Abstract
The feline T-cell lymphotropic lentivirus (feline immunodeficiency virus) is a recently described feline-specific retrovirus that can produce chronic immunodeficiency-like disorders in cats. A microdilution plate format enzyme-linked immunosorbent assay has been developed to detect the presence of antibody to the virus in feline serum or plasma. Temporal studies performed with experimentally infected animals show that seroconversion can be demonstrated 3 to 4 weeks after exposure to the virus. Results of a serosurvey (n = 1,556 samples) indicate that infection is fairly common in both clinic (5.2%) and sick cat (15.2%) populations. Western blot (immunoblot) and sodium dodecyl sulfate radioimmunoprecipitation assays were developed to confirm microdilution plate test results and to identify peptides specific for the feline immunodeficiency virus. All microdilution plate test positive results and selected negative results were confirmed by one or both of these procedures. These data demonstrate that this microassay plate enzyme-linked immunosorbent assay is a very sensitive and specific test for detection of antibody to the feline immunodeficiency virus.
Full text
PDF


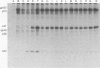
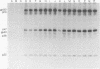
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain J. P., Laurian Y., Paul D. A., Senn D. Serological markers in early stages of human immunodeficiency virus infection in haemophiliacs. Lancet. 1986 Nov 29;2(8518):1233–1236. doi: 10.1016/s0140-6736(86)92673-5. [DOI] [PubMed] [Google Scholar]
- Barin F., McLane M. F., Allan J. S., Lee T. H., Groopman J. E., Essex M. Virus envelope protein of HTLV-III represents major target antigen for antibodies in AIDS patients. Science. 1985 May 31;228(4703):1094–1096. doi: 10.1126/science.2986291. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Arthur L. O., Tsai C. C., Sowder R., Copeland T. D., Henderson L. E., Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986 Nov;60(2):483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Ruprecht R., Simpson R. W., Spiegelman S. Deoxyribonucleic acid polymerase of Rous sarcoma virus: reaction conditions and analysis of the reaction product nucleic acids. J Virol. 1971 Nov;8(5):730–741. doi: 10.1128/jvi.8.5.730-741.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. S., Redfield R. R., Putman P., Alexander S. S. Variations in Western blot banding patterns of human T-cell lymphotropic virus type III/lymphadenopathy-associated virus. J Clin Microbiol. 1987 Jan;25(1):81–84. doi: 10.1128/jcm.25.1.81-84.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Gaskin J. M., Perk K. Morphological and immunological comparison of caprine arthritis encephalitis and ovine progressive pneumonia viruses. J Virol. 1981 Sep;39(3):914–919. doi: 10.1128/jvi.39.3.914-919.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Braun M. J., Carter S. G., Kost T. A., Bess J. W., Jr, Arthur L. O., Van der Maaten M. J. Characterization and molecular cloning of a bovine lentivirus related to human immunodeficiency virus. 1987 Nov 26-Dec 2Nature. 330(6146):388–391. doi: 10.1038/330388a0. [DOI] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Ishida T., Washizu T., Toriyabe K., Motoyoshi S. Detection of feline T-lymphotropic lentivirus (FTLV) infection in Japanese domestic cats. Nihon Juigaku Zasshi. 1988 Feb;50(1):39–44. doi: 10.1292/jvms1939.50.39. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Lui K. J., Lawrence D. N., Morgan W. M., Peterman T. A., Haverkos H. W., Bregman D. J. A model-based approach for estimating the mean incubation period of transfusion-associated acquired immunodeficiency syndrome. Proc Natl Acad Sci U S A. 1986 May;83(10):3051–3055. doi: 10.1073/pnas.83.10.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael J. C., Stiers S., Coffin S. Prevalence of feline leukemia virus infection among adult cats at an animal control center: association of viremia with phenotype and season. Am J Vet Res. 1986 Apr;47(4):765–768. [PubMed] [Google Scholar]
- Montelaro R. C., Lohrey N., Parekh B., Blakeney E. W., Issel C. J. Isolation and comparative biochemical properties of the major internal polypeptides of equine infectious anemia virus. J Virol. 1982 Jun;42(3):1029–1038. doi: 10.1128/jvi.42.3.1029-1038.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Chase J. Antigenic shift of visna virus in persistently infected sheep. Science. 1977 Jul 22;197(4301):376–378. doi: 10.1126/science.195339. [DOI] [PubMed] [Google Scholar]
- Parekh B., Issel C. J., Montelaro R. C. Equine infectious anemia virus, a putative lentivirus, contains polypeptides analogous to prototype-C oncornaviruses. Virology. 1980 Dec;107(2):520–525. doi: 10.1016/0042-6822(80)90319-0. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Ho E. W., Brown M. L., Yamamoto J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987 Feb 13;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J. K., Sparger E., Ho E. W., Andersen P. R., O'Connor T. P., Mandell C. P., Lowenstine L., Munn R., Pedersen N. C. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988 Aug;49(8):1246–1258. [PubMed] [Google Scholar]