Abstract
Cytokines are crucial in host defence against pathogens such as bacteria, viruses, fungi and parasites. A newly described cytokine, interleukin-32 (IL-32), induces various proinflammatory cytokines (tumour necrosis factor-α, IL-1β, IL-6) and chemokines in both human and mouse cells through the nuclear factor-κB and p38 mitogen-activated protein kinase inflammatory signal pathway. The IL-32 primarily acts on monocytic cells rather than T cells. In an attempt to isolate the IL-32 soluble receptor, we used an IL-32 ligand-affinity column to purify neutrophil proteinase 3, which is a serine proteinase involved in many inflammatory diseases. IL-32 has biological activity associated with Mycobacterium tuberculosis and chronic proinflammatory diseases such as rheumatoid arthritis. IL-32 is transcribed as six alternative splice variants and the biological activity of each individual isoform remains unknown. Here, we cloned the complementary DNA of the four IL-32 isoforms (α, β, γ and δ) that are the most representative IL-32 transcripts. To produce recombinant protein with a high yield, the amino acids of two cysteine residues were mutated to serine residues, because serine residues are not conserved among different species. The multi-step purified recombinant IL-32 isoform proteins were assessed for their biological activities with different cytokine assays. The γ isoform of IL-32 was the most active, although all isoforms were biologically active. The present study will provide a specific target to neutralize endogenous IL-32, which may contribute to basic and clinical immunology.
Keywords: autoimmunity, cytokine, endotoxin/lipopolysaccharide, inflammation, innate immunity
Introduction
Interleukin-32 (IL-32) is a new cytokine, originally described as a transcript (NK4), that is selectively expressed in activated natural killer or T cells.1 Although IL-32 does not share sequence homology with the known inflammatory cytokine families, it has a proinflammatory effect by inducing different inflammatory cytokines.1 Proinflammatory cytokines have the ability of inducing other inflammatory cytokines, leading a reciprocal induction. Reciprocal induction of cytokines in local areas of the body causes chronic inflammation and escalates disease severity.
Interleukin-18 is a well-studied proinflammatory cytokine associated with various chronic inflammatory diseases such as rheumatoid arthritis, Crohn’s disease, psoriasis and inflammatory bowel disease.2–4 In attempting to identify the genes regulated by IL-18, we discovered a new cytokine, IL-32,1 that stimulates monocytic cells to produce proinflammatory cytokines such as IL-1β, IL-6, tumour necrosis factor-α (TNF-α) and chemokines.1 The biological properties of IL-32 are typical of proinflammatory cytokines in activating the signal pathway of nuclear factor-κB (NF-κB) and p38 mitogen-activate protein kinase (MAPK).1
In an attempt to identify a soluble IL-32 receptor, an IL-32 ligand-affinity column was used to isolate a specific 30 000 molecular weight (MW) protein from concentrated human urine. Mass spectrometric analysis identified the 30 000 MW band as neutrophil proteinase 3 (PR3);5 this exists in a soluble or membrane-associated form and is the major autoantigen for autoantibodies in the systemic vasculitic disease, Wegener’s granulomatosis.6,7 The affinity of IL-32α for PR3 was determined by surface plasmon resonance. The dissociation constants were 2.65 ± 0.4 nm for urinary PR3 and 1.2 ± 0.05 nm for commercial PR3. Interestingly, inactivation of PR3 enzymatic activity did not change the binding affinity between the two molecules. Moreover, after limited cleavage by PR3, IL-32α had enhanced biological activity in inducing mouse macrophage inflammatory protein 2 (MIP-2) and human IL-8.5 The primary function of neutrophil-derived serine proteases, such as PR3, is the degradation of extracellular proteins at sites of inflammation; however, the excessive proteolytic activity causes untoward effects. In addition to this function, PR3 exerts an active function in immune regulation.8
Interleukin-32 is highly expressed in rheumatoid arthritis synovial tissue biopsies, but was not detected in synovial tissues from patients with osteoarthritis.3 After the administration of human IL-32 into the knee joints of naïve mice, joint swelling with an influx of inflammatory cells and cartilage damage was observed. The IL-32-driven joint swelling and cell influx were markedly reduced in TNF-α-deficient mice, but loss of proteoglycan was unaffected.3 These data suggest that IL-32-induced rheumatoid arthritis is dependent on TNF-α. The overexpression mouse model of human IL-32β by bone marrow transplantation (BM-hIL-32) showed increased expression and secretion of TNF-α, IL-1β and IL-6 in spleen cells after lipopolysaccharide (LPS) stimulation. The BM-hIL-32β mice showed an onset of collagen/antibody-induced arthritis and trinitrobenzene sulphonic acid-induced colitis, and a TNF-α blockade reduced the inflammatory effects of human IL-32β.9 In addition, IL-32 was a potent inducer of prostaglandin E2 release in mouse macrophages and human blood monocytes, an important property for inflammation.3
