Abstract
Although allogeneic bone marrow transplantation (BMT) plus donor lymphocyte infusion (DLI) is performed for solid tumours to enhance graft-versus-tumour (GVT) effects, a graft-versus-host reaction (GVHR) is also elicited. We carried out intra-bone marrow–bone marrow transplantation (IBM-BMT) plus adult thymus transplantation (ATT) from the same donor to supply alloreactive T cells continually. Normal mice treated with IBM-BMT + ATT survived for a long time with high donor-derived thymopoiesis and mild GVHR. The percentage of CD4+ FoxP3+ regulatory T cells in the spleen of the mice treated with IBM-BMT + ATT was lower than in normal B6 mice or mice treated with IBM-BMT alone, but higher than in mice treated with IBM-BMT + DLI; the mice treated with IBM-BMT + DLI showed severe GVHR. In tumour-bearing mice, tumour growth was more strongly inhibited by IBM-BMT + ATT than by IBM-BMT alone. Mice treated with IBM-BMT + a high dose of DLI also showed tumour regression comparable to that of mice treated with IBM-BMT + ATT but died early of GVHD. By contrast, mice treated with IBM-BMT + a low dose of DLI showed longer survival but less tumour regression than the mice treated with IBM-BMT + ATT. Histologically, significant numbers of CD8+ T cells were found to have infiltrated the tumour in the mice treated with IBM-BMT + ATT. The number of terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labelling (TUNEL)-positive apoptotic tumour cells also significantly increased in the mice treated with IBM-BMT + ATT. Allogeneic IBM-BMT + ATT thus can induce high thymopoiesis, preserving strong GVT effects without severe GVHR.
Keywords: graft-versus-host, graft-versus-tumour, intra-bone marrow–bone marrow transplantation, regulatory T cells, thymopoiesis, thymus transplantation
Introduction
Allogeneic bone marrow transplantation (BMT) has been used as a potentially curative therapy for patients with a wide variety of diseases, including haematological disorders, congenital immunodeficiencies, metabolic disorders, autoimmune diseases, and solid tumours.1–7 However, BMT alone is not wholly effective against tumours, which tend to recur, particularly in the absence of T cells.7 To enhance graft-versus-leukemia (GVL) or graft-versus-tumour (GVT) effects, donor lymphocyte infusion (DLI) is often performed following allogeneic BMT.8–10 Although DLI can produce remission of leukemia11 or the regression of solid tumours, GVL and GVT effects unfortunately seem to be closely associated with graft-versus-host disease (GVHD), which remains a major cause of post-transplantation morbidity and mortality.12–14 New cellular-based methods are thus desired.
We have developed various new BMT methods. To supply recipients with major histocompatibility complex (MHC)-matched bone marrow (BM) stromal cells, we previously performed BMT plus bone grafts from the same donor.5 For aging hosts with thymic involution, we performed thymus grafts with BMT.15 To induce extramedullary haematopoiesis in the liver, we injected bone marrow cells (BMCs) from the portal vein.16 Finally, we have recently developed intra-bone marrow (IBM)-BMT, in which BMCs are directly injected into the BM cavity.17
We have found that IBM-BMT not only allows us to use low-dose irradiation as a pre-conditioning regimen17 but also helps to suppress GVHD,18 as this IBM-BMT method can efficiently recruit donor-derived stromal cells [including mesenchymal stem cells (MSCs)], which can support donor-derived haemopoietic stem cells.1,19–21 In addition, it has recently been shown, even in humans, that stromal cells or MSCs suppress GVHD.22,23
The thymus is an organ in which T cells can be induced to differentiate from precursor T cells. In addition, to maintain homeostasis during events such as autoimmune disease, infection, graft rejection and the growth of malignant tumours, the thymus itself regulates the production, proliferation and function of T cells not only by producing cytokines and hormones such as interleukin (IL)-4, IL-5 and IL-7, stem cell factor, thymopoietin and thymic stromal lymphopoietin,24–26 but also by inducing functional subsets of T cells, including CD4+ CD25+ forkhead-box transcription factor p3 (FoxP3)+ regulatory T cells (Treg), CD4+ CD25− FoxP3− effector T cells and CD8+ T cells.27 Recently, Tregs have also been shown to preserve GVT effects while inhibiting GVH reactions (GVHRs).28,29
We have previously reported that fetal thymus transplantation in conjunction with allogeneic BMT from the same donor is successful for aged hosts who show low T-cell function.15 In addition, we have also recently found that allogeneic BMT plus adult thymus transplantation (ATT) can be used to treat autoimmune diseases in chimeric-resistant MRL/Mp-Ipr/Ipr (MRL/Ipr) mice.30 Interestingly, although T-cell functions were well restored or enhanced, concomitant GVHD was not observed. Thymus transplantation may thus represent an attractive method for improving T-cell functions.31,32 However, thymus transplantation has only been clinically applied to patients with DiGeorge syndrome or human immunodeficiency virus infection who show hypoplasia of the thymus.33,34 Its effectiveness in the treatment of other intractable diseases, including cancers, has not been examined in any detail.
In the present study, we attempt to carry out allogeneic IBM-BMT + ATT from the same donor for cancer therapy to recruit naïve allogeneic T cells continuously in vivo. We found that the high thymopoiesis induces strong GVT effects without inducing severe GVHR.
Materials and methods
Mice
Male C57BL/6 (B6:H-2b) and female BALB/c (H-2d) mice were obtained from Shimizu Laboratory Supplies (Kyoto, Japan). All mice were kept in our animal facilities under specific pathogen-free conditions. B6 mice were used as donors and BALB/c mice were used as recipients at the age of 6–8 weeks. All protocols for these animal experiments were approved under the Guideline for Animal Experimentation, Kansai Medical University.
Cell lines
Meth-A cells (H-2d) are derived from methylcholanthrene-induced sarcoma in BALB/c mice. The cells were kindly provided by Dr Junko Yoshida of Kanazawa Medical School (Kanazawa, Japan). Cells were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum with antibiotics.
Inoculation of tumour cells
One day before inoculation of tumour cells, recipients (BALB/c mice) underwent total body irradiation (3 Gy) using a 137Cs irradiator (Gammacell 40 Exactor; MDS Nordion International, Ottawa, Ontario, Canada). The next day, 2 × 106 Meth-A cells were subcutaneously inoculated into the right flank of the mice. We also examined the influence of 3-Gy irradiation in the mice before IBM-BMT. The lymphocytes recovered well after 2 weeks, which is the time required to grow the tumour sufficiently for IBM-BMT (described below). Therefore, the influence of irradiation was minimal.
BMT and thymus transplantation (TT)
Recipient 6- to 8-week-old BALB/c mice were irradiated (4·5 Gy × 2, at a 4-hr interval) using the 137Cs irradiator 1 day before BMT. Bone marrow cells were flushed from the shafts of the femurs and tibias of donor 6- to 8-week-old B6 mice and single-cell suspensions were prepared. B6 BMCs (2 × 107) were directly injected into the BM cavity of the tibia, as previously described for the IBM-BMT method.17 Simultaneously, a quarter of each of the removed thymic lobes from the same donor B6 mice was grafted under the renal capsule of the left kidney, or transplanted splenocytes (1 × 107 or 3 × 106) from the same donor were injected intravenously into some mice as DLI. As thymic function is significantly age-dependent, we used young thymus grafts from the same donor 6- to 8-week-old B6 mice. We previously carried out TT in the muscle (intramuscle) of the thigh.30 Although this is an effective method, grafting under the renal capsule is preferable because of the higher success rate. Therefore, we carried out TT under the renal capsule in the present study.
