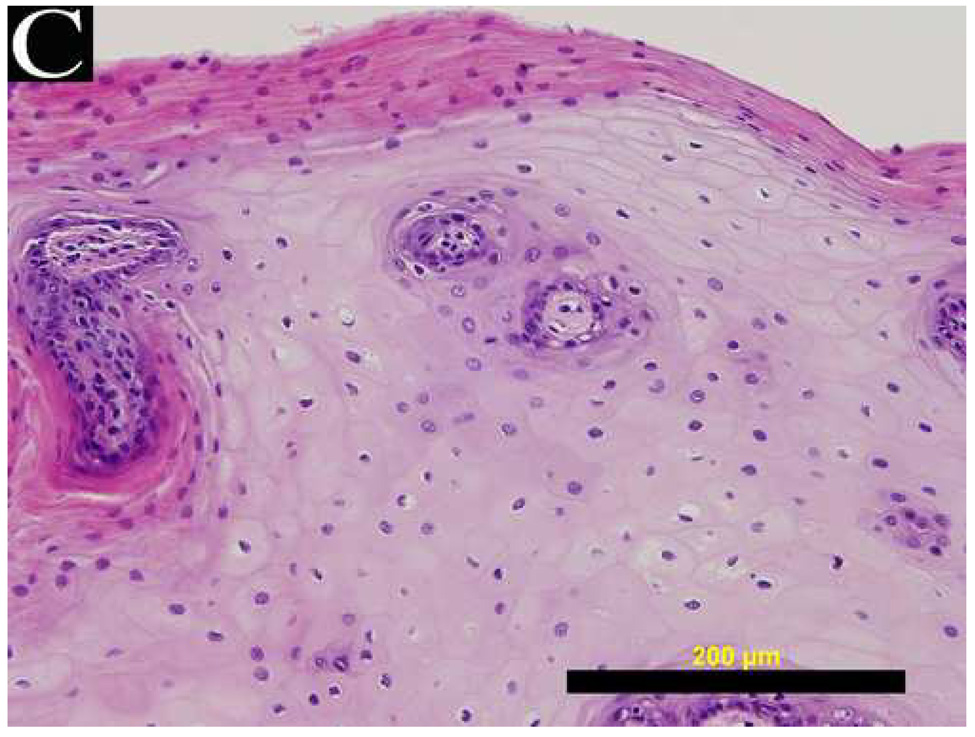
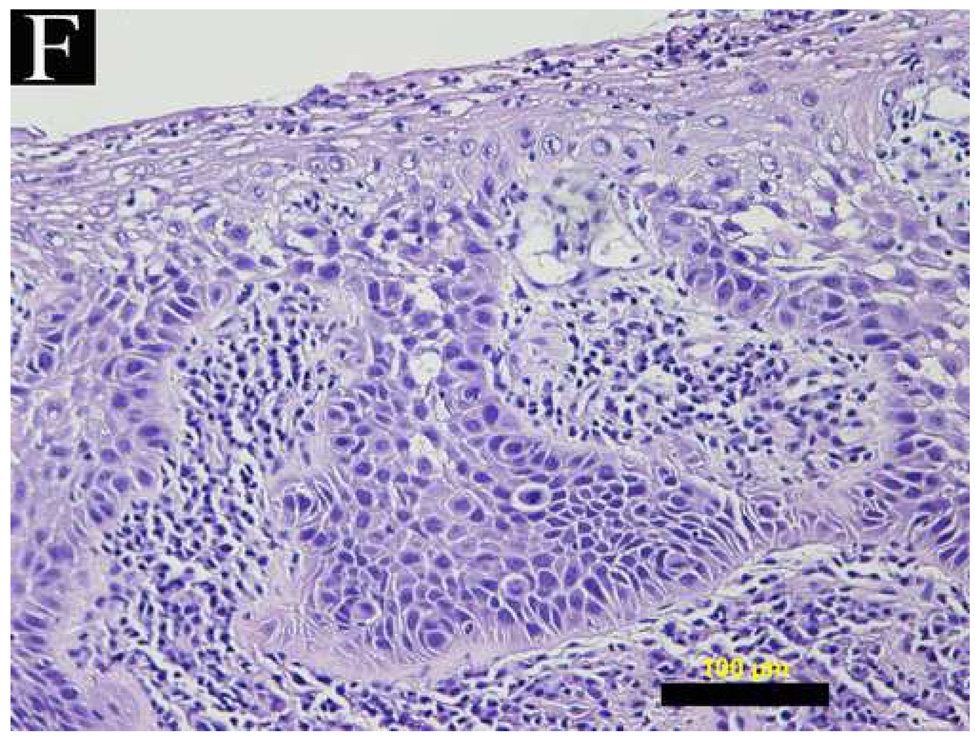
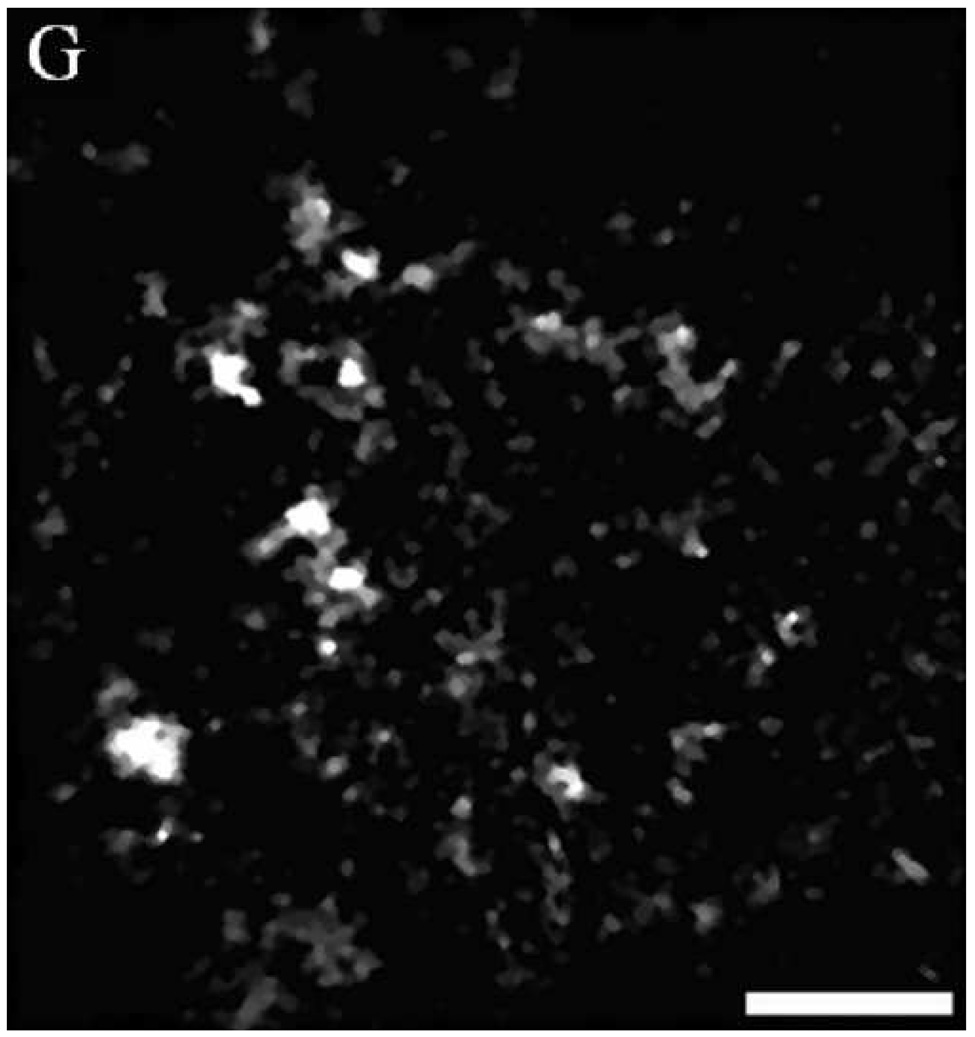
Figure 4 shows a comparison of reflectance confocal images acquired in vivo and H&E stained histology sections of normal, dysplastic, and SCC sites. The distribution of nuclei in confocal images (A and B) of normal ventral tongue site #8A compares well with the evenly spaced nuclei in the histology section (C) of a biopsy obtained from the imaging site. (Confocal video included in supplemental material.) In contrast to the transverse histology section, the confocal image plane is oriented parallel to the epithelial surface. Although the exact depths of the confocal images are unknown, Figure 4A is located in the superficial epithelium and Figure 4B is located in the intermediate epithelium. The white circular features in the confocal images are epithelial nuclei, as indicated with arrows. With low density of nuclei in the superficial layer and regular spacing of nuclei in the intermediate layer, this confocal image shows very typical organization and structure of normal epithelial tissue. The cell morphology and epithelial structure assessed by in vivo confocal microscopy compares well with histology.
