Abstract
AIM: To assess the effect of notoginsenoside R1 on hepatic microcirculatory disturbance induced by gut ischemia/reperfusion (I/R) in mice.
METHODS: The superior mesenteric artery (SMA) of C57/BL mice was ligated for 15 min to induce gut ischemia followed by 30-min reperfusion. In another set of experiments, R1 was continuously infused (10 mg/kg per hour) from 10 min before I/R until the end of the investigation to study the influence of R1 on hepatic microcirculatory disturbance induced by gut I/R. Hepatic microcirculation was observed by inverted microscopy, and the vascular diameter, red blood cell (RBC) velocity and sinusoid perfusion were estimated. Leukocyte rolling and adhesion were observed under a laser confocal microscope. Thirty and 60 min after reperfusion, lactate dehydrogenase (LDH), alanine aminotransferase (ALT) and aspartate transaminase (AST) in peripheral blood were determined. The expression of adhesion molecules CD11b/CD18 in neutrophils and tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and monocyte chemotactic protein-1 (MCP-1) in plasma were evaluated by flow cytometry. E-selectin and intercellular adhesion molecule-1 (ICAM-1) in hepatic tissue were examined by immunofluorescence.
RESULTS: After gut I/R, the diameters of terminal portal venules and central veins, RBC velocity and the number of perfused sinusoids were decreased, while the leukocyte rolling and adhesion, the expression of E-selectin in hepatic vessels and CD18 in neutrophils, IL-6, MCP-1, LDH, ALT and AST were increased. R1 treatment attenuated these alterations except for IL-6 and MCP-1.
CONCLUSION: R1 prevents I/R-induced hepatic microcirculation disturbance and hepatocyte injury. The effect of R1 is related to its inhibition of leukocyte rolling and adhesion by inhibiting the expression of E-selectin in endothelium and CD18 in neutrophils.
Keywords: Ischemia/reperfusion, Notoginsenoside R1, Leukocytes adhesion, E-selectin, Hepatic injury
INTRODUCTION
It is well recognized that gut ischemia/reperfusion (I/R) induces injury of distant organs, such as liver and lung[1–3]. A rate-limiting step in the pathogenesis of I/R injury of liver and other organs is the recruitment of leukocytes to vascular endothelium[2,4,5]. Oxygen free radicals produced by gut I/R activate nuclear factor kappa-B (NF-κB)[6,7], initiate expression of selectin and adhesion molecules[8–10], and elicit release of proinflammatory mediators like tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6)[10]. The expression of L-selectin on leukocytes and E-selectin on endothelial cells induces the rolling of leukocytes along the vascular endothelium[11,12], which further promotes the expression of adhesion molecules CD11b, CD18 on leukocytes and intercellular adhesion molecule-1 (ICAM-1) on endothelial cells, resulting in adhesion of leukocytes to vascular endothelial cells[2,4,13,14]. In addition, proinflammatory mediators produced by I/R enhance the expression of selectins and adhesion molecules, and aggravate the rolling and adhesion of leukocytes[8,15]. The adhesion of leukocytes to the vascular endothelium results in release of oxygen free radicals and proteinase, thus inducing hepatic injury[16]. In line with these findings, studies indicate that inhibition of the expression of selectins and adhesion molecules on leukocytes and endothelial cells ameliorates the adhesion of leukocytes to the hepatic vascular endothelium after gut I/R and attenuates hepatic microcirculation disturbance and injury[2,4,17].
Panax notoginseng (PN) is the dried root of Panax notoginseng (Araliaceae), a Chinese herb medicine widely used in China, Korea, Japan and other Asian countries in the treatment of microcirculatory disturbance-related diseases, such as cardiovascular disease, cerebral vascular diseases and liver dysfunction[18,19]. PN contains more than 30 different types of saponin, of which ginsenoside Rg1 (Rg1), ginsenoside Rb1 (Rb1) and notoginsenoside R1 (R1) are the eminent members[20]. Previous studies have proved that Panax notoginseng saponins (PNS) improve I/R-induced hepatic microcirculation disturbance[19], inhibit platelet aggregation and adhesion molecule expression, and improve vascular endothelium function[21]. It was also reported that PNS inhibit adhesion of leukocytes to rat mesentery venules and expression of neutrophil adhesion molecules CD11b and CD18 induced by lipopolysaccharide (LPS)[22]. The expression of LPS-induced vascular endothelial TNF-α is inhibited by R1 by inhibiting degradation of the inhibitor kappa-B (I-κB)[23]. It has been shown that cardiotonic pills (CP, a traditional Chinese medicine containing PN, salvia miltiorrhiza and borneol) can inhibit adhesion of leukocytes to the hepatic vascular endothelium in rats induced by gut I/R and chronical ethanol feed, and blunt the concentration increment of peripheral blood alanine aminotransferase (ALT), TNF-α and LPS[24]. However, whether R1 improves gut I/R-induced hepatic microcirculation disturbance has not yet been reported, although it is highly anticipated as a major component of PNS, and the structure of R1 has been identified (Figure 1). Therefore, by virtue of intravital microscopy the present study explored the dynamic effects of R1 on a mouse hepatic microcirculation disturbance model induced by gut I/R, especially on leukocyte rolling and adhesion on the vascular endothelium. The expression of E-selectin and ICAM-1 was also determined by immunofluorescent technique. The expression of neutrophil adhesion molecules CD11b and CD18, and the concentration of proinflammatory mediators such as TNF-α, IL-6 and monocyte chemotactic protein-1 (MCP-1) was measured by flow cytometry. The concentrations of lactate dehydrogenase (LDH), ALT and aspartate aminotransferase (AST) were also determined.
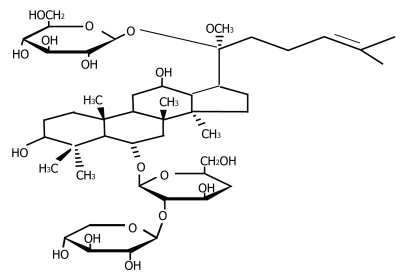
Figure 1.
Chemical structure of notoginsenoside (R1).