Histology
A histological study was performed on the liver, intestine and grafted tumour obtained from recipients 3 weeks after BMT. Tissues were fixed in 10% formaldehyde and embedded in paraffin. Serial tissue sections (4 μm thick) were prepared and stained using haematoxylin and eosin (HE). The degree of GVHD was evaluated using a semiquantitative scoring system for abnormalities known to be associated with GVHD, as previously described.35,36 In the scoring system, for each parameter, 0 denotes normal, 0·5 focal and rare, 1 focal and mild, 2 diffuse and mild, 3 diffuse and moderate, and 4 diffuse and severe in GVHD. The maximum score for the liver was thus 40, and for the small intestine it was 28. We examined five to seven slides of tissue samples measuring > 10 × 5 mm from different sites of each organ in five or six mice from each group. The average scores were compared between the respective groups.
Analyses of tumour-cell apoptosis
Apoptosis of tumour cells was measured with the in situ terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labelling (TUNEL) method, using an in situ Apoptosis Detection Kit (Takara, Shiga, Japan), as previously described.37 Tumour cells with TUNEL-positive nuclei were interpreted as displaying apoptotic changes. Positively stained cells were counted in 10 high-power fields (HPFs; ×400) in a blinded manner by two researchers, and the average was calculated as the number of apoptotic cells per HPF.38 We examined five slides of tissue samples measuring > 5 × 5 mm from different sites of the tumours in five mice from each group. The average scores were compared between groups.
Immunohistochemistry
Tumour tissues were embedded in Tissue-Tek optimal cutting temperature (OCT) compound (Sakura Finetek, Tokyo, Japan) and stored at −40°. Cryosections (4 μm thick) were air-dried and fixed with acetone for 10 min. Specimens were treated using 0·5% bovine serum albumin in Tris-buffered saline (TBS) for 10 min, and then stained with biotin-conjugated H-2Kb or H-2Kd monoclonal antibodies (mAbs) and phycoerythrin (PE)-conjugated rat anti-mouse CD45, or with fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD8 or CD4 mAbs (Pharmingen, San Diego, CA) for 1 hr. The reaction of avidin–FITC was followed by H-2 staining. Expressions were evaluated by confocal microscopy using an LSM 510 META microscope (Carl Zeiss, Minneapolis, MN). Numbers of positive cells per HPF were calculated using the same methods as those described above.
Analyses of surface marker antigens and intracellular FoxP3 and cytokines by flow cytometry and numbers of lymphocytes
Surface markers on lymphocytes from peripheral blood and the spleen were analysed with three-colour fluorescence staining using a FACScan system (Becton Dickinson, Franklin Lakes, NJ). FITC-conjugated anti-H-2Kb (Pharmingen) was used to determine chimerism. Phycoerythrin- or biotin-conjugated CD4 or CD8 (Pharmingen) was used to analyse lymphocyte subsets. Avidin-Cy5 (Dako, Kyoto, Japan) was used as the third colour in the avidin/biotin system. The percentage of T cells was evaluated by determining the per cent of CD4+ plus CD8+ T cells. Intracytoplasmic FoxP3 staining was performed using an FITC-anti mouse/rat FoxP3 staining set (eBioscience, San Diego, CA). The procedure was performed in accordance with the instructions of the manufacturer. Intracellular cytokines [IL-2, IL-4, IL-10, and interferon (IFN)-γ] were detected using an Intracellular Cytokine Staining Kit (Pharmingen). The procedure was also performed in accordance with the instructions of the manufacturer. The numbers of lymphocytes in the peripheral blood were calculated as the total numbers of white blood cells measured by SF-3000 with the SFVU-1 unit (Sysmex, Kobe, Japan). The numbers of T cells were calculated by the percentages of T cells.
Relative evaluation of T-cell receptor excision circle
The T-cell receptor rearrangement excision circle (TREC) was evaluated using real-time polymerase chain reaction (PCR), as previously described.39 The method was modified in some parts for relative evaluation. A standard curve was obtained using thymocytes from donor B6 mice. A total of 1 × 107 thymocytes were stored at −80°. These cells were then lysed by incubation at 55° for 1 hr in 25 μl of 100 μg/ml proteinase K (TaKaRa, Tokyo, Japan) in 10 mm Tris. The sample was assayed at 5 μl per PCR reaction. In the samples, T cells enriched (purity > 98%) from 1 × 107 splenocytes using magnetic beads with anti-mouse CD45R, CD11b and Gr-1 Abs (BD Pharmingen) were used for assays. Cells were lysed with 25 μl of 100 μg/ml proteinase K (TaKaRa) in 10 mm Tris. For DNA purification, 5-μl aliquots of the resulting samples were used. DNA was obtained from standard and experimental samples using a Puregene Cell and Tissue DNA purification kit (Gentra Systems, Minneapolis, MN). Real-time quantitative PCR was performed with standard thymocyte DNAs (diluted to 1/10, 1/100, 1/1000 and 1/10 000) and samples from chimeric mice containing 0·5 μm forward (CAT TGC CTT TGA ACC AAG CTG) and reverse (TTA TGC ACA GGG TGC AGG TG) primers of the T-cell receptor (TCR) α/δ locus gene, 0·3 μm fluorescent probe (FAM-CAG GGC AGG TTT TTG TAA AGG-QSY) and iQ Supermix (Bio-Rad, Hercules, CA). Amplifications were performed in duplicate on a DNA Engine OPTICON2 (MJ Research, Waltham, MA) and analysed using associated opticon monitor2 software (MJ Research). PCR conditions were 95° for 3 min followed by 50 cycles at 95° for 30 seconds and 63° for 30 seconds. A standard curve for TREC was obtained using serially diluted DNA samples from thymocytes of B6 mice, and the relative quantity of TREC in the spleen from chimeric mice was determined. Every assay was performed at least twice to confirm the results.
Mixed lymphocyte reaction
T cells that had been enriched (to a purity > 98%) using magnetic beads (Invitrogen, Carlsbad, CA) with anti-mouse CD45R, CD11b and Gr-1 (Pharmingen) were used for responders. The enriched T cells were incubated with 2 × 105 splenocytes irradiated at 15 Gy from various strains of mice including donor (B6) mice, recipient (BALB/c) mice, and third-party (C3H) mice as stimulators for 96 hr. Twenty millilitres of 0·5 μCi [3H]thymidine (3H-TdR; New England Nuclear, Cambridge, MA) was introduced during the last 18 hr of the culture period. The incorporation of 3H-TdR was measured using Microbeta TriLux (PerkinElmer, Wellesley, MA). The stimulator index for the mixed lymphocyte reaction (MLR) was calculated as the average of 3H-TdR incorporation (stimulator in medium)/3H-TdR incorporation (medium) in triplicate wells.
Statistical analyses
Non-parametric analyses (Mann–Whitney U-test and log rank test) were performed using statview software (Abacus Concepts, Berkley, CA). Values of P < 0·05 were considered statistically significant.
Results
Effects of IBM-BMT + ATT on survival rate, body weight, chimerism and T-cell count in peripheral blood
First, we carried out conventional intravenous (IV)-BMT (intravenous injection of marrow cells) using low-dose irradiation (4·5Gy × 2) and radio-sensitive BALB/c mice as recipients. However, most of the (B6→BALB/c) chimeric mice (produced by IV-BMT) died of infection resulting from graft failure. Some chimeric mice survived but no donor-derived cells could be found. We therefore carried out IBM-BMT, as we know that IBM-BMT allows us to use low-dose irradiation, as previously described.17,18,40,41
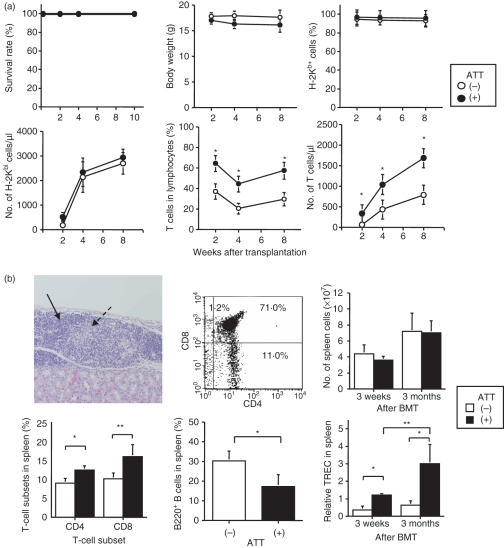
We examined the effects of IBM-BMT + ATT on survival rate, weight, chimerism and T-cell count in the peripheral blood (Fig. 1a). BALB/c mice reconstituted with B6 BMCs by IBM-BMT with or without B6 ATT survived for a long time (> 100 days) and there was no significant difference in weight between mice with and without ATT. Regarding chimerism, all mice, regardless of ATT, showed approximately 100% donor-derived chimerism by 2 weeks after BMT. The number of lymphocytes increased to the same extent with and without ATT. Interestingly, both the percentages of T cells and the cell counts of T cells in the peripheral blood from the mice treated with IBM-BMT + ATT were significantly higher than in the mice treated with IBM-BMT alone. T-cell counts in the mice treated with IBM-BMT + ATT were about 1·5-fold higher than in the mice treated with IBM-BMT alone, and the counts remained elevated for up to 8 weeks after BMT.