Fig. 4.
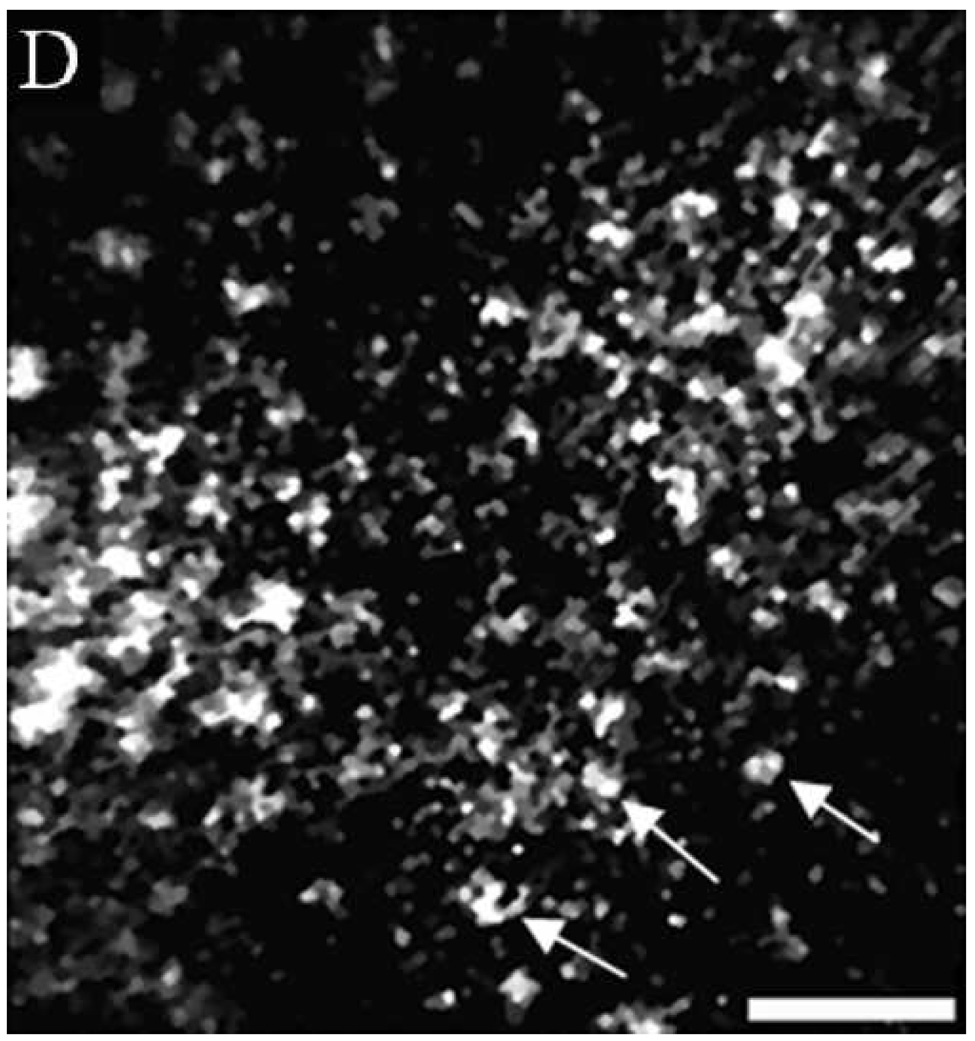
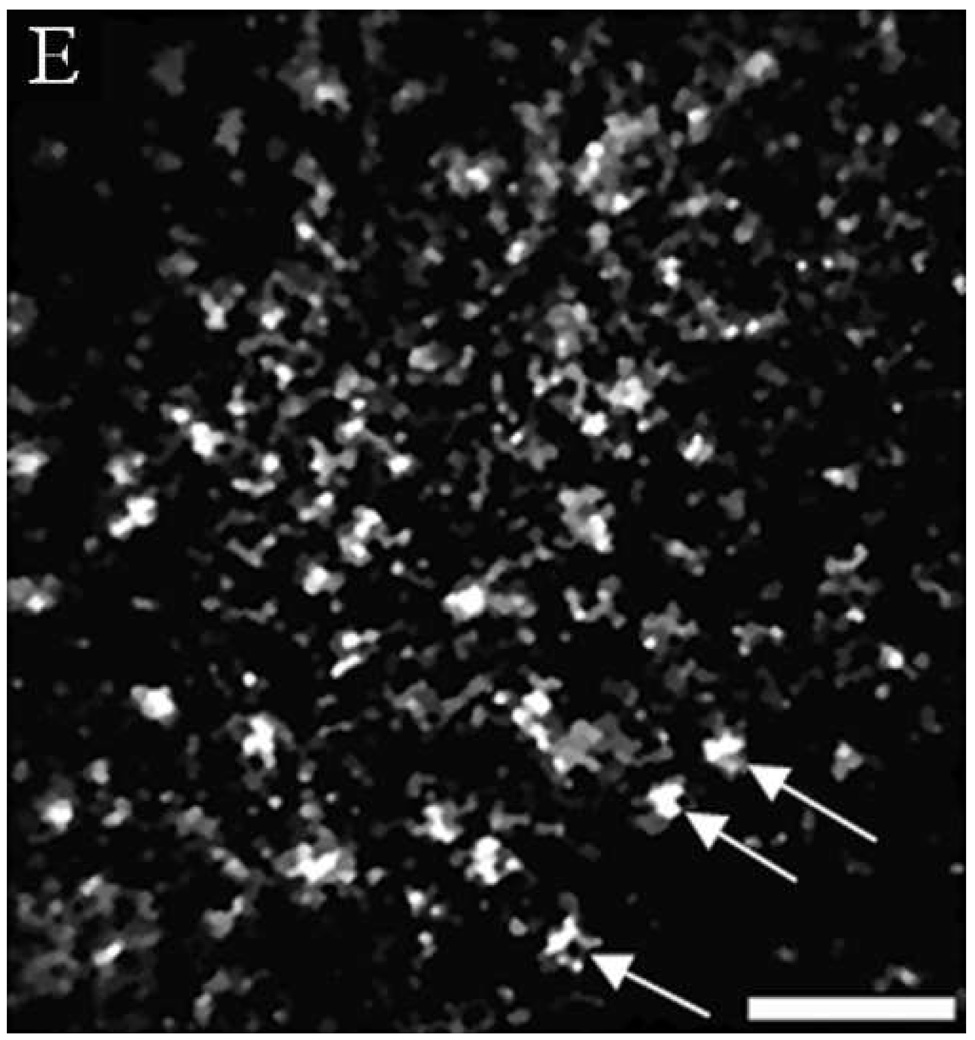
(A, B, D, E, G, H) In vivo confocal reflectance images and (C, F, I) photomicrographs of (A–C) normal ventral tongue site #8A, (D–F) floor of mouth epithelium pathologically diagnosed as moderate dysplasia (site #4B), and (G–I) tongue SCC lesion site #1A. Nuclei, which appear white in the confocal images and dark in the histology images, are identified by arrows in the confocal images. Epithelial cell nuclei are clearly visible and orderly spaced in (A) the superficial keratinizing epithelium and (B) the intermediate squamous epithelium of the normal site. (C) The histopathological diagnosis for this site was hyperplasia and hyperkeratosis. Nuclear crowding disorganization can be seen throughout the epithelium in confocal images of the (D) superficial and (E) intermediate layers and (F) histology section of dysplastic floor of mouth epithelium. (G, H) Confocal images of SCC tissue are characterized by disordered tissue structure. Clusters of nuclei within host stromal and inflammatory cells can be seen in the corresponding histology section (I) of tongue lesion site diagnosed as SCC. Scale bars: (A, B, D, E, G, H) 50 µm, (F) 100 µm, and (C, I) 200 µm.
