Abstract
Cytoscape is a bioinformatic data analysis and visualization platform that is well-suited to the analysis of gene expression data. To facilitate the analysis of yeast microarray data using Cytoscape, we constructed an interaction network (interactome) using the curated interaction data available from the Saccharomyces Genome Database (www.yeastgenome.org) and the database of yeast transcription factors at YEASTRACT (www.yeastract.com). These data were formatted and imported into Cytoscape using semi-automated methods, including Linux-based scripts, that simplified the process while minimizing the introduction of processing errors. The methods described for the construction of this yeast interactome are generally applicable to the construction of any interactome. Using Cytoscape, we illustrate the use of this interactome through the analysis of expression data from a recent yeast diauxic shift experiment. We also report and briefly describe the complex associations among transcription factors that result in the regulation of thousands of genes through coordinated changes in expression of dozens of transcription factors. These cells are thus able to sensitively regulate cellular metabolism in response to changes in genetic or environmental conditions through relatively small changes in the expression of large numbers of genes, affecting the entire yeast metabolome.
INTRODUCTION
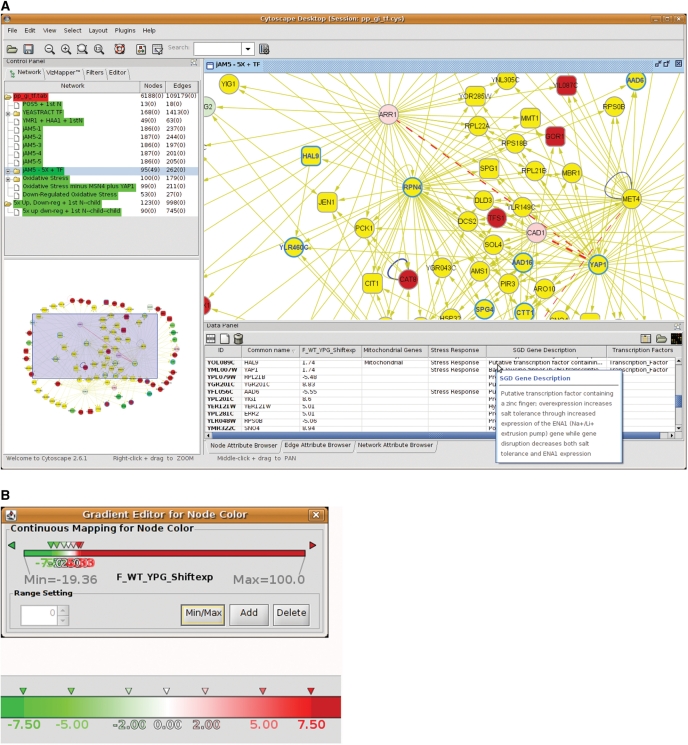
Cytoscape (www.cytoscape.org) is an open source bioinformatics software platform originally intended for, but not limited to, the analysis of molecular interaction data associated with changes in gene expression and other data (1). Cytoscape's core distribution provides a basic set of features for data integration and visualization, with additional features available as plugins. Additionally, the visual display properties are highly customizable, including the use of annotation files that allow additional information to be visually represented in a more meaningful manner (Figure 1).
Figure 1.
Selected illustration from the yeast interactome, using a screen capture from a Cytoscape ‘session’ (Cytoscape sessions can be saved, preserving the work for future use). (A) This panel illustrates part of the subnetwork identified by the jActiveModules plugin, as described in the text and summarized in Table 1. The main, upper right frame displays a close-up view of some of the genes present in this subnetwork, while the lower left frame shows an overview of the entire subnetwork, with the part shown in the main frame indicated by the shaded region. The upper left frame contains a list of the subnetworks arising from various analyses within Cytoscape, including the numbers of nodes and edges, and the number of these currently selected, that are highlighted in yellow in the displayed subnetworks. In this example, genes directly associated with (i.e. regulated by) transcription factor Rpn4 are selected and shown in yellow (these selections are made from the Select menu). The data panel at the lower right of the image displays information associated with each of these nodes, imported from various user-defined annotation files, including (as shown) common gene names, the expression values, genes associated with the mitochondrion and the response to stress, descriptions of the genes from SGD and genes encoding transcription factors. In this example, transcription factor Hal9 is a mitochondrial stress response gene that was up-regulated 1.7-fold in wild-type diauxic-shifted cells. Also illustrated is a a box containing the SGD gene description, that automatically pops up when the cursor is placed on top of a gene description, here showing the complete SGD gene description for Hal9. Mitochondrial-associated nodes are shown as rectangles, and nodes associated with the response to stress are drawn with emphasized, blue borders with the gene names also displayed in blue text. Node–node interactions (edges) are color-coded with blue edges indicating protein–protein interactions, gold edges indicating transcription factors with the arrow pointing from the transcription factor toward the regulated gene, and broken red edges indicating genetic (rather than physical) interactions, e.g. synthetic lethality—the loss of viability when both alleles are inactivated. Nodes in which the blue edge loops back on itself indicate self-regulated genes. The thickness of the edges represent weights, i.e. heavier edges indicate more (multiple) interactions, of the indicated type, between two nodes, as described in the Materials and Methods section. (B) The ‘Gradient Editor for Node Color’ editor from the Cytoscape VizMapper tool. In each of the Cytoscape displays in this article, up-regulated genes (nodes) are colored red, while down-regulated genes are colored green, with the extent of shading proportional to the level of expression as indicated in an expanded view in the image in the lower part of this panel.