MATERIALS AND METHODS
Notoginsenoside and reagents
R1 (purity > 98%) was supplied by Tianjin Talsy Group (Tianjin, China). Other reagents used in experiments were as follows: rhodamine 6G (purity > 99.0%, Lot No.2350994, Fluka Co., Switzerland), FITC-rat anti-mouse CD18 monocolonal antibody (Lot No.553293, BD Biosciences PharMingen, USA), FITC-rat anti-mouse CD11b monoclonal antibody (Lot No.557396, BD Biosciences PharMingen, USA), goat polyclonal antibody against mouse E-selectin (M-20) (sc-6939, Santa Cruz Biotechnology, Inc. USA), goat polyclonal antibody against mouse ICAM-1 (M-19) (sc-1511, Santa Cruz Biotechnology, Inc. USA), rhodamine conjugated rabbit anti-goat lgG-R (Lot No.B1006, Santa Cruz Biotechnology, Inc. USA), Hoechst33342 (Lot No.6538, Santa Cruz Biotechnology, Inc. USA), mouse MCP-1 flex set (Lot No.558342, BD Biosciences, USA), mouse TNF flex set (Lot No.558299, BD Biosciences, USA), mouse IL-6 flex set (Lot No.558301, BD Biosciences, USA).
Animals
C57/BL mice, weighing 22-26 g and aged 8-10 wk (the animal certificate number was SCXK 2002-2001) were purchased from the Animal Center of Peking University Health Science Center. The animals were caged at 24°C ± 1°C with a humidity of 50% ± 5% in a 12 h light/dark cycle, and starved with free access to water for 12 h before the experiment. All animals were handled according to the Guidelines of the Peking University Animal Research Committee.
Intravital microscopy
C57/BL mice were anesthetized with 20% urethane (10 mL/kg body wt, im), as previously described[25]. The left jugular vein was cannulated for drug administration with a polyethylene pipe (0.96 mm in diameter). Immediately after laparotomy, the mice were placed on an observation board in lateral position. The liver was placed on an adjustable Plexiglas microscope stage within a thermo-controlled (37°C) observation box and carefully handled to minimize the influence of respiratory movements. The left lateral lobe of liver was observed under an inverted intravital microscope (DM-IRB, Leica, Germany) assisted by a 3CCD colour camera (JK-TU53H, 3CCD camera, Toshiba, Japan). Areas (400 μm × 320 μm) were selected that included both terminal portal venules and central veins without adherent leukocytes for observation. Images of the microcirculation of liver surface were monitored through a × 20 objective, and the dynamics of hepatic microcirculation was recorded on DVD discs using a DVD recorder (DVR-R25, Malata, China). The liver surface for observation was moisturized with 37°C physiological saline drops throughout the whole procedure, and the liver surface around the observation region was covered with saline-soaked cotton gauze[26].
Procedure for ischemia and reperfusion
The surface of liver was observed for 10 min before ligation of the superior mesenteric artery (SMA) to ensure that all parameters measured were in a steady state. The SMA was then ligated with a snare created from polyethylene tubing (1.00 mm) for 15 min. After ischemia, the ligation was gently released for reperfusion. Venular diameter, RBC velocity, sinusoidal reperfusion, and leukocyte rolling and adhesion were determined immediately before ischemia (baseline) and every 15 min after reperfusion for half an hour.
Experimental protocols
Mice in the I/R group were continuously infused with vehicle saline through the jugular vein from 10 min before I/R until the end of the observation. Mice in the R1 + I/R group were continuously infused with R1 (10 mg/kg per hour) from 10 min before I/R until the end of the observation. Mice in the sham-operated control group were treated in an identical fashion as those in the I/R group, but not subjected to ligation of the SMA. Six mice (three males, three females) were included in each group.
Determination of microcirculatory parameters
The diameters of terminal portal venules and central veins were on replayed DVD images using Image-Pro Plus 5.0 software[27]. The result was presented as the ratio of the value determined at 15 min or 30 min to the baseline.
To access the sinusoidal perfusion, the number of hepatic sinusoids with red blood cells (RBCs) flowing through in the hepatic terminal portal venule and central vein regions was scored on the DVD replay, and presented as the perfused hepatic sinusoids/field of view (250 μm × 300 μm)[26]. The result was presented as the ratio of the value determined at 15 min or 30 min to the baseline.
The RBC velocity in hepatic terminal portal venule and central vein was recorded at a rate of 1000 frames/s by changing the monitor from CCD to a high speed video camera system (FASTCAM-ultima APX, photon, Japan), and the recordings were replayed from the high speed stored images at a rate of 25 frames/s. The RBC velocity in venules was measured with Image-Pro Plus 5.0 software[26,27]. The velocity was presented as μm/s. The result was presented as the ratio of the value determined at 15 min or 30 min to the baseline.
To evaluate the leukocyte rolling and adhesion the fluorescence tracer 0.2 mL Rhodamine 6G (0.5 mg/mL in physiological saline) was administrated via the left jugular vein for the selective staining of white blood cells in vivo[28–32]. Under the inverted laser confocal microscope system (BIO-RAD, Radiance 2100, A xiovert 200, Carl Zeiss Shanghai Co, Ltd, German), with 20 × fluorescent object lens, irradiated with the argon laser beam (wavelength = 543 nm), the rolling and adhesion of leukocytes in hepatic terminal portal venules and central veins were recorded. At each time point, a total of 10 successive frames were recorded at a scanning speed of 1 frame/s, and adhered leukocytes were defined as those that appeared at the same position in the 10 successive frames[24]. The adhered leukocytes in hepatic terminal portal venules and central veins were presented as the number of leukocytes/200 μm, while those within the hepatic sinusoid were counted as the number per field of view of 200 μm2. The leukocytes that stayed at the same position in hepatic terminal portal venules and central veins for less than 10 s were designated as rolling leukocytes, and presented as the number/200 μm.
