Abstract
Current theories on drug resistance in epilepsy include the drug transporter hypothesis, the drug target hypothesis, and a novel approach called the inherent severity model of epilepsy, which posits that the severity of the disease determines its relative response to medication. Valuable as each of these hypotheses is, none is currently a stand-alone theory that is able to convincingly explain drug resistance in human epilepsy. As a consequence, it may be of interest to update and integrate the various hypotheses of drug resistance and to explore possible links to the severity of epilepsy. The observation that a high frequency of seizures prior to onset of treatment is a prognostic signal of increased severity and future drug failure suggests that common neurobiological factors may underlie both disease severity and pharmacoresistance. Such a link has been proposed for depression; however, the evidence for a direct mechanistic link, genetic or otherwise, between drug response and disease severity of human epilepsy is still elusive. Although emerging data from experimental studies suggest that alterations in GABAA receptors may present one example of a mechanistic link, clearly more work is needed to explore whether common neurobiological factors may underlie both epilepsy severity and drug failure.
Much to the disappointment of physicians, patients, and care providers, extensive antiepileptic drug (AED) resistance continues to be a major public health issue, affecting one in three patients with epilepsy despite the introduction of modern AEDs with new mechanisms of action (1). The development of innovative and possibly more effective drug treatment strategies to deal with drug resistance has been hampered by the fact that none of the prevailing pharmacological hypotheses, including the drug target and transporter hypotheses, is able to fully explain the neurobiological basis for drug resistance (2). Efforts to explain pharmacoresistance by one hypothesis alone may suffer the fate of blind men trying to describe an elephant. Perhaps not surprisingly, these hypotheses have not led yet to the development of new pharmacological strategies that can successfully treat patients with drug-resistant epilepsy (1–3), which at least in part, may be due to the fact that current hypotheses for the mechanism of epilepsy pharmacoresistance are often discussed in an exclusive rather than integrative fashion. Investigators recently proposed a novel approach that considers pharmacoresistance in terms of intrinsic disease severity (2). This review will update and integrate the pharmacological, neurobiological, and clinical hypotheses that endeavor to explain the relationships between the intrinsic severity of epilepsy and drug resistance.
Inherent Disease Severity as a Mechanism of AED Failure: Clinical and Experimental Evidence
The theory, termed the inherent severity model of epilepsy, proposes that there is a continuum in severity of the disease, which determines its relative response to medication (2). Prospective studies of outcome in populations of patients with newly treated epilepsy have consistently shown that the single most important factor associated with the chance of remission of seizures is the frequency of seizures in the early phase of epilepsy, with an association between increased number of seizures in this period and poorer outcome (4–6). In a study of 780 patients newly diagnosed with epilepsy and followed up at a single center over a 20-year period, the numbers of pretreatment seizures were greater for patients with drug-resistant epilepsy. Those reporting more than ten seizures prior to initiation of therapy were more than twice as likely to develop drug-resistant epilepsy (6). In addition, clusters of three or more seizures occurring within a 24-hour period during treatment also were found to be associated with drug resistance (7). In an earlier prospective community-based cohort study of 792 patients recruited at the time of their first diagnosis of epileptic seizures, the number of seizures in the first 6 months after presentation was the single most important predictive factor for both early and long-term remission of seizures (8). Indeed, according to a recent review, a high frequency of seizures in the early phase of epilepsy is the dominant risk factor influencing the chance of remission of seizures, outweighing the contribution from other factors associated with prognosis, including etiology of epilepsy, seizure type, or the results of either EEG or imaging (2). These epidemiological data support the intrinsic severity epilepsy model, which seeks to explain AED failure by assessing differences in inherent epilepsy severity by the frequency of seizures in the early phase of epilepsy.
