Abstract
A protective immune response to a respiratory viral infection requires a series of coordinated cellular and molecular responses. We have previously demonstrated that increased expression of airway epithelial cell interleukin (IL)-12 p80, a macrophage chemoattractant, is associated with human respiratory viral infection and mediates post-viral mortality in the mouse. To better understand the role of IL-12 p80-dependent macrophage chemotaxis in mediating viral immunity, we generated a transgenic mouse strain utilizing a promoter to drive IL-12 p40 gene expression in the airway epithelium. This transgenic strain secreted biologically active IL-12 p80 in a lung-specific manner, and demonstrated a selective increase in the number of resident, unactivated airway macrophages at baseline. Following infection with a sublethal dose of mouse parainfluenza virus type 1 (Sendai virus), the transgenic mice demonstrated an earlier peak and decline in the number of airway inflammatory cells. The transgenic mice were resistant to a lethal dose of virus and this viral resistance was dependent on the increased number of airway macrophages at baseline as partial depletion prior to infection abrogated this phenotype. The survival advantage in the transgenic mice was independent of viral load but was associated with a more rapid decline in the number of airway inflammatory cells and concentrations of multiple chemokines including the CC chemokine ligand 2 (CCL2)/JE, CCL3/macrophage inflammatory protein (MIP)-1α, CCL4/MIP-1β, and CCL5/RANTES. Collectively, these results suggest that IL-12 p80-driven increases in the number of resident airway macrophages prime the host for a protective immune response that can confer increased survival following a lethal respiratory viral infection.
Keywords: infection, lung immunology/disease, macrophages/monocytes, transgenics/knockouts, viruses/viral immunity
Introduction
An appropriate immune response is required to protect the host from infectious pathogens; however, an exuberant response can produce detrimental immunopathology. In general, this response is tightly regulated by the coordinated expression of inflammatory mediators from immune and non-immune cells. For the specific case of the immune response to a respiratory viral infection, we have previously shown that both airway epithelial cells and airway macrophages provide early signals for a protective antiviral immune response.1–4 In the context of human airway disease, we have demonstrated induction of interleukin (IL)-12 p80 in the airway epithelial cell during a respiratory viral infection as well as in airway inflammatory diseases such as asthma and lung allograft rejection. Interestingly, in these conditions there was a positive correlation between the concentration of IL-12 p80 and the degree of airway macrophage accumulation.1,2,5 Collectively, these findings demonstrated that airway epithelial cell IL-12 p80 expression and the consequent recruitment of macrophages are critical regulatory processes for an appropriate antiviral immune response and suggested that IL-12 p80 overexpression may contribute to the excessive inflammation seen in certain diseases.
IL-12 p80 is one of four cytokines that contain the IL-12p40 subunit: IL-12 (a p40 and p35 heterodimer), IL-12 p80 (a p40 homodimer), p40 (a p40 monomer), and IL-23 (a p40 and p19 heterodimer).6–14 Our laboratory and others have demonstrated that IL-12 p80 interacts directly with IL-12 receptor β1 (IL-12Rβ1) to function as a macrophage chemoattractant as well as a competitive antagonist of IL-12.1,2,12,13,15–18 In this regard, we previously showed that airway epithelial cell expression of IL-12 p80 was induced following a respiratory viral infection in the mouse and modulation of its function altered post-viral macrophage accumulation and mortality. Specifically, IL-12 p35-deficient mice that overexpressed IL-12 p80 during an infection mounted a more robust immune response that was associated with increased numbers of airway macrophages and an increased mortality.1 Conversely, IL-12 Rβ1-deficient mice that are incapable of IL-12 p80-dependent macrophage chemotaxis displayed a blunted immune response, decreased numbers of airway macrophages, and lower mortality.2 Although these experiments demonstrated that IL-12 p80-dependent macrophage chemotaxis is a critical determinant of a protective antiviral immune response, they were performed in mice with genetic deletions in IL-12 family members or their receptors. Accordingly, the role of IL-12 p80 overexpression in the antiviral immune response in the setting of undisturbed IL-12 family member genes remains to be determined.
The present studies were undertaken to better define the influence of airway epithelial cell IL-12 p80 overexpression on macrophage accumulation and the antiviral immune response in the context of intact IL-12 family member expression. To accomplish this, we generated and characterized a transgenic mouse strain that utilized the rat Clara Cell 10-kD protein (CC-10) promoter to selectively drive IL-12 p40 gene expression in airway epithelial cells. This transgenic overexpression of IL-12 p40 resulted in a lung-specific increase in IL-12 p80, which specifically increased the number of resident airway macrophages. We then used this transgenic strain as a tool to examine the role of enhanced IL-12 p80-dependent macrophage recruitment on the accumulation of immune cells during a viral challenge. As increased expression of IL-12 p80 in human airway epithelial cells was associated with airway inflammatory conditions and IL-12 p80-driven macrophage accumulation during a viral infection increased mortality in the mouse, we anticipated that the transgenic mice would also display increased post-viral inflammation and mortality. Indeed, the transgenic mice demonstrated an earlier peak in post-viral immune cell accumulation; however, this exaggerated early response was also associated with an earlier decline in the inflammatory response and an unexpected decrease in post-viral mortality. Taken together, these results suggest that overexpression of IL-12 p80 prior to a viral infection increases the number of resident airway macrophages and this primes the host for a protective response against a lethal respiratory viral infection.
Materials and methods
Generation of IL-12 p80/p40 transgenic strain
The mouse IL-12 p40 cDNA was obtained from the plasmid pBSK-mp40 [American Type Culture Collection (ATCC), Rockville, MD; #87595]. Amino acids 2 and 159 were changed to a cystine and serine, respectively, to achieve exact protein homology with the murine IL-12 p40 GenBank entry (accession number M86671). A HindIII site and modified Kozak sequence (CACC) were inserted immediately upstream of the mouse p40 start codon and a BamHI site was inserted immediately downstream of the mouse p40 stop codon by incorporating the appropriate nucleotide sequences into polymerase chain reaction (PCR) primers.19 The mouse p40 cDNA was liberated with HindIII and BamHI digestion and inserted into a HindIII- and BamHI-digested pcDNA3.1/Zeo vector creating pcDNA3.1/Zeo/mp40. A 2·2-kb fragment of the rat Clara Cell 10-kD protein (CC-10) promoter was HindIII-released from the pcDNA3.1/Zeo rat CC-10 plasmid (a gift from J. Whitsett, University of Cincinnati College of Medicine, Cincinnati, OH) and inserted into the HindIII site of pcDNA3.1/Zeo mp40 to generate pcDNA3.1/Zeo/CC-10/mp40.20 An 862-bp fragment containing the SV40 intron and polyadenylation sequence was NotI- and XhoI-released from the pBSKII SV40IPA plasmid and inserted into the NotI and XhoI sites of pcDNA3.1/Zeo/CCSP/mp40 to generate pcDNA3.1/Zeo/CC-10/mp40/SV40IPA. Correct orientation of the CC-10 promoter, mouse IL-12 p40 cDNA, and the SV40 intron and polyadenylation sequence was verified by restriction mapping and sequencing. The 4·5-kb transgene fragment was gel-purified, resuspended at 2 μg/ml in transgene buffer [5 mm Tris, pH 7·4, 0·25 mm ethylenediaminetetraacetic acid (EDTA) and 5 mm NaCl], microinjected into the pronuclei of C57BL/6J zygotes, and transferred into pseudopregnant Swiss Webster foster mothers as described previously21. Potential founder pups were screened for incorporation of the transgene using genomic tail DNA and PCR primers that amplified a 190-bp fragment of the SV40 intron and polyadenylation sequence (upstream 5′-GCCGCGGATCTTTGTGAAGGAACCTTACTT-3′ and downstream 5′-ATTCCACCACTGCTCCCATTCATCAGTTCC-3′). Germline incorporation was confirmed by identification of transgene transmission in pups from a cross between a transgene-positive and wild-type C57BL/6J mouse. Of 83 potential founder pups, four demonstrated incorporation of the transgene fragment, two of these demonstrated germline incorporation, and one demonstrated increased IL-12 p40 protein expression in the bronchoalveolar lavage (BAL) fluid. This male founder was used to generate the IL-12 p80/p40 transgenic strain and experiments were performed with age- and gender-matched transgene-positive (referred to as p80/p40 Tg) and wild-type transgene-negative (referred to as wild-type) littermates as controls. A cross between transgene-positive and wild-type C57BL/6J mice resulted in a normal number of live births and transgene transmission in a gender-independent heterozygote manner (27% transgene-negative males, 24% transgene-negative females, 26% transgene-positive males, and 22% transgene-positive females). All pups continued to develop normally through adulthood and survival at 6 months was independent of transgene expression. Mice were maintained under pathogen-free conditions for study at 7 weeks of age. Sentinel mice and experimental control mice for Sendai virus infection were handled identically to Sendai virus-infected mice and exhibited no serological or histological evidence of exposure to 11 rodent pathogens (including Sendai virus). The Animal Studies Committee of Washington University School of Medicine approved all animal studies.