Analysis of immnofluorescent staining of hepatic endothelial adhesion molecules E-selectin and ICAM-1
After 30-min reperfusion, liver was fixed with 4% paraformaldehyde perfusion, removed and frozen with liquid nitrogen, then cut into sections of 6 μm by a cryostat (LEICA CM 1800, Leica Co., German). The sections were further fixed with 4% paraformaldehyde at room temperature for 10 min and washed with PBS. The samples were then immunohistochemically stained as routing. Goat polyclonal antibody against mouse E-selectin or goat polyclonal antibody against mouse ICAM-1 was applied at dilution of 1:50. The secondary antibody (Rhodamine-labeled rabbit anti goat IgG diluted at 1:200) was added and incubated at 37°C for 30 min, followed by washing with PBS and incubation with Hoechst 33342 (2 μg/mL) at room temperature for 3 min[33]. After washed with PBS, the specimen was sealed and observed under Laser confocal microscope with 63 × object lens. Fluorescence intensity was detected at excitation wavelength 543 nm for R-phycoerythrin and 405 nm for Hoechst (nuclear staining). Five fields of view (1.6 × 104 μm2 each) were evaluated in mouse hepatic sinusoids for each condition. Fluorescence intensities of E-selectin or ICAM-1 were estimated by Image Pro Plus software and expressed as an average proportion, positive area/area of one field of view (1.6 × 104 μm2).
Assessment of the expression of adhesion molecules CD11b and CD18 in peripheral neutrophils
After 30-min reperfusion, blood was collected via inferior vena cava and anticoagulated with heparin (20 unit/mL whole blood). The sample was incubated with 1 μg FITC-labeled antibody against CD18 or CD11b for 20 min at room temperature in dark. RBCs were lysed by addition of hemolysin and the samples were then washed twice with PBS. Flow cytometry (FACS Calibur, B.D. Co, USA) was used to assess the mean fluorescence intensity of CD11b or CD18 for 5000 neutrophils in each condition[22].
Peripheral blood hepatic enzyme assay
In some mice, at 30 min and 60 min, respectively after reperfusion, blood samples were withdrawn via inferior vena cava and anticoagulated with heparin (20 unit/mL whole blood). The blood serum was isolated by centrifugation (AllegraTM 64R Centrifuge, Beckman Coultertm, German) at 4000 r/min for 10 min at 4°C and stored at -20°C. The activities of LDH, ALT, and AST were measured respectively using lactate dehydrogenase, alanine aminotransferase and aspartate aminotransferase kits with parameter rate-A[34], following their manufacturer’s instructions, with an automatic enzyme analyzer (7170A Automatic Analyzer, Hitachi, Japan).
Peripheral blood TNF-α, IL-6 and MCP-1 assay
At 30 min after reperfusion, blood was collected via inferior vena cava, and anticoagulated with heparin (20 unit/mL whole blood). The blood serum was isolated by centrifugation (AllegraTM 64R Centrifuge, Beckman Coultertm, German) at 4000 r/min for 10 min at 4°C and stored at -20°C. The concentrations of TNF-α, IL-6 and MCP-1 were measured by flow cytometry with a BD cytometric bead array kit (BD Biosciences Pharmingen, USA)[35]. Fifty μL bead was added into 50 μL blood plasma or standard substance and incubated at room temperature in dark for 1 h for bead capture. Fifty μL PE-labelled detecting antibody was then added and incubated at room temperature for 2 h to form a sandwich complex. After incubation, the samples were washed thoroughly with 1 mL washing buffer (BD Biosciences Pharmingen, USA). The mean fluorescence intensity of TNF-α, IL-6 and MCP-1 was detected respectively by flow cytometry (FACS Calibur, B.D. Co., USA) and the data were analyzed using the BD cytometric bead array analysis software.
Statistical analysis
Values are presented as mean ± SE (n = 6), F-test was performed using SPSS 10.0 statistical software. P < 0.05 was considered statistically significant.
RESULTS
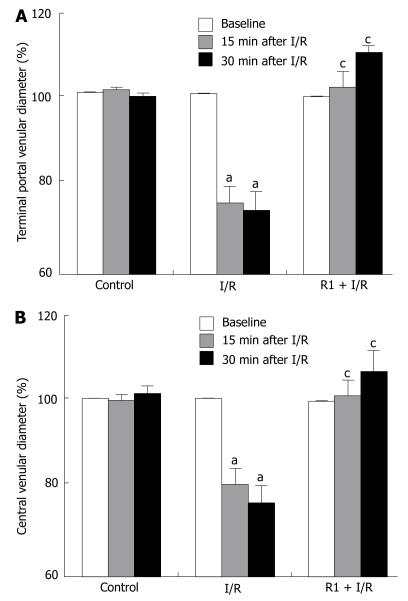
The effect of R1 on the diameter of hepatic terminal portal venule and central vein of mice subjected to SMA I/R are shown in Figure 2. In the control group, the diameters of both terminal portal venules and central veins remained nearly constant over the entire observation period. SMA I/R decreased the diameter of vessels in a time-dependent manner, while treatment with R1 significantly relieved SMA I/R-induced decrease in the vessel diameters.
Figure 2.
Effect of R1 on the terminal portal venular diameter (A) and central venular diameter (B) of hepatic venules of mice subjected to SMA I/R at 0 min before ischemia (Baseline) and 15 min, 30 min after reperfusion. Abscissa represents the ratio of the diameter value at a time point to the baseline. The results are presented as mean ± SE from 6 animals. aP < 0.05 vs control, cP < 0.05 vs I/R.
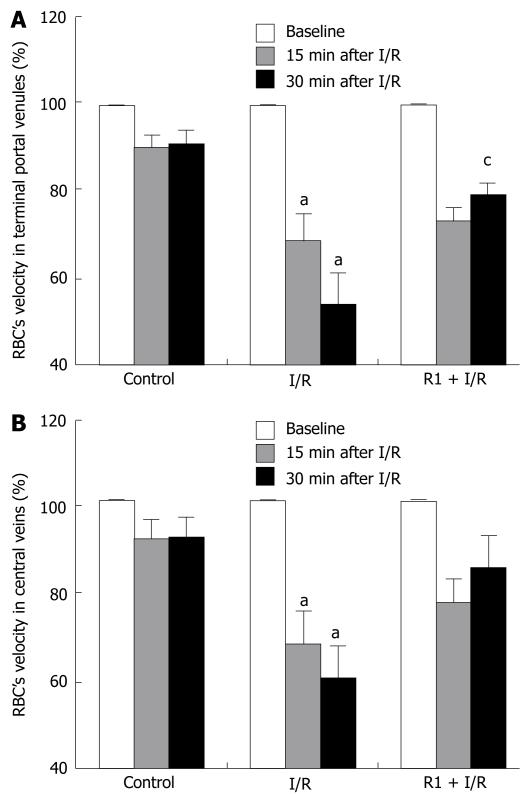
The influence of R1 on the RBC velocity in hepatic terminal portal venules and central veins of mice after SMA I/R is shown in Figure 3. In the control group, no significant change was observed in the RBC velocity of both types of vessels during the period of observation. SMA I/R significantly decreased the RBC velocity of vessels in a time-dependent fashion. R1 treatment blunted the SMA I/R-induced decrease in the RBC velocity of both types of vessels at 30 min after reperfusion, being significant in terminal portal venules (Figure 3A) but not in central veins (Figure 3B), in comparison with the I/R group.
