Abstract
AIM: To evaluate the role of positron emission tomo-graphy using 18F-fluorodeoxyglucose (FDG-PET) in the surgical management of patients with pancreatic cancer, including the diagnosis, staging, and selection of patients for the subsequent surgical treatment.
METHODS: This study involved 53 patients with proven primary pancreatic cancer. The sensitivity of diagnosing the primary cancer was examined for FDG-PET, CT, cytological examination of the bile or pancreatic juice, and the serum levels of carcinoembrionic antigens (CEA) and carbohydrate antigen 19-9 (CA19-9). Next, the accuracy of staging was compared between FDG-PET and CT. Finally, FDG-PET was analyzed semiquantitatively using the standard uptake value (SUV). The impact of the SUV on patient management was evaluated by examining the correlations between the SUV and the histological findings of cancer.
RESULTS: The sensitivity of FDG-PET, CT, cytological examination of the bile or pancreatic juice, and the serum levels of CEA and CA19-9 were 92.5%, 88.7%, 46.4%, 37.7% and 69.8%, respectively. In staging, FDG-PET was superior to CT only in diagnosing distant disease (bone metastasis). For local staging, the sensitivity of CT was better than that of FDG-PET. The SUV did not correlate with the pTNM stage, grades, invasions to the vessels and nerve, or with the size of the tumor. However, there was a statistically significant difference (4.6 ± 2.9 vs 7.8 ± 4.5, P = 0.024) in the SUV between patients with respectable and unresectable disease.
CONCLUSION: FDG-PET is thus considered to be useful in the diagnosis of pancreatic cancer. However, regarding the staging of the disease, FDG-PET is not considered to be a sufficiently accurate diagnostic modality. Although the SUV does not correlate with the patho-histological prognostic factors, it may be useful in selecting patients who should undergo subsequent surgical treatment.
Keywords: Pancreatic cancer, Fluorodeoxyglucose positron emission tomography, Computed tomography, Standard uptake value, Carcinoembrionic antigens, Carbohydrate antigen 19-9, Prognostic factor
INTRODUCTION
Today, despite advances in diagnosing modalities, most patients with pancreatic cancer are still unresectable at the time of diagnosis. Surgery remains the only potential for long-term survival, with a resectability rate of around 15%-20% in the latest review[1]. Even in patients with resectable disease, the 5-year survival rate is still around 20%[1–3]. For patients with unresectable disease, chemotherapy with or without radiation therapy is usually chosen, and the survival benefit of chemotherapy with Gemcitabine over 5-fluorouracil has recently been reported[4]. Clearly, an effort has to be made for diagnosing early stage cancer, also by determining its clinical stage and by accurately predicting the prognosis, thus avoiding unnecessary surgical explorations.
Recently, the advantages of positron emission tomography using 18F-fluorodeoxyglucose (FDG-PET) on diagnosing pancreatic cancer, especially small lesions less than 2 cm in size, over the conventional modalities, including computed tomography (CT), have been reported[2]. Furthermore, FDG-PET has also been reported to possibly play a role in predicting the prognosis of pancreatic cancer[2,5,6]. However, the significance of FDG-PET imaging in the management of pancreatic cancer, including diagnosis, staging, or predicting prognosis, has not yet been established. In this study, we explored the role of FDG-PET in surgery for pancreatic cancer by examining its sensitivity in diagnosing and staging, and its potential for predicting prognosis by comparing the standard uptake value (SUV) and the histological findings.
MATERIALS AND METHODS
Patients
This study involved 53 patients with histologically (30 patients) or clinically (23 patients) proven primary pancreatic cancer who had undergone FDG-PET from January 2004 to January 2007. In clinically proven pancreatic cancer patients metastatic lesions were detected in the liver, lymph nodes, or the other organs, by the following up CT retrospectively, and the patients died of the primary pancreatic cancer. The patients' mean age was 70.1 years (range from 44 to 84 years). The patients consisted of 33 males and 20 females. The localisation of the cancer was in the head of the pancreas in 32 patients and in the body and tail in 21 patients. Twenty-eight patients presented with diabetes requiring insulin therapy. Among the 53 patients, 28 patients were diagnosed to be unresectable, and 25 patients eventually underwent surgery with a curative intention, although in 7 of them the cancer turned out to be unresectable because of the intraoperative findings.