Figures 4D and 4E show similar results for dysplastic tissue; nuclei are visible in the confocal images. The images from the floor of mouth epithelium from clinically abnormal and histologically dysplastic site #4B exhibit characteristic dense nuclei in the in vivo confocal images in both the superficial (Figure 4D) and intermediate (Figure 4E) layers of tissue and the histology section (Figure 4F). (Confocal video included in supplemental material.) In contrast, confocal images of SCC typically are very disorganized and have irregular structure. Nuclei can occasionally be distinguished in confocal images of SCC; however, it is usually difficult to identify or differentiate various tissue and cell structures. Figures 4G and 4H show examples of confocal images of tongue lesion site #1A. This site had a histologic diagnosis of SCC (Figure 4I).
Other tissue structures of interest were visible in some of the confocal images. We were able to image a section of a blood vessel, just beneath the epithelium of normal site #6B. We also detected structures that appeared to be keratin pearls. (Confocal videos of these sites are included in the supplemental material.)
DISCUSSION
Our results show that in vivo confocal microscopy has significant potential to noninvasively identify changes in tissue structure and cell morphology associated with neoplastic development in the oral mucosa. By qualitatively analyzing the confocal images obtained clinically, one can anticipate the histological appearance of the surgically removed, fixed, sectioned and stained tissue. For normal tissue, the nuclei in the confocal image appear regularly organized and spaced. As the tissue transitions to dysplasia, the confocal image shows disrupted and overlapping nuclei throughout the epithelium, as is seen in the histology sections of dysplastic tissue. The confocal images of cancerous oral tissue show markedly disorganized features. If nuclei are visible, they appear haphazardly distributed. As illustrated in Table 1, confocal images of SCC contain abnormal reflective tissue structure that has not been seen in any images of normal tissue. Obviously, these new images, which appear in black and white, with a different orientation and lower resolution, are not comparable to the standard H&E stained pathology images. However, they do not require biopsy, histological processing, and lateral microscopic examination. Continued advances in imaging technology should enable improvements in the quality and resolution of in vivo confocal microscopy images 32. These confocal images, which were acquired non-invasively and in real-time, demonstrate the potential clinical applications for non-invasively identifying changes in tissue structure and cell morphology associated with neoplastic development. Immediate assessment of the mucosal edges of resection for any residual microscopic disease by the surgeon at the time of cancer resection is another potential clinical application.
Confocal reflectance microscopy has previously been demonstrated as a method to image morphologic features of head and neck surgical specimens without fixing, staining, and sectioning of tissue required for histology and without the artifacts associated with histologic processing.33 As a real-time imaging modality, it has been proposed as an adjunct to frozen-section examination in intraoperative consultation.34 A study published by White et al. in 1999 comparing in vivo confocal reflectance imaging of normal oral tissue with histology established the foundation for in vivo confocal imaging for disease detection in oral mucosa.34 The confocal images presented by White et al. correlated well, both qualitatively and quantitatively, with histology. Epithelial cell nuclei and cell borders, circulating blood cells, and extracellular matrix were imaged with confocal microscopy. However, with the large microscope objective, access inside the oral cavity was limited to the lip and tongue mucosa. In this study, we presented in vivo images of various sites of normal, pre-neoplastic, and neoplastic oral tissue, including lip, anterior and posterior ventral tongue, buccal mucosa, floor of mouth, gum, and retrimolar trigone using a miniaturized confocal microscope. Similar to the in vivo confocal images presented previously by White, et al., our in vivo confocal images have correlated well to histology obtained from the imaging sites.
While conducting this study, we identified several technological difficulties that need to be resolved to make in vivo confocal imaging practical for diagnosing early neoplastic changes in the clinical setting. With the current fiber optic confocal microscope, we are restricted to imaging specific regions of the oral cavity. We were not able to image gingiva or hard palate sites due to the inability of the suction mechanism to move the rigid tissue through the focal plane. Modifications to move the miniature objective lens relative to the tissue, rather than the tissue relative to the lens, would be mechanically more complicated, but should allow imaging of these sites. Alternatively, a fixed plane imaging system that assessed tissue at one specific depth below the surface would provide cellular resolution of intact tissue but only limited information about all other epithelial layers. Another oral site that is difficult to image is the dorsal tongue. The highly keratinized and irregular surface is extremely reflective and difficult to image with safe laser power levels. Fortunately, tumors of the dorsal tongue are extremely rare, and the areas that have the highest incidence of SCC, the lateral and ventral surfaces, are easily imaged with the fiber optic confocal microscope.