Figure 3.
Effect of R1 on the RBC velocity in terminal portal venules (A) and in central veins (B) of mice subjected to SMA I/R at 0 min before ischemia (Baseline) and 15 min, 30 min after reperfusion. Abscissa represents the ratio of the RBC velocity value at a time point to the baseline. The results are presented as mean ± SE from 6 animals. aP < 0.05 vs control, cP < 0.05 vs I/R.
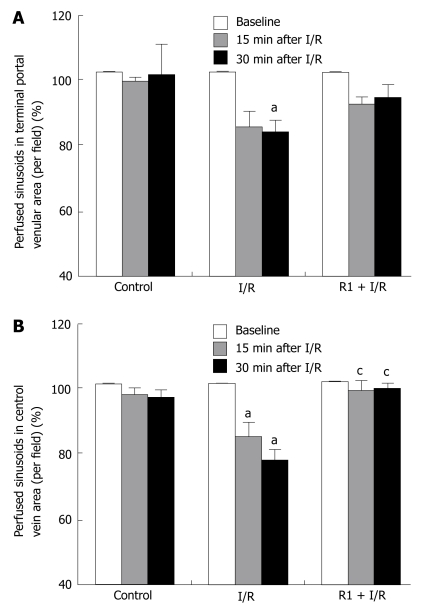
The effect of R1 on the number of reperfused sinusoids in hepatic terminal portal venule and central vein areas after SMA I/R is depicted in Figure 4. In the control group, no significant change in the number of reperfused sinusoids was detected either in the terminal portal venule area or in the central vein area through the entire observation. SMA I/R exposure elicited a time-dependent decrease in the number of reperfused sinusoids, which became statistically significant at 15 min of reperfusion in the central vein areas and at 30 min of reperfusion in the terminal portal venule areas compared to the control group. I/R-induced decrease in the number of reperfused sinusoids in central vein areas was attenuated significantly after treatment with R1 (Figure 4B), and this effect was not observed in the terminal portal venule areas (Figure 4A).
Figure 4.
Effect of R1 on the perfused hepatic sinusoids in the areas of terminal portal venules (A) and in the area of central veins (B) of mice subjected to SMA I/R at 0 min before ischemia (Baseline) and 15 min, 30 min after reperfusion. Abscissa represents the ratio of perfused sinusoids at a time point to the baseline. The results are presented as mean ± SE from 6 animals. aP < 0.05 vs control, cP < 0.05 vs I/R.
The effect of R1 on the number of rolling leukocytes in mouse hepatic terminal portal venules and central veins after SMA I/R is depicted in Figure 5. I/R challenge significantly increased the number of rolling leukocytes in both terminal portal venules and central veins when compared with the control group, although a small number of rolling leukocytes could be observed in the control group during the period of examination. R1 treatment reduced the enhancement in the leukocyte rolling induced by I/R, which was statistically significant in the central veins (Figure 5B), but not in terminal portal venules (Figure 5A).
Figure 5.
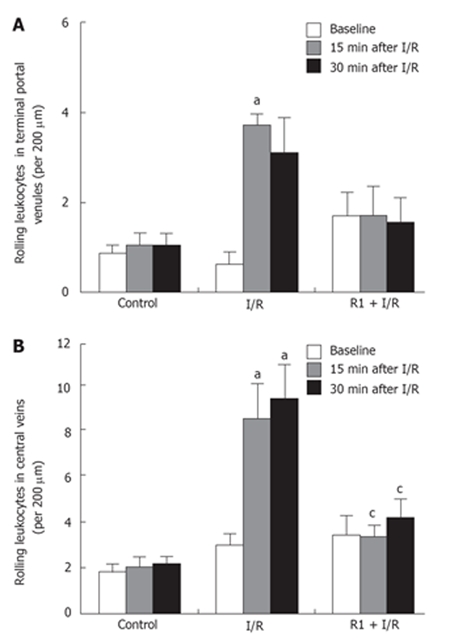
Effect of R1 on the rolling leukocytes in the areas of terminal portal venules (A) and central veins (B) of mice subjected to SMA I/R at 0 min before ischemia (Baseline) and 15 min, 30 min after reperfusion. Abscissa represents the number of rolling leukocytes per 200 μm. The results are presented as mean ± SE from 6 animals. aP < 0.05 vs control, cP < 0.05 vs I/R.
Figure 6 shows the effect of R1 on the adhesion of leukocytes induced by SMA I/R. SMA I/R increased the number of adherent leukocytes in both hepatic terminal portal venule and central vein regions, which was attenuated after treatment with R1, being significant in the terminal portal venules (Figure 6A), but not in the central veins (Figure 6B).
Figure 6.
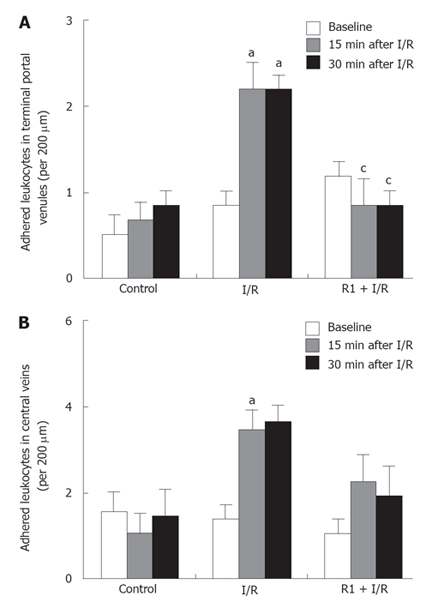
Effect of R1 on the adherent leukocytes in terminal portal venules (A) and central veins (B) of mice subjected to SMA I/R at 0 min before ischemia (Baseline) and 15 min, 30 min after reperfusion. Abscissa represents the number of adherent leukocytes per 200 μm. The results are presented as mean ± SE from 6 animals. aP < 0.05 vs control, cP < 0.05 vs I/R.