Several years ago, we determined that disruption of the POS5 gene in Saccharomyces cerevisiae results in a 50-fold increase in the reversion of a frameshift deletion in mtDNA and demonstrated that POS5 encodes a NAD(H) kinase, the sole source of NADP+ and NADPH in the mitochondrion of S. cerevisiae (2). In a recent follow-up study, we used a yeast microarray to evaluate the changes in gene expression in S. cerevisiae due to genetic and environmental factors associated with oxidative stress (3). To facilitate those analyses, we created a high-quality yeast interaction network (interactome) suitable for use in Cytoscape, illustrated through the analysis of data from a recent diauxic shift experiment in wild-type yeast cells that serves as an in-house reference source of yeast expression data. Analyses of these data additionally revealed that transcription factors and their target genes form highly complex, interconnected networks affecting all aspects of cellular metabolism in S. cerevisiae.
MATERIALS AND METHODS
Strain
Saccharomyces cerevisiae strain YPH925 (ade2-101 cyh2 his3-Δ200 kar1-Δ15 leu2-Δ1 lys2-801 trp1-Δ63 ura3-52) (4) was employed for this work, and for convenience is referred to as being ‘wild-type’.
Bioinformatic platforms
Microarray fluorescence data (3) were imported into Rosetta Resolver (Rosetta Biosoftware, Seattle, WA, USA) for the estimation of random error by application of an error model that calculated the confidence limits (P-values) for the expression values. The Agilent GeneSpring Analysis Platform (Agilent Technologies, Palo Alto, CA, USA) was used for LOWESS data normalization. To aid the visualization and the analysis of the expression data, a S. cerevisiae protein–protein and protein–DNA interactome was constructed as described in the following section. The microarray expression data were then mapped onto this interactome using Cytoscape.
Genes associated with the highest scoring subnetworks were identified using the Cytoscape jActiveModules plugin. Active Modules are connected subnetworks within the interaction network whose genes show significant coordinated changes in mRNA expression over particular experimental conditions (1). The algorithm iteratively reduces network complexity by pinpointing regions whose states are perturbed by the conditions of interest, while removing false-positive interactions and interactions not involved in the perturbation response. Genes present in each of the five top-scoring networks in wild-type cells shifted to growth in glycerol were identified using the jActiveModules algorithm. Since many of the genes in each of these five subnetworks (186–187 genes each) were present in two or more of these subnetworks, for simplicity these groups of genes were combined, resulting in a single jActiveModules gene list.
Construction of a high-quality yeast interactome
The interactome described in this article was constructed in April 2008 using semi-automated methods to format the interaction data from the Saccharomyces Genome Database (SGD: www.yeastgenome.org) and the transcription factor data from YEASTRACT (5) (yeastract.com) into a form suitable for use with Cytoscape, as described in detail in the Supplementary Material (Supplementary file: Constructing a Yeast Interactome). Briefly, the interactions.tab file downloaded from SGD was processed by deleting unnecessary text (e.g. ‘Bait’ and ‘Hit’) and columns and reclassifying the various interaction types as either ‘pi’ (physical interactions, e.g. protein–protein) or ‘gi’ (genetic interactions, e.g. synthetic lethality). This step dramatically simplified the visual display of the interaction types (edges) between the various nodes (genes; proteins), while allowing us to assign ‘weights’ to each of the edges, reflecting the numbers of interactions documented between nodes. These interaction weights provided a measure of the number of times that two genes were found to interact with one another in a specific manner, from among the data curated at SGD from various sources.