Any neurobiological and pharmacological hypothesis of intrinsic severity of epilepsy and drug response, based on long-term studies in childhood-onset epilepsy and short-term studies in adults with epilepsy, has to consider that approximately 7 percent of newly treated patients is extensively drug resistant and never enters 1-year remission from the start of treatment, despite the use of many drugs (4). However, only an additional 15 to 20 percent of patients switch in and out of drug resistance during the course of their epilepsy (4). This observation is supported by several studies showing that approximately one in five patients previously considered to be drug resistant, eventually becomes seizure-free with a change of medical regimen (9–11). These data show that drug resistance is reversible, and the intrinsic severity of the epilepsy can decrease over time—at least in a significant subgroup of patients with drug-resistant epilepsy. These findings allow revision of earlier suggestions that a patient who does not achieve seizure control with the first two or three drug regimens (including combinations) within the first 2 or 3 years of starting treatment is unlikely ever to achieve remission and usually can be considered to have drug-resistant epilepsy (12).
Although intuitively instructive, the inherent severity model, if based solely on early seizure frequency, has its limitations. These limitations include: 1) A lack of studies on the neurobiological basis of the severity hypothesis (2). Surprisingly, no molecular genetic studies have been performed for patients with low, versus high, seizure frequency at presentation or early in the course of the disease (2). 2) The clinical observation that a subgroup of patients with a higher seizure frequency at the onset of treatment will become seizure-free but require higher serum concentrations of AEDs to do so than those with a lower seizure frequency (13). This finding suggests that the intrinsic epilepsy severity concept alone is not able to fully explain or predict drug resistance of the epilepsy. 3) The evidence from a community-based cohort of 77 children with new-onset temporal lobe epilepsy (TLE), who were followed prospectively with formal reviews at 7 and 14 years after seizure onset, showed that there were lesions on MRI but neither initial seizure frequency nor early seizure remissions were predictive of seizure outcome (14). The undeniable success of resective epilepsy surgery in drug-resistant epilepsy, also apparent in those patients with a low seizure frequency, suggests that structural lesions play a direct and important role in determining AED failure (1). Thus, it is of interest not only to examine more closely if drug failure is determined by seizure frequency alone, but also to study the interaction of seizure frequency and other potential contributing factors that may lead to drug resistance.
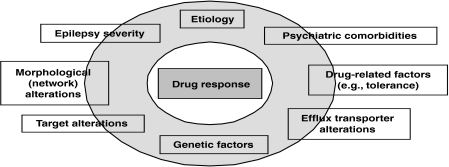
Univariate and multivariate logistic regression analyses demonstrated that drug resistance also is associated with family history of epilepsy, previous febrile seizures, traumatic brain injury as the cause of the epilepsy, intermittent recreational drug use, and an interesting new finding, prior or current psychiatric comorbidity, particularly depression (6). The data provide a preliminary basis for the integrative hypothesis that the deleterious neurobiological processes that underpin psychiatric comorbidity and an intrinsic severity of epilepsy may interact with those producing drug resistance in epilepsy (see Figure 1).
FIGURE 1.
Possible determinants of drug resistance in human epilepsy.
Animal models of epilepsy that offer direct comparison of pharmacoresistant and pharmacoresponsive animals, in the absence of the various confounding factors that complicate epidemiological studies, may be valuable tools in the effort to understand the biological basis of drug resistance and whether or not and how it can be predicted (15). Using an animal model of spontaneous recurrent seizures that develop after electrically induced status epilepticus, the difference in seizure frequency between pharmacoresistant and pharmacoresponsive rats, prior to AED treatment, was investigated (W. Löscher and C. Brandt, unpubl. ms.). In this model, about 30 to 40 percent of epileptic rats did not respond to prolonged treatment with either phenobarbital or phenytoin (16,17), thus meeting the operational definition of pharmacoresistance in animal models (i.e., persistent seizure activity not responding to at least two AEDs at maximum tolerated doses) (18). A meta-analysis of three drug trials involving a total of 33 epileptic rats showed that before onset of AED treatment, average seizure frequency in AED nonresponders (n= 13) was significantly higher than seizure frequency in responders (n= 20); however, the outcome actually was due to a subgroup of nonresponders (n= 6) that exhibited >3 seizures/day (W. Löscher and C. Brandt, unpubl. ms.). Such high seizure frequency was not observed in responders, demonstrating that high seizure frequency predicted pharmacoresistance, but did not occur in all nonresponders. In other words, in this animal model, as in human epilepsy, intrinsic disease severity alone is not sufficient to explain AED resistance.