Mouse specimen harvest
BAL collection, lung tissue harvest and procurement, peritoneal macrophage harvest, and intranasal inoculation of Sendai virus were performed as described previously.1–3 Briefly, for BAL and lung fixation, mice underwent intraperitoneal (i.p.) injection with ketamine (80 mg/kg) and xylazine (16 mg/kg) followed by anterior neck dissection. The trachea was cannulated with a 22-gauge angiocatheter and lungs lavaged with 1 ml of phosphate-buffered saline (PBS) for BAL, fixed (25 cm water pressure) with 10% formalin for haematoxylin and eosin staining or immunolabelling, or removed and placed in RNA Later (Ambion, Austin, TX; #76104) for RNA analysis. For collection of peritoneal macrophages mice underwent i.p. injection with 1·0 ml of sterile 3% [weight/volume (w/v)] thioglycollate (Sigma-Aldrich, St. Louis, MO). Five days later the peritoneal cavity was flushed with 10 ml of Dulbecco’s modified Eagle’s minimal essential medium (DMEM) containing 0·01% bovine serum albumin (BSA) resulting in a cell population that contained > 98% macrophages using light microscopic criteria.2 Mice underwent anaesthesia and intranasal inoculation with Sendai virus (Fushimi strain; ATCC #VR-105) or ultraviolet (UV)-inactivated Sendai virus at 5000 or 50 000 egg infectious dose 50% (EID50) diluted in 30 μl of PBS. For collection of whole blood, anaesthetized mice underwent a terminal cardiac puncture using a 25-gauge needle and syringe without heparin for serum or with heparin for analysis of blood leucocytes. Serum was isolated from whole blood using a serum separator tube according to the manufacturer’s recommendation (BD Biosciences, San Diego, CA; #365956). To generate a single-cell suspension of parenchyma lung cells for flow cytometric analysis, BAL was performed and then the lungs were inflated with complete DMEM supplemented with hyaluronidase (0·01% w/v), collagenase I (250 U/ml), and DNase I (50 U/ml), minced, passed through a cell strainer, and treated with hypotonic saline to remove red blood cells. The total viable lung cell number was determined as described previously.22
BAL and immune cell analysis
BAL fluid was centrifuged and the cell-free supernatant was collected and stored for cytokine analysis, while the cell pellet was resuspended in 1 ml of PBS for total cell count, cytospin preparation, and Wright-Giemsa staining. Two blinded observers determined the immune cell differential using standard light microscopy criteria as described previously.1–3 Murine IL-12, IL-12 p80/p40 and IL-23 were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits. Sensitivity for the mouse IL-12 p80/p40 ELISA kit (R&D Systems Inc., Minneapolis, MN) was 4 pg/ml. We noted a 20% cross-reactivity to mouse IL-12 at 1000 pg/ml, and we measured 6·4 and 5·3% cross-reactivity to recombinant mouse IL-23 at 250 and 1500 pg/ml, respectively. Sensitivity for the mouse IL-12 ELISA kit (R&D Systems Inc.) was 2·5 pg/ml with no cross-reactivity to mouse p80 or IL-23 at 50 000 pg/ml. Sensitivity for the mouse IL-23 ELISA kit (eBioscience, San Diego, CA) was 15 pg/ml with no cross-reactivity to mouse p80 or IL-12 at 100 ng/ml. For detection of murine IL-12 family members by western immunoblotting, cell-free BAL fluid was concentrated 10-fold using a Centriprep YM-3 concentrator (Millipore Corp., Billerica, MA) and subjected to 10% polyacrylamide gel electrophoresis (PAGE) under non-reducing conditions; protein was then transferred to polyvinylidene difluoride (PVDF) membrane for blotting against rat anti-mouse IL-12 p40 immunoglobulin G (IgG) (hybridoma clone 17·8; 0·1 μg/ml; a gift from G. Trinchieri, National Cancer Institute, Frederick, MD) for 1 hr at 25° followed by incubation with goat anti-rat IgG conjugated to peroxidase (1 : 5000 v/v; Boehringer Mannheim, Indianapolis, IN; #605 190) and peroxidase detection with enhanced chemiluminescence (ECL-Plus; Amersham, Piscataway, NJ). To detect mouse IgG, concentrated BAL was subjected to 10% PAGE under reducing (10 mm dithiothreitol) conditions and incubated with goat anti-mouse IgG conjugated to peroxidase (1 : 5000 v/v; Roche, Indianapolis, IN; #100831), and peroxidase detection by enhanced chemiluminescence (ECL-Plus). Image acquisition was performed using a UMAX Powerlook 1120 scanner (UMAX Data Systems, Inc., Taipei, Taiwan). Peripheral blood leucocyte counts were performed using an automated veterinary haematological analyser with a preprogrammed murine calibration mode (Hemavet 950FS; Drew Scientific, Waterbury, CT). To quantify immune cells per lung by flow cytometry, the total lung cell number was multiplied by the percentage of total cells that were conventional dendritic cells (appropriate scatter characteristics with high CD11c+ and B220−), plasmacytoid dendritic cells (low CD11c expression and high PDCA-1+ and B220+) or T cells (CD4+ or CD8+) as previously described.4,22
To quantify multiple inflammatory mediators in cell-free BAL from uninfected or Sendai-infected mice, we analysed cell-free supernatant in a multiplex flow cytometry-based assay according to the manufacturer’s recommendations (Bio-Rad Laboratories, Hercules, CA). Unique beads each conjugated with a distinct capture antibody were incubated with 50 μl of BAL and 0·5% BSA or a serially diluted standard mix with a known concentration of all measured inflammatory mediators. The beads were then washed and sequentially incubated with a detection antibody mixture that contained cytokine-specific biotin-conjugated antibodies and then streptavidin-phycoerythrin (PE). The PE intensity in each of the unique bead regions from the serially diluted standards was used to generate standard curves to calculate the concentration of each inflammatory mediator in the BAL samples. The detection limit for the Bio-plex mouse cytokine 23-plex panel (Bio-Rad) is: IL-1α, 2 pg/ml; IL-1β, 2 pg/ml; IL-2, 3 pg/ml; IL-3, 2 pg/ml; IL-4, 3 pg/ml; IL-5, 2 pg/ml; IL-6, 2 pg/ml; IL-9, 15 pg/ml; IL-10, 2 pg/ml; IL-12 (p40), 2 pg/ml; IL-12 (p70), 4 pg/ml; IL-13, 9 pg/ml; IL-17, 1 pg/ml; eotaxin, 148 pg/ml; granulocyte colony-stimulating factor (G-CSF), 1 pg/ml; granulocyte–macrophage colony-stimulating factor (GM-CSF), 7 pg/ml; interferon (IFN)-γ, 6 pg/ml; keratinocyte-derived chemokine (KC), 3 pg/ml; CC chemokine ligand 2 (CCL2)/JE, 14 pg/ml; CCL3/macrophage inflammatory protein (MIP)-1α, 24 pg/ml; CCL4/MIP-1β, 2 pg/ml; CCL5/RANTES, 5 pg/ml; and tumour necrosis factor (TNF)-α, 6 pg/ml.