Figure 1.
Effect of intra-bone marrow–bone marrow transplantation (IBM-BMT) + adult thymus transplantation (ATT) on survival rate, body weight, chimerism, percentage of T cells in peripheral blood and thymopoiesis. Lethally irradiated BALB/c mice underwent transplantation with 2 × 107 B6 bone marrow cells (BMCs) by IBM-BMT with or without ATT from the same donor. (a) The survival rate (upper left), body weight (upper middle), and percentages and numbers of donor-type H-2Kb+ cells (upper left and lower left) and T cells (lower middle and right) in the peripheral blood are shown. The percentages and numbers of T cells in mice treated with IBM-BMT + ATT were significantly higher than in mice treated with IBM-BMT alone, whereas there were no differences in survival rate, body weight and chimerism. IBM-BMT alone, n = 5; IBM-BMT + ATT, n = 6. Data are shown as mean ± standard deviation (SD). *P < 0·05 compared with IBM-BMT alone at the same time. (b) Histology of the thymus, fluorescence-activated cell sorter (FACS) profiles for CD4 and CD8 double-staining in thymocytes and the number of cells, T-cell receptor rearrangement excision circle (TREC) analyses and percentages of CD4 and CD8 T and B cells in the spleen. Thymus tissue was engrafted, and cortical (arrow) and medullary (dotted arrow) areas displayed fine construction [haematoxylin and eosin (HE) staining, ×400, upper left] with sufficient CD4 and CD8 subsets in thymocytes 3 months after transplantation (upper middle). Numbers of spleen cells (upper right) and relative TRECs in spleen cells (lower right) were determined 3 weeks and 3 months after transplantation. Percentages of CD4 and CD8 T cells and B220 B cells 3 weeks after transplantation in the spleen are shown (lower left and middle). Numbers of both CD4 and CD8 T cells and relative TRECs in mice treated with IBM-BMT + ATT were significantly higher than in mice treated with IBM-BMT alone, whereas the number of spleen cells was no different. IBM-BMT alone, n = 5; IBM-BMT + ATT, n = 5. Data are shown as mean ± SD. *P < 0·05; **P < 0·01.
Analyses of thymopoiesis induced by IBM-BMT + ATT
Next, we investigated thymopoiesis in the mice treated with IBM-BMT + ATT (Fig. 1b). Histologically, the transplanted thymus showed a normal appearance with both cortex and medullary constructions under the renal capsule 3 months after transplantation. Almost normal thymocyte differentiation was seen in CD4− CD8−, CD4+ CD8+, CD4+ CD8− and CD4− CD8+ cells. In the spleen, although total cell counts did not differ between 3 weeks and 3 months after BMT, the numbers of both CD4 and CD8 T cells in the mice treated with IBM-BMT + ATT were significantly higher than in the mice treated with IBM-BMT alone 3 weeks after BMT. A significant number of T-cell subsets by IBM-BMT + ATT also increased 3 months after treatment (data not shown). Conversely, the number of B220+ B cells was significantly lower in the mice treated with IBM-BMT + ATT than in the mice treated with IBM-BMT alone. The lymphocyte subset in the lymph nodes showed a similar tendency (data not shown). However, as it is unclear whether the high number of T cells was a result of peripheral proliferation or production by the transplanted thymus, we performed TREC analyses using real-time PCR on the spleens of chimeric mice. The relative quantity of TREC in the spleen cells of the mice treated with IBM-BMT + ATT was significantly greater than in the mice treated with IBM-BMT alone, both 3 weeks and 3 months after BMT. In addition, TREC at 3 months was higher than that at 3 weeks after IBM-BMT + ATT but not after IBM-BMT alone. These results indicate that the increase in T-cell numbers induced by IBM-BMT + ATT was attributable to continuous production by the transplanted thymus.
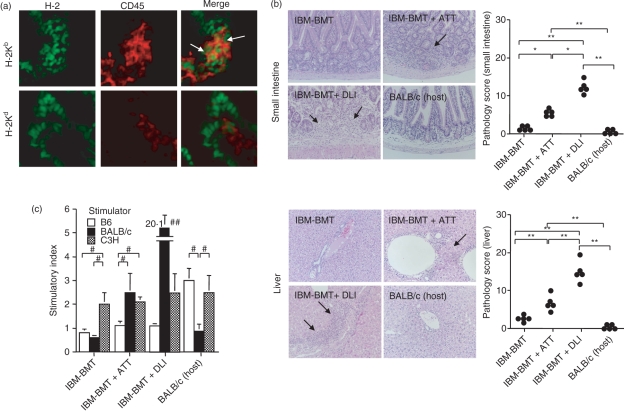
Induction of mild GVHR by IBM-BMT + ATT
If the increase in the number of T cells after allogeneic IBM-BMT + ATT is the result of ATT, GVHR should occur. In the analysis of donor-derived lymphocytes, we found a small number of donor-derived H-2Kb+ CD45+ cells, but no host-derived H-2Kd+ CD45+ cells in the small intestine and liver from mice treated with IBM-BMT + ATT (Fig. 2a). Histologically, a small number of lymphocytes infiltrated the portal area of the liver (Fig. 2a) and the mucosa of the small intestine (Fig. 2b) with some fibrosis in the mice treated with IBM-BMT + ATT, indicating the development of mild GVHD. However, the degree of GVHD was much less than that in the group treated with IBM-BMT plus 1 × 107 B6 spleen cell injection (DLI), which induced severe GVHD with mortality by 3 weeks after the transplantation.41 The mice treated with IBM-BMT alone showed no pathological findings. We also investigated the specific responses for MHC determinants in MLR assays. The MLR showed a slight response to host (BALB/c mice) in the mice treated with IBM-BMT + ATT, but not in the mice treated with IBM-BMT alone (Fig. 2c). However, the level was significantly lower than that in the mice treated with IBM-BMT + DLI. All the mice showed comparable responses to the third party (C3H).
Figure 2.