Other factors associated with pharmacoresistance in the same model include behavioral abnormalities (19), hippocampal damage (20,21), overexpression of the efflux transporter P-glycoprotein (P-gp) at the blood–brain barrier (BBB) (22), and alterations in GABAA receptors that form the target for several AEDs, including phenobarbital (20,21). Thus, these experimental data suggest that multifactorial rather than single alterations underlie pharmacoresistance (see Figure 1).
Update of the Transporter and Drug Target Hypotheses
Two hypotheses have gained considerable favor in recent years, the transporter hypothesis, which suggests that AED levels are decreased at their brain targets because of overexpression of drug efflux transporters, such as P-gp, in epileptogenic brain regions, and the target hypothesis, which suggests that intrinsic (genetic) and acquired (disease-related) alterations to the structure and/or functionality of AED targets in epileptogenic brain regions lead to reduced drug effects (23,24).
Similar to the famous Koch's postulates, which originally were used to establish the role of bacteria in infectious disease, Sisodiya suggested that at least four criteria must be satisfied for a proposed drug-resistance mechanism of epilepsy to be accepted. The mechanism must: 1) be detectable in epileptogenic brain tissue; 2) have appropriate functionality; 3) be active in drug resistance; and 4) result in reduced drug resistance when the mechanism is overcome (25). All of these criteria recently have been satisfied for the transporter hypothesis; however, as yet, only in rodent models of epilepsy. Accordingly, P-gp expression is 1) increased in epileptogenic brain tissue of rodents (23), 2) associated with lower brain levels of AEDs (26,27), 3) higher in AED-resistant rats than in responsive animals (22), and most importantly, 4) coadministration of the highly selective P-gp inhibitor, tariquidar, reverses AED resistance (28,29). Thus, it is reasonable to conclude that P-gp plays a significant role in mediating resistance to AEDs in rodent models of TLE and that inhibition of P-gp can circumvent this mechanism; however, whether or not this phenomenon extends to the human species remains unclear (30).
The major caveat of the transporter hypothesis is the lack of evidence that AEDs are substrates for efflux by human P-gp (2). The in vitro model systems used in this respect have not been validated for highly permeable compounds, such as AEDs, and it is not clear if P-gp levels in the model systems accurately represent P-gp levels in brain capillaries of patients with drug-resistant epilepsy (31). It thus is important to note that two independent studies recently reported transport of AEDs by human P-gp, using modified model systems (32,33). First, investigators developed a new, dynamic in vitro BBB model that recapitulates several of the functional and structural properties of the BBB, including shear stress (32,34). By using cocultures of human microvascular endothelial cells and astrocytes from normal and drug-resistant epileptic brain tissue, the investigators found that the permeability of the in vitro BBB to phenytoin was tenfold less when using brain capillary endothelial cells from drug-resistant patients (32). This decreased permeability could be partially counteracted by the selective P-gp inhibitor, tariquidar, indicating that P-gp was involved in the reduced permeability of endothelial cells from epileptic patients (32). Transport of phenytoin by human P-gp was substantiated in another study that used MDR1-transfected cells in a modified transport assay, which allows evaluation of active transport independently of the passive permeability component (33). In addition to phenytoin, several other major AEDs were transported by human P-gp, and this transport could be inhibited by tariquidar.