Tissue immunolabelling
To identify lung macrophages, tissue sections were blocked with non-immune rabbit serum, and then incubated with rat anti-mouse Mac-3 IgG (2 μg/ml; clone M3/84; BD PharMingen, San Diego, CA) for 18 hr at 4°, biotinylated rabbit anti-rat IgG and streptavidin-conjugated horseradish peroxidase (HRP) complex, and detected with the brown chromogen 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA).1,23,24 For Sendai virus and lung macrophage dual identification, tissue sections were sequentially incubated with streptavidin and biotin blocking solution (Vector Laboratories) and 2% fish gel [diluted in PBS with 0·2% Triton × (PBST)] for 1 hr at 25°, and chicken anti-Sendai virus serum (1 : 500 v/v; Charles River, Wilmington, MA; #529501) and rat anti-mouse CD68 conjugated to biotin (2 μg/ml; clone FA-11; Serotec, Raleigh, NC; #MCA1957B) or rat IgG control (2 μg/ml; Pharmingen, San Diego, CA; #11011D), all for 16 hr at 4°. Tissue was then incubated with donkey anti-chicken immunoglobulin conjugated with fluorescein isothiocyanate (FITC; 1 : 200 v/v; Jackson Immunoresearch, West Grove, PA; #703-096-155) and Alexa Fluor 555 conjugated to streptavidin (1 : 700 v/v; Invitrogen, Carlsbad, CA; #S-32355) diluted in 0·2% PBST with 3% BSA for 30 min at 25°. Cover-slips were mounted with vectastain (Vector Laboratories) and tissue images were obtained with an epifluorescent microscope (Model BX-51; Olympus, Center Valley, PA) interfaced to a digital photomicrographic system (Optronix CCD camera, Orlando, FL and MagnaFire2.1C software, Melville, NY). Final images were pseudocoloured and merged using MagnaFire2.1C and formatted using Adobe Photoshop CS and Illustrator CS (Adobe Systems, San Jose, CA).
Alveolar macrophage depletion
To test the effect of partial alveolar macrophage depletion in the p80/p40 Tg mice on Sendai viral mortality, we administered intranasal clodronate-liposomes to partially depleted airway macrophages as previously described.4,25,26 As the p80/p40 Tg mice contained approximately twice as many alveolar macrophages as wild-type mice, we titrated the clodronate-liposome dose in the p80/p40 Tg strain to deplete alveolar macrophages by 50%. Following intraperitoneal anaesthesia, intranasal administration of 0·5 mg of clodronate-liposome compared with 0·5 mg of empty control liposome (both in 30 μl of PBS) resulted in a 52% reduction in the number of alveolar macrophages 5 days later. Accordingly, we administered 0·5 mg of clodronate-liposomes or empty control liposomes intranasally 5 days prior to inoculation of Sendai virus.
Quantification of Sendai virus
To quantify Sendai virus, a plaque assay was performed as described previously.3 Confluent Vero E6 cells in six-well plates were incubated with serial dilutions of whole-lung homogenate for 2 hr at 37°. Following two PBS washes, wells were covered with DMEM containing 0·5% agarose and 2 μg/ml trypsin. After 4 days at 37°, cells were fixed with 10% formalin for 1 hr at 25°, agar overlay was removed, cells were incubated with 0·5% crystal violet for 10 min at 25°, plaque-forming units (PFU) were enumerated from duplicate wells and the mean value for two observers was reported as PFU per gram of lung tissue. The quantity of Sendai virus-specific RNA was determined from whole lung using a TaqMan one-step fluorogenic RT-PCR reaction according to the manufacturer’s recommendation (Applied Biosystems, Foster City, CA).3 Lung tissue was placed in RNA Later, homogenized with stainless steel beads for 3 min (Biospect Products Inc., Bartlesville, OK), and column purified with an RNeasy mini kit according to the manufacturer’s recommendations (Qiagen, Alencia, CA). Cellular samples were placed in Stat-60 solution and RNA was purified according to the manufacturer’s recommendations (Tel-Test, Inc., Friendswood, TX). Duplicate serial 10-fold dilutions of total RNA from Sendai-infected lung tissue underwent one-step fluorogenic RT-PCR detection of Sendai virus nucleocapsid protein (upstream primer 5′-TCCACCCTGAGGAGCAGG-3′; downstream primer 5′-ACCCGGCCATCGTGAACT-3′; probe 5′-6FAM-TGGCAGCAAAGCAAAGGGTCTGGA-TAMRA-3′) and murine GAPDH-specific RNA (proprietary primer/probe combination from Applied Biosystems; #MM99999915G1) to construct standard curves. Sendai values were calculated as the mean of duplicate samples from reactions with a cycle threshold between 20 and 25 and final results were normalized to GAPDH and reported as the Sendai/GAPDH ratio.
Statistical analysis
Comparisons of BAL cytokine measurements, BAL cell counts, chemotaxis results, Sendai PFU/g of lung tissue, and Sendai/GADPH ratio between wild-type and p80/p40 Tg mice were made with the Mann–Whitney test. Survival rates for wild-type and p80/p40 Tg mice were determined from multiple individual experiments and the composite curves were compared using the Wilcoxon rank-sum test. For all tests a P-value less than 0·05 was considered significant and data were analysed with spss 13 (SPSS, Chicago, IL).
Results
p80/p40 transgenic mice demonstrate lung-specific expression of biologically active IL-12 p80
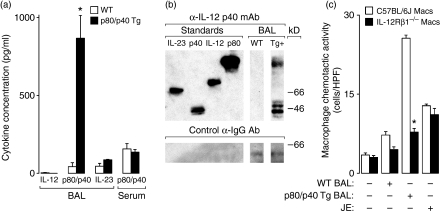
As airway epithelial cell IL-12 p80 (an IL-12 p40 homodimer) expression is induced in humans during a respiratory viral infection and is a critical determinant of post-viral mortality in mice, we set out to examine the effects of lung-specific IL-12 p40 overexpression on the antiviral immune response. Accordingly, we generated a transgenic mouse strain using the lung-specific rat CC-10 promoter to drive murine IL-12 p40 protein expression in C57BL/6J mice. In view of the fact that IL-12 p40 can be secreted as a monomer (p40), homodimer (p80) or heterodimer (IL-12 and IL-23), we compared IL-12 family member expression in the BAL fluid from wild-type transgene-negative and transgene-positive (p80/p40 Tg) littermates. We observed a specific increase in the expression of IL-12 p80/p40, but not IL-12 or IL-23, in 7-week-old mice that was still present in 6-month-old mice. This increase in the expression of IL-12 p80/p40 is approximately twofold more than observed after infection with a sublethal dose of Sendai virus.1 The IL-12 p80/p40 expression was compartmentalized to the lung because the serum concentration was unchanged in the transgenic mice (Fig. 1a). Western blot analysis of the BAL indicated expression of IL-12 p80 and p40, but not IL-12 or IL-23 (Fig. 1b). To determine if the IL-12 p80 was biologically active, we relied on the previous observation that IL-12 p80, but not p40, acted as a macrophage chemoattractant and that IL-12Rβ1 expression on the macrophage was necessary for this chemoattraction to occur.2 The BAL fluid from p80/p40 Tg mice resulted in chemotaxis of macrophages isolated from wild-type C57BL/6J mice, but not IL-12Rβ1 deficient mice (Fig. 1c). On the basis of these findings, we conclude that the p80/p40 Tg mice expressed biologically active IL-12 p80 in a lung-specific manner.