Interleukin-32 synergized with the nucleotide oligomerization domain 1 (NOD1) and two specific muropeptides of peptidoglycans for the release of IL-1β and IL-6.1 The synergistic effect of IL-32 and the muramyl dipeptide on cytokine production was deleted in the cells of patients with Crohn’s disease bearing the NOD2 mutation with insertion of cytidine at position 2030. In addition, the synergistic effect of IL-32 on cytokine induction by the muropeptide (FK-156) was absent in the macrophage cells of NOD1-deficient mice.1 The IL-32 was induced by Mycobacterium tuberculosis and Mycobacterium bovis bacillus Calmette–Guérin (BCG) as well as by either LPS or mycobacteria. Moreover, IL-32 production induced by M. tuberculosis is dependent on endogenous interferon-γ (IFN-γ).3
Splice variants exist in other cytokines, such as IL-15, IL-1F7 and vascular endothelial growth factor, although cytokine gene splicing events are unusual.10–14 Interleukin-15 exists as two isoforms because of an alternative splicing in exon 5.15 Although both IL-15 isoforms possess identical biological properties, each one has a distinct regulation and expression pattern. Four isoforms of IL-32 exist because of messenger RNA (mRNA) alternative splicing as described in our previous study.1 Additionally, two more IL-32 isoforms, ε and ζ, were reported recently, but these two isoforms were not abundantly expressed in other cell types except activated T cells.16
In the present study, four different human IL-32 isoforms were cloned and each recombinant IL-32 (rIL-32) isoform protein was produced. The biological activities for each of the four rIL-32 isoforms were examined with multiple bioassays. All four rIL-32 isoforms were biologically active and reacted with PR3, although there is deviation in amino acid sequences between the different isoforms. The rIL-32γ, which is the longest isoform, exhibited the highest biological activity, inducing cytokines both in vitro and in vivo.
Materials and methods
Reagents
The PR3 was purchased from Athens Research and Technology (Athens, GA). To isolate immunoglobulin G (IgG) of rabbit and goat anti-IL-32 antibodies, a diethylaminoethyl (DEAE) blue affi-gel column was obtained from BioRad (Hercules, CA). Affi-15 agarose beads were purchased from BioRad to produce an IL-32 ligand-affinity column. TALON affinity beads and tobacco etch virus (AcTEV) protease were obtained from Invitrogen (Carlsbad, CA). The TA cloning vector pGEMT-Easy was obtained from Promega (Madison, WI).
Cloning of four isoforms, mutation of the cysteine residue and construction of the Escherichia coli expression vector
Total RNA was isolated from a human natural killer (NK) cell line with Tri-Reagent (Sigma, St Louis, MO). The human NK cell line was kindly provided by Dr Hans Klingerman (Rush Medical Center, Chicago, IL). Superscript II reverse transcriptase (Invitrogen) converted 2 μg total RNA to first-strand complementary DNA (cDNA) at 42° for 30 min. A pair of sense primers, 5′-CTGTCCCGAGTCTGGACTTT-3′ and reverse primers, 5′-GCAAAGGTGGTGTCAGTATC-3′ was used for the polymerase chain reaction (PCR) which was performed at 94° for 45 seconds, 70° for 2 min, 59° for 1 min for 30 cycles. The PCR products were ligated into pGEMT-Easy (Promega) for DNA sequencing. We cloned four IL-32 splice variants and then the open reading frame of IL-32 isoform cDNAs was transferred into the E. coli pPROEX/HTa expression vector (Invitrogen) using EcoRI and XabI restriction enzyme sites. We used a PCR mutation strategy using a pair of sense and reverse primers to mutate the two cysteine residues in N-terminal and C-terminal.17,18 A pair of primers (sense: 5′-ATGAATTCATGTCCTTCCCGAAGGTCC-3′ and reverse: 5′-TATCTAGATCATTTTGAGGATTGGGGTTCAGAGGACTT-3′) were applied for PCR and then ligated into the pPROEX/HTa vector. The mutation of two cysteine residues was confirmed by DNA sequencing analysis.
Reverse transcription–PCR
Human epithelial WISH cells (3 × 106 per 9 cm plate) were treated with IFN-γ (5 ng/ml) at different time-points and then harvested for total RNA isolation as described above. The different pairs of primers for the specific IL-32 splice variant were designed using a particular intron and exon border where a distinct splicing event takes place in each isoform.3 The PCR products can be distinguished by their sizes in addition to their sequences.