The effect of R1 on the leukocyte adhesion in sinusoids of hepatic terminal portal venule and central vein areas was determined (Figure 7). As in the hepatic terminal portal venules and central veins, only a small number of adherent leukocytes were visualised within sinusoids either of either the terminal portal venule area or the central vein area in the control group. A remarkable increase in the number of adherent leukocytes within sinusoids of both areas was observed when the mice were subjected to SMA I/R, which was inhibited significantly after treatment with R1 (Figure 7) starting from 15 min after reperfusion.
Figure 7.
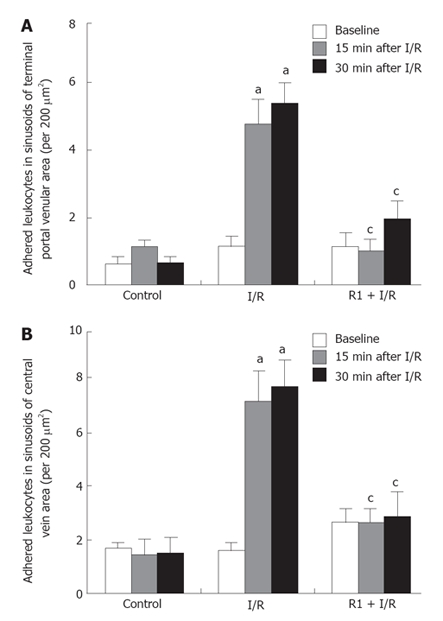
Effect of R1 on the adherent leukocytes in the hepatic sinusoids in the areas of terminal portal veins (A) and central veins (B) of mice subjected to SMA I/R at 0 min before ischemia (Baseline) and 15 min, 30 min after reperfusion. Abscissa represents the number of adherent leukocytes per field of view of 200 μm2. The results are presented as mean ± SE from 6 animals. aP < 0.05 vs control, cP < 0.05 vs I/R.
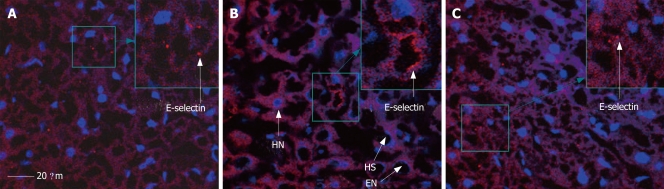
Figure 8 shows the expression of E-selectin in mouse hepatic sinusoids in the sham, I/R and R1 + I/R groups after 30 min of reperfusion. Thirty minutes after reper-fusion, the expression of E-selectin increased (Figure 8B) compared with the sham group (Figure 8A), and treatment with R1 (Figure 8C) suppressed the increase in I/R-elicited E-selectin expression.
Figure 8.
Expression of E-selectin in mouse hepatic sinusoid in sham (A), I/R (B) and R1 + I/R (C) after 30 min of reperfusion. HN: hepatocyte nucleus; EN: endothelium nucleus; HS: hepatic sinusoid; Bar indicates 20 μm.
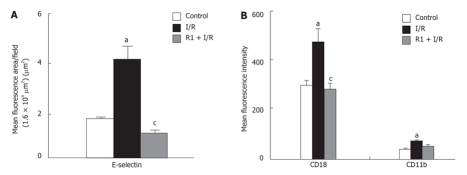
The influence of R1 on the expression of E-selectin in mouse hepatic sinusoids after 30 min of reperfusion was quantitatively evaluated (Figure 9A). Thirty-minute reperfusion significantly enhanced the expression of E-selectin. The SMA I/R-induced increase in the expression of E-selectin was completely ablated after treatment with R1.
Figure 9.
Effect of R1 on the expression of E-selectin in hepatic vessels (A) and CD18 and CD11b in neutrophils (B) of mice subjected to SMA I/R. The results are presented as mean ± SE from 6 animals. aP < 0.05 vs control, cP < 0.05 vs I/R.
The expression of ICAM-1 in mouse hepatic sinusoids after 30 min of reperfusion had no significant change. Treatment with R1 had no significant influence on the expression of ICAM-1 either (data not shown).
After 30 min of reperfusion, the blood was collected and used to evaluate the role of R1 in the expression of adhesion molecules CD18 and CD11b of mouse peripheral neutrophils. As illustrated in Figure 9B, 30-minute reperfusion significantly enhanced the expression of both CD18 and CD11b compared to the control group. R1 treatment significantly inhibited the increment in the mean fluorescence intensity of CD18 induced by I/R, and also diminished, although not significantly, the increment in the fluorescence intensity of CD11b induced by I/R.
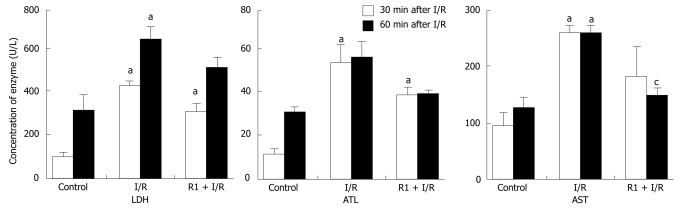
The influence of R1 on the concentration of enzymes in mouse peripheral blood was determined after 30 and 60 min of reperfusion (Figure 10). Thirty-minute or 60-minute reperfusion obviously increased the concentration of LDH, ALT and AST. However, treatment with R1 could not significantly blunt these increases, except for the activity of AST after 60-min reperfusion. I/R significantly increased the concentrations of IL-6 and MCP-1, but not TNF-α compared to the control group. R1 did not influence the increase in the concentration of IL-6 and MCP-1 induced by I/R (data not shown).
Figure 10.
Effect of R1 on the concentration of LDH, ALT and AST in serum of mice subjected to SMA I/R. The results are presented as mean ± SE from 6 animals. aP < 0.05 vs control, cP < 0.05 vs I/R.