Figure 1.
p80/p40 transgenic mice demonstrate lung-specific expression of biologically active interleukin (IL)-12 p80. (a) Bronchoalveolar lavage (BAL) or serum from 7-week-old wild-type (WT) or IL-12 p80/p40 transgenic (p80/p40 Tg) littermates was analysed for IL-12, IL-12 p80/40 and IL-23 by enzyme-linked immunosorbent assay (ELISA). Values represent mean ± standard deviation (SD) of duplicate samples (n = 5–6). (b) Recombinant IL-23, IL-12 p40 monomer (p40), IL-12 and IL-12 p80 (p80) standards, and concentrated BAL fluid from WT and p80/p40 Tg (Tg+) mice were subjected to western analysis against anti-IL-12 p40 antibody (Ab) under non-reducing conditions (top) or anti-mouse immunoglobulin G (IgG) Ab under reducing conditions (bottom). (c) Wild-type C57BL/6J or C57BL/6J IL-12 receptor β-1-deficient (IL-12Rβ1 −/−) peritoneal macrophages were placed in the upper compartment of a modified Boyden chamber apparatus. Media, concentrated BAL from WT or p80/p40 Tg mice, or media with the chemoattractant JE (10−8 m) were placed in the lower compartment and the apparatus was incubated for 2 hr at 37°. Migrating cells were counted and values represent mean ± SD for five high-power fields (HDF) (magnification × 400) (n = 9–12). For panels (b) and (d) a significant difference from WT is indicated (*P < 0·05).
Increased resident airway macrophages in the p80/p40 transgenic mice
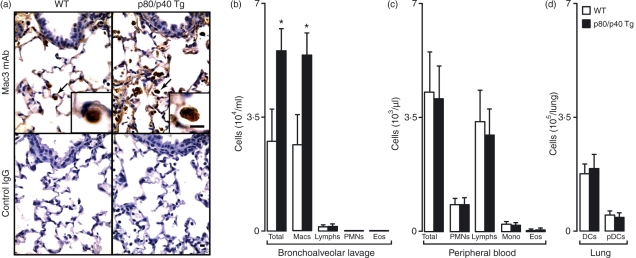
We next examined the lungs of the p80/p40 Tg mice to determine whether any changes in lung development or immune cell composition had occurred. We noted normal airway, vascular and alveolar architecture; however, consistent with the observation that IL-12 p80 is a macrophage chemoattractant, we observed an increase in the number of airway macrophages (Fig. 2a). Quantification of BAL immune cells revealed a selective increase in airway macrophages, but not lymphocytes, neutrophils or eosinophils (Fig. 2b). No differences were noted in total peripheral blood leucocyte counts or the number of peripheral blood monocytes, lymphocytes, neutrophils or eosinophils in the p80/p40 Tg mice. Thus, the increased lung macrophage number was not explained by an increase in peripheral blood monocytes or any other type of leucocyte (Fig. 2c).
Figure 2.
Increased numbers of resident airway macrophages in the p80/p40 transgenic mice. (a) Lung tissue from wild-type (WT) or p80/p40 transgenic (p80/p40 Tg) littermates was immunolabelled with anti-Mac3 antibody (Ab) (top row) or control immunoglobulin G (IgG) (bottom row) and detected with 3,3′-diaminobenzidine (brown colour). Arrows indicate alveolar macrophages shown at higher magnification in the insert. Representative photomicrographs are shown (n = 5). Bar, 20 μm. (b) Bronchoalveolar lavage (BAL) fluid from WT or p80/p40 Tg littermates was analysed for total and differential cell numbers. Values represent mean ± standard deviation (SD) (n = 4–6). (c) Blood from WT or p80/p40 Tg littermates underwent total and differential cell counts using an automated veterinary haematological analyser. Values represent mean ± SD (n = 10). (d) Total lung cells from WT or p80/p40 Tg littermate lungs were counted, immunolabelled, and examined by flow cytometry for conventional dendritic cells (DCs; based on scatter characteristics, high CD11c expression and B220−) and plasmacytoid dendritic cells (pDCs; scatter characteristics, low CD11c+ and high B220 expression). Values represent mean ± SD of cells per lung (n = 4). Macs, macrophages; Lymphs, lymphocytes; Mono, monocytes; PMNs, polymorphonuclear leucocytes; Eos, eosinophils.
To determine if the increased number of airway macrophages was associated with an alteration in dendritic cell and lymphocyte accumulation in the lung, we quantified lung dendritic cells (conventional and plasmacytoid) and T cells (CD4+ and CD8+). We found no changes with respect to dendritic cells or T cells in the p80/p40 Tg mice (Fig. 2d and data not shown). As activated macrophages can secrete inflammatory mediators, we next aimed to investigate the activation status of airway macrophages in the p80/p40 Tg mice by quantifying multiple inflammatory mediators in the BAL. Despite the increased number of airway macrophages in the p80/p40 Tg mice, the BAL concentrations of 23 cytokines and chemokines in p80/p40 Tg mice were at or below the lower limit of detection and remained unchanged from wild-type concentrations (data not shown). In further support of the conclusion that the macrophages were not activated, the histological appearance of the lungs from 6-month-old p80/p40 Tg mice was normal, except for an increase in the number of airway macrophages (data not shown). Collectively, these results indicate lung-specific expression of IL-12 p80 produced a selective increase in the number of resident unactivated lung macrophages.
Enhanced resolution of viral-dependent airway inflammation in the p80/p40 transgenic mice
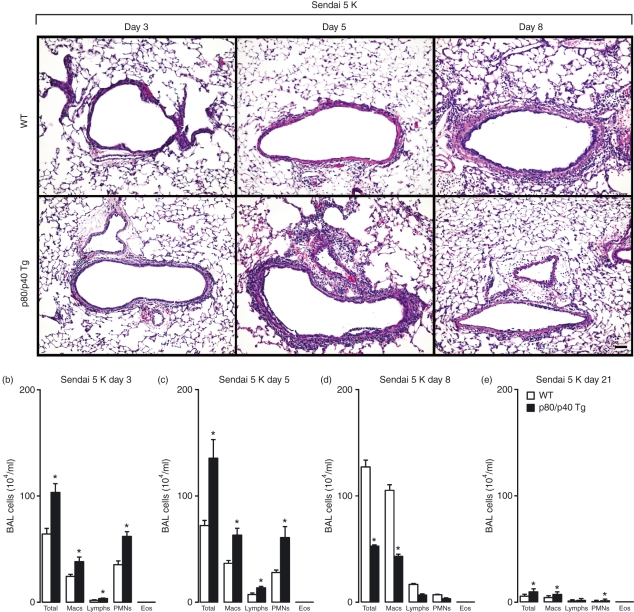
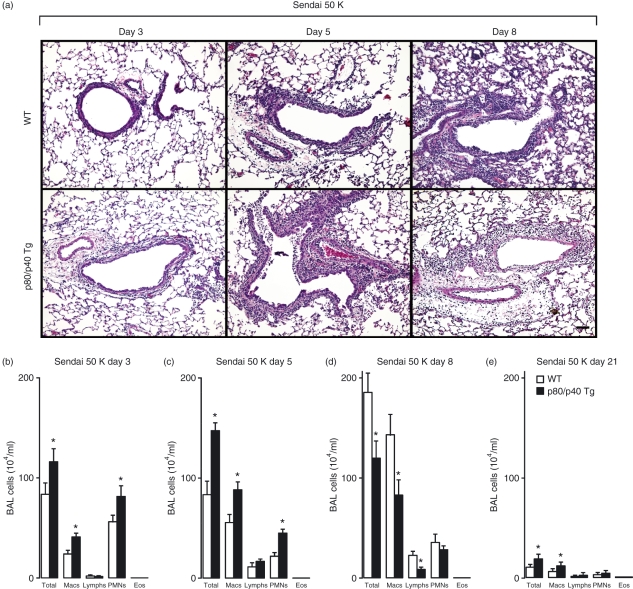
To assess the role of this increased number of resident airway macrophages in the accumulation of immune cells during a respiratory viral infection, we inoculated the p80/p40 Tg mice with Sendai virus and quantified the BAL immune cells. Using a sublethal dose of virus that generates a reversible bronchitis/bronchiolitis with peak immune cell accumulation at day 8 post-viral inoculation1, the lung histology of the p80/p40 Tg mice demonstrated an earlier peak and decline in immune cell accumulation (Fig. 3a). We confirmed this observation by demonstrating more immune cells in the BAL at days 3 and 5 and fewer at day 8 post-viral inoculation. At day 21 post-viral inoculation, the BAL cell counts decreased towards baseline values in both the p80/p40 Tg and wild-type mice (Fig. 3b–e).