Recombinant protein and purification
Four rIL-32α, β, γ and δ proteins were expressed in E. coli and purified with a TALON affinity column (Invitrogen) using his6-tag at the N-terminus of recombinant proteins. The TALON affinity-purified proteins were subjected to size exclusion chromatography (Superdex 75, ÄKTAFPLC) and then digested with AcTEV protease (Invitrogen) for 16 hr at 4° to remove the his6-tag fusion protein. The AcTEV-cleaved recombinant proteins were dialysed in Tris-base buffer (50 mm, pH 8), and the resulting materials were subjected to ion exchange chromatography (HiTrapQFF, ÄKTAFPLC). The three-step purified rIL-32 proteins were tested for biological activities.
The production and purification of a polyclonal antibody for Western blotting
A polyclonal antibody produced in goat against rIL-32α was used for Western blotting. Goat anti-rIL-32α polyclonal antibody was produced in Rockland (Gilbertsville, PA) according to the manufacturer’s protocol. The anti-rIL-32α IgG fraction was isolated using DEAE blue affi-gel column and then the IgG fraction was further affinity-purified with an IL-32α-immobilized agarose bead column of Affi-gel 15. The rIL-32 isoform proteins were applied to 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and then transferred to a nitrocellulose membrane. The membrane was probed with affinity-purified goat anti-IL-32α polyclonal antibody (1 : 1000 with 5% skimmed milk in phosphate-buffered saline containing 0.05% Tween-20) for 18 hr and then probed with the secondary antibody (rabbit anti-goat IgG–horseradish peroxidase). The Western blot was exposed to X-ray film after soaking in chemiluminescence solution.
Cell culture and cytokine assays
Human peripheral blood mononuclear cells (PBMC) were isolated through density centrifugation of blood that was diluted 1 : 1 in pyrogen-free saline over Histopaque (Sigma). The PBMC were washed twice with culture medium (RPMI-1640) containing penicillin (100 U/ml), streptomycin (100 U/ml) and glutamine (29.2 μg/ml) and was suspended in the same culture medium.
The PBMC (5 × 105) were seeded in 96-well plates in a 100-μl volume, which was then incubated with either 100 μl of culture medium (control) or 100 μl of the different stimuli: LPS (20 ng/ml), 40 ng/ml of each rIL-32α, IL-32β, IL-32γ and IL-32δ: staphylococcus (1 × 107/ml). After an 18-hr stimulation, the cell culture supernatants were accessed for cytokine measurement.
To exclude the possibility that the effects of IL-32 were the result of microbial contaminants, we stimulated C3H/HeNj peritoneal macrophages (LPS-resistant) with purified rIL-32 and compared them with control macrophages from C57-Black/6 mice. The cells were harvested 10 min after the intraperitoneal injection of 4 ml sterile PBS containing 0.38% sodium citrate. After centrifugation and washing, the cells were suspended in RPMI-1640 medium. The cells were cultured in 96-well plates (1 × 105 cells/well) in a volume of 100 μl. After stimulation for 18 hr with various stimuli, cytokines were measured in the culture supernatant.
The liquid-phase enhanced chemiluminescence (ECL) method was used to measure human IL-6 and TNF-α as well as mouse MIP-2 and TNF-α in cell culture media and serum samples (BioVeris, Gaithersburg, MD). The amount of electrochemiluminescence was determined using Origen Analyzer (BioVeris).
In vivo assay
Five wild-type (C57-Black/6) and Toll-like-receptor-2-deficient (TLR-2−/−) mice were intraperitoneally injected with rIL-32γ (10 μg per mouse), while an additional five wild-type (C57-Black/6) and TLR-2−/− mice received an injection of fresh buffer (50 mm phosphate buffer pH 8), as a negative control. The mice were killed for cytokine assays 4 hr later and then the mouse sera were accessed for cytokine measurement. The ECL assay was used to measure mouse MIP-2 and TNF-α as described above.
Results
The regulation of four different IL-32 isoform genes in human epithelial cells
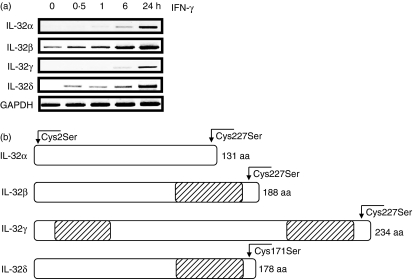
We previously described that human IL-32 was expressed in human epithelial WISH cells stimulated with IFN-γ. To examine the mRNA expression of different IL-32 variants, reverse transcription-PCR (RT-PCR) analysis was performed in WISH cells in a time-dependent manner using specific primers for each isoform (α, β, γ and δ). As shown in Fig. 1a, mRNA levels of all isoforms were increased by IFN-γ in human epithelial cells in a time-dependent manner, although the IL-32β isoform mRNA was constitutively expressed. The present experiment legitimates the study of human IL-32 splice variants with recombinant proteins in E. coli, because human IL-32 isoforms exist and are induced by different stimuli in various cell types. Determination of the expression pattern of IL-32 isoforms and its regulation in different type of cells will contribute to the understanding of the functional regulation of endogenous IL-32 in immune responses.