We applied vinegar before imaging the oral mucosa to enhance contrast between nuclei and cytoplasm based on results of previous confocal imaging studies that used acetic acid as a contrast agent in imaging human epithelial tissue.5, 9, 31, 35 Indeed, while imaging normal volunteers before and after application of vinegar, significant improvement in the ability to visualize nuclei throughout the epithelium after vinegar application was achieved. However, we have found that the application of vinegar to the oral tissue is the most uncomfortable component of the imaging procedure. To alleviate the discomfort of the taste and smell, we used apple cider vinegar, which yields the same imaging results and was found to be more palatable. In this research protocol, we performed imaging on patients in the operating room under general anesthesia, in order to avoid patient discomfort from the vinegar and subsequent biopsy. However, for this technology to be clinically applicable, it will need to be acceptable to patients in the outpatient clinic setting.
In conclusion, we believe that in vivo fiber optic confocal reflectance microscopy has significant clinical potential to provide a sensitive and specific method for non-invasive detection of precancer and cancer in the oral cavity and this technology should be easily translated to other mucosal sites through the body.
Supplementary Material
In vivo confocal video of blood vessel identified below the epithelium in the histology section. Field of view approximately 230 × 230 µm.
In vivo confocal video of dysplastic floor of mouth epithelium demonstrating “dence nuclei” in dysplastic oral epithelium. Field of view approximately 230 × 230 µm.
In vivo confocal video scanning up through the epithelium showing round shadows. Corresponding histology included keratin pearls within the tissue. Field of view approximately 230 × 230 µm.
In vivo confocal video of normal ventral tongue site demonstrating “dispersed nuclei” in normal oral epithelium. Nuclei appear white in video. Field of view approximately 230 × 230 µm.
ACKNOWLEDGMENTS
This work was supported by NIH Grant R01 EB002179.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.American Cancer Society. Cancer Facts and Figures. Atlanta: American Cancer Society; 2007. 500807. [Google Scholar]
- 2.Wilson T. Confocal Microscopy. San Diego: Academic Press; 1990. [Google Scholar]
- 3.Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104(6):946–952. doi: 10.1111/1523-1747.ep12606215. [DOI] [PubMed] [Google Scholar]
- 4.Smithpeter CL, Dunn AK, Drezek R, Collier T, Richards-Kortum R. Near real time confocal microscopy of cultured amelanotic cells: sources of signal, contrast agents, and limits of contrast. J Biomed Opt. 1998;3(4):429–436. doi: 10.1117/1.429853. [DOI] [PubMed] [Google Scholar]
- 5.Collier T, Shen P, de Pradier B, Sung KB, Richards-Kortum R. Near real time confocal microscopy of amelanotic tissue: dynamics of aceto-whitening enable nuclear segmentation. Opt Express. 2000;6(2):40–48. doi: 10.1364/oe.6.000040. [DOI] [PubMed] [Google Scholar]
- 6.Petroll WM, Jester JV, Cavanagh HD. In vivo confocal imaging. International review of experimental pathology. 1996;36:93–129. [PubMed] [Google Scholar]
- 7.Rajadhyaksha M, Anderson RR, Webb RH. Video-rate confocal scanning laser microscope for imaging human tissues in vivo. Appl Opt. 1999;38(10):2105–2115. doi: 10.1364/ao.38.002105. [DOI] [PubMed] [Google Scholar]