A next logical step in the assessment of the transporter hypothesis in epilepsy patients will be the use of 11C-labeled AEDs and PET to determine if AED resistance is associated with lower brain uptake and increased brain efflux of AEDs. A pilot PET study assessing the P-gp ligand (R)-11C-verapamil by patients with TLE showed increased brain efflux of this ligand in parahippocampal regions of the ipsilateral hemisphere in five of seven patients, but, because of its low brain uptake, (R)-11C-verapamil is not an ideal substance to identify alterations in function or expression of P-gp in the brain (35). Recent experimental data have indicated that (R)-11C-verapamil combined with the P-gp inhibitor tariquidar is a more promising approach to measure P-gp function at the BBB (36), and clinical trials are underway to evaluate this approach in patients.
Increased brain expression of efflux transporters, such as P-gp, could be either a result of prolonged or frequent seizures, as demonstrated in rodent models of epilepsy, or of genetic factors, such as polymorphisms in the MDR1 gene or, theoretically, of both (23). Several pharmacogenetic studies have indicated that MDR1 polymorphisms that affect the expression or functionality of P-gp, such as the 3435C>T polymorphism, are more frequent in AED nonresponders than responders, but the finding could not be reproduced in other studies (37). A likely explanation for these inconsistent data is that most previous genetic association studies included patients treated with various AEDs, and for several of these drugs, it is not known whether or not they are transported by P-gp (37). More recent monotherapy studies in human patients with epilepsy treated with AEDs that are transported by human P-gp (phenytoin or phenobarbital) demonstrated a significant association between the MDR1 3435C>T polymorphism and pharmacoresistance (38,39). Furthermore, in the phenobarbital study, the CC genotype of 3435 was associated with significantly lower drug levels in CSF and a significantly lower CSF/plasma ratio than the CT or TT genotypes (39). However, causality has not been proven in any of these studies, thus all reported findings remain associations. Finally, it is of interest to note that polymorphisms in MDR1 also have been shown to predict antidepressant treatment response in depression (40).
The target hypothesis of drug failure is based primarily on studies indicating reduced sensitivity of voltage-gated sodium channels to carbamazepine in epileptogenic brain tissue from patients who were not seizure-free while receiving this AED and underwent resective surgery (41,42). Surprisingly, compared to the transporter hypothesis, only a few studies have explored the target hypothesis; therefore the criteria proposed by Sisodiya have not been met, either experimentally or clinically, as of yet. Furthermore, it remains unknown if target alterations in epileptogenic brain tissue from AED-resistant patients alter only the efficacy of carbamazepine or whether they also can affect other AEDs that act at sodium channels or ones that have a different mechanism of action (2). Alterations in voltage-gated sodium channels and other AED targets, including GABAA receptors, have been determined in animal models of TLE (3,24). In line with the target hypothesis, GABAA receptor subunit alterations were associated with resistance to phenobarbital in a rat model of TLE (20,21). Furthermore, alterations in GABAA receptors are likely to explain resistance of status epilepticus to benzodiazepines (43,44). Chen and Wasterlain have proposed the interesting concept that alterations in GABAA receptors, including a decrease in the number of functional receptors because of receptor trafficking, are an explanation for both the transition from isolated seizures to status epilepticus and for the progressive loss of efficacy of the AED to terminate status epilepticus, suggesting that the same neurobiological mechanism may underlie the evolution of both severity of seizures and AED failure (44).
Conclusion: The Need for an Integrative View
In addition to the transporter and target hypotheses of epilepsy drug resistance, a novel approach, the inherent severity model of epilepsy, recently has been proposed by Rogawski and Johnson (2). As valuable as each of the three hypotheses may be, neither is able to explain drug resistance in human epilepsy as a stand-alone theory. The transporter hypothesis, which has a solid base in experimental epilepsy, needs more evidence from human epilepsy studies. The target hypothesis, although intuitively attractive, is based on very few studies in human epilepsy with only one AED (carbamazepine), although more recent experimental data indicate that target alterations also may be involved in resistance to other AEDs. Although the novel third theory is a welcome approach, it also has its limitations. High seizure frequency often is associated with drug resistance, yet a subgroup of these patients eventually becomes seizure-free after a change of medication, and conversely, a subgroup of epilepsy patients starting with few seizures, turn out to have drug-resistant epilepsy. Finally, another caveat of the inherent severity model is the lack of studies on the neurobiological, molecular, and genetic basis of the severity hypothesis. It is possible that each of the three theories applies to one pure subgroup of patients, yet in many patients these mechanisms may overlap.