Figure 1.
The regulation of four different human interleukin-32 (IL-32) messenger RNA splice variants in human epithelial WISH cells and the diagram of four IL-32 isoforms with the indicated position for cysteine mutation. (a) WISH cells were stimulated with human interferon-γ (IFN-γ; 5 ng/ml) and the cells were harvested over time, as indicated at the top of the figure. Total RNA was isolated from the cells and semi-quantitative reverse transcription–polymerase chain reaction was performed. The bottom line, the GAPDH housekeeping gene, depicts equal amounts of total RNA template in each sample. (b) Four different IL-32 isoforms were shown with the amino acid (aa) positions, cysteine residues changed to serine residues. The hatched bar displays the additional amino acid sequence as a result of the absence of exon splicing in IL-32 isoforms β, γ, and δ. The N-terminal Cys/Ser-mutated residue is consistent as the second amino acid residue, however the C-terminal Cys/Ser-mutated residue is varied, and is listed by amino acid position because of distinct splicing in each isoform.
Mutation of two cysteine residues in four IL-32 isoforms
To increase the production of rIL-32 protein in E. coli, we mutated cysteine residues in the N-terminus as well as in the C-terminus. These residues are not conserved among species (not shown), as indicated in Fig. 1b. The mutations were performed with a pair of sense and reverse primers in which a cysteine codon ‘TGC’ was changed to a serine codon ‘TCC’ at the second amino acid ‘Cys2Ser’ for all isoforms. Another cysteine residue in the C-terminus was changed in a similar manner; however, the position of the cysteine residue varies because of the distinct length of each IL-32 polypeptide; for instance, Cys124Ser for IL-32α; Cys181Ser for IL-32β; Cys227Ser for IL-32γ; Cys171Ser for IL-32δ, respectively. The mutation of the IL-32 isoform cDNA sequence was confirmed by DNA sequencing.
The expression of four rIL-32 isoforms
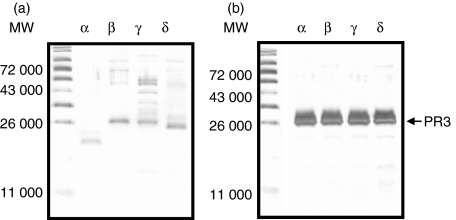
Four human IL-32 isoform cDNAs were cloned from the human NK cell line using RT-PCR followed by TA cloning. After sequencing the complete open reading frame of each isoform, the gene was inserted into pPROEX/HTa for expression in E. coli. The multi-step (his-tag protein purification, size exclusion chromatography and ion exchange chromatography) purification was employed to obtain a pure rIL-32 isoform protein. The rIL-32 proteins were assessed by 10%-SDS–PAGE and the purity of multi-step-purified rIL-32 isoforms α, β, γ and δ was verified with silver staining (Fig. 2a). The IL-32 isoforms α, β and δ appeared as a dominant band corresponding to each molecular size, however the γ isoform of IL-32 appeared as multiple bands. The overexpression of recombinant protein often migrates with various molecular sizes. To examine whether the multiple bands are rIL-32, we incubated rIL-32 isoform proteins with PR3, which is a specific enzyme for IL-32 cleavage. As depicted in Fig. 2(b), the PR3 cleaved the multiple bands and PR3 also elaborately cleaved other IL-32 isoforms.
Figure 2.
The expression of four recombinant interleukin-32 (rIL-32) isoform proteins and verification of their specificity by neutrophil proteinase 3 (PR3). (a) Four rIL-32 isoform proteins, indicated by letter at the top of the lane, were purified by a multi-step purification procedure and then each rIL-32 protein (50 ng/lane) was applied for sodium dodecyl sulphate–polyacrylamide gel electrophoresis/silver stain to evaluate their purity. (b) The verification of rIL-32 isoform protein by PR3, which is a specific binding protein for IL-32 and has the ability to digest IL-32 in a very specific manner.
The specificity of the multi-step purified rIL-32 isoform proteins
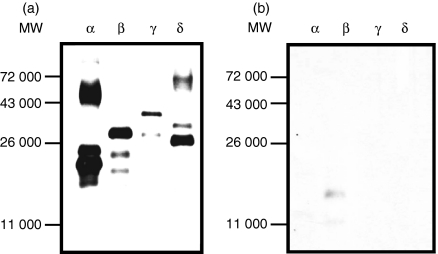
To demonstrate the specificity of rIL-32 isoform proteins, the four IL-32 isoform proteins were probed with affinity-purified rabbit anti-IL-32α polyclonal antibody. The major band of each isoform was detected after only a short exposure (Fig. 3a), while the multi-bands appeared after longer exposures (data not shown). The same amount of each rIL-32 isoform protein was incubated with PR3 and probed with anti-IL-32α antibody. As seen in Fig. 3(b), PR3 completely abolished the IL-32 band. We also confirmed the specificity of rIL-32 isoform proteins with a monoclonal antibody against IL-32α, which recognizes all four isoforms. The result was precisely similar to the affinity-purified polyclonal antibody (data not shown).