Next, an interaction file containing a list of S. cerevisiae transcription factors and their documented target genes, RegulationTwoColumnTable_Documented_2008410_1839_1043605408.tsv, was downloaded from YEASTRACT. In preparation for use in Cytoscape, the yeast common gene names provided by YEASTRACT were converted to their systematic names, as found at SGD (e.g. POS5 was converted to YPL188W). Next, all letters in the gene names appearing in lowercase were converted to uppercase, again a requirement for Cytoscape. This list of genes was then processed through the ‘Batch Download’ tool at SGD to identify ‘rogue’ genes (e.g. MAL63 is not present in the in the systematic sequence of the SGD reference strain S288c; or, two or more genes sharing the same common name). Next, a column of interactions weights (all equal to 1) was appended to the transcription factor interaction file, for compatibility with the weighted SGD interaction data.
The SGD and YEASTRACT plain-text, tab-delimited interaction files were then concatenated as a single file (Supplementary file: pp_gi_tf.tab) and imported into Cytoscape using the import tool located under the File menu. At this time various annotation files, including gene-expression data and lists of stress response and mitochondrial genes, were also imported into Cytoscape. Lastly, the visual display properties of the nodes and edges were defined using the Cytoscape VizMapper tool.
The computer used for this work employed an Intel Pentium 4 CPU operating at 3.0 GHz, 1.5 GB of RAM, and the Microsoft Windows XP Professional Version 2002 Service Pack 2 operating system. To facilitate the steps summarized above associated with manipulating and formatting the raw interaction data files, simple perl and awk scripts were employed using Cygwin (http://www.cygwin.com/), a Linux-like environment for Windows [GNU bash shell, version 3.2.33(18)-release (i686-pc-cygwin]. On Macintosh and Linux-based operating systems, the awk and perl programming languages can be implemented directly in a command shell. Cytoscape is available for any major computer platform, including the Windows, Macintosh and Linux operating systems. All of the tools and source data described in this article are freely available from the indicated sources, while the yeast interactome described in this article is provided as Supplementary Material (Supplementary file: pp_gi_tf.cys) a Cytoscape session file with 6,188 nodes (genes/proteins) and 109,179 edges (interactions), that also includes the sample microarray expression data from our yeast diauxic shift experiment, plus the VizMapper visual display settings. For use with older versions of Cytoscape (or for use with other platforms), data files separately containing the diauxic shift expression data, the interactome (Supplemental file: pp_gi_tf.tab), node annotation files (lists of genes associated with the mitochondrion, the response to stress or transcription factors; common gene names; SGD gene descriptions), as well as the VizMapper visual display properties (vizmap.props) file, and lastly an Excel look-up table that can be used to convert common yeast names to their systematic gene name are included in the supplementary interactome files: (Supplementary interactome files: Mitochondrial_Gene_Names.txt; Stress_Response_Genes.txt; Transcription_Factor_Gene_List.txt; Common_Gene_Names.txt; SGD_Gene_Descriptions.txt; vizmap.props; Common_to_Systematic_Name_Lookup_Table.xls; WT_YPG_Shift_Expression_Data.pvals).
RESULTS
Changes in expression occurring during a glycerol-induced diauxic shift
In a related study (3), we examined and compared changes in gene expression in cells containing a deletion of the POS5 gene using Cytoscape (1), a bioinformatic data analysis and visualization tool. Here, we describe the construction of the robust yeast interactome used in those analyses. To better describe the application and versatility of this interactome, we describe the results from a parallel study from our laboratory that examined gene expression in a wild-type yeast strain grown to the mid-logarithmic phase of growth, then shifted to growth in glycerol for 2 h.
Similar to previous reports (6–12), we observed profound changes in gene expression following the switch from a fermentable carbon source (glucose) to the nonfermentable carbon source (glycerol), as summarized in Figure 2 and Table 1. Specifically, 3777 of the 6256 genes on the Agilent yeast chip (60.4%) were found to be differentially expressed at a significance level of <10−4 (at this level of significance, ∼0.6 false-positives were expected). To reduce this list of genes to a more meaningful and manageable dataset, we used the Cytoscape jActiveModules plugin to identify genes showing coordinated, significant changes in expression. The five top-scoring Active Modules subnetworks (jAM5-1 through jAM5-5) each contained 186 or 187 genes, as indicated in Figure 1. Since these subnetworks partially overlapped, these genes were combined into a single list which was further simplified by selecting genes that were greater than 5-fold up- or down-regulated, plus their associated transcription factors (Table 1).