Experimentally, it has been shown that pharmacoresistance is associated with high seizure frequency, target alterations, and P-gp overexpression in the same epileptic rats—although, as discussed, not all AED-resistant rats display high seizure frequency. Inhibition of P-gp selectively increases AED concentrations in epileptogenic brain regions that share overexpression of P-gp and reduced target sensitivity (21,27), which may explain the surprisingly high efficacy of the P-gp inhibitor tariquidar to counteract pharmacoresistance in epileptic rats (27,28). In other words, P-gp inhibition may counteract the consequences of both P-gp overexpression and loss of target sensitivity in epileptogenic brain regions by increasing AED concentrations at target sites. The European Union is currently supporting a “European research initiative to develop imaging probes for early in vivo diagnosis and evaluation of response to therapeutic substances” (EURIPIDES; cf., http://www.euripides-europe.com/) that will explore the causes of drug resistance for patients with major neurological diseases, including epilepsy. A key aim of this initiative is the development of new PET ligands, including 11C-labeled AEDs, to study alterations in functionality and expression of P-gp in epilepsy that could ultimately permit identification of patients who would benefit from coadministration of P-gp inhibitors.
Finally, the ultimate integrative hypothesis would be one in which the mechanisms thought to be involved in regulating drug response also would play a role in determining disease severity, which as yet remains unproven for epilepsy. It is of interest to note that similar links have been proposed for depression: it was reported that polymorphisms in FKBP5, a glucocorticoid receptor-regulating cochaperone of Hsp90, are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment (45). Although promising data from experimental epilepsy studies suggest target modification may present such a link, as reviewed, the evidence for a direct mechanistic link, genetic or otherwise, between drug response and human disease severity, if it exists, is still elusive. Clearly, more work is needed to explore whether or not common neurobiological factors may underlie both epilepsy severity and drug failure.
References
- 1.Schmidt D, Löscher W. Drug resistance in epilepsy: Putative neurobiologic and clinical mechanisms. Epilepsia. 2005;46:858–877. doi: 10.1111/j.1528-1167.2005.54904.x. [DOI] [PubMed] [Google Scholar]
- 2.Rogawski MA, Johnson MR. Intrinsic severity as a determinant of antiepileptic drug refractoriness. Epilepsy Currents. 2008;8:127–30. doi: 10.1111/j.1535-7511.2008.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Löscher W. Current knowledge on basic mechanisms of drug-resistance. In: Kahane P, Berg A, Löscher W, Nordli D, Perucca E, editors. Progress in Epileptic Disorders, Vol. 7. Drug-Resistant Epilepsies. Montrouge: John Libbey; 2008. pp. 47–62. [Google Scholar]
- 4.Sillanpää M, Schmidt D. Natural history of treated childhood-onset epilepsy: Prospective, long-term population-based study. Brain. 2006;129:617–624. doi: 10.1093/brain/awh726. [DOI] [PubMed] [Google Scholar]
- 5.Sillanpää M, Schmidt D. Early seizure frequency and aetiology predict long-term medical outcome in childhood-onset epilepsy. Brain. 2009 doi: 10.1093/brain/awn357. in press. [DOI] [PubMed] [Google Scholar]