Methods
The sensitivity of diagnosing pancreatic cancer was examined for FDG-PET, CT, cytological examination of the bile or pancreatic juice, and the serum levels of carcinoembrionic antigens (CEA) and carbohydrate antigen 19-9 (CA19-9). Next, the accuracy of staging of the disease and the impact on patient management was evaluated and compared between FDG-PET and CT. Finally, FDG-PET was analyzed semiquantitatively using the standard uptake value (SUV). In the patients who underwent surgery, a correlation was found between the preoperatively obtained SUV and the histological findings of the specimen in regard to the pathological stagings of cancer classified based on the UICC TNM classification (6th edition, 2002), histological differentiation, lymphatic invasions, vascular invasion, and intrapancreatic nerve invasion. The SUV was compared between the resectable and unresectable disease patient groups. A correlation between the SUV and the maximum diameter of the primary lesion, which was determined on CT, was examined. All of the studies were performed retrospectively by collecting and analyzing data from the patient records.
Cytological examination of the bile or pancreatic juice
The bile or pancreatic juice, which was collected from 28 patients after brushing endoscopic retrograde cholangio-pancreatography (ERCP), was subjected to a cytological examination. This was performed using the Papanicolaou staining, and the diagnosis established according to the Papanicolaou classification. The results in Classes I , II, or III were counted as negative in this study; the results in Classes IV or V were counted as positive.
CT
For multidetector CT scans, a contrast enhancement was performed for each patient. Helical images of the abdomen were routinely obtained and reconstructed with 5 mm thickness. The CT images were interpreted independently and consecutively by two radiologists with extensive experience of more than 10 years in CT scanning. The findings of the CT scans were considered positive when both of the radiologists strongly suspected malignant disease due to a discrete low-attenuation mass within the pancreas, and/or involving the adjacent vessels, and/or swelling of the regional lymph nodes.
FDG-PET
The FDG-PET images were acquired with PET machine (Siemens EXACT HR+, CTI, Knoxville, TN, USA). The patients were required to fast for at least 4 h before PET imaging. One hour after the intravenous administration of 5 mCi of FDG, the emission images were acquired. The transmission images were acquired to correct for attenuation. The FDG-PET images were interpreted independently and consecutively by two radiologists with extensive experience in FDG-PET imaging. The findings were considered to be positive when both of the radiologists strongly suspected malignant disease. In addition, the images were analyzed semiquantitatively using the SUV, as reported elsewhere[7]. Briefly, regions of interest measuring 1.0 cm2 were drawn over the area of maximum activity in a lesion. The SUV was calculated as follows:
SUV = (activity in region of interest in mCi)/(injected dose in mCi/weight in kg).
Statistical analysis
The chi-square test was employed for a statistical comparison of the sensitivity of FDG-PET and CT. The Student’s t test was used to compare the values of the SUV between the two groups. Finally, correlations between the SUV and the maximum diameter of the primary lesion determined on CT were examined by the Pearson’s correlation test. All statistical analyses were performed using the SPSS software program (SPSS, Chicago, USA). A P value < 0.05 was considered statistically significant.
RESULTS
Sensitivity for diagnosing pancreatic cancer
The sensitivity of FDG-PET, CT, cytological examination of the bile or pancreatic juice, and the serum levels of CEA and CA19-9 were 92.5% (49/53), 88.7% (47/53), 46.4% (13/28), 37.7% (20/53) and 69.8% (37/53), respectively.