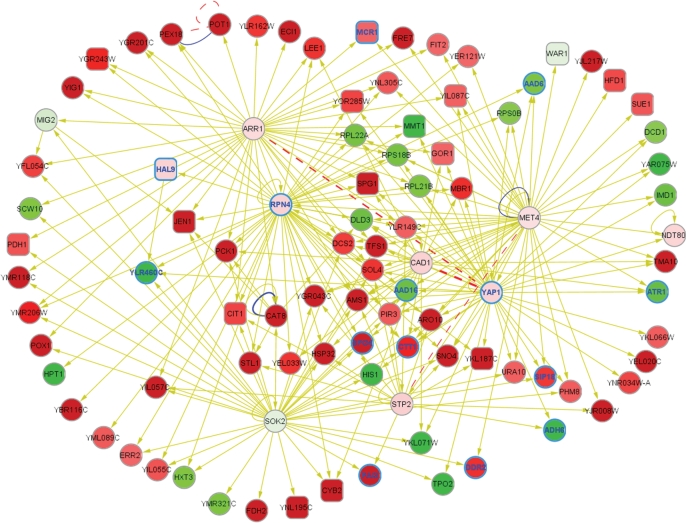
Figure 2.
A schematic showing the 95 genes present among the five top-scoring Cytoscape jActiveModules subnetworks from YPG-shifted cells with changes in expression ≥5-fold, plus the associated transcription factors, displayed using Cytoscape, and also shown at lower magnification in the lower left frame in Figure 1A. For convenience, these genes are also summarized in Table 1. For a description of the visual display elements (node colors, etc.), please refer to the Figure 1 legend.
Table 1.
Expression levels of genes in glycerol-shifted wild-type cells identified by the Cytoscape jActiveModules, with expression values ≥5-fold up- or down-regulated, plus their associated transcription factors
| Systematic name | Common name | Fold-changea | Saccharomyces Genome Database gene description (abbreviated) |
|---|---|---|---|
| YIL160C | POT1 | 74.67 | 3-ketoacyl-CoA thiolase; cleaves 3-ketoacyl-CoA into acyl-CoA and acetyl-CoA during beta-oxidation of fatty acids |
| YPL276W | FDH2 | 62.63 | NAD(+)-dependent formate dehydrogenase; may protect cells from exogenous formate |
| YKL217W | JEN1 | 57.30 | Lactate transporter (uptake of lactate, pyruvate); derepressed by Cat8p under nonfermentative growth conditions |
| YMR107W | SPG4 | 52.99 | Required for survival at high temperature during stationary phase; not required for growth on nonfermentable carbon sources |
| YIL057C | YIL057C | 50.37 | Hypothetical protein |
| YGR236C | SPG1 | 38.51 | Required for survival at high temperature during stationary phase; not required for growth on nonfermentable carbon sources |
| YKR097W | PCK1 | 36.18 | Phosphoenolpyruvate carboxykinase; gluconeogenesis; repressed by glucose; regulated by Mcm1p and Cat8p |
| YKL187C | YKL187C | 36.13 | Putative protein of unknown function; detectable in highly purified mitochondria |
| YGL205W | POX1 | 24.28 | Fatty-acyl coenzyme A oxidase, involved in the fatty acid beta-oxidation pathway; localized to the peroxisomal matrix |
| YDR536W | STL1 | 21.12 | Plasma membrane glycerol proton symporter; subject to glucose-induced inactivation; transiently induced by osmotic shock |
| YML054C | CYB2 | 19.79 | Cytochrome b2; mitochondrial intermembrane space; required for lactate utilization; repressed by glucose |
| YMR280C | CAT8 | 17.69 | Transcriptional activator; derepresses a variety of genes under non-fermentative growth conditions, active after diauxic shift |
| YGR043C | NQM1 | 16.42 | Putative protein of unknown function; transcription is repressed by Mot1p and induced during diauxic shift |
| YBR116C | YBR116C | 14.90 | Hypothetical protein |
| YHR160C | PEX18 | 14.68 | Part of a two-member peroxin family (Pex18p and Pex21p) |
| YMR174C | PAI3 | 13.67 | Cytoplasmic proteinase A inhibitor, dependent on Pbs2p and Hog1p protein kinases for osmotic induction |
| YMR118C | YMR118C | 13.42 | Protein of unknown function with similarity to succinate dehydrogenase cytochrome b subunit; nonessential gene |
| YNL195C | YNL195C | 13.34 | Hypothetical protein |
| YLR327C | TMA10 | 12.62 | Protein of unknown function that associates with ribosomes |
| YLR178C | TFS1 | 10.10 | Carboxypeptidase Y inhibitor; phosphatidylethanolamine-binding protein involved in protein kinase A signaling pathway |