Figure 3.
The Western blot of four recombinant interleukin-32 (rIL-32) isoforms in the presence or absence of preincubation with proteinase 3 (PR3). (a) Four rIL-32 proteins (5 ng in each lane) were loaded and probed with rabbit anti-IL-32α polyclonal antibody. (b) The rIL-32 protein (5 ng in each lane) was preincubated with 10 ng of human PR3 for 30 min at 37°, loaded for Western blotting, and then probed with the same antibody as in (a).
Recombinant IL-32-induced cytokines
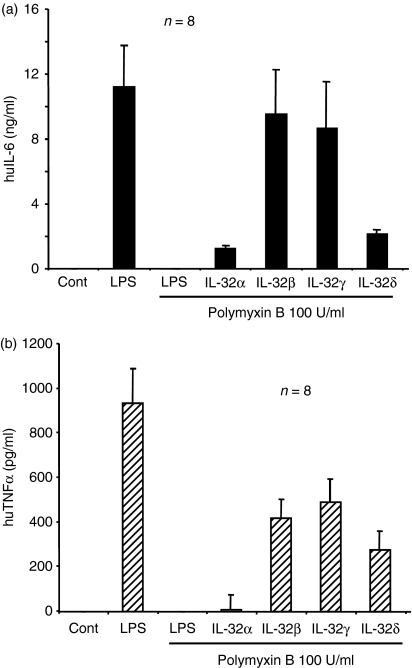
The four multi-step-purified rIL-32 isoform proteins were tested for biological activities in human PBMC. Freshly isolated human PBMC were stimulated with four different rIL-32 proteins in the presence of polymyxin B to eliminate a possible endotoxin activity, although the rIL-32 proteins were purified by multi-steps. As shown in Fig. 4(a), all four IL-32 isoforms induced prominent IL-6 production. Both IL-32β and IL-32γ exerted more potent activity than IL-32α and IL-32δ. The same cell culture supernatants were assessed for human TNF-α production, and were found to be similar to IL-6 production, whereas IL-32α-induced human TNF-α was much less than that of the other IL-32 isoforms (Fig. 4b).
Figure 4.
The biological activity of four recombinant interleukin-32 (rIL-32) isoforms in human peripheral blood mononuclear cells (PBMC). Human PBMC were stimulated with four different rIL-32 isoforms (40 ng/ml) in the presence of polymyxin B (100 U/ml) for 18 hr, after which the cell culture supernatant was harvested for cytokine measurement. (a) IL-6 and (b) tumour necrosis factor-α (TNF-α), were measured by enhanced chemiluminescence. As shown in the second lane, lipopolysaccharide (LPS) induced a large amount of cytokine in both IL-6 and TNF-α, whereas polymyxin B (100 U/ml) in the third lane, abolished LPS activity.
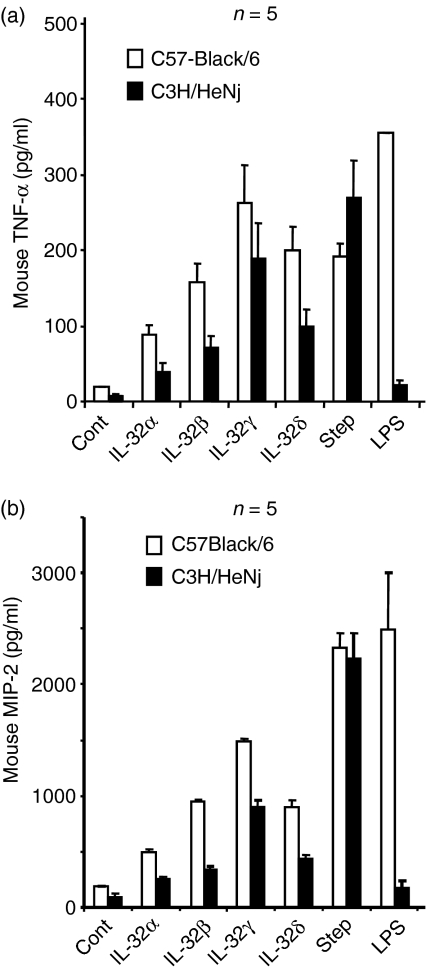
IL-32-induced cytokines in C3H/HeNj mouse-derived macrophages
Peritoneal macrophage cells were isolated from C3H/HeNj and C57-Black/6 mice. The C3H/HeNj mouse is resistant to endotoxin responsiveness as the result of a single point mutation in the Toll/IL-1R domain at the intracellular part of TLR-4. Freshly isolated peritoneal macrophage cells were treated with rIL-32α, β, γ and δ for 18 hr and the cytokines in the cell culture supernatants were measured. Recombinant IL-32γ isoform-induced mouse TNF-α production was much higher than that of the rIL-32α isoform, which is similar to the human PBMC results, in both C3H/HeNj and C57-Black/6 peritoneal macrophage cells (Fig. 5a). The results show that wild-type C57-Black/6 peritoneal macrophage cells are more sensitive responders in the production of cytokines compared to the TLR-4 mutant, C3H/HeNj, even in the untreated control cells (Fig. 5). The LPS-treated C57-Black/6 mouse macrophages produced a prominent amount of mouse TNF-α, whereas C3H/HeNj peritoneal macrophages did not respond to LPS. In addition, staphylococcus-stimulated cytokine production was not defective in C3H/HeNj peritoneal macrophages. These data demonstrate that rIL-32-induced mouse TNF-α was not the result of LPS contamination. We measured mouse MIP-2 in the same cell culture supernatants and the result was similar to that for TNF-α (Fig. 5b).