Figure 3.
Enhanced resolution of viral-dependent airway inflammation in the p80/p40 transgenic mice. (a) Wild-type (WT) or p80/p40 transgenic (p80/p40 Tg) littermates were inoculated with Sendai virus 5000 egg infectious dose 50% (Sendai 5 K) and day 3, 5 and 8 post-inoculation lung sections were stained with haematoxylin and eosin. Representative photomicrographs are shown (n = 5). Bar, 100 μm. (b–e) BAL from day 3, 5, 8 and 21 post viral inoculation was analysed for total and differential cell numbers. Values represent mean ± standard deviation (n = 4–6), and a significant difference from WT is indicated (*P < 0·05). Macs, macrophages; Lymphs, lymphocytes; PMNs, polymorphonuclear leucocytes; Eos, eosinophils.
Resistance to a lethal Sendai virus infection in the p80/p40 transgenic mice is abrogated by macrophage depletion
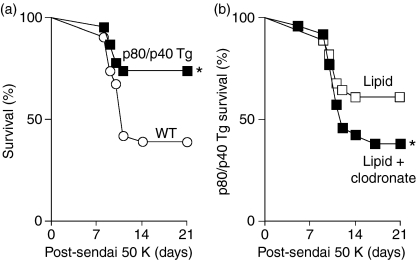
We previously demonstrated that viral-driven overexpression of IL-12 p80 in the IL-12 p35-deficient mice enhanced the immune response and was detrimental to host survival, whereas blockade of IL-12 p80 function using IL-12Rβ1-deficient mice was beneficial.1,2 Accordingly, we reasoned the p80/p40 Tg mice that overexpress IL-12 p80 would demonstrate an increased mortality following inoculation with a lethal dose, 50% (LD50) of Sendai virus. However, with this dose of virus the p80/p40 Tg mice demonstrated significantly lower mortality, indicating that transgene expression conferred viral resistance (Fig. 4a).
Figure 4.
Resistance to a lethal Sendai virus infection in the p80/p40 transgenic mice is abrogated by macrophage depletion. (a) Wild-type (WT; n = 25) or p80/p40 Tg (n = 21) littermates were inoculated with Sendai virus 50 000 egg infectious dose 50% (Sendai 50 K) and monitored for survival by Kaplan–Meier analysis. A significant increase in survival of p80/p40 Tg mice (by Wilcoxon rank-sum test) is indicated (*P < 0·05). (b) p80/p40 Tg mice were inoculated with empty liposomes (lipid; n = 28) or clodronate-containing liposomes (lipid + clodronate; n = 26) 5 days prior to inoculation with Sendai virus 50 000 EID50 (Sendai 50 K). Mice were monitored for survival by Kaplan–Meier analysis and a significant decrease in survival of mice treated with clodronate (by Wilcoxon rank-sum test) is indicated (*P < 0·05).
In prior experiments we showed that macrophage depletion in wild-type mice resulted in a higher post-viral mortality, indicating that a decrease in macrophage number is detrimental for post-viral survival.4 To determine if the increased number of resident airway macrophages mediated the viral resistance seen in the p80/p40 Tg mice, we attempted to reverse the survival advantage in the p80/p40 Tg mice by depleting alveolar macrophages prior to lethal infection. Compared with p80/p40 Tg mice inoculated with empty liposomes, the transgenic mice that were treated with clodronate-containing liposomes to partially deplete their macrophages demonstrated a higher mortality (Fig. 4b). Although the p80/p40 Tg mice that received empty liposome demonstrated a slightly higher mortality than untreated p80/p40 Tg mice (39% versus 29%) this difference was not statistically significant (P = 0·48).
Resistance to a lethal Sendai virus infection in the p80/p40 transgenic mice is associated with enhanced resolution of viral-dependent airway inflammation
To further examine the mechanisms underlying the resistance to a lethal Sendai virus infection in the p80/p40 transgenic mice, we evaluated recruitment of immune cells to the airway following lethal viral infection. The lung histology revealed an earlier peak and decline in the immune cells in the lung tissue of the p80/p40 Tg mice (Fig. 5a). Quantification of BAL immune cells demonstrated an increase in immune cell accumulation at days 3 and 5 as well as a more rapid decline at day 8. At day 21 post-viral inoculation, the BAL cell count decreased towards baseline values in both the p80/p40 Tg and wild-type mice, with p80/p40 Tg mice again demonstrating more total cells and macrophages (Fig. 5b–e). Taking together, we conclude that the p80/p40 Tg mice with increased numbers of resident airway macrophages resolved the post-viral immune response more rapidly and this enhanced resolution was associated with resistance to a lethal viral inoculation.
Figure 5.
Resistance to a lethal Sendai virus infection in the p80/p40 transgenic mice is associated with enhanced resolution of viral-dependent airway inflammation. (a) Wild-type (WT) or p80/p40 transgenic (p80/p40 Tg) littermates were inoculated with Sendai virus 50 000 egg infectious dose 50% (Sendai 50 K) and day 3, 5 and 8 post-inoculation lung sections were stained with haematoxylin and eosin. Representative photomicrographs are shown (n = 8). Bar, 100 μm. (b–e) BAL from day 3, 5, 8 and 21 post viral inoculation was analysed for total and differential cell numbers. Values represent mean ± standard deviation (n = 6–8), and a significant difference from WT is indicated (*P < 0·05). Macs, macrophages; Lymphs, lymphocytes; PMNs, polymorphonuclear leucocytes; Eos, eosinophils.
Resistance to a lethal Sendai virus infection in the p80/p40 transgenic mice is independent of viral load
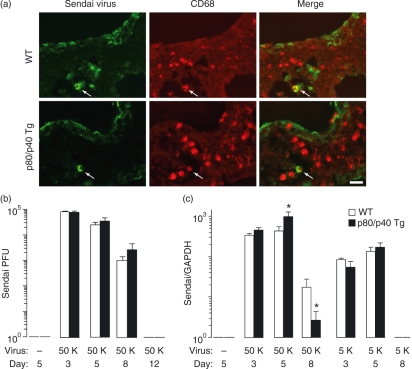
We proposed that the viral resistance in the p80/p40 Tg mice might be related to an alteration in viral replication or clearance. To assess viral load, we initially performed immunolabelling of lung tissue for Sendai virus. In wild-type mice we noted that immunolabelling of Sendai virus occurred predominantly in airway epithelial cells and to a lesser extent in macrophages. In comparison to wild-type, the p80/p40 Tg mice demonstrated a similar pattern of epithelial cell and macrophage Sendai virus immunolabelling (Fig. 6a). To further quantify viral protein in the lung, we performed western analysis of whole-lung lysate from wild-type and p80/p40 Tg mice 5 days following viral inoculation. In agreement with the immunolabelling observations, we noted similar levels of total Sendai protein (data not shown). To further quantify Sendai virus load, we measured Sendai PFU and Sendai virus-specific RNA in whole-lung homogenate. There were no differences in Sendai virus load or clearance between wild-type and p80/p40 Tg mice with respect to viral PFU following inoculation of a lethal dose of virus (Fig. 6b). Although the p80/p40 Tg mice demonstrated a statistical increase in Sendai virus-specific RNA at day 5 and a decrease at day 8 following a lethal Sendai infection, these differences were relatively small and probably not biologically significant. We next quantified Sendai virus-specific RNA following a sublethal infection, and again we noted no differences between wild-type and p80/40 Tg mice (Fig. 6c). Collectively, we conclude that the resistance to a lethal viral infection in the p80/p40 Tg mice was independent of viral load.