- 6.Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75:192–196. doi: 10.1016/j.eplepsyres.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Sillanpää M, Schmidt D. Seizure clustering during drug treatment affects seizure outcome and mortality of childhood-onset epilepsy. Brain. 2008;131:938–944. doi: 10.1093/brain/awn037. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald BK, Johnson AL, Goodridge DM, Cockerell OC, Sander JW, Shorvon SD. Factors predicting prognosis of epilepsy after presentation with seizures. Ann Neurol. 2000;48:833–841. [PubMed] [Google Scholar]
- 9.Schiller Y, Najjar Y. Quantifying the response to antiepileptic drugs: Effect of past treatment history. Neurology. 2008;70:54–65. doi: 10.1212/01.wnl.0000286959.22040.6e. [DOI] [PubMed] [Google Scholar]
- 10.Selwa LM, Schmidt SL, Malow BA, Beydoun A. Long-term outcome of nonsurgical candidates with medically refractory localization-related epilepsy. Epilepsia. 2003;44:1568–1572. doi: 10.1111/j.0013-9580.2003.15003.x. [DOI] [PubMed] [Google Scholar]
- 11.Callaghan BC, Anand K, Hesdorffer D, Hauser WA, French JA. Likelihood of seizure remission in an adult population with refractory epilepsy. Ann Neurol. 2007;62:382–389. doi: 10.1002/ana.21166. [DOI] [PubMed] [Google Scholar]
- 12.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt D, Haenel F. Therapeutic plasma levels of phenytoin, phenobarbital, and carbamazepine: Individual variation in relation to seizure frequency and type. Neurology. 1984;34:1252–1255. doi: 10.1212/wnl.34.9.1252. [DOI] [PubMed] [Google Scholar]
- 14.Spooner CG, Berkovic SF, Mitchell LA, Wrennall JA, Harvey AS. New-onset temporal lobe epilepsy in children: Lesion on MRI predicts poor seizure outcome. Neurology. 2006;67:2117–2118. doi: 10.1212/01.wnl.0000248189.93630.4f. [DOI] [PubMed] [Google Scholar]
- 15.Löscher W. Animal models of drug-refractory epilepsy. In: Pitkänen A, Schwartzkroin PAMSL, editors. Models of Seizures and Epilepsy. Amsterdam: Elsevier; 2006. pp. 551–567. [Google Scholar]
- 16.Brandt C, Volk HA, Löscher W. Striking differences in individual anticonvulsant response to phenobarbital in rats with spontaneous seizures after status epilepticus. Epilepsia. 2004;45:1488–1497. doi: 10.1111/j.0013-9580.2004.16904.x. [DOI] [PubMed] [Google Scholar]
- 17.Bethmann K, Brandt C, Löscher W. Resistance to phenobarbital extends to phenytoin in a rat model of temporal lobe epilepsy. Epilepsia. 2007;48:816–826. doi: 10.1111/j.1528-1167.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- 18.Stables JP, Bertram E, Dudek FE, Holmes G, Mathern G, Pitkänen A, White HS. Therapy discovery for pharmacoresistant epilepsy and for disease-modifying therapeutics: Summary of the NIH/NINDS/AES models II workshop. Epilepsia. 2003;44:1472–1478. doi: 10.1111/j.0013-9580.2003.32803.x. [DOI] [PubMed] [Google Scholar]
- 19.Gastens AM, Brandt C, Bankstahl JP, Löscher W. Predictors of pharmacoresistant epilepsy: Pharmacoresistant rats differ from pharmacoresponsive rats in behavioral and cognitive abnormalities associated with experimentally induced epilepsy. Epilepsia. 2008;49:1759–1776. doi: 10.1111/j.1528-1167.2008.01659.x. [DOI] [PubMed] [Google Scholar]