Sensitivity for preoperative staging
Among the 53 patients, 28 patients were diagnosed to be unresectable based on the following preoperative imaging findings: invasion to major arteries such as the supramesenteric artery, celiac trunks, or common hepatic artery in 9 patients, para-aortic lymph node metastasis in 12 patients, detection of hepatic metastasis in 14 patients, detection of bone metastasis in 7 patients, strongly suspected peritoneal dissemination in 5 patients, general complications associated with aging in 4 patients. Among the 25 patients who eventually underwent surgery with a curative intention, the cancer turned out to be unresectable in 7 of them because of the following intraoperative findings: detection of hepatic metastasis (5 to 8 mm in diameter) in 5 patients, histologically confirmed metastases in the para-aortic lymph node (less than 1 cm in diameter) in 2 patients, and cytologically positive ascites in 2 patients. Therefore, the staging diagnostic sensitivity of FDG-PET and CT was 2/9 (22.2%) and 9/9 (100%) for invasion into the major arteries, 8/14 (57.1%) and 11/14 (78.6%) for para-aortic regional lymph nodes metastases, 10/19 (52.6%) and 14/19 (73.7%) for hepatic metastases, 8/8 (100%) and 1/8 (12.5%) for bone metastases, 3/7 (42.9%) and 4/7 (57.1%) for peritoneal dissemination, respectively. There was a statistically significant difference in the sensitivity detecting invasion into the major arteries (P = 0.001) and bone metastases (P = 0.001), based on the chi-square test.
Correlation between the SUV and histological findings
In 18 patients, a surgical resection with a lymphadenectomy was performed. A pancreaticoduodenectomy was performed in 11 patients, while a distal pancreatectomy was performed in 7 patients. In these patients, a pathohistological examination was performed. As Table 1 shows, no statistically significant difference was found in any of the histological findings listed in Table 1.
Table 1.
Histological findings of the patients
| Histological findings | Number of patients | SUV (mean ± SD) | Difference | ||
| Pathological stage (TNM) | |||||
| pT | pN | stage | |||
| 1 | 0 | IA | 1 | 3.59 | |
| 1 | 1 | IB | 1 | 3.14 | |
| 3 | 0 | IIA | 8 | 4.1 ± 0.9 | NS |
| 1, 2, 3 | 1 | IIB | 8 | 5.9 ± 4.5 | |
| Histological differentiation | |||||
| Well | 7 | 3.7 ± 0.7 | NS | ||
| Moderately-poorly | 11 | 5.6 ± 3.6 | |||
| Lymphatic invasions | |||||
| Negative | 9 | 5.3 ± 3.9 | NS | ||
| Positive | 9 | 4.5 ± 2.1 | |||
| Venous invasions | |||||
| Negative | 6 | 6.2 ± 5.1 | NS | ||
| Positive | 12 | 4.4 ± 1.9 | |||
| Intrapancreatic nerve invasions | |||||
| Negative | 11 | 4.8 ± 3.5 | NS | ||
| Positive | 7 | 5.1 ± 2.3 | |||
SUV: Standard uptake value; NS: Not significant.
Comparison of SUV between resectable and unresectable
disease
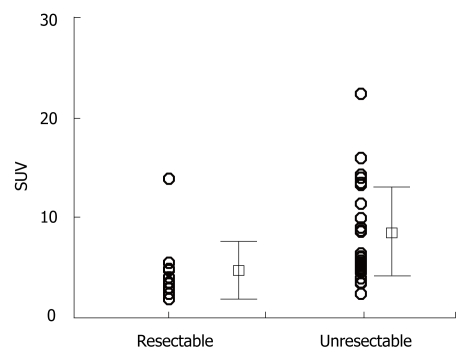
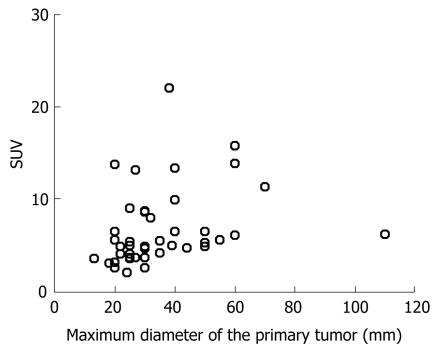
As Figure 1 shows, the SUV of the primary lesion was 4.6 ± 2.9 (mean ± SD) in the resectable disease cases, while it was 7.8 ± 4.5 in the unresectable cases, with a statistically significant difference (P = 0.024) by the Student’s t-test. On the other hand, the maximum diameter of the primary lesion measured on CT was 27.9 ± 10.9 mm in the resectable cases, and 40.5 ± 21.4 mm in the unresectable ones (P = 0.053). Furthermore, we found no correlation between the SUV and the maximum diameter of the primary lesion measured on CT by using Pearson’s correlation test (P = 0.274), as shown in Figure 2.