Analyses of donor-derived lymphocytes, histology and mixed lymphocyte reaction (MLR) for graft-versus-host disease (GVHD) induced by intra-bone marrow–bone marrow transplantation (IBM-BMT) + adult thymus transplantation (ATT). Lethally irradiated BALB/c mice underwent transplantation with 2 × 107 B6 bone marrow cells (BMCs) by IBM-BMT with or without ATT, or injection of 1 × 107 spleen cells from the same donor. At 3 weeks after transplantation, donor-derived lymphocytes (H-2Kb+ CD45+) and host-derived lymphocytes (H-2Kd+ CD45+) in the mice treated with IBM-BMT + ATT (a) and histology (b) in the small intestine (upper) and the liver (lower) were analysed. Donor-derived lymphocytes (H-2Kb+ CD45+ cells), but not host-derived lymphocytes (H-2Kd+ CD45+ cells), were observed in the small intestine from the mice treated with IBM-BMT + ATT (arrows) (×1000) (a). H-2Kd+ CD45− cells may be epithelial cells in the intestine. (b) Representative histology is shown [left; haematoxylin and eosin (HE) staining; ×200]: lymphocytes infiltrate the mucosa of the small intestine with fibrosis and the portal area of the liver as GVHD (arrows) in mice with IBM-BMT + ATT or 1 × 107 spleen cell injection [donor lymphocyte infusion (DLI)] from the same donor. However, the degree of GVHD in mice treated with IBM-BMT + ATT was significantly lower than in mice treated with IBM-BMT + DLI (right; pathology scores). Few or no specific pathological findings were observed in mice treated with IBM-BMT alone or untreated host BALB/c mice, and the degree of GVHD in these mice was significantly lower than in the case of IBM-BMT + ATT or DLI. IBM-BMT alone, n = 5; IBM-BMT + ATT, n = 5; IBM-BMT + DLI, n = 5; untreated donor B6 spleen cells, n = 5. Data are shown as mean ± standard deviation (SD). *P < 0·01, **P < 0·0005. (c) MLRs in splenocytes are shown for mice treated with IBM-BMT, IBM-BMT + ATT or IBM-BMT + DLI or BALB/c mice. The stimulatory index was calculated as the average [3H]thymidine (3H-TdR) incorporation of triplicate samples of responding cells with either mitogen or stimulating cells/3H-TdR incorporation of responding cells in medium alone. #P < 0·05; ##P < 0·01 compared with B6 and C3H stimulators and the BALB/c stimulator in mice treated with IBM-BMT + ATT.
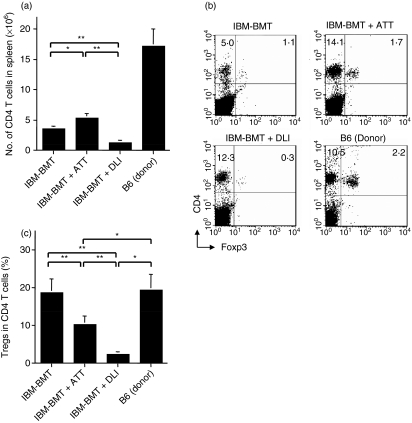
Induction of Tregs by IBM-BMT + ATT
To explore the mechanism underlying GVHD, we next analysed CD4+ FoxP3+ Tregs and CD4+ FoxP3− T effector cells. We first analysed the number of CD4 T cells in the spleen (Fig. 3a). Three weeks after the transplantation, there was a significantly greater number of these cells in the mice treated with IBM-BMT + ATT than in the mice treated with IBM-BMT alone. In contrast, the number of these cells in the mice treated with IBM-BMT + DLI was, as a result of GVHD, significantly lower than in the mice treated with IBM-BMT alone. Interestingly, although the numbers of both CD4+ FoxP3+ Tregs and CD4+ FoxP3− effector T cells were low in the mice treated with IBM-BMT alone (Fig. 3a,b), the percentages of Tregs in total CD4+ T cells of the spleen were comparable to those in the untreated donor B6 spleen (Fig. 3c). In contrast, although numbers of both CD4+ FoxP3+ Tregs and CD4+ FoxP3− effector T cells increased in the spleen of mice treated with IBM-BMT + ATT, the number of effector T cells increased to a greater extent. However, the number of Tregs markedly decreased in the spleen of mice treated with IBM-BMT + DLI. As a result, the percentages of Tregs in total CD4+ T cells in the IBM-BMT + DLI groups were significantly lower than in the IBM-BMT group and in untreated donor B6 mice (Fig. 3c). In contrast, the percentage of Tregs in total CD4+ T cells in the mice treated with IBM-BMT + ATT was still significantly higher than in the mice treated with IBM-BMT + DLI. There was thus a negative correlation between the Tregs in total CD4+ T cells and the degree of GVHD.
Figure 3.
Number of CD4 T cells and percentages of CD4+ FoxP3+ regulatory T cells (Tregs) and CD4+ FoxP3− effector cells induced by intra-bone marrow–bone marrow transplantation (IBM-BMT) + adult thymus transplantation (ATT). Lethally irradiated BALB/c mice underwent transplantation with 2 × 107 B6 bone marrow cells (BMCs) by IBM-BMT with or without ATT, or injection of 1 × 107 spleen cells [donor lymphocyte infusion (DLI)] from the same donor. At 3 weeks after transplantation, the numbers of CD4 T cells (a), CD4+ FoxP3+ Tregs and CD4+ FoxP3− effector cells (b) were analysed in spleen cells. Representative fluorescence-activated cell sorter (FACS) profiles for CD4+ FoxP3+ Tregs and CD4+ FoxP3− effector cells in the spleen (b) and the analysis for the percentage of Treg cells in CD4+ cells (c) are shown: the number of CD4 T cells in the mice treated with IBM-BMT + ATT was significantly higher than in those treated with IBM-BMT alone 3 weeks after transplantation. In addition, the cell number in the mice treated with IBM-BMT + DLI was significantly reduced compared with that in the mice treated with IBM-BMT alone or plus ATT (a). The percentage of CD4+ FoxP3+ Tregs in the mice treated with IBM-BMT alone and B6 mice (donor) was significantly higher than in the mice treated with IBM-BMT + ATT or DLI. In the latter, the percentage of cells in those treated with IBM-BMT + ATT was significantly higher than in those treated with IBM-BMT + DLI (b). IBM-BMT alone, n = 6; IBM-BMT + ATT, n = 6; IBM-BMT + DLI, n = 5; untreated donor B6 spleen cells, n = 5. Data are shown as mean ± standard deviation (SD). *P < 0·05; **P < 0·005.
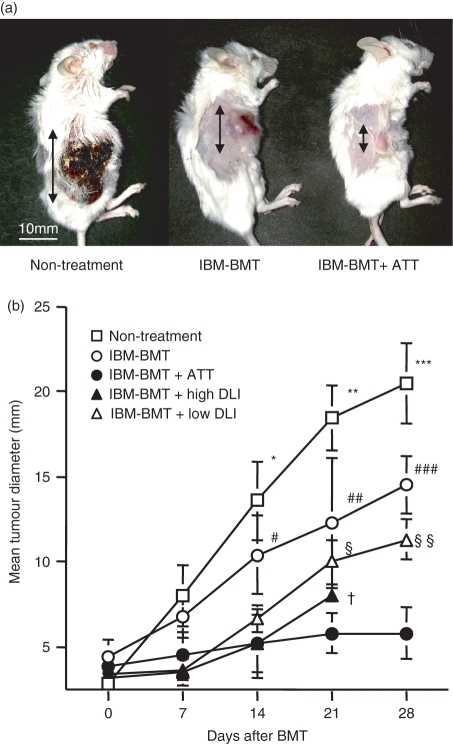
GVT effects of IBM-BMT + ATT
Next, we examined GVT effects in the mice treated with IBM-BMT + ATT. Meth-A sarcoma cells were subcutaneously inoculated into BALB/c mice, and IBM-BMT was performed when tumours had reached 5 mm in diameter. Interestingly, IBM-BMT + ATT significantly inhibited tumour growth, compared with non-treatment and IBM-BMT alone, after 14 days (Fig. 4a,b). Moreover, all mice that had a high dose of DLI (1 × 107 of spleen cells) died within 21 days from severe GVHD even with a strong GVT effect comparable to that of IBM-BMT + ATT. In contrast, the mice with a low dose of DLI (3 × 106 of spleen cells) survived for a long time, but showed weaker GVT effects than those treated with IBM-BMT + ATT.
Figure 4.