- 8.Rajadhyaksha M, Gonzalez S, Zavislan JM, Anderson RR, Webb RH. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113(3):293–303. doi: 10.1046/j.1523-1747.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- 9.White WM, Rajadhyaksha M, Gonzalez S, Fabian RL, Anderson RR. Noninvasive imaging of human oral mucosa in vivo by confocal reflectance microscopy. Laryngoscope. 1999;109(10):1709–1717. doi: 10.1097/00005537-199910000-00029. [DOI] [PubMed] [Google Scholar]
- 10.Collier T, Lacy A, Richards-Kortum R, Malpica A, Follen M. Near real-time confocal microscopy of amelanotic tissue: detection of dysplasia in ex vivo cervical tissue. Acad Radiol. 2002;9(5):504–512. doi: 10.1016/s1076-6332(03)80326-4. [DOI] [PubMed] [Google Scholar]
- 11.Sung KB, Liang C, Descour M, Collier T, Follen M, Malpica A, et al. Near real time in vivo fibre optic confocal microscopy: sub-cellular structure resolved. J Microsc. 2002;207(2):137–145. doi: 10.1046/j.1365-2818.2002.01049.x. [DOI] [PubMed] [Google Scholar]
- 12.Boudoux C, Yun SH, Oh WY, White WM, Iftimia NV, Shishkov M, et al. Rapid wavelength-swept spectrally encoded confocal microscopy. Opt Express. 2005;13(20):8214–8221. doi: 10.1364/opex.13.008214. [DOI] [PubMed] [Google Scholar]
- 13.Dabbs T, Glass M. Fiber-optic confocal microscope: FOCON. Appl Opt. 1992;31(16):3030–3035. doi: 10.1364/AO.31.003030. [DOI] [PubMed] [Google Scholar]
- 14.Gmitro AF, Aziz D. Confocal microscopy through a fiber-optic imaging bundle. Opt Lett. 1993;18(8):565–567. doi: 10.1364/ol.18.000565. [DOI] [PubMed] [Google Scholar]
- 15.Sung KB, Liang C, Descour M, Collier T, Follen M, Richards-Kortum R. Fiber-optic confocal reflectance microscope with miniature objective for in vivo imaging of human tissues. IEEE Trans Biomed Eng. 2002;49(10):1168–1172. doi: 10.1109/TBME.2002.803524. [DOI] [PubMed] [Google Scholar]
- 16.Carlson KD, Chidley MD, Sung KB, Descour M, Gillenwater A, Follen M, et al. In vivo fiber-optic confocal reflectance microscope with an injection-molded plastic miniature objective lens. Appl Opt. 2005;44(10):1792–1797. doi: 10.1364/ao.44.001792. [DOI] [PubMed] [Google Scholar]
- 17.Knittel J, Schnieder L, Buess G, Messerschmidt B, Possner T. Endoscope-compatible confocal microscope using a gradient index-lens system. Opt Commun. 2001;188:267–273. [Google Scholar]
- 18.Dickensheets DL, Kino GS. Micromachined scanning confocal optical microscope. Opt Lett. 1996;21(10):764–766. doi: 10.1364/ol.21.000764. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez S, Rajadhyaksha M, Anderson RR. Non-invasive (real-time) imaging of histologic margin of a proliferative skin lesion in vivo. J Invest Dermatol. 1998;111(3):538–539. doi: 10.1046/j.1523-1747.1998.00309.x. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez S, Rajadhyaksha M, Rubinstein G, Anderson RR. Characterization of psoriasis in vivo by reflectance confocal microscopy. J Med. 1999;30(5–6):337–356. [PubMed] [Google Scholar]
- 21.Masters BR, Gonnord G, Piérard GE, Leveque In vivo confocal microscopy of human skin: a new design for cosmetology and dermatology. Scanning. 1996;18:351–355. doi: 10.1002/sca.1996.4950180505. [DOI] [PubMed] [Google Scholar]
- 22.Cavanagh HD, Jester JV, Essepian J, Shields W, Lemp MA. Confocal microscopy of the living eye. Clao J. 1990;16(1):65–73. [PubMed] [Google Scholar]
- 23.Masters BR, Thaer AA. In vivo human corneal confocal microscopy of identical fields of subepithelial nerve plexus, basal epithelial, and wing cells at different times. Microsc Res Tech. 1994;29(5):350–356. doi: 10.1002/jemt.1070290505. [DOI] [PubMed] [Google Scholar]
- 24.Sung KB, Richards-Kortum R, Follen M, Malpica A, Liang C, Descour M. Fiber optic confocal reflectance microscopy: a new real-time technique to view nuclear morphology in cervical squamous epithelium in vivo. Opt Express. 2003;11(24):3171–3181. doi: 10.1364/oe.11.003171. [DOI] [PubMed] [Google Scholar]