Figure 1.
Comparison of the SUV of the primary lesion between patients with resectable and unresectable disease. SUV: Standard uptake value.
Figure 2.
Relationship between the SUV and the maximum diameter of the primary lesion.
DISCUSSION
Pancreatic cancer is one of the most lethal human cancers and it continues to be a major unsolved health problem worldwide. Despite efforts in the past 50 years, conventional treatment approaches, such as surgery, radiation, chemotherapy, or combination of these, have had little impact on the course of this aggressive neoplasm[1]. Although recent progress in systemic chemotherapy has been reported[8], surgery remains the only hope for long-term survival[9]. In surgery for pancreatic cancer, a great deal of effort has been made to expand the resection with an extended lymphadenectomy in order to improve the outcome[10]. However, only patients with localized disease and a tumor size less than 2 cm with no lymph node metastases can expect long-term survival after surgery[1,2,11]. Therefore, increased efforts should be focusing not only on diagnosing the early stage disease, but also on staging and predicting prognosis so that unnecessary surgical exploration may be avoided. Recently, PET has been reported to have superiority to CT, US, and EUS in its sensitivity and specificity in diagnosing pancreatic cancer[2,12]. Furthermore, regarding the role of PET in diagnosing the disease, the metabolic activity of the tumor may be of prognostic significance[5,6]. However, the role of PET in the management of pancreatic cancer has yet to be established.
The role of FDG-PET in diagnosis of primary cancer
The sensitivity of FDG-PET was 92.5%, and it was better than that of CT, cytological examinations of the bile or pancreatic juice, and the serum levels of CEA and CA19-9. Our findings also correlate with those of previous reports, in which the sensitivity of FDG-PET, CT, and US has been reported to be 94%, 89%, and 89%, respectively[12]. On the other hand, in another study, the sensitivity of FDG-PET was found to be lower than or equal to CT[13–15] and these findings thus remain controversial. In particular, when a multidetector CT is performed routinely with thin sections (1 mm), the sensitivity of the CT may further improve[2]. This study was conducted among patients with the histologically or clinically proven pancreatic cancer, and therefore the specificity was not evaluated.
The role of FDG-PET in staging of the disease
This study showed that, as far as staging is considered, FDG-PET was superior to CT only in diagnosing distant disease. On the other hand for local staging the sensitivity of CT was better than that of FDG-PET. As described[2], the poor spatial resolution of FDG-PET limits the local staging of pancreatic cancer. Therefore, the anatomical imaging modalities with CT are better suited to demonstrate the relationship of the tumor, adjacent organs, and vascular structure.
In the nodal staging of the disease, there was no difference between FDG-PET and CT, since both performed poorly. The reported sensitivities of FDG-PET have varied between 46% and 71%[2,16]; thus the results of this study are considered to correlate with these previous reports. It has been said that one possible reason for the apparent low sensitivity of FDG-PET is the close proximity of the peripancreatic and paraaortic lymph node basins to the primary tumor, which can obscure their detection[2,16].