Graft-versus-tumour (GVT) effect induced by intra-bone marrow–bone marrow transplantation (IBM-BMT) with or without adult thymus transplantation (ATT). Lethally irradiated BALB/c mice with Meth-A sarcoma underwent transplantation with 2 × 107 B6 bone marrow cells (BMCs) by IBM-BMT with or without ATT or spleen cell injection [donor lymphocyte infusion (DLI)] from the same donor. (a) Representative findings for tumours (arrows) in non-treated mice, or in mice treated with IBM-BMT with or without ATT 28 days after BMT. (b) The time-course of tumour growth after transplantation in mice following IBM-BMT with or without ATT or DLIs (high dose, 1 × 107; low dose, 3 × 106 B6 spleen cells) or non-treatment. The mice treated with IBM-BMT + ATT showed significant tumour regression, in contrast to the non-treated mice and the mice treated with IBM-BMT or IBM-BMT + a low DLI. The mice treated with IBM-BMT + a high DLI showed similar results, but they died early as a result of GVHD. IBM-BMT alone, n = 8; IBM-BMT + ATT, n = 12; IBM-BMT + a high DLI, n = 8; IBM-BMT + a low DLI, n = 10; non-treatment, n = 7. Data are shown as mean ± standard deviation (SD). *P < 0·05 compared with IBM-BMT + a low dose of DLI, IBM-BMT + a high dose of DLI and IBM-BMT + ATT; **P < 0·05 compared with IBM-BMT, IBM-BMT + a low dose of DLI, IBM-BMT + a high dose of DLI and IBM-BMT + ATT; ***P < 0·05 compared with IBM-BMT, IBM-BMT + a low dose of DLI and IBM-BMT + ATT. #P < 0·05 compared with IBM-BMT + a low dose of DLI, IBM-BMT + a high dose of DLI and IBM-BMT + ATT; ##P < 0·05 compared with IBM-BMT + ATT; ###P < 0·05 compared with IBM-BMT + a low dose of DLI and IBM-BMT + ATT. §P < 0·05 compared with IBM-BMT + ATT; §§P < 0·05 compared with IBM-BMT + ATT. †P < 0·05 for short survival time in IBM-BMT + a high dose of DLI compared with other groups.
Mechanisms underlying tumour regression induced by IBM-BMT + ATT
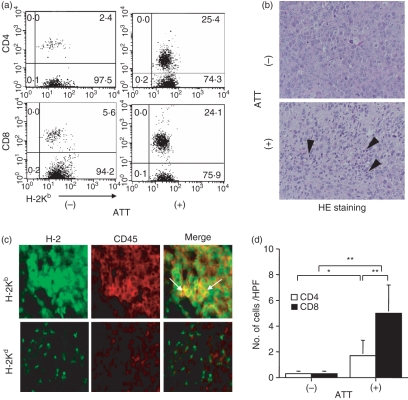
We analysed the mechanisms of tumour regression. Although the tumour-bearing mice treated with IBM-BMT with or without ATT clearly displayed donor-derived chimerism in both CD4 and CD8 T cells in the spleen (Fig. 5a), the percentages of both subsets in the mice treated with IBM-BMT + ATT were higher than those in the mice treated with IBM-BMT alone. Histologically, in contrast to the infiltration of a few lymphocytes in the tumours of the mice treated with IBM-BMT alone, numerous lymphocytes had infiltrated the tumours in the mice treated with IBM-BMT + ATT (Fig. 5b; HE staining). The cells were H-2Kb+ CD45+ (not H-2Kd+ CD45+), indicating that they were donor-derived lymphocytes (Fig. 5c). The analyses of T-cell subsets in the tumour revealed that numbers of both CD4 and CD8 T cells were significantly higher in the mice treated with IBM-BMT + ATT than in the mice treated with IBM-BMT alone (Fig. 5d). Interestingly, CD8 T cells predominantly infiltrated the tumours in the mice treated with IBM-BMT + ATT.
Figure 5.
Analysis of donor-derived T-cell subsets in the spleen and infiltrated T-cell subsets in tumours following intra-bone marrow–bone marrow transplantation (IBM-BMT) with or without adult thymus transplantation (ATT). Lethally irradiated BALB/c mice with Meth-A sarcoma underwent transplantation with 2 × 107 B6 bone marrow cells (BMCs) by IBM-BMT with or without ATT from the same donor. Tumours were removed 4 weeks after transplantation. (a) Fluorescence-activated cell sorter (FACS) profile for donor-derived H-2Kb+ and CD8 or CD4 T cells in the spleen. Elevated numbers of both CD4 and CD8 T cells were found in the mice treated with IBM-BMT + ATT compared with those treated with IBM-BMT alone. (b) Representative findings for tumour cells with haematoxylin and eosin (HE) staining (upper) and the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labelling (TUNEL) method (lower) following IBM-BMT with or without ATT (×800). Numerous lymphocytes had infiltrated the tumours in the mice treated with IBM-BMT + ATT compared with those treated with IBM-BMT alone (arrowhead). Donor-derived lymphocytes (H-2Kb+ CD45+) and host-derived lymphocytes (H-2Kd+ CD45+) in tumours from the mice treated with IBM-BMT + ATT were examined (×1000) (c). Donor-derived lymphocytes (H-2Kb+ CD45+ cells), but not host-derived lymphocytes (H-2Kd+ CD45+ cells), were observed in the tumour from the mice treated with IBM-BMT + ATT (arrows). H-2Kb+ CD45− cells may be tumour cells. (d) Comparison of the CD4 and CD8 T-cell subsets in tumours by IBM-BMT with or without ATT. Both subsets in the mice treated with IBM-BMT + ATT were significantly higher than those in the mice treated with IBM-BMT, and predominantly CD8 T cells infiltrated the tumour compared with CD8 T cells in those treated with IBM-BMT + ATT. IBM-BMT + ATT, n = 5; IBM-BMT alone, n = 5. Data are shown as mean ± standard deviation (SD). *P < 0·05; **P < 0·01.
Cytokine production and apoptosis in tumours from mice treated with IBM-BMT + ATT
We next investigated cytokine production in spleen cells from tumour-bearing non-treated mice and tumour-bearing mice treated with IBM-BMT with or without ATT (Fig. 6a). The production of IFN-γ was higher in the mice treated with IBM-BMT + ATT than in the mice treated with IBM-BMT alone. The non-treated mice showed the lowest level of IFN-γ. Although IL-2 was slightly elevated in these mice, no significant difference was observed. IL-4 and IL-10 production was very low compared with IFN-γ and IL-2. Finally, we performed TUNEL staining for the detection of apoptotic cells in the tumour. The positive cell counts were much higher in the mice treated with IBM-BMT + ATT than in the mice treated with IBM-BMT alone (Fig. 6b,c). The non-treated mice showed few apoptotic cells in the tumour (data not shown).
Figure 6.