DISCUSSION
Terminal portal venules and central veins are two major types of vessels consisting of hepatic microvasculature, in addition to sinusoids. Using intravital microscopy, the present study demonstrated that the mouse SMA I/R induced a variety of disorders in hepatic microcirculation, including the decreased diameters of terminal portal venules and central veins, RBC velocity in venules and the number of perfused sinusoids. Besides, leukocyte rolling and adhesion in hepatic venules and sinusoids were also promoted by the SMA I/R challenge. These results are in agreement with previous findings[2,4,24–26,30]. In the current study, treatment with R1 could remarkably attenuate hepatic microcirculatory disturbances in mice evoked by SMA I/R. PNS improved gut I/R-induced hepatic microcirculation disturbances and CP inhibited the adhesion of leukocytes to the hepatic vascular endothelium in rats induced by gut I/R, suggesting that R1 is at least one of the components of PNS that are responsible for its beneficial effect on hepatic microcirculation[19,24].
The concentration of ET-1 increases in serum and hepatic parenchyma in response to I/R[36], while NO is depleted by combination with I/R-evoked -O-[37], which concurs to bring about unbalance between ET-1 and NO, resulting in contraction of hepatic vessels. The present study revealed that administration of R1 attenuated I/R-elicited contraction of hepatic terminal portal venules and central veins that profoundly improved microcirculation in mice, suggesting that R1 may exert its action on the production of ET-1 and NO in the situation of I/R, which merits further study.
I/R-induced microcirculatory dysfunction and subsequent tissue injury are a complicated process consisting of multiple reactions, among which leukocyte recruitment is a crucial step mediated by the expression of a group of adhesion molecules on neutrophils and endothelial cells. It was reported that peroxide produced by SMA I/R degrades I-κB for activation of NF-κB and induces the expression of vascular endothelial E-selectin and ICAM-1[6–10], and also transfers L-selectin and adhesion molecules CD11b and CD18 from leukocyte cytoplasm to the cell surface[11–13,38], which initiates leukocyte rolling and adhesion ultimately. The ability of R1 to attenuate gut I/R-induced leukocyte rolling and adhesion in hepatic venules found in the present study is most probably due to its inhibiting effect on the expression of adhesion molecules on both leukocytes and endothelial cells, as suggested by the fact that pre-treatment with R1 could significantly blunt SMA I/R- induced expression of E-selectin on endothelium and CD18 on neutrophils.
It has been demonstrated that SMA I/R provokes a surge of peroxide release which activates NF-κB[6,7], leading not only to the expression of adhesion molecules on leukocytes and endothelium and subsequent leukocyte rolling and adhesion, but also to the explosive release of cytokines from leukocytes, endothelial and Kupffer cells[39–41], including TNF-α, IL-6 and MCP-1. The present experiment revealed that mouse gut I/R significantly increased the concentration of IL-6 and MCP-1, but not TNF-α in plasma. The reason is so far unknown. Moreover, no study is available to date concerning the antioxidant effect of R1 in I/R-induced hepatic microcirculatory disturbance, and it merits clarification if taking into account the fact that R1 depresses I/R-induced expression of adhesion molecules, but does not interfere with the increased concentrations of IL-6 and MCP-1 induced by I/R.
As expected, results of the present study show that the activities of ALT, AST and LDH of peripheral blood could slightly increased in response to 60-min SMA I/R, indicating that intestine I/R-induced microcirculatory disturbance in liver leads to injury of hepatic cells and dysfunction of the liver. Treatment with R1 significantly suppressed the activity of AST 60 min after reperfusion, suggesting that R1 can protect against hepatocyte injury induced by I/R. This beneficial effect is most likely related to its improving effect on hepatic microcirculatory disturbance, as mentioned above.
In summary, treatment with R1 considerably attenuates SMA I/R-induced hepatic microcirculatory disturbances, including decreased venular diameters, RBC velocity, the number of perfused sinusoids, as well as the increased leukocyte rolling and adhesion. R1 ameliorates SMA I/R-induced increase in the leukocyte rolling and adhesion in hepatic venules by inhibiting the expression of adhesion molecules on endothelial cells and neutrophils. We propose that it is the improving effect of R1 on microcirculatory disturbance that underlines its protecting function against I/R-induced hepatic injury.
COMMENTS
Background
Major abdomen surgery or organ transplantation initiates gut ischemia and reperfusion (I/R) leading to hepatic microcirculatory disturbance and subsequent liver injury, a manifestation that is closely correlated to the outcome of operation or transplantation and the living quality of patients as well. Thus, attenuating hepatic microcirculatory disturbance and liver injury elicited by gut I/R is of pivotal significance in clinic. Notoginsenoside R1 (R1), one of the saponins derived from Panax notoginseng, is reported to attenuate endotoxin-induced mesenteric microcirculatory disturbance in rats by inhibiting oxygen peroxide production and expression of adhesion molecules CD11b/CD18. It has been shown that R1 containing compound Chinese medicine preparation (cardiotonic pills) is able to ameliorate hepatic microcirculatory disturbance and liver injury elicited by gut I/R. R1 containing Chinese medicines is extensively applied in treatment of microcirculatory disturbance related diseases in China. However, no report is available regarding its attenuating effect on hepatic microcirculatory disturbance and liver injury induced by gut I/R.
Research frontiers
In present study, an animal model of hepatic microcirculatory disturbance was established by ligation of the superior mesenteric artery (SMA) in C57/BL mice for 15 min followed by 30-min reperfusion. The dynamics of vascular diameter, RBC velocity, rolling and adherent leukocytes was investigated in the hepatic terminal portal venule and central vein regions under inverted intravital microscope assisted by a 3CCD color camera and high speed video camera as well as a laser confocal microscope. Thirty minutes after reperfusion, the expression of adhesion molecules CD11b and CD18 on leukocytes in peripheral blood was estimated, and the expression of E-selectin and ICAM-1 in hepatic tissue was determined by immunohistochemistry. Sixty minutes after reperfusion, the levels of LDH, ALT, AST in peripheral blood were measured to explore the possible protective effect of R1 on hepatic microcirculatory disturbance and liver injury elicited by gut I/R.