The important impact of FDG-PET on staging has been in its ability to identify distant metastases[17]. According to the previous reports[18], the sensitivity of FDG-PET for detecting hepatic metastases is about 70%, while that of this study was 52.6%. In particular, small lesions less than 1 cm could not be detected. It has also been reported that the sensitivity for lesions less than or greater than 1 cm is 43% and 97%, respectively[19]. Direct spread into the peritoneum is also not uncommon and often is missed on conventional imaging. However, in this study, both FDG-PET and CT failed to demonstrate a high degree of accuracy in these diagnostic analyses.
The prognostic significance of FDG-PET
Some researchers have shown the SUV in FDG-PET to be an independent prognostic factor in a subpopulation of patients with pancreatic cancer. Nakata et al[5] showed that in patients with unresectable disease, a high SUV correlated with a shorter survival. Maemura et al[20] reported that pancreatic tumors with distant metastasis showed significantly higher SUV levels than tumors without metastasis. Sperti et al[6] also demonstrated that a high SUV (> 4.0) was associated with shorter survival. For prognostic factors for pancreatic cancer, the tumor stage and grade[21], R0 resection[21], levels of serum tumor marker[22], size of the primary lesion[3], and status of nodes metastases[3,11], have been reported. On the other hand, the increased glycolytic activity of the tumor detected by the SUV may represent tumor growth and also resemble the tumor’s biological behavior[23–25]. Therefore, in this study we examined the correlation between the SUV and the patho-histological findings. The results were that the SUV did not correlate with the pTNM stage, grades, invasions to the vessels and nerve, or size of the tumor. Sperti et al[6] also found no difference between a high SUV (> 4.0) and a low SUV patients group in regard with TNM staging and histological grading, as shown in this study. Nevertheless, they found SUV to be an independent prognostic factor in patients with pancreatic cancer, and therefore advocated that the different biological aggressiveness of the tumor, detected by the SUV, might explain the difference in survival in patients with otherwise similar prognostic valuables. In the present study, there was a statistically significant difference in the SUV between the patients with resectable and unresectable disease. This may support the findings of previous reports which describe the SUV to be an independent prognostic factor. Therefore, although in staging FDG-PET did not perform precisely enough except for detecting bone metastases, by evaluating the SUV, FDG-PET may provide such additional information on the biological aggressiveness of the tumor, and thus play an important role in helping to select patients for subsequent surgical therapy. Some small studies reported that the tumor SUVs were useful in predicting the effectiveness of chemotherapy for unresectable pancreatic cancer[17,26,27]. However, conclusions must await further studies including larger population of patients.
In conclusion, FDG-PET was found to be useful for diagnosing pancreatic cancer. However, in staging of the disease, FDG-PET does not perform precisely enough. Although the SUV does not correlate with the patho-histological prognostic factors, it may be useful in selecting patients who should undergo the subsequent surgical treatment. Therefore, FDG-PET may play an important role in the decision making process and surgical management for patients with pancreatic cancer. However, this study suffers from the limitation of a small population of patients. Therefore, a definite conclusion may have to wait for further studies involving a larger population of patients. Furthermore, the use of image fusion with PET/CT[28,29] or with PET/MRI[30] may improve the accuracy when staging pancreatic cancer.
COMMENTS
Background
Pancreatic cancer is one of the most lethal human cancers and it continues to be a major unsolved health problem worldwide. Only patients with localized disease and a tumor size less than 2 cm with no lymph node metastases can expect long-term survival after surgery. Therefore, increased efforts should be focusing not only on diagnosing the early stage disease, but also on staging and predicting prognosis so that unnecessary surgical exploration may be avoided. Recently, positron emission tomography (PET) has been reported to be superior to computed tomography (CT), ultrasound (US), and endoscopic US (EUS) in its sensitivity and specificity in diagnosing pancreatic cancer. Furthermore, the metabolic activity of the tumor evaluated by uptake value of fluorodeoxyglucose (FDG) (standardized uptake value: SUV) may be of prognostic significance. However, the role of PET in the management of pancreatic cancer has yet to be established.