Figure 5.
The examination of recombinant interleukin-32 (rIL-32) isoform protein with the peritoneal macrophages of lipopolysaccharide (LPS)-resistant C3H/HeNj mice. The peritoneal macrophages of normal C57-Black/6 and LPS-resistant C3H/HeNj mice were stimulated four rIL-32 isoforms and then mouse (a) macrophage inflammatory protein 2 (MIP-2) and (b) tumour necrosis factor-α (TNF-α) were measured in the cell culture supernatant after 18 hr of stimulation. (a) and (b), Lane 6, staphylococcus stimulates a normal response in both normal C57-Black/6 and LPS-resistant C3H/HeNj peritoneal macrophages, whereas LPS, lane 7, shows a lack of response in LPS-resistant C3H/HeNj peritoneal macrophages.
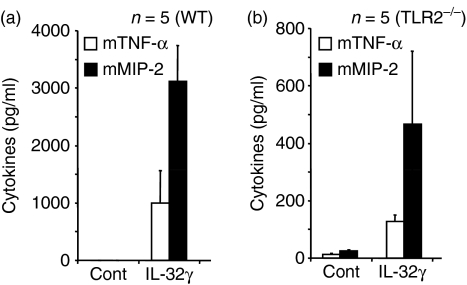
Examination of recombinant IL-32 in vivo with TLR-2−/− mouse
Five wild-type mice were injected with the most active rIL-32 proteins, IL-32γ, in the peritoneal cavity and five other mice received fresh buffer (50 mm of phosphate buffer pH 8) as negative controls. The rIL-32γ induced a large amount of mouse MIP-2 (Fig. 6a) as well as mouse TNF-α. To demonstrate that the biological activity of rIL-32 is not the result of contamination by the agonist of TLR-2, an additional five TLR-2−/− mice were administered with the rIL-32γ isoform in the same manner. We observed significant induction of mouse TNF-α and MIP-2 in TLR-2−/− mice (Fig. 6b), although the cytokine levels were much lower compared to the wild-type (Fig. 6a).
Figure 6.
Τhe biological activity of recombinant interleukin-32γ (rIL-32γ) in vivo with Toll-like receptor-2-deficient (TLR-2−/−). The most active rIL-32γ isoform was administrated to five C57-Black/6 and TLR-2−/− and then the mice were killed and cytokines were measured from sera. In both (a) C57-Black/6 and (b) TLR-2−/− mice, tumour necrosis factor-α (TNF-α) and MIP-2 were sufficiently produced although TLR-2−/− showed fewer cytokines.
Discussion
The regulation of cytokines is an axis of the immune response for host defence against the invasion of various pathogens during infections. The regulation of specific cytokine production is crucial in developing a proper immune response to an antigen. Regulating cytokines maintain the homeostasis of immune responses: cytokines are dramatically increased when the host is infected by pathogens. However, when the host recovers from infection, the cytokines return to quiescent levels. A non-preceded up-regulation of cytokines often leads to various autoimmune diseases and chronic inflammatory diseases, as a high level of cytokine expression is a hallmark of autoimmune and inflammatory disease diagnosis.
In the present study we demonstrated the regulation and biological activity of four different IL-32 isoforms both in vitro and in vivo. Four different IL-32 isoform genes were cloned from human NK cells and expressed as recombinant proteins in E. coli. The multi-step purification procedure yielded a pure recombinant protein and then each recombinant IL-32 was assessed for its biological activity. The longest IL-32 isoform, γ, exerted the greatest biological activity inducing different cytokines in human PBMC and mouse macrophage cells.