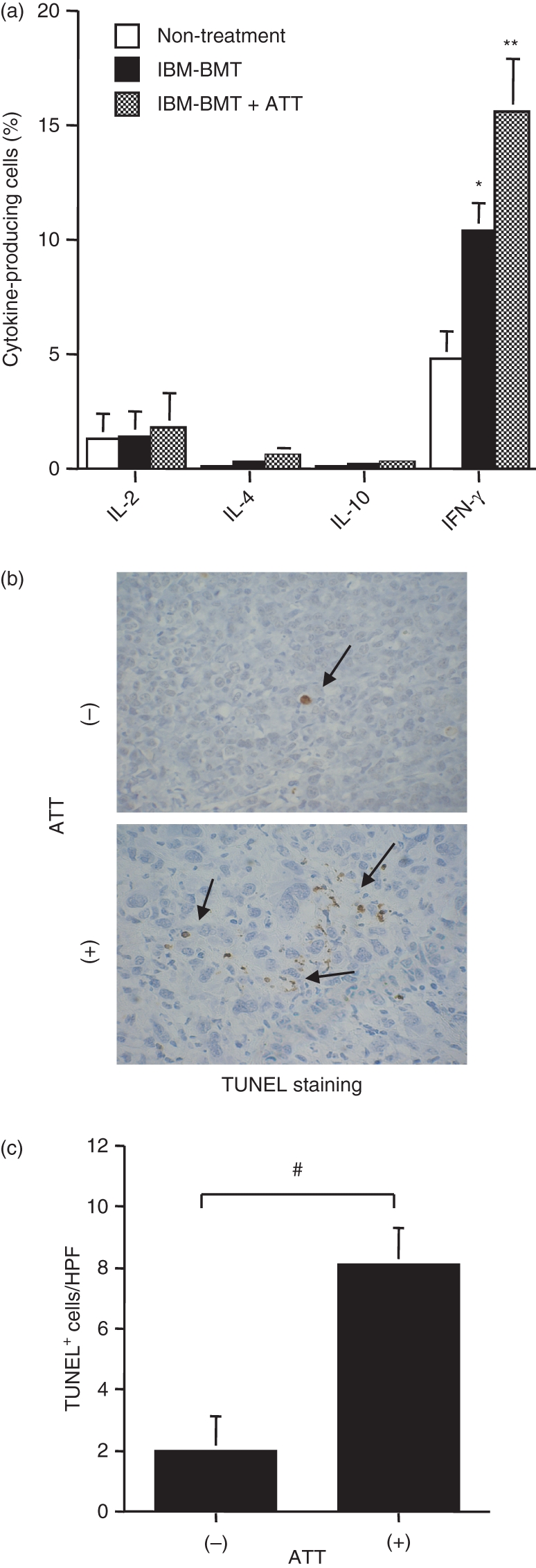
Analysis of cytokine production and apoptotic cells in tumours following intra-bone marrow–bone marrow transplantation (IBM-BMT) with or without adult thymus transplantation (ATT). (a) Spleen cells were intracytoplasmically stained with phycoerythrin-anti-interleukin (IL)-2, IL-4, IL-10 or interferon (IFN)-γ monoclonal antibodies (mAbs) to determine the per cent of IL-2-, IL-4- or IL-10-producing cells. Non-treatment, n = 4; IBM-BMT alone, n = 4; IBM-BMT + ATT, n = 4. *P < 0·05 compared with non-treatment; **P < 0·05 compared with non-treatment and IBM-BMT alone. (b) Representative findings for tumour cells with terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labelling (TUNEL) staining (×400). TUNEL-positive tumour cells were observed (arrows). Numbers of TUNEL+ cells after treatment with IBM-BMT with and without ATT were compared. Numbers of TUNEL-positive tumour cells were significantly elevated in the mice treated with IBM-BMT + ATT in comparison with those treated with IBM-BMT. IBM-BMT + ATT, n = 5; IBM-BMT alone, n = 5. Data are shown as mean ± standard deviation (SD). #P < 0·01.
Discussion
In the present study, we have demonstrated that allogeneic IBM-BMT + ATT successfully induces high thymopoiesis. Although this treatment induced mild GVHR, survival rate, weight and donor-derived chimerism did not differ from those in mice that received allogeneic IBM-BMT alone. Interestingly, when IBM-BMT + ATT was performed in tumour-bearing mice, tumour growth was significantly inhibited, compared with non-treatment or IBM-BMT alone. IBM-BMT + DLI did not produce a longer survival time than IBM-BMT + ATT. The donor-derived CD8 T cells markedly infiltrated the tumour after IBM-BMT + ATT, and the tumour cells underwent apoptosis as a result of lymphocyte infiltration with elevation of IFN-γ. These findings strongly indicate that IBM-BMT + ATT induces high thymopoiesis, thereby eliciting strong GVT effects with mild GVHR.
We first examined the effects of IBM-BMT + ATT on normal mice. The transplanted thymus showed a normal structure under the renal capsule and normal thymocyte differentiation. Although the number of spleen cells did not differ between the mice treated with IBM-BMT alone and the mice treated with IBM-BMT + ATT, the numbers of both CD4 and CD8 T-cell subsets were significantly higher with ATT than without ATT. In addition, the number of TRECs in the mice treated with IBM-BMT + ATT was significantly higher than in the mice treated with IBM-BMT alone, with the number increasing over time. These results indicate the successful induction of high and continuous thymopoiesis by IBM-BMT + ATT, and that most T cells are derived from TT.
As some T cells were produced by the transplanted allogeneic thymus in the mice treated with IBM-BMT + ATT, the cells should display anti-host activity, as seen in GVHR. However, in contrast to IBM-BMT + DLI, which induces severe GVHD with rapid mortality,41 IBM-BMT + ATT elicits only mild GVHD.
We next attempted to explain the present data by the frequency of Tregs, detected as FoxP3+ cells in CD4+ T cells; this frequency was slightly lower in the recipients of IBM-BMT + ATT than in the recipients of IBM-BMT alone or in control B6 mice, but significantly higher than in the mice treated with IBM-BMT + DLI. The low number of CD4 T cells in mice treated with IBM-BMT + DLI may result from the progression of GVHD. Much evidence has recently accumulated that Tregs are involved in the regulation of GVHD in mice,42,43 and that, in humans, the reduced frequency of Tregs is also observed in patients with chronic GVHD and is negatively correlated with its severity.44 This was the case in the present study, in which severe GVHD was observed in recipients with few Tregs after IBM-BMT + DLI, while only mild GVHD was observed in recipients possessing relatively high numbers of Tregs in the spleen after IBM-BMT + ATT. IBM-BMT alone produced no GVHD and a high proportion of Tregs, comparable to that in non-treated mice. These findings strongly suggest the participation of Tregs in the inhibition or negative regulation of GVHD.
Next, we investigated GVT effects associated with IBM-BMT + ATT. Interestingly, the growth of Meth-A tumours was most significantly inhibited by IBM-BMT + ATT, compared with non-treatment. IBM-BMT alone also produced significant regression, compared with non-treatment. This may be partially attributable to the effects of irradiation. In addition, IBM-BMT plus a high dose of DLI also induced strong tumour regression, but death from severe GVHD, whereas IBM-BMT plus a low dose of DLI elicited less tumour regression but mild GVHD with a long survival time. In histological analysis, IBM-BMT + ATT produced marked lymphocyte infiltration inside the tumour involving significant high numbers of donor-derived CD8 T cells compared with IBM-BMT alone. In addition, IFN-γ, which is a T helper type 1 (Th1) cytokine, also showed significantly high production in the mice with IBM-BMT + ATT, compared with the non-treated mice and the mice treated with IBM-BMT alone. Because IFN-γ itself has a GVT effect, it may not only promote an immune response with GVH effects, but also facilitate tumour regression. The mice treated with IBM-BMT alone also showed higher IFN-γ production than did the non-treated mice, suggesting that not only irradiation but also IFN-γ may play a role in tumour regression in these mice, too. As a result, the tumour cells also displayed significantly increased apoptosis. Although other tumour cell lines should be examined, these findings indicate that IBM-BMT + ATT induces strong GVT effects with donor cytotoxic CD8+ T lymphocytes against the tumour.
In the MLR analyses, T cells showed a low anti-host response in the mice treated with IBM-BMT + ATT. As the mice treated with IBM-BMT alone showed no response to host cells, the difference is probably derived from ATT. Notably, the anti-host response was lower than in the mice treated with IBM-BMT + DLI. In addition, there was a positive correlation with the degree of GVHD and a negative correlation with the percentage of Tregs.