Research frontiers
As is described above, pancreatic cancer is one of the most lethal human cancers and it continues to be a major unsolved health problem worldwide. Despite efforts in the past 50 years, conventional treatment approaches, such as surgery, radiation, chemotherapy, or combination of these, have had little impact on the course of this aggressive neoplasm. Although recent progress in systemic chemotherapy has been reported, surgery remains the only hope for long-term survival. In surgery for pancreatic cancer, a great deal of effort has been made to expand the resection with an extended lymphadenectomy in order to improve the outcome. However, only patients with localized disease and a tumor size less than 2 cm with no lymph node metastases can expect long-term survival after surgery. Therefore, breakthrough for pancreatic cancer may be achieved in diagnosing an early stage disease.
Innovations and breakthroughs
The present study showed that FDG-PET was useful for diagnosing pancreatic cancer. However, in staging of the disease, FDG-PET does not perform precisely enough. Although the SUV does not correlate with the patho-histological prognostic factors, it may be useful in selecting patients who should undergo the subsequent surgical treatment. Therefore, FDG-PET may play an important role in the decision making process and surgical management for patients with pancreatic cancer.
Applications
By using FDG-PET in combination with conventional diagnostic modalities, such as computed tomography (CT), ultrasound, magnetic resonance imaging, and endoscopic pancreatography, the ability to diagnose the early stage pancreatic cancer, or to select candidates for surgery can be improved. At the same time, as the increased glycolytic activity of the tumor detected by SUV may represent tumor growth and resemble the tumor biological behavior, the SUV may provide additional information on the biological aggressiveness of the tumor.
Terminology
FDG-PET: 18F-fluorodeoxyglucose (FDG) is a positron-emitting radio-tracer that is transported intracellularly via glucose transporters which are highly expressed in various cancer cells, then FDG is phosphorylated by hexokinase to FDG-6-PO4. However, further metabolism of FDG-6-PO4 is not possible in the neoplastic cells due to insufficient phosphatase levels and tracer accumulation. Therefore, FDG-PET can image cancer cells based on such specific tissue metabolism. SUV: To perform a quantitative analysis for accumulation of FDG, the standardized uptake value (SUV) is calculated in the suspected neoplastic foci. The SUV was calculated as follows:
SUV = (activity in region of interest in mCi)/(injected dose in mCi/weight in kg).
Peer review
This is a good clinical research on the diagnosis, staging and choice of operation in patients with pancreatic carcinoma.
Footnotes
S- Editor Zhu LH L- Editor Negro F E- Editor Yin DH
References
- 1.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 2.Pakzad F, Groves AM, Ell PJ. The role of positron emission tomography in the management of pancreatic cancer. Semin Nucl Med. 2006;36:248–256. doi: 10.1053/j.semnuclmed.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, Dooley WC, Coleman J, Pitt HA. Pancreatico-duodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–731; discussion 731-733. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 5.Nakata B, Nishimura S, Ishikawa T, Ohira M, Nishino H, Kawabe J, Ochi H, Hirakawa K. Prognostic predictive value of 18F-fluorodeoxyglucose positron emission tomography for patients with pancreatic cancer. Int J Oncol. 2001;19:53–58. doi: 10.3892/ijo.19.1.53. [DOI] [PubMed] [Google Scholar]