Mycobacterial species such as M. tuberculosis or M. bovis BCG are potent stimuli for the production of the proinflammatory cytokine IL-32. The IL-32 production by mycobacteria is dependent on active IL-18 induced by a caspase-1-dependent pathway.3 Interleukin-18 is a key factor for the production of IFN-γ, which in turn is the definitive mediator of IL-32 synthesis induced by mycobacteria.12 Investigation of IL-32 regulation by IFN-γ in epithelial cells will contribute to understanding a specific role of cytokines in tuberculosis pathogenesis.
The IL-32 mRNA transcripts exist as six different isoforms. IL-32 isoforms α, β, γ, and δ were initially described1 and recently two more isoforms, ε and ζ, have been described in the absence of detailed characterization.16 In human NK cells, the most abundant IL-32 isoform transcript was isoform α, which is the shortest isoform because of two additional splicing sites in exon 3–4 and exon 7–8. When four different recombinant IL-32 isoforms were expressed in E. coli, IL-32 isoform α produced the highest yield whereas the other three isoforms were poorly expressed as soluble proteins (data not shown). The recombinant human IL-18 protein is prone to inactivation through the formation of multimers by disulphide bonds during its expression in E. coli. A highly stable human recombinant IL-18 was produced through the replacement of cysteine by serine based on the tertiary structure.19 To increase the yield of recombinant IL-32 protein, we mutated two non-conserved cysteine residues. The two cysteine mutations improved the solubility of recombinant IL-32 isoform β, γ, and δ resulting in an increased amount of recombinant protein.
Interleukin-32 has arthritogenic effects in mice and its expression was associated with the severity of inflammation in synovial biopsies from patients with rheumatoid arthritis.3 IL-32 expression strongly correlated with the expression of other proinflammatory cytokines, TNF-α, IL-1β and IL-18, in the synovial tissues of rheumatoid arthritis patients. The induction of cytokines and the recruitment of activated cells resulting in cartilage and bone destruction20,21 is probably part of the mechanism explaining IL-32-induced rheumatoid arthritis. However, it remains unknown which IL-32 isoform is actively involved in the process of rheumatoid arthritis. Interestingly, the overexpression mouse model of human IL-32β by bone marrow transplantation (i.e. BM-hIL-32) was exacerbated in response to anti-collagen antibodies and LPS stimulation or trinitrobenzene sulphonic acid-induced colitis.16 These independent reports strongly suggest that IL-32 might have an important role in chronic inflammatory diseases such as rheumatoid arthritis, colitis and psoriasis.
Interleukin-32 specifically synergizes with the muropeptide agonist for NOD1 and NOD2, which is common to all clinically-relevant bacteria, including mycobacteria. The synergism was observed for the production of IL-1β and IL-6. Mutations of the NOD2 gene lead to an increased risk of developing Crohn’s disease,22–24 and NOD2-deficient mice have an increased susceptibility to lethality when infected with intracellular pathogen.25 The synergistic effect of IL-32 on NOD2 implies that this cytokine might have an important role in Crohn’s disease.
Examining the biological activity of different isoforms in vivo is extremely difficult, although it is pertinent to determine the most active isoform. The blockade of cytokine with a soluble receptor or antibody against a ligand and its receptor is a powerful clinical approach for therapeutic purposes.26–28 Therefore, it is of interest to identify the most active IL-32 isoform to identify a target isoform for the blockade of IL-32, which will be beneficial for autoimmune and inflammatory diseases.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (No. R01-2006-000-10837). Do-Young Yoon is supported by the Korea Research Foundation (2006-E00119). Charles A. Dinarello is supported by National Institutes of Health grants AI-15614, HL-68743, CA-04 6934, and Amgen, Inc.