- 25.McLaren W, Tan J, Quinn M. Detection of cervical neoplasia using non-invasive fibre-optic confocal microscopy. Paper presented at: 5th International Multidisciplinary Congress EUROGIN 2003; April 13–16, 2003; Paris. 2003. [Google Scholar]
- 26.Zheng W, Harris M, Kho KW, Thong PS, Hibbs A, Olivo M, et al. Confocal endomicroscopic imaging of normal and neoplastic human tongue tissue using ALA-induced-PPIX fluorescence: a preliminary study. Oncol Rep. 2004;12(2):397–401. [PubMed] [Google Scholar]
- 27.Thong PS, Olivo M, Kho KW, Zheng W, Mancer K, Harris M, Soo KC. Laser confocal endomicroscopy as a novel technique for fluorescence diagnostic imaging of the oral cavity. J Biomed Opt. 2007;12 doi: 10.1117/1.2710193. 014007. [DOI] [PubMed] [Google Scholar]
- 28.Clark AL, Gillenwater AM, Collier TG, Alizadeh-Naderi R, El-Naggar AK, Richards-Kortum RR. Confocal microscopy for real-time detection of oral cavity neoplasia. Clin Cancer Res. 2003;9(13):4714–4721. [PubMed] [Google Scholar]
- 29.Liang C, Sung KB, Richards-Kortum RR, Descour MR. Design of a high-numerical-aperture miniature microscope objective for an endoscopic fiber confocal reflectance microscope. Appl Opt. 2002;41(22):4603–4610. doi: 10.1364/ao.41.004603. [DOI] [PubMed] [Google Scholar]
- 30.Balas CJ, Themelis GC, Prokopakis EP, Orfanudaki I, Koumantakis E, Helidonis ES. In vivo detection and staging of epithelial dysplasias and malignancies based on the quantitative assessment of acetic acid-tissue interaction kinetics. J Photochem Photobiol B. 1999;53(1–3):153–157. doi: 10.1016/s1011-1344(99)00133-5. [DOI] [PubMed] [Google Scholar]
- 31.Drezek R, Collier T, Brookner C, Malpica A, Lotan R, Richards-Kortum R. Laser scanning confocal microscopy of cervical tissue before and after application of acetic acid. AM J Obstet Gynecol. 2000;182:1135–1139. doi: 10.1067/mob.2000.104844. [DOI] [PubMed] [Google Scholar]
- 32.Luck BL, Carlson KD, Bovik AC, Richards-Kortum RR. An image model and segmentation algorithm for reflectance confocal images of in vivo cervical tissue. IEEE Trans. Image Proc. 2005;14(9):1265–1276. doi: 10.1109/tip.2005.852460. [DOI] [PubMed] [Google Scholar]
- 33.Yelin D, Rizvi I, White WM, Motz JT, Hasan T, Bouma BE, et al. Three-dimensional miniature endoscopy. Nature. 2006;443(7113):765. doi: 10.1038/443765a. [DOI] [PubMed] [Google Scholar]
- 34.White WM, Baldassano M, Rajadhyaksha M, Gonzalez S, Tearney GJ, Anderson RR, et al. Confocal reflectance imaging of head and neck surgical specimens. A comparison with histologic analysis. Arch Otolaryngol Head Neck Surg. 2004;130(8):923–928. doi: 10.1001/archotol.130.8.923. [DOI] [PubMed] [Google Scholar]
- 35.Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9(2):323–331. doi: 10.1117/1.1646175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vivo confocal video of blood vessel identified below the epithelium in the histology section. Field of view approximately 230 × 230 µm.
In vivo confocal video of dysplastic floor of mouth epithelium demonstrating “dence nuclei” in dysplastic oral epithelium. Field of view approximately 230 × 230 µm.
In vivo confocal video scanning up through the epithelium showing round shadows. Corresponding histology included keratin pearls within the tissue. Field of view approximately 230 × 230 µm.
In vivo confocal video of normal ventral tongue site demonstrating “dispersed nuclei” in normal oral epithelium. Nuclei appear white in video. Field of view approximately 230 × 230 µm.