Innovations and breakthroughs
By using a visualized microcirculatory research, results of the present study provide evidence for the first time that a pulse prior administration of R1 is able to prevent hepatic microcirculatory disturbance in mice induced by intestine I/R, including ameliorating contraction of hepatic terminal portal venule and central vein, resuming reduced velocity of RBCs, and inhibiting leukocyte rolling and adhesion in hepatic terminal portal venule and central vein, the later may be correlated to the inhibition of the expression of adhesion molecules and E-selectin on vascular endothelial cells and CD18 on leukocytes. R1 treatment could suppress AST level in peripheral blood, suggesting that R1 is able to prevent intestine I/R-induced hepatic microcirculatory disturbance and subsequent liver injury.
Applications
The findings in the present work support the utilization of R1 as a remedy in clinic to prevent hepatic microcirculatory disturbance and liver injury following major abdomen surgery or transplantation.
Peer review
This is a very interesting paper examining the effects of notoginsenoside R1 (R1) on hepatic microvascular function after gut I/R. The data are largely convincing. R1 has both vasodilatory and anti-inflammatory functions.
Supported by Tianjin Tasly Group, Tianjin, China
S- Editor Zhu LH L- Editor Wang XL E- Editor Yin DH
References
- 1.Horie Y, Ishii H. Liver dysfunction elicited by gut ischemia-reperfusion. Pathophysiology. 2001;8:11–20. doi: 10.1016/s0928-4680(01)00063-3. [DOI] [PubMed] [Google Scholar]
- 2.Horie Y, Wolf R, Miyasaka M, Anderson DC, Granger DN. Leukocyte adhesion and hepatic microvascular responses to intestinal ischemia/reperfusion in rats. Gastroenterology. 1996;111:666–673. doi: 10.1053/gast.1996.v111.pm8780571. [DOI] [PubMed] [Google Scholar]
- 3.Carden DL, Young JA, Granger DN. Pulmonary microvascular injury after intestinal ischemia-reperfusion: role of P-selectin. J Appl Physiol. 1993;75:2529–2534. doi: 10.1152/jappl.1993.75.6.2529. [DOI] [PubMed] [Google Scholar]
- 4.Horie Y, Wolf R, Anderson DC, Granger DN. Hepatic leukostasis and hypoxic stress in adhesion molecule-deficient mice after gut ischemia/reperfusion. J Clin Invest. 1997;99:781–788. doi: 10.1172/JCI119224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill J, Lindsay T, Rusche J, Valeri CR, Shepro D, Hechtman HB. A Mac-1 antibody reduces liver and lung injury but not neutrophil sequestration after intestinal ischemia-reperfusion. Surgery. 1992;112:166–172. [PubMed] [Google Scholar]
- 6.Turnage RH, Bagnasco J, Berger J, Guice KS, Oldham KT, Hinshaw DB. Hepatocellular oxidant stress following intestinal ischemia-reperfusion injury. J Surg Res. 1991;51:467–471. doi: 10.1016/0022-4804(91)90166-j. [DOI] [PubMed] [Google Scholar]
- 7.Hur GM, Ryu YS, Yun HY, Jeon BH, Kim YM, Seok JH, Lee JH. Hepatic ischemia/reperfusion in rats induces iNOS gene transcription by activation of NF-kappaB. Biochem Biophys Res Commun. 1999;261:917–922. doi: 10.1006/bbrc.1999.1143. [DOI] [PubMed] [Google Scholar]
- 8.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 9.Toledo-Pereyra LH, Lopez-Neblina F, Lentsch AB, Anaya-Prado R, Romano SJ, Ward PA. Selectin inhibition modulates NF-kappa B and AP-1 signaling after liver ischemia/reperfusion. J Invest Surg. 2006;19:313–322. doi: 10.1080/08941930600889474. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 11.Giannaki G, Rizos D, Xyni K, Sarandakou A, Protonotariou E, Phocas I, Creatsas G. Serum soluble E- and L-selectin in the very early neonatal period. Early Hum Dev. 2000;60:149–155. doi: 10.1016/s0378-3782(00)00115-8. [DOI] [PubMed] [Google Scholar]
- 12.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 13.Furie MB, Tancinco MC, Smith CW. Monoclonal antibodies to leukocyte integrins CD11a/CD18 and CD11b/CD18 or intercellular adhesion molecule-1 inhibit chemoattractant-stimulated neutrophil transendothelial migration in vitro. Blood. 1991;78:2089–2097. [PubMed] [Google Scholar]
- 14.Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989;83:2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pober JS. Warner-Lambert/Parke-Davis award lecture. Cytokine-mediated activation of vascular endothelium. Physiology and pathology. Am J Pathol. 1988;133:426–433. [PMC free article] [PubMed] [Google Scholar]
- 16.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–166. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi A, Imamura H, Isobe M, Matsuyama Y, Soeda J, Matsunaga K, Kawasaki S. Mac-1 (CD11b/CD18) and intercellular adhesion molecule-1 in ischemia-reperfusion injury of rat liver. Am J Physiol Gastrointest Liver Physiol. 2001;281:G577–G585. doi: 10.1152/ajpgi.2001.281.2.G577. [DOI] [PubMed] [Google Scholar]
- 18.Zhang HG, Li XH, Yang ZC. Effects of Panax notoginseng saponins on myocardial Gsalpha mRNA expression and ATPase activity after severe scald in rats. Burns. 2003;29:541–546. doi: 10.1016/s0305-4179(03)00143-8. [DOI] [PubMed] [Google Scholar]
- 19.Park WH, Lee SK, Kim CH. A Korean herbal medicine, Panax notoginseng, prevents liver fibrosis and hepatic microvascular dysfunction in rats. Life Sci. 2005;76:1675–1690. doi: 10.1016/j.lfs.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Du Q, Jerz G, Waibel R, Winterhalter P. Isolation of dammarane saponins from Panax notoginseng by high-speed counter-current chromatography. J Chromatogr A. 2003;1008:173–180. doi: 10.1016/s0021-9673(03)00988-9. [DOI] [PubMed] [Google Scholar]