References
- 1.Netea MG, Azam T, Ferwerda G, et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci USA. 2005;102:16309–14. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bombardieri M, McInnes IB, Pitzalis C. Interleukin-18 as a potential therapeutic target in chronic autoimmune/inflammatory conditions. Expert Opin Biol Ther. 2007;7:31–40. doi: 10.1517/14712598.7.1.31. [DOI] [PubMed] [Google Scholar]
- 3.Joosten LA, Netea MG, Kim SH, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci USA. 2006;103:3298–303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McInnes IB, Liew FY, Gracie JA. Interleukin-18: a therapeutic target in rheumatoid arthritis? Arthritis Res Ther. 2005;7:38–41. doi: 10.1186/ar1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novick D, Rubinstein M, Azam T, Rabinkov A, Dinarello CA, Kim SH. Proteinase 3 is an IL-32 binding protein. Proc Natl Acad Sci USA. 2006;103:3316–21. doi: 10.1073/pnas.0511206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frosch M, Foell D. Wegener granulomatosis in childhood and adolescence. Eur J Pediatr. 2004;163:425–34. doi: 10.1007/s00431-004-1464-3. [DOI] [PubMed] [Google Scholar]
- 7.Lamprecht P, Gross WL. Wegener's granulomatosis. Herz. 2004;29:47–56. doi: 10.1007/s00059-004-2525-0. [DOI] [PubMed] [Google Scholar]
- 8.Sugawara S. Immune functions of proteinase 3. Crit Rev Immunol. 2005;25:343–60. doi: 10.1615/critrevimmunol.v25.i5.10. [DOI] [PubMed] [Google Scholar]
- 9.Shoda H, Fujio K, Yamaguchi Y, Okamoto A, Sawada T, Kochi Y, Yamamoto K. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res Ther. 2006;8:R166. doi: 10.1186/ar2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beutler B, Bazzoni F. TNF, apoptosis and autoimmunity: a common thread? Blood Cells Mol Dis. 1998;24:216–30. doi: 10.1006/bcmd.1998.0187. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Hanning CR, Brigham-Burke MR, et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine. 2002;18:61–71. doi: 10.1006/cyto.2002.0873. [DOI] [PubMed] [Google Scholar]
- 12.Meazza R, Verdiani S, Biassoni R, et al. Identification of a novel interleukin-15 (IL-15) transcript isoform generated by alternative splicing in human small cell lung cancer cell lines. Oncogene. 1996;12:2187–92. [PubMed] [Google Scholar]
- 13.Nishimura H, Washizu J, Nakamura N, Enomoto A, Yoshikai Y. Translational efficiency is up-regulated by alternative exon in murine IL-15 mRNA. J Immunol. 1998;160:936–42. [PubMed] [Google Scholar]
- 14.Pufe T, Petersen W, Tillmann B, Mentlein R. The splice variants VEGF121 and VEGF189 of the angiogenic peptide vascular endothelial growth factor are expressed in osteoarthritic cartilage. Arthritis Rheum. 2001;44:1082–8. doi: 10.1002/1529-0131(200105)44:5<1082::AID-ANR188>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 15.Tagaya Y, Kurys G, Thies TA, Losi JM, Azimi N, Hanover JA, Bamford RN, Waldmann TA. Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. Proc Natl Acad Sci USA. 1997;94:14444–9. doi: 10.1073/pnas.94.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goda C, Kanaji T, Kanaji S, Tanaka G, Arima K, Ohno S, Izuhara K. Involvement of IL-32 in activation-induced cell death in T cells. Int Immunol. 2006;18:233–40. doi: 10.1093/intimm/dxh339. [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Azam T, Novick D, Yoon DY, Reznikov LL, Bufler P, Rubinstein M, Dinarello CA. Identification of amino acid residues critical for biological activity in human interleukin-18. J Biol Chem. 2002;277:10998–1003. doi: 10.1074/jbc.M108311200. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Azam T, Yoon DY, Reznikov LL, Novick D, Rubinstein M, Dinarello CA. Site-specific mutations in the mature form of human IL-18 with enhanced biological activity and decreased neutralization by IL-18 binding protein. Proc Natl Acad Sci USA. 2001;98:3304–9. doi: 10.1073/pnas.051634098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Kato Z, Matsukuma E, Li A, Omoya K, Hashimoto K, Ohnishi H, Kondo N. Generation of highly stable IL-18 based on a ligand-receptor complex structure. Biochem Biophys Res Commun. 2004;317:181–6. doi: 10.1016/j.bbrc.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191:303–12. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwerina J, Hayer S, Tohidast-Akrad M, et al. Single and combined inhibition of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction. Arthritis Rheum. 2004;50:277–90. doi: 10.1002/art.11487. [DOI] [PubMed] [Google Scholar]
- 22.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 23.Hugot JP, Laurent-Puig P, Gower-Rousseau C, et al. Mapping of a susceptibility locus for Crohn's disease on chromosome 16. Nature. 1996;379:821–3. doi: 10.1038/379821a0. [DOI] [PubMed] [Google Scholar]
- 24.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–4. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 26.Darlington C. PEG-sTNF-RI. Amgen. Curr Opin Invest Drugs. 2003;4:583–7. [PubMed] [Google Scholar]
- 27.Geletka RC, St Clair EW. Infliximab for the treatment of early rheumatoid arthritis. Expert Opin Biol Ther. 2005;5:405–17. doi: 10.1517/14712598.5.3.405. [DOI] [PubMed] [Google Scholar]
- 28.Nahar IK, Shojania K, Marra CA, Alamgir AH, Anis AH. Infliximab treatment of rheumatoid arthritis and Crohn's disease. Ann Pharmacother. 2003;37:1256–65. doi: 10.1345/aph.1C039. [DOI] [PubMed] [Google Scholar]