Figure 6.
Resistance to a lethal Sendai virus infection in the p80/p40 transgenic mice is independent of viral load. (a) Wild-type (WT; top) or p80/p40 Tg (bottom) littermates were inoculated with Sendai virus 50 000 egg infectious dose 50% (50 K) and day 5 post-inoculation lung sections were immunolabelled with anti-Sendai antibody (Ab) [detected with fluorescein isothiocyanate (FITC); green] and anti-CD68 Ab (detected with Alexa Fluor 555; red). Top and bottom rows are identical views photographed with a filter for the green channel (column 1), a filter for the red channel (column 2), and a merged image (column 3). Control immunoglobulin G (IgG) Ab gave no signal above background (not shown). Arrows indicate dual-labelled cells. Representative photomicrographs are shown (n = 4). Bar, 20 μm. (b) Wild-type (WT) and p80/p40 Tg littermates were inoculated without Sendai virus or with Sendai virus 50 K and day 3, 5, 8 and 12 whole-lung homogenates were analysed for Sendai plaque-forming units (PFU)/g of lung tissue. Values represent mean ± standard deviation for duplicate samples (n = 6–8). (c) Wild-type and p80/p40 Tg littermates were inoculated without Sendai virus or with Sendai virus 50 K or 5 K and whole-lung RNA from day 3, 5 and 8 post-inoculation was analysed for Sendai virus-specific and GAPDH RNA by one-step fluorogenic reverse transcriptase–polymerase chain reaction (RT-PCR). The mean of duplicate measurements of Sendai virus-specific RNA was normalized to GAPDH and reported as the Sendai to GAPDH ratio. A significant difference from WT is indicated (*P < 0·05).
Enhanced resolution of viral-dependent airway inflammation in the p80/p40 transgenic mice is associated with altered expression of airway chemokines
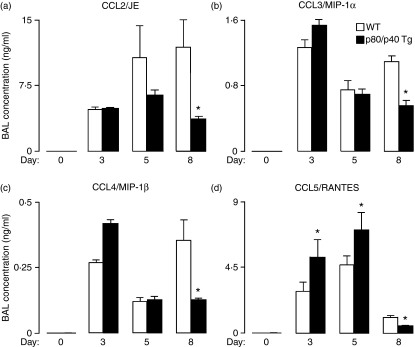
As the resistance to lethal viral infection in the p80/p40 Tg mice was associated with an early increase and enhanced resolution of the post-viral accumulation of inflammatory cells, we considered that differences in inflammatory mediators might account for this phenotype. Comparison of multiple inflammatory mediators demonstrated that the early increase and enhanced resolution in airway immune cells in the p80/p40 Tg mice was associated with corresponding differences in BAL concentrations of monocyte and T-cell chemokines. Specifically, in p40/p80 Tg mice we observed an increase in CCL5/RANTES expression at days 3 and 5, and a trend towards an increase in CCL4/MIP-1β at day 3 (P = 0·08). Whereas the wild-type mice demonstrated an increase in CCL2/JE [the murine homologue to human monocyte chemoattractant protein-1 (MCP-1)], CCL3/MIP-1α, and CCL4/MIP-1β concentrations at day 8, the p80/p40 Tg mice had significantly lower concentrations of CCL2, CCL3, CCL4 and CCL5 at this time-point (Fig. 7a–d). We noted no differences between p80/p40 Tg and wild-type mice with respect to additional chemokines (KC and eotaxin), interleukins (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-12, IL-13 and IL-17), cytokines (IFN-γ and TNF-α), or growth factors (G-CSF and GM-CSF). We conclude that the resistance to a lethal Sendai virus infection in the p80/p40 transgenic mice was associated with an enhanced resolution of viral-dependent airway inflammation and this correlated with a decrease in airway chemokine concentrations.
Figure 7.
Enhanced resolution of viral-dependent airway inflammation in the p80/p40 transgenic mice is associated with altered expression of airway chemokines. (a–d) Wild-type (WT) or p80/p40 transgenic (p80/p40 Tg) littermates were inoculated without or with Sendai virus 50 000 egg infectious dose 50% (EID50) and at days 0, 3, 5 and 8 cell-free bronchoalveolar lavage (BAL) supernatants were analysed for CC chemokine ligand 2 (CCL2)/JE [murine homologue of human monocyte chemoattractant protein (MCP)-1] (a), CCL3/macrophage inflammatory protein (MIP)-1α (b), CCL4/MIP-1β (c) and CCL5/RANTES (d). Values represent mean ± standard deviation for duplicate samples (n = 6–8) and a significant difference from WT is indicated (*P < 0·05).
Discussion
Previously, we reported that increased expression of IL-12 p80 during a viral infection mediated an enhanced immune response marked by increased macrophage accumulation and increased mortality. Based on these findings, we predicted that the IL-12 p80/40 Tg mouse might have increased mortality in response to respiratory virus, but we now show that lung-specific overexpression of IL-12 p80 prior to the infection increased the number of resident unactivated airway macrophages and this was associated with a decrease in post-viral mortality. Compared with wild-type mice, this protective response was characterized by an earlier peak and decline in immune cell accumulation as well as a decrease in local chemokine expression. These findings illustrate that the temporal regulation of IL-12 p80 expression and macrophage accumulation in the lung is critical for an appropriate antiviral inflammatory response. Increased macrophage accumulation during the infection is detrimental, whereas increased airway macrophage accumulation prior to infection exerts a beneficial priming effect that confers protection against a lethal viral infection. Thus, this report provides novel information regarding the immunomodulatory roles of IL-12 p80 and supports a paradigm whereby resident airway macrophages enhance the antiviral immune response.
Our results support a growing body of literature that suggest airway macrophage number is a critical determinant of an appropriate immune response. We associate a selective increase in the number of resident airway macrophages at the time of respiratory viral infection with an accelerated and protective antiviral immune response. In line with our observations, a recent report demonstrated that lung-specific overexpression of the chemokine CCL2 using the surfactant protein C promoter resulted in an increased number of BAL macrophages, monocytes and lymphocytes at baseline. This was associated with a more robust immune response and survival advantage following a lethal Streptococcus pneumoniae inoculation.27,28 As in our report, these mice demonstrated an earlier peak in immune cell accumulation. Unlike our results, these CCL2-overexpressing mice did not demonstrate a more rapid decline in the immune response, which may be related to the development of an abnormal chronic phenotype, described as post-infectious obliterative bronchiolitis.28 Although a selective increase in macrophage number has not been previously shown to provide a survival advantage in wild-type mice post-viral infection, transfer of wild-type alveolar macrophages to severe combined immunodeficient mice prior to intranasal inoculation of Pneumocyctis carinii restored clearance of the infectious organisms to levels seen in wild-type animals.29
While the present study illustrates that an increase in resident macrophage number prior to infection was associated with a protective antiviral immune response, our laboratory and others have also made the corollary observation that a decrease in macrophage number prior to and during a viral or bacterial infection is detrimental to survival.4,30–35 Specifically, alveolar macrophage depletion in Sendai, influenza A (1918 HA/NA:Tx/91), and vaccinia virus infection models increased mortality. In all cases, higher viral titres were observed, and following influenza A and vaccinia infection, the virus was detected in the brain and spleen, respectively, indicating a role for the alveolar macrophage in controlling systemic spread of the virus.4,34,35 Interestingly, in the influenza A model, alveolar macrophage depletion initiated during the infection (on either day 3 or day 5 post-inoculation) was again associated with higher subsequent viral titres; however, this depletion regimen did not alter mortality.34 This observation further supports the concept that the resident alveolar macrophage is a critical determinant of the antiviral immune response and post-viral mortality. Our current results, taken together with these prior findings, demonstrate a positive correlation between resident macrophage number and a protective antiviral immune response. In addition, these observations also indicate that resident airway macrophages are necessary for an appropriate immune response and that increased numbers may be sufficient to increase survival from a lethal respiratory viral infection.