- 21.Chen SW, Li XH, Ye KH, Jiang ZF, Ren XD. Total saponins of Panax notoginseng protected rabbit iliac artery against balloon endothelial denudation injury. Acta Pharmacol Sin. 2004;25:1151–1156. [PubMed] [Google Scholar]
- 22.Sun K, Wang CS, Guo J, Liu YY, Wang F, Liu LY, He JG, Fan JY, Han JY. Effect of Panax notoginseng saponins on lipopolysaccharide-induced adhesion of leukocytes in rat mesenteric venules. Clin Hemorheol Microcirc. 2006;34:103–108. [PubMed] [Google Scholar]
- 23.Zhang WJ, Wojta J, Binder BR. Notoginsenoside R1 counteracts endotoxin-induced activation of endothelial cells in vitro and endotoxin-induced lethality in mice in vivo. Arterioscler Thromb Vasc Biol. 1997;17:465–474. doi: 10.1161/01.atv.17.3.465. [DOI] [PubMed] [Google Scholar]
- 24.Horie Y, Han JY, Mori S, Konishi M, Kajihara M, Kaneko T, Yamagishi Y, Kato S, Ishii H, Hibi T. Herbal cardiotonic pills prevent gut ischemia/reperfusion-induced hepatic microvascular dysfunction in rats fed ethanol chronically. World J Gastroenterol. 2005;11:511–515. doi: 10.3748/wjg.v11.i4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horie Y, Kajihara M, Yamagishi Y, Kimura H, Tamai H, Kato S, Ishii H. A Japanese herbal medicine, Sho-saiko-to, prevents gut ischemia/reperfusion-induced hepatic microvascular dysfunction in rats. J Gastroenterol Hepatol. 2001;16:1260–1266. doi: 10.1046/j.1440-1746.2001.02622.x. [DOI] [PubMed] [Google Scholar]
- 26.Yamagishi Y, Horie Y, Kato S, Kajihara M, Tamai H, Granger DN, Ishii H. Ethanol modulates gut ischemia/reperfusion-induced liver injury in rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G640–G646. doi: 10.1152/ajpgi.00171.2001. [DOI] [PubMed] [Google Scholar]
- 27.Han JY, Miura S, Akiba Y, Higuchi H, Kato S, Suzuki H, Yokoyama H, Ishii H. Chronic ethanol consumption exacerbates microcirculatory damage in rat mesentery after reperfusion. Am J Physiol Gastrointest Liver Physiol. 2001;280:G939–G948. doi: 10.1152/ajpgi.2001.280.5.G939. [DOI] [PubMed] [Google Scholar]
- 28.Phillis JW, Estevez AY, O'Regan MH. Protective effects of the free radical scavengers, dimethyl sulfoxide and ethanol, in cerebral ischemia in gerbils. Neurosci Lett. 1998;244:109–111. doi: 10.1016/s0304-3940(98)00139-6. [DOI] [PubMed] [Google Scholar]
- 29.Horie Y, Kimura H, Kato S, Ohki E, Tamai H, Yamagishi Y, Ishii H. Role of nitric oxide in endotoxin-induced hepatic microvascular dysfunction in rats chronically fed ethanol. Alcohol Clin Exp Res. 2000;24:845–851. [PubMed] [Google Scholar]
- 30.Horie Y, Yamagishi Y, Kato S, Kajihara M, Tamai H, Granger DN, Ishii H. Role of ICAM-1 in chronic ethanol consumption-enhanced liver injury after gut ischemia-reperfusion in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G537–G543. doi: 10.1152/ajpgi.00098.2002. [DOI] [PubMed] [Google Scholar]
- 31.Horie Y, Kato S, Ohki E, Tamai H, Ishii H. Role of endothelin in endotoxin-induced hepatic microvascular dysfunction in rats fed chronically with ethanol. J Gastroenterol Hepatol. 2001;16:916–922. doi: 10.1046/j.1440-1746.2001.02544.x. [DOI] [PubMed] [Google Scholar]
- 32.Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- 33.Lawson JA, Burns AR, Farhood A, Lynn Bajt M, Collins RG, Smith CW, Jaeschke H. Pathophysiologic importance of E- and L-selectin for neutrophil-induced liver injury during endotoxemia in mice. Hepatology. 2000;32:990–998. doi: 10.1053/jhep.2000.19068. [DOI] [PubMed] [Google Scholar]
- 34.Bergmeyer HU. Methods of Enzymatic Analysis. 3rd ed. Weinheim: Verlag Chemie; 1983. pp. 126–132. [Google Scholar]
- 35.Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, Lit LC, Hui DS, Chan MH, Chung SS, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peralta C, Bulbena O, Bargallo R, Prats N, Gelpi E, Rosello CJ. Strategies to modulate the deleterious effects of endothelin in hepatic ischemia-reperfusion. Transplantation. 2000;70:1761–1770. doi: 10.1097/00007890-200012270-00016. [DOI] [PubMed] [Google Scholar]
- 37.Taniai H, Hines IN, Bharwani S, Maloney RE, Nimura Y, Gao B, Flores SC, McCord JM, Grisham MB, Aw TY. Susceptibility of murine periportal hepatocytes to hypoxia-reoxygenation: role for NO and Kupffer cell-derived oxidants. Hepatology. 2004;39:1544–1552. doi: 10.1002/hep.20217. [DOI] [PubMed] [Google Scholar]
- 38.Terada LS, Hybertson BM, Connelly KG, Weill D, Piermattei D, Repine JE. XO increases neutrophil adherence to endothelial cells by a dual ICAM-1 and P-selectin-mediated mechanism. J Appl Physiol. 1997;82:866–873. doi: 10.1152/jappl.1997.82.3.866. [DOI] [PubMed] [Google Scholar]
- 39.Funaki H, Shimizu K, Harada S, Tsuyama H, Fushida S, Tani T, Miwa K. Essential role for nuclear factor kappaB in ischemic preconditioning for ischemia-reperfusion injury of the mouse liver. Transplantation. 2002;74:551–556. doi: 10.1097/00007890-200208270-00021. [DOI] [PubMed] [Google Scholar]
- 40.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 41.Uchinami H, Yamamoto Y, Kume M, Yonezawa K, Ishikawa Y, Taura K, Nakajima A, Hata K, Yamaoka Y. Effect of heat shock preconditioning on NF-kappaB/I-kappaB pathway during I/R injury of the rat liver. Am J Physiol Gastrointest Liver Physiol. 2002;282:G962–G971. doi: 10.1152/ajpgi.00466.2001. [DOI] [PubMed] [Google Scholar]