We could thus induce GVT effects without inducing severe GVHD by treatment with allogeneic IBM-BMT + ATT. Although the details of the mechanisms are still unknown, there are a number of possibilities. One is that Tregs also function to create the GVT effect in recipients treated with IBM-BMT + ATT, as it has been reported that Tregs suppress GVHR induced by CD4 T cells, but do not reduce GVT effects induced by CD8 T cells;29,45 Tregs suppress the peripheral proliferation of CD8 T cells but do not inhibit cytotoxic T lymphocyte (CTL) activity. Thus, the intermediate proportion of CD4+ FoxP3+ Tregs induced by IBM-BMT + ATT can only achieve incomplete – but continuous – inhibition of GVHR but can maintain CTL activity, which leads to strong GVT effects. The other possibility, from the viewpoint of effector T-cell development, is that, in the case of IBM-BMT + DLI, the mature T cells with allo-MHC reactivity that were present easily induced severe GVHD. In the case of IBM-BMT + ATT, the reactivity of allo-specific T cells derived from the grafted thymus might be insufficient as a result of incomplete repertoire formation, as the thymic dendritic cells in the engrafted B6 thymus may present BALB/c-derived molecules (including their MHC) in negative selection during thymopoiesis. However, tumour-specific antigens are difficult to detect in the transplanted thymus because the tumour itself is located far from the thymus. As a result, the small number of allo-specific T cells (but not Tregs) may have proliferated peripherally and led to the low ratio of Tregs in CD4 T cells. To confirm these findings, further studies are required of the expression of CD4, CD8 and Foxp3 staining in GVHD and GVT sites. In addition, direct transfer and/or deletion experiments using Tregs should be carried out.
The continuous supplementation of T cells from the allogeneic ATT may induce mild GVHD and strong GVT effects with well-balanced effector and regulatory T cells. Alternatively, the transplanted thymus itself may regulate homeostasis of the cells. Thymus transplantation thus initially appears to be a simple method, but may prove to be an effective approach in that it supplies the organ in which T cells are differentiated, produced and functionally regulated. The method may be adequate to cure slow progressive diseases such as cancers, whereas the direct transfer of T cells, including Tregs, may be adequate for acute diseases such as infection and in the case of acute rejection.
Overall, we have found that allogeneic IBM-BMT + ATT induces high thymopoiesis with a mild GVH reaction and elicits strong GVT effects. Although it may clinically be difficult to obtain adequate thymus ethically and technically (with problems including donor age), grafts could be obtained from patients with congenital heart diseases or from aborted fetuses, as previously utilized.33 In this respect, we have recently found that, even if the thymus donor is different from the donor of BMCs, the effect is comparable to that seen with transplantation from the same donor using triple chimeric mice.46 In addition, a method of regenerating the thymus has also been developed dramatically.47 We thus believe that IBM-BMT + ATT could become a viable strategy for the treatment of malignant tumours in humans.
Acknowledgments
This work was supported by a grant from the Haiteku Research Center of the Ministry of Education, a grant from the Millennium programme of the Ministry of Education, Culture, Sports, Science and Technology, a grant from the Science Frontier programme of the Ministry of Education, Culture, Sports, Science and Technology, a grant from the 21st Century Center of Excellence (COE) programme of the Ministry of Education, Culture, Sports, Science and Technology, a Research Grant C from Kansai Medical University, Health and Labour Sciences research grants (Research on Human Genome, Tissue Engineering and Food Biotechnology), a grant from the Department of Transplantation for Regeneration Therapy (sponsored by Otsuka Pharmaceutical Company, Ltd), a grant from the Molecular Medical Science Institute, Otsuka Pharmaceutical Company, Ltd, and a grant from Japan Immunoresearch Laboratories Co., Ltd (JIMRO). We thank Ms Y. Tokuyama, Ms K. Hayashi and Ms A. Kitajima for their technical assistance and Mr Hilary Eastwick-Field and Ms K. Ando for their help with the preparation of the manuscript.
Glossary
Abbreviations:
- ATT
adult thymus transplantation
- BM
bone marrow
- BMC
bone marrow cell
- BMT
bone marrow transplantation
- DLI
donor lymphocyte infusion
- FITC
fluorescein isothiocyanate
- FoxP3
forkhead-box transcription factor p3
- GVHD
graft-versus-host disease
- GVT
graft-versus-tumour
- HE
haematoxylin and eosin
- HPF
high-power field
- IBM-BMT
intra-bone marrow–bone marrow transplantation
- IFN
interferon
- IL
interleukin
- IV-BMT
intravenous bone marrow transplantation
- MHC
major histocompatibility complex
- MLR
mixed lymphocyte reaction
- MSC
mesenchymal stem cell
- PE
phycoerythrin
- TREC
T-cell receptor rearrangement excision circle
- Treg
regulatory T cell
- TT
thymus transplantation
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labelling
References
- 1.Thomas ED. Landmarks in the development of hematopoietic cell transplantation. World J Surg. 2000;24:815–8. doi: 10.1007/s002680010130. [DOI] [PubMed] [Google Scholar]
- 2.Bacigalupo A, Oneto R, Bruno B, et al. Current results of bone marrow transplantation in patients with acquired severe aplastic anemia. Report of the European Group for Blood and Marrow transplantation. On behalf of the Working Party on Severe Aplastic Anemia of the European Group for Blood and Marrow Transplantation. Acta Haematol. 2000;103:19–25. doi: 10.1159/000041000. [DOI] [PubMed] [Google Scholar]
- 3.Chen RL, Hou JW, Chang PY, Tsai FJ, Wang PJ. Matched unrelated bone marrow transplantation without splenectomy for a child with Gaucher disease caused by homozygosity of the L444P mutation, who also suffered from schizencephaly. J Pediatr Hematol Oncol. 2007;29:57–9. doi: 10.1097/MPH.0b013e3180308793. [DOI] [PubMed] [Google Scholar]
- 4.Sato T, Kobayashi R, Toita N, Kaneda M, Hatano N, Iguchi A, Kawamura N, Ariga T. Stem cell transplantation in primary immunodeficiency disease patients. J Pediatr Hematol Oncol. 2007;29:57–9. doi: 10.1111/j.1442-200X.2007.02468.x. [DOI] [PubMed] [Google Scholar]
- 5.Kanamori H, Tanaka M, Kawaguchi H, Yamaji S, Fujimaki K, Tomita N, Fujisawa S, Ishigatsubo Y. Resolution of psoriasis following allogeneic bone marrow transplantation for chronic myelogenous leukemia: case report and review of the literature. Am J Hematol. 2002;71:41–4. doi: 10.1002/ajh.10169. [DOI] [PubMed] [Google Scholar]
- 6.Saba N, Flaig T. Bone marrow transplantation for nonmalignant diseases. J Hematother Stem Cell Res. 2002;11:377–87. doi: 10.1089/152581602753658565. [DOI] [PubMed] [Google Scholar]
- 7.Ueno NT, Rizzo JD, Demirer T, et al. Allogeneic hematopoietic cell transplantation for metastatic breast cancer. Bone Marrow Transplant. 2008;41:537–45. doi: 10.1038/sj.bmt.1705940. [DOI] [PubMed] [Google Scholar]
- 8.Kolb HJ, Schattenberg A, Goldman JM, et al. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–50. [PubMed] [Google Scholar]
- 9.Saito TI, Rubio MT, Sykes M. Clinical relevance of recipient leukocyte infusion as antitumor therapy following nonmyeloablative allogeneic hematopoietic cell transplantation. Exp Hematol. 2006;34:1271–7. doi: 10.1016/j.exphem.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Ohmori I, Hayamizu K, Oishi K, Yoshimitsu M, Itamoto T, Asahara T. Inhibition of tumor growth in immunocompromised hosts by restoring type-2 immunity using infusion of G-CSF-treated allogeneic CD8+ leukocytes. Cytokine. 2005;32:255–62. doi: 10.1016/j.cyto.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Russell NH, Byrne JL, Faulkner RD, Gilyead M, Das-Gupta EP, Haynes AP. Donor lymphocyte infusions can result in sustained remissions in patients with residual or relapsed lymphoid malignancy following allogeneic haemopoietic stem cell transplantation. Bone Marrow Transplant. 2005;36:437–41. doi: 10.1038/sj.bmt.1705074. [DOI] [PubMed] [Google Scholar]