- 20.Volk HA, Arabadzisz D, Fritschy JM, Brandt C, Bethmann K, Löscher W. Antiepileptic drug resistant rats differ from drug responsive rats in hippocampal neurodegeneration and GABAA-receptor ligand-binding in a model of temporal lobe epilepsy. Neurobiol Dis. 2006;21:633–646. doi: 10.1016/j.nbd.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Bethmann K, Fritschy JM, Brandt C, Löscher W. Antiepileptic drug resistant rats differ from drug responsive rats in GABAA-receptor subunit expression in a model of temporal lobe epilepsy. Neurobiol Dis. 2008;31:169–187. doi: 10.1016/j.nbd.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Volk HA, Löscher W. Multidrug resistance in epilepsy: Rats with drug-resistant seizures exhibit enhanced brain expression of P-glycoprotein compared with rats with drug-responsive seizures. Brain. 2005;128:1358–1368. doi: 10.1093/brain/awh437. [DOI] [PubMed] [Google Scholar]
- 23.Löscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nature Rev Neurosci. 2005;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 24.Remy S, Beck H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain. 2006;129:18–35. doi: 10.1093/brain/awh682. [DOI] [PubMed] [Google Scholar]
- 25.Sisodiya SM. Mechanisms of antiepileptic drug resistance. Curr Opin Neurol. 2003;16:197–201. doi: 10.1097/01.wco.0000063771.81810.6c. [DOI] [PubMed] [Google Scholar]
- 26.Rizzi M, Caccia S, Guiso G, Richichi C, Gorter JA, Aronica E, Aliprandi M, Bagnati R, Fanelli R, D'Incalci M, Samanin R, Vezzani A. Limbic seizures induce P-glycoprotein in rodent brain: Functional implications for pharmacoresistance. J Neurosci. 2002;22:5833–5839. doi: 10.1523/JNEUROSCI.22-14-05833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Vliet EA, van Schaik R, Edelbroek PM, Voskuyl RA, Redeker S, Aronica E, Wadman WJ, Gorter JA. Region-specific overexpression of P-glycoprotein at the blood-brain barrier affects brain uptake of phenytoin in epileptic rats. J Pharmacol Exp Ther. 2007;322:141–147. doi: 10.1124/jpet.107.121178. [DOI] [PubMed] [Google Scholar]
- 28.Brandt C, Bethmann K, Gastens AM, Löscher W. The multidrug transporter hypothesis of drug resistance in epilepsy: Proof-of-principle in a rat model of temporal lobe epilepsy. Neurobiol Dis. 2006;24:202–211. doi: 10.1016/j.nbd.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 29.van Vliet EA, van Schaik R, Edelbroek PM, Redeker S, Aronica E, Wadman WJ, Marchi N, Vezzani A, Gorter JA. Inhibition of the multidrug transporter P-glycoprotein improves seizure control in phenytoin-treated chronic epileptic rats. Epilepsia. 2006;47:672–680. doi: 10.1111/j.1528-1167.2006.00496.x. [DOI] [PubMed] [Google Scholar]
- 30.Löscher W, Sills GJ. Drug resistance in epilepsy: Why is a simple explanation not enough? Epilepsia. 2007;48:2370–2372. doi: 10.1111/j.1528-1167.2007.01260_2.x. [DOI] [PubMed] [Google Scholar]
- 31.Robey RW, Lazarowski A, Bates SE. P-glycoprotein—A clinical target in drug-refractory epilepsy? Mol Pharmacol. 2008;73:1343–1346. doi: 10.1124/mol.108.046680. [DOI] [PubMed] [Google Scholar]
- 32.Cucullo L, Hossain M, Rapp E, Manders T, Marchi N, Janigro D. Development of a humanized in vitro blood-brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia. 2007;48:505–516. doi: 10.1111/j.1528-1167.2006.00960.x. [DOI] [PubMed] [Google Scholar]