The IL-12 p40 protein is common to four members of the IL-12 p40 family: IL-12 p40 monomer (p40), IL-12-p80 (p40 homodimer), IL-12 (p40 and p35 heterodimer), and IL-23 (p40 and p19 heterodimer). Although the precise biochemical mechanisms that regulate IL-12 p40 partnering and the selective secretion of these four members are unknown, our results suggest that, in the case of a respiratory viral infection and transgenic overexpression, the airway epithelial cell is intrinsically programmed to secrete IL-12 p80 and p40.1,5 We have previously observed regulatory crosstalk among the IL-12 family members. For example, in the setting of a Sendai virus respiratory infection we observed that the IL-12Rβ1- and IL-12 p35-deficient mice overexpressed IL-12 p80 and p40, suggesting the presence of a p35- or IL-12-dependent negative feedback loop that down-regulated IL-12 p80 expression.1,2 In the current IL-12 p40 overexpression model, we observed a selective increase in IL-12 p80 and p40 without a significant change in IL-12 or IL-23 concentrations. As the overexpression of IL-12 p80 and p40 did not change IL-12 or IL-23, it is unlikely that IL-12 p40 or p80-dependent positive or negative feedback loops regulate the secretion of these other IL-12 family members.
The results from our p80/p40 Tg mice corroborate and extend previous results regarding the immunomodulatory properties of IL-12 p80. The increase in the number of resident airway macrophages in the IL-12 p80/p40 Tg mice is consistent with in vitro experiments that demonstrated IL-12 p80 is a macrophage chemoattractant.2,17 Others have also demonstrated that IL-12 p80 can induce TNF-α and nitric oxide synthase gene induction in resident peritoneal macrophages and microglial cells.36,37 At baseline, we did not observe an increase in the BAL concentration of TNF-α (or multiple other inflammatory mediators) and we failed to observe any histological evidence of airway injury consistent with macrophage activation. The apparent discrepancy in IL-12 p80-dependet TNF-α induction may be related to differences in cell behaviour between the in vivo and in vitro settings, different local concentrations of IL-12 p80, or intrinsic differences between airway macrophages and resident peritoneal macrophages/microglial cells.
Direct comparison of our results with previous IL-12 p40 transgene reports is not possible because of differences in transgenic promoters, immunological challenges, and analytic end-points. However, our results are consistent with a closely related report that assessed the role of transgenic IL-12 p40 overexpression in airway epithelial cells using the surfactant protein C promoter.38 In this report, inoculation of Mycobacterium tuberculosis in IL-12 p40 transgenic mice resulted in fewer pulmonary immune cells 2 weeks following infection. Survival rates, systemic IL-12 family member levels in the serum, and the early response to the M. tuberculosis infection were not provided, so a direct comparison of these parameters cannot be made. We note that a transgenic strain utilizing the Eαd MHC class II promoter to drive IL-12 p40 expression demonstrated increased serum IL-12 p40, IL-12 p80, IL-12 and IL-23 and no differences in the immune response to Mycobacterium bovisBacille Calmette-Guérin infection or post-infection M. tuberculosis load.39 We speculate that the lack of a phenotype in this study may be related to alterations in the expression ratios of the IL-12 family members or systemic IL-12 p80 expression as transgenic overexpression of other chemokines (CCL2 and KC) in the serum has also failed to elicit a phenotype in previous reports.40,41
We demonstrated that lung-specific IL-12 p40 and p80 overexpression conferred a survival advantage following a Sendai virus infection. In contrast, others reported IL-12 p40 and p80 overexpression in the serum, using the serum amyloid P component (SAP) promoter, was detrimental during Plasmodium berghei and Leishmania major infections.42,43 These susceptibility differences may again be explained by systemic compared with local transgene expression or the differential requirement of IFN-γ for host survival. IFN-γ production is required for survival of P. berghei and L. major infections, and IFN-γ expression was decreased in SAP-IL-12 p40 transgenic mice,42–46 whereas we have shown that mortality from a Sendai viral infection is independent of IFN-γ production.3 Lastly, overexpression of IL-12 p40 using the keratinocyte-specific (K14) promoter resulted in the spontaneous accumulation of immune cells (macrophages, lymphocytes, eosinophils, mast cells and neutrophils) in the skin and the development of an inflammatory skin disease.47 Although we observed an increase in the number of airway macrophages, we did not see an increase in numbers of other inflammatory cells, nor did we see the spontaneous development of an inflammatory lung disease. We postulate that the development of a spontaneous skin disease may be related to a differential effect of IL-12 p80 on keratinocytes or resident immune cells in the skin. Alternatively, tissue-specific factors may dictate the development of spontaneous disease following transgenic chemokine overexpression. In that context, CCL2 overex pression in the lung resulted in the accumulation of immune cells in the lung without spontaneous disease, whereas heart-specific CCL2 overexpression resulted in a chronic myocarditis.27,48
In summary, the present study has demonstrated that lung-specific IL-12 p80 transgenic overexpression resulted in a selective increase in the number of resident airway macrophages. This IL-12 p80-driven augmentation of airway macrophages was associated with an accelerated immune response that protected the host from a lethal viral infection. These findings support a paradigm whereby increasing the number of resident airway macrophages may enhance antiviral respiratory immunity and provide a therapeutic approach to protect against a lethal viral infection.
Acknowledgments
This work was supported by National Institutes of Health grants R01 HL/AI 71947 and R01 HL83894 (to MJW). The authors gratefully thank Hong Chen, Loryn Rikimaru, Le Yan, Jin Y. Norris, Ronald McCarthy, Dr J. Michael Shipley and Dr Matthew J. Walter for their technical assistance. Additionally, the authors thank Drs Michael Holtzman, Jeffrey Whitsett and Giorgio Trinchieri for helpful advice and the use of their reagents.