- 12.Chao NJ. Graft-versus-host disease. The view point from the donor T cell. Biol Blood Marrow Transplant. 1997;3:1–10. [PubMed] [Google Scholar]
- 13.Goulmy E, Schipper R, Blokland E, Falkenburg F. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host-disease after bone marrow transplantation. N Engl J Med. 1996;334:281–5. doi: 10.1056/NEJM199602013340501. [DOI] [PubMed] [Google Scholar]
- 14.Murphy WJ, Blazar BR. New strategies for preventing graft-versus-host disease. Curr Opin Immunol. 1999;11:509–15. doi: 10.1016/s0952-7915(99)00002-3. [DOI] [PubMed] [Google Scholar]
- 15.Hosaka N, Nose M, Kyogoku M, Nagata N, Miyashima S, Good RA, Ikehara S. Thymus transplantation, a critical factor for correction of autoimmune disease in aging MRL/+mice. Proc Natl Acad Sci USA. 1996;93:8558–62. doi: 10.1073/pnas.93.16.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kushida T, Inaba M, Takeuchi K, Sugiura K, Ogawa R, Ikehara Y. Treatment of intractable autoimmune disease in MRL/lpr mice using a new strategy for allogeneic bone marrow cells transplantation. Blood. 2000;95:1862–8. [PubMed] [Google Scholar]
- 17.Kushida T, Inaba M, Hisha H, Ichioka N, Esumi T, Ogawa R, Iida H, Ikehara S. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood. 2001;97:3292–9. doi: 10.1182/blood.v97.10.3292. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Inaba M, Sugiura K, Yoshimura T, Kwon AH, Kamiyama Y, Ikehara S. Enhancement of allogeneic hematopoietic stem cell engraftment and prevention of graft-versus-host diseases (GvHD) by intra-bone marrow-bone marrow transplantation plus donor lymphocyte infusion. Stem Cells. 2004;22:125–34. doi: 10.1634/stemcells.22-2-125. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto F, Sugiura K, Inoue K, Ikehara S. Major histocompatibility complex restriction between hematopoietic stem cells and stromal cells in vivo. Blood. 1997;89:49–54. [PubMed] [Google Scholar]
- 20.Sugiura K, Hisha H, Ishikawa J, Adachi Y, Taketani S, Lee S, Nagahama T, Ikehara S. Major histocompatibility complex restriction between hematopoietic stem cells and stromal cells in vitro. Stem Cells. 2001;19:46–58. doi: 10.1634/stemcells.19-1-46. [DOI] [PubMed] [Google Scholar]
- 21.Ikehara S. A novel strategy for allogeneic stem cell transplantation: perfusion method plus intra-bone marrow injection of stem cells. Exp Hematol. 2003;31:1142–6. doi: 10.1016/j.exphem.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 23.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 24.Moore NC, Anderson G, Smith CA, Owen JJ, Jenkinson EJ. Analysis of cytokine gene expression in subpopulations of freshly isolated thymocytes and thymic stromal cells using semiquantitative polymerase chain reaction. Eur J Immunol. 1993;23:922–7. doi: 10.1002/eji.1830230424. [DOI] [PubMed] [Google Scholar]
- 25.Bodey B, Bodey B, Jr, Siegel SE, Kaiser HE. Review of thymic hormones in cancer diagnosis and treatment. Int J Immunopharmacol. 2000;22:261–73. doi: 10.1016/s0192-0561(99)00084-3. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006;7:709–14. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 28.Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, Cohen JL. Recipient-type specific CD4+ CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112:1688–96. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+ CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 30.Hosaka N, Ryu T, Miyake T, Cui W, Nishida T, Takaki T, Inaba M, Ikehara S. Treatment of autoimmune diseases in MRL/lpr mice by allogeneic bone marrow transplantation plus adult thymus transplantation. Clin Exp Immunol. 2007;147:555–63. doi: 10.1111/j.1365-2249.2006.03310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryu T, Hosaka N, Miyake T, et al. Transplantation of newborn thymus plus hematopoietic stem cells can rescue supralethal irradiated mice. Bone Marrow Transplant. 2008;41:659–66. doi: 10.1038/sj.bmt.1705957. [DOI] [PubMed] [Google Scholar]
- 32.van den Brink MR, Alpdogan O, Boyd RL. Strategies to enhance T-cell reconstitution in immunocompromised patients. Nat Rev Immunol. 2004;4:856–67. doi: 10.1038/nri1484. [DOI] [PubMed] [Google Scholar]
- 33.Markert ML, Boeck A, Hale LP, Kloster AL, McLaughlin TM. Transplantation of thymus tissue in complete DiGeorge syndrome. N Engl J Med. 1999;341:1180–9. doi: 10.1056/NEJM199910143411603. [DOI] [PubMed] [Google Scholar]
- 34.Markert ML, Hicks CB, Bartlett JA, Harmon JL. Hale Effect of highly active antiretroviral therapy and thymic transplantation on immunoreconstitution in HIV infection. AIDS Res Hum Retroviruses. 2000;16:403–13. doi: 10.1089/088922200309061. [DOI] [PubMed] [Google Scholar]
- 35.Hill GR, Cooke KR, Teshima T, Crawford JM, Keith JC, Jr, Brinson YS, Bungard D, Ferrara JL. Interleukin-11 promotes T cell polarization and prevents acute graft-versus-host disease after allogeneic bone marrow transplantation. J Clin Invest. 1998;102:115–23. doi: 10.1172/JCI3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–13. [PubMed] [Google Scholar]
- 37.Hosaka N, Minato T, Yoshida S, Toki J, Yang G, Hisha H, Ikehara S. Kimura’s disease with unusual eosinophilic epithelioid granulomatous reaction: a finding possibly related to eosinophil apoptosis. Hum Pathol. 2002;33:561–4. doi: 10.1053/hupa.2002.124037. [DOI] [PubMed] [Google Scholar]
- 38.Hosaka N, Kitajiri S, Hiraumi H, Nogaki H, Toki J, Yang G, Hisha H, Ikehara S. Ectopic pituitary adenoma with malignant transformation. Am J Surg Pathol. 2002;26:1078–82. doi: 10.1097/00000478-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 39.Sempowski GD, Gooding ME, Liao HX, Le PT, Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol. 2002;38:841–8. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 40.Takada K, Inaba M, Ichioka N, et al. Treatment of senile osteoporosis in SAMP6 mice by intra-bone marrow injection of allogeneic bone marrow cells. Stem Cells. 2006;24:399–405. doi: 10.1634/stemcells.2005-0068. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki Y, Adachi Y, Minamino K, Zhang Y, Iwasaki M, Nakano K, Koike Y, Ikehara S. A new strategy for treatment of malignant tumor: intra-bone marrow-bone marrow transplantation plus CD4− donor lymphocyte infusion. Stem Cells. 2005;23:365–70. doi: 10.1634/stemcells.2004-0258. [DOI] [PubMed] [Google Scholar]
- 42.Miura Y, Thoburn CJ, Bright EC, Arai S, Hess AD. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104:2187–93. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 43.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–99. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann P, Edinger M. CD4+ CD25+ regulatory T cells and graft-versus-host disease. Semin Hematol. 2006;43:62–9. doi: 10.1053/j.seminhematol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Sakaguchi S. Taming transplantation with T cells. Nat Med. 2003;9:1117–8. doi: 10.1038/nm0903-1117. [DOI] [PubMed] [Google Scholar]
- 46.Cui W, Hosaka N, Miyake T, et al. Analysis of tolerance induction using triple chimeric mice: MHC-disparate thymus, hemopoietic cells and microenvironment. Transplantation. 2008;85:1151–8. doi: 10.1097/TP.0b013e31816a8f1f. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Sun L, Zhao Y. Thymic epithelial progenitor cells and thymus regeneration: an update. Cell Res. 2007;17:50. doi: 10.1038/sj.cr.7310114. [DOI] [PubMed] [Google Scholar]