- 33.Luna-Tortos C, Fedrowitz M, Löscher W. Several major antiepileptic drugs are substrates for human P-glycoprotein. Neuropharmacology. 2008;5:1364–1375. doi: 10.1016/j.neuropharm.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 34.Santaguida S, Janigro D, Hossain M, Oby E, Rapp E, Cucullo L. Side by side comparison between dynamic versus static models of blood-brain barrier in vitro: A permeability study. Brain Res. 2006;1109:1–13. doi: 10.1016/j.brainres.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 35.Langer O, Bauer M, Hammers A, Karch R, Pataraia E, Koepp MJ, Abrahim A, Luurtsema G, Brunner M, Sunder-Plassmann R, Zimprich F, Joukhadar C, Gentzsch S, Dudczak R, Kletter K, Müller M, Baumgartner C. Pharmacoresistance in Epilepsy: A Pilot PET Study with the P-Glycoprotein Substrate R-[C]verapamil. Epilepsia. 2007;48:1774–1784. doi: 10.1111/j.1528-1167.2007.01116.x. [DOI] [PubMed] [Google Scholar]
- 36.Bankstahl JP, Kuntner C, Abrahim A, Karch R, Stanek J, Wanek T, Wadsak W, Kletter K, Müller M, Löscher W, Langer O. Tariquidar-induced P-glycoprotein inhibition at the rat blood-brain barrier studied with (R)-11C-verapamil and PET. J Nucl Med. 2008;49:1328–1335. doi: 10.2967/jnumed.108.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Löscher W, Klotz U, Zimprich F, Schmidt D. The clinical impact of pharmacogenetics on the treatment of epilepsy. Epilepsia. 2009;50:1–23. doi: 10.1111/j.1528-1167.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 38.Ebid AH, Ahmed MM, Mohammed SA. Therapeutic drug monitoring and clinical outcomes in epileptic Egyptian patients: A gene polymorphism perspective study. Ther Drug Monit. 2007;29:305–312. doi: 10.1097/FTD.0b013e318067ce90. [DOI] [PubMed] [Google Scholar]
- 39.Basic S, Hajnsek S, Bozina N, Filipcic I, Sporis D, Mislov D, Posavec A. The influence of C3435T polymorphism of ABCB1 gene on penetration of phenobarbital across blood-brain barrier in patients with generalized epilepsy. Seizure Eur J Epilepsy. 2008;17:524–530. doi: 10.1016/j.seizure.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Uhr M, Tontsch A, Namendorf C, Ripke S, Lucae S, Ising M, Dose T, Ebinger M, Rosenhagen M, Kohli M, Kloiber S, Salyakina D, Bettecken T, Specht M, Pütz B, Binder EB, Müller-Myhsok B, Holsboer F. Polymorphisms in the drug transporter gene ABCB1 predict antidepressant treatment response in depression. Neuron. 2008;57:203–209. doi: 10.1016/j.neuron.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Remy S, Gabriel S, Urban BW, Dietrich D, Lehmann TN, Elger CE, Heinemann U, Beck H. A novel mechanism underlying drug resistance in chronic epilepsy. Ann Neurol. 2003;53:469–479. doi: 10.1002/ana.10473. [DOI] [PubMed] [Google Scholar]
- 42.Jandova K, Pasler D, Antonio LL, Raue C, Ji S, Njunting M, Kann O, Kovács R, Meencke HJ, Cavalheiro EA, Heinemann U, Gabriel S, Lehmann TN. Carbamazepine-resistance in the epileptic dentate gyrus of human hippocampal slices. Brain. 2006;129:3290–3306. doi: 10.1093/brain/awl218. [DOI] [PubMed] [Google Scholar]
- 43.Macdonald RL, Kapur J. Acute cellular alterations in the hippocampus after status epilepticus. Epilepsia. 1999;40:S9–S20. doi: 10.1111/j.1528-1157.1999.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 44.Chen JW, Wasterlain CG. Status epilepticus: Pathophysiology and management in adults. Lancet Neurol. 2006;5:246–256. doi: 10.1016/S1474-4422(06)70374-X. [DOI] [PubMed] [Google Scholar]
- 45.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Pütz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Künzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Köhnlein O, Dabitz H, Brückl T, Müller N, Pfister H, Lieb R, Mueller JC, Löhmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]