- 6.Sperti C, Pasquali C, Chierichetti F, Ferronato A, Decet G, Pedrazzoli S. 18-Fluorodeoxyglucose positron emission tomography in predicting survival of patients with pancreatic carcinoma. J Gastrointest Surg. 2003;7:953–959; discussion 959-960. doi: 10.1016/j.gassur.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Nishiyama Y, Yamamoto Y, Monden T, Sasakawa Y, Tsutsui K, Wakabayashi H, Ohkawa M. Evaluation of delayed additional FDG PET imaging in patients with pancreatic tumour. Nucl Med Commun. 2005;26:895–901. doi: 10.1097/00006231-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Huguet F, Andre T, Hammel P, Artru P, Balosso J, Selle F, Deniaud-Alexandre E, Ruszniewski P, Touboul E, Labianca R, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 9.Eloubeidi MA, Desmond RA, Wilcox CM, Wilson RJ, Manchikalapati P, Fouad MM, Eltoum I, Vickers SM. Prognostic factors for survival in pancreatic cancer: a population-based study. Am J Surg. 2006;192:322–329. doi: 10.1016/j.amjsurg.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Capussotti L, Massucco P, Ribero D, Vigano L, Muratore A, Calgaro M. Extended lymphadenectomy and vein resection for pancreatic head cancer: outcomes and implications for therapy. Arch Surg. 2003;138:1316–1322. doi: 10.1001/archsurg.138.12.1316. [DOI] [PubMed] [Google Scholar]
- 11.Shimada K, Sakamoto Y, Sano T, Kosuge T. The role of paraaortic lymph node involvement on early recurrence and survival after macroscopic curative resection with extended lymphadenectomy for pancreatic carcinoma. J Am Coll Surg. 2006;203:345–352. doi: 10.1016/j.jamcollsurg.2006.05.289. [DOI] [PubMed] [Google Scholar]
- 12.Inokuma T, Tamaki N, Torizuka T, Magata Y, Fujii M, Yonekura Y, Kajiyama T, Ohshio G, Imamura M, Konishi J. Evaluation of pancreatic tumors with positron emission tomography and F-18 fluorodeoxyglucose: comparison with CT and US. Radiology. 1995;195:345–352. doi: 10.1148/radiology.195.2.7724751. [DOI] [PubMed] [Google Scholar]
- 13.Koyama K, Okamura T, Kawabe J, Nakata B, Chung KH, Ochi H, Yamada R. Diagnostic usefulness of FDG PET for pancreatic mass lesions. Ann Nucl Med. 2001;15:217–224. doi: 10.1007/BF02987835. [DOI] [PubMed] [Google Scholar]
- 14.Lytras D, Connor S, Bosonnet L, Jayan R, Evans J, Hughes M, Garvey CJ, Ghaneh P, Sutton R, Vinjamuri S, et al. Positron emission tomography does not add to computed tomography for the diagnosis and staging of pancreatic cancer. Dig Surg. 2005;22:55–61; discussion 62. doi: 10.1159/000085347. [DOI] [PubMed] [Google Scholar]
- 15.Sendler A, Avril N, Helmberger H, Stollfuss J, Weber W, Bengel F, Schwaiger M, Roder JD, Siewert JR. Preoperative evaluation of pancreatic masses with positron emission tomography using 18F-fluorodeoxyglucose: diagnostic limitations. World J Surg. 2000;24:1121–1129. doi: 10.1007/s002680010182. [DOI] [PubMed] [Google Scholar]
- 16.Bares R, Klever P, Hauptmann S, Hellwig D, Fass J, Cremerius U, Schumpelick V, Mittermayer C, Bull U. F-18 fluorodeoxyglucose PET in vivo evaluation of pancreatic glucose metabolism for detection of pancreatic cancer. Radiology. 1994;192:79–86. doi: 10.1148/radiology.192.1.8208970. [DOI] [PubMed] [Google Scholar]