References
- 1.Walter MJ, Kajiwara N, Karanja P, Castro M, Holtzman MJ. IL-12 p40 production by barrier epithelial cells during airway inflammation. J Exp Med. 2001;193:339–51. doi: 10.1084/jem.193.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell TD, Yan Q, Fan G, Khalifah AP, Bishop DK, Brody SL, Walter MJ. IL-12 p40 homodimer-dependent macrophage chemotaxis and respiratory viral inflammation are mediated through IL-12 receptor beta1. J Immunol. 2003;171:6866–74. doi: 10.4049/jimmunol.171.12.6866. [DOI] [PubMed] [Google Scholar]
- 3.Walter MJ, Morton JD, Kajiwara N, Agapov E, Holtzman MJ. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J Clin Invest. 2002;110:165–75. doi: 10.1172/JCI14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyner JW, Uchida O, Kajiwara N, et al. CCL5/CCR5 interaction provides anti-apoptotic signals for macrophage survival during viral infection. Nat Med. 2005;11:1180–7. doi: 10.1038/nm1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikols CL, Yan L, Norris JY, et al. IL-12 p80 is an innate epithelial cell effector that mediates chronic allograft dysfunction. Am J Respir Crit Care Med. 2006;174:461–70. doi: 10.1164/rccm.200512-1886OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–31. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 7.Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–8. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 9.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–4. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 10.Stern AS, Podlaski FJ, Hulmes JD, et al. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci USA. 1990;87:6808–12. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling P, Gately MK, Guble U, et al. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1995;154:116–27. [PubMed] [Google Scholar]
- 13.Gillessen S, Carvajal D, Podlaski FJ, et al. Mouse Interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–6. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 14.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biologic activities similar as well as distict from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 15.Heinzel FP, Hujer AM, Ahmed FN, Rerko RM. In vivo production and function of IL-12 p40 homodimers. J Immunol. 1997;158:4381–8. [PubMed] [Google Scholar]
- 16.Wang X, Wilkinson VL, Podlaski FJ, Wu C, Stern AS, Presky DH, Magram J. Characterization of mouse interleukin-12 p40 homodimer binding to the interleukin-12 receptor subunits. Eur J Immunol. 1999;29:2007–13. doi: 10.1002/(SICI)1521-4141(199906)29:06<2007::AID-IMMU2007>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Ha SJ, Lee CH, Lee SB, Kim CM, Jang KL, Shin HS, Sung YC. A novel function of IL-12p40 as a chemotactic molecule for macrophages. J Immunol. 1999;163:2902–8. [PubMed] [Google Scholar]
- 18.Khader SA, Partida-Sanchez S, Bell G, et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203:1805–15. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–92. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 20.Stripp BR, Sawaya PL, Luse DS, et al. Cis-acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem. 1992;267:14703–12. [PubMed] [Google Scholar]
- 21.Zhang Y, Huang G, Shornick LP, Roswit WT, Shipley JM, Brody SL, Holtzman MJ. A transgenic FOXJ1-Cre system for gene inactivation in ciliated epithelial cells. Am J Respir Cell Mol Biol. 2007;36:515–9. doi: 10.1165/rcmb.2006-0475RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grayson MH, Ramos MS, Rohlfing MM, Kitchens R, Wang HD, Gould A, Agapov E, Holtzman MJ. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J Immunol. 2007;179:1438–48. doi: 10.4049/jimmunol.179.3.1438. [DOI] [PubMed] [Google Scholar]
- 23.Ho MK, Springer TA. Tissue distribution, structural characterization, and biosynthesis of Mac-3, a macrophage surface glycoprotein exhibiting molecular weight heterogeneity. J Biol Chem. 1983;258:636–42. [PubMed] [Google Scholar]
- 24.Springer TA. Monoclonal antibody analysis of complex biological systems. Combination of cell hybridization and immunoadsorbents in a novel cascade procedure and its application to the macrophage cell surface. J Biol Chem. 1981;256:3833–9. [PubMed] [Google Scholar]
- 25.Roscic-Mrkic B, Schwendener RA, Odermatt B, Zuniga A, Pavlovic J, Billeter MA, Cattaneo R. Roles of macrophages in measles virus infection of genetically modified mice. J Virol. 2001;75:3343–51. doi: 10.1128/JVI.75.7.3343-3351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seiler P, Aichele P, Odermatt B, Hengartner H, Zinkernagel RM, Schwendener RA. Crucial role of marginal zone macrophages and marginal zone metallophils in the clearance of lymphocytic choriomeningitis virus infection. Eur J Immunol. 1997;27:2626–33. doi: 10.1002/eji.1830271023. [DOI] [PubMed] [Google Scholar]
- 27.Gunn MD, Nelken NA, Liao X, Williams LT. Monocyte chemoattractant protein-1 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice but requires an additional stimulus for inflammatory activation. J Immunol. 1997;158:376–83. [PubMed] [Google Scholar]
- 28.Winter C, Taut K, Srivastava M, et al. Lung-specific overexpression of CC chemokine ligand (CCL) 2 enhances the host defense to Streptococcus pneumoniae infection in mice: role of the CCL2-CCR2 axis. J Immunol. 2007;178:5828–38. doi: 10.4049/jimmunol.178.9.5828. [DOI] [PubMed] [Google Scholar]
- 29.Martin WJ, 2nd, Wu M, Pasula R. A novel approach to restore lung immunity during systemic immunosuppression. Trans Am Clin Climatol Assoc. 2005;116:221–6. discussion 6–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R, 3rd, Standiford TJ. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–46. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cote CK, Van Rooijen N, Welkos SL. Roles of macrophages and neutrophils in the early host response to Bacillus anthracis spores in a mouse model of infection. Infect Immun. 2006;74:469–80. doi: 10.1128/IAI.74.1.469-480.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldmann O, Rohde M, Chhatwal GS, Medina E. Role of macrophages in host resistance to group A streptococci. Infect Immun. 2004;72:2956–63. doi: 10.1128/IAI.72.5.2956-2963.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, van Rooijen N, van der Poll T. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2003;167:171–9. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- 34.Tumpey TM, Garcia-Sastre A, Taubenberger JK, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–44. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivera R, Hutchens M, Luker KE, Sonstein J, Curtis JL, Luker GD. Murine alveolar macrophages limit replication of vaccinia virus. Virology. 2007;363:48–58. doi: 10.1016/j.virol.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pahan K, Sheikh FG, Liu X, Hilger S, McKinney M, Petro TM. Induction of nitric-oxide sythnase and activation of NF-kB by interleukin-12 p40 in microglial cells. J Biol Chem. 2001;276:7899–905. doi: 10.1074/jbc.M008262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jana M, Dasgupta S, Saha RN, Liu X, Pahan K. Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J Neurochem. 2003;86:519–28. doi: 10.1046/j.1471-4159.2003.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leemans JC, Wieland CW, Florquin S, van der Poll T, Vervoordeldonk MJ. Mice overexpressing p40 in lungs have reduced leucocyte influx and slightly impaired resistance during tuberculosis. Immunology. 2006;117:409–18. doi: 10.1111/j.1365-2567.2005.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olleros ML, Vesin D, Martinez-Soria E, et al. Interleukin-12p40 overexpression promotes interleukin-12p70 and interleukin-23 formation but does not affect bacille Calmette-Guerin and Mycobacterium tuberculosis clearance. Immunology. 2007;122:350–61. doi: 10.1111/j.1365-2567.2007.02646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grewal IS, Rutledge BJ, Fiorillo JA, Gu L, Gladue RP, Flavell RA, Rollins BJ. Transgenic monocyte chemoattractant protein-1 (MCP-1) in pancreatic islets produces monocyte-rich insulitis without diabetes: abrogation by a second transgene expressing systemic MCP-1. J Immunol. 1997;159:401–8. [PubMed] [Google Scholar]
- 41.Wiekowski MT, Chen SC, Zalamea P, et al. Disruption of neutrophil migration in a conditional transgenic model: evidence for CXCR2 desensitization in vivo. J Immunol. 2001;167:7102–10. doi: 10.4049/jimmunol.167.12.7102. [DOI] [PubMed] [Google Scholar]
- 42.Nigg AP, Zahn S, Ruckerl D, et al. Dendritic cell-derived IL-12p40 homodimer contributes to susceptibility in cutaneous leishmaniasis in BALB/c mice. J Immunol. 2007;178:7251–8. doi: 10.4049/jimmunol.178.11.7251. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimoto T, Wang C, Yoneto T, et al. Reduced T helper 1 responses in IL-12 p40 transgenic mice. J Immunol. 1998;160:588–94. [PubMed] [Google Scholar]
- 44.Swihart K, Fruth U, Messmer N, et al. Mice from a genetically resistant background lacking the interferon gamma receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. J Exp Med. 1995;181:961–71. doi: 10.1084/jem.181.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang ZE, Reiner SL, Zheng S, Dalton DK, Locksley RM. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med. 1994;179:1367–71. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoneto T, Yoshimoto T, Wang CR, Takahama Y, Tsuji M, Waki S, Nariuchi H. Gamma interferon production is critical for protective immunity to infection with blood-stage Plasmodium berghei XAT but neither NO production nor NK cell activation is critical. Infect Immun. 1999;67:2349–56. doi: 10.1128/iai.67.5.2349-2356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kopp T, Kieffer D, Rot A, Strommer S, Stingl G, Kupper TS. Inflammatory skin disease in K14/p40 transgenic mice: evidence for interleukin-12-like activities of p40. J Invest Dermatol. 2001;117:618–26. doi: 10.1046/j.1523-1747.2001.01441.x. [DOI] [PubMed] [Google Scholar]
- 48.Kolattukudy PE, Quach T, Bergese S, et al. Myocarditis induced by targeted expression of the MCP-1 gene in murine cardiac muscle. Am J Pathol. 1998;152:101–11. [PMC free article] [PubMed] [Google Scholar]