- 17.Bang S, Chung HW, Park SW, Chung JB, Yun M, Lee JD, Song SY. The clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the differential diagnosis, staging, and response evaluation after concurrent chemoradiotherapy for pancreatic cancer. J Clin Gastroenterol. 2006;40:923–929. doi: 10.1097/01.mcg.0000225672.68852.05. [DOI] [PubMed] [Google Scholar]
- 18.Diederichs CG, Staib L, Vogel J, Glasbrenner B, Glatting G, Brambs HJ, Beger HG, Reske SN. Values and limitations of 18F-fluorodeoxyglucose-positron-emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas. 2000;20:109–116. doi: 10.1097/00006676-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Frohlich A, Diederichs CG, Staib L, Vogel J, Beger HG, Reske SN. Detection of liver metastases from pancreatic cancer using FDG PET. J Nucl Med. 1999;40:250–255. [PubMed] [Google Scholar]
- 20.Maemura K, Takao S, Shinchi H, Noma H, Mataki Y, Kurahara H, Jinnouchi S, Aikou T. Role of positron emission tomography in decisons on treatment strategies for pancreatic cancer. J Hepatobiliary Pancreat Surg. 2006;13:435–441. doi: 10.1007/s00534-006-1102-8. [DOI] [PubMed] [Google Scholar]
- 21.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperti C, Pasquali C, Catalini S, Cappellazzo F, Bonadimani B, Behboo R, Pedrazzoli S. CA 19-9 as a prognostic index after resection for pancreatic cancer. J Surg Oncol. 1993;52:137–141. doi: 10.1002/jso.2930520302. [DOI] [PubMed] [Google Scholar]
- 23.Higashi T, Tamaki N, Honda T, Torizuka T, Kimura T, Inokuma T, Ohshio G, Hosotani R, Imamura M, Konishi J. Expression of glucose transporters in human pancreatic tumors compared with increased FDG accumulation in PET study. J Nucl Med. 1997;38:1337–1344. [PubMed] [Google Scholar]
- 24.Reske SN, Grillenberger KG, Glatting G, Port M, Hildebrandt M, Gansauge F, Beger HG. Overexpression of glucose transporter 1 and increased FDG uptake in pancreatic carcinoma. J Nucl Med. 1997;38:1344–1348. [PubMed] [Google Scholar]
- 25.Maher JC, Savaraj N, Priebe W, Liu H, Lampidis TJ. Differential sensitivity to 2-deoxy-D-glucose between two pancreatic cell lines correlates with GLUT-1 expression. Pancreas. 2005;30:e34–e39. doi: 10.1097/01.mpa.0000153327.46945.26. [DOI] [PubMed] [Google Scholar]
- 26.Maisey NR, Webb A, Flux GD, Padhani A, Cunningham DC, Ott RJ, Norman A. FDG-PET in the prediction of survival of patients with cancer of the pancreas: a pilot study. Br J Cancer. 2000;83:287–293. doi: 10.1054/bjoc.2000.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshioka M, Sato T, Furuya T, Shibata S, Andoh H, Asanuma Y, Hatazawa J, Shimosegawa E, Koyama K, Yamamoto Y. Role of positron emission tomography with 2-deoxy-2-[18F]fluoro-D-glucose in evaluating the effects of arterial infusion chemotherapy and radiotherapy on pancreatic cancer. J Gastroenterol. 2004;39:50–55. doi: 10.1007/s00535-003-1244-2. [DOI] [PubMed] [Google Scholar]
- 28.Goh BK, Tan YM, Chung YF. Utility of fusion CT-PET in the diagnosis of small pancreatic carcinoma. World J Gastroenterol. 2005;11:3800–3802. doi: 10.3748/wjg.v11.i24.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbaum SJ, Stergar H, Antoch G, Veit P, Bockisch A, Kuhl H. Staging and follow-up of gastrointestinal tumors with PET/CT. Abdom Imaging. 2006;31:25–35. doi: 10.1007/s00261-005-0031-3. [DOI] [PubMed] [Google Scholar]
- 30.Ruf J, Lopez Hanninen E, Bohmig M, Koch I, Denecke T, Plotkin M, Langrehr J, Wiedenmann B, Felix R, Amthauer H. Impact of FDG-PET/MRI image fusion on the detection of pancreatic cancer. Pancreatology. 2006;6:512–519. doi: 10.1159/000096993. [DOI] [PubMed] [Google Scholar]