| YDR380W | ARO10 | 9.62 | Phenylpyruvate decarboxylase (decarboxylation of phenylpyruvate to phenylacetaldehyde); first specific step of Ehrlich pathway |
| YJL217W | YJL217W | 9.59 | Cytoplasmic protein of unknown function; induced by copper sensing transcription factor Mac1p during copper deficiency |
| YEL020C | YEL020C | 9.37 | Hypothetical protein with low sequence identity to Pdc1p |
| YLR284C | ECI1 | 9.04 | Peroxisomal delta3,delta2-enoyl-CoA isomerase; essential for the beta-oxidation of unsaturated fatty acids, oleate-induced |
| YMR322C | SNO4 | 8.94 | Possible chaperone and cysteine protease; similar to Hsp31p, Hsp32p, and Hsp33p; possible role in pyridoxine metabolism |
| YGR201C | YGR201C | 8.83 | Putative protein of unknown function |
| YPL201C | YIG1 | 8.60 | Protein that interacts with glycerol 3-phosphatase and plays a role in anaerobic glycerol production |
| YOL152W | FRE7 | 8.60 | Putative ferric reductase with similarity to Fre2p; expression induced by low copper levels |
| YJR008W | YJR008W | 8.27 | Putative protein of unknown function; expression induced by mild heat-stress on a nonfermentable carbon source. |
| YGL156W | AMS1 | 8.05 | Vacuolar alpha mannosidase, involved in free oligosaccharide (fOS) degradation |
| YPL280W | HSP32 | 7.95 | Possible chaperone and cysteine protease; similar to Hsp31p, Hsp33p and Sno4p |
| YMR206W | YMR206W | 7.39 | Putative protein of unknown function; YMR206W is not an essential gene |
| YGR088W | CTT1 | 7.18 | Cytosolic catalase T, has a role in protection from oxidative damage by hydrogen peroxide |
| YGR243W | FMP43 | 7.02 | The authentic, nontagged protein was localized to mitochondria |
| YKL093W | MBR1 | 6.70 | Involved in mitochondrial function and stress response; overexpression suppresses hap2, hap3, and hap4 defects |
| YOL052C-A | DDR2 | 6.65 | Multistress response protein; activated by xenobiotic agents and environmental or physiological stresses |
| YPL054W | LEE1 | 6.54 | Zinc-finger protein of unknown function |
| YGR248W | SOL4 | 6.47 | 6-phosphogluconolactonase with similarity to Sol3p |
| YEL033W | YEL033W | 6.42 | Predicted to have metabolic role based on analysis of gene networks |
| YLR162W | YLR162W | 6.38 | Putative protein of unknown function; overexpression confers resistance to the antimicrobial peptide MiAMP1 |
| YMR175W | SIP18 | 6.33 | Protein of unknown function whose expression is induced by osmotic stress |
| YOR173W | DCS2 | 6.20 | Non-essential protein; regulated by Msn2p, Msn4p; accumulates under glucose limitation, similar to Dcs1p |
| YFL054C | YFL054C | 6.06 | Putative channel-like protein; similar to Fps1p; mediates passive diffusion of glycerol in the presence of ethanol |
| YOR285W | YOR285W | 5.98 | Protein of unknown function, localized to the mitochondrial outer membrane |
| YNR001C | CIT1 | 5.93 | Citrate synthase (condensation of acetyl coenzyme A and oxaloacetate to citrate); rate-limiting TCA cycle enzyme |
| YNR034W-A | YNR034W-A | 5.91 | Hypothetical protein |
| YMR110C | HFD1 | 5.78 | Putative fatty aldehyde dehydrogenase, located in the mitochondrial outer membrane and also in lipid particles |
| YPR002W | PDH1 | 5.55 | Mitochondrial protein that participates in respiration, induced by diauxic shift |
| YIL055C | YIL055C | 5.53 | Hypothetical protein |
| YPR151C | SUE1 | 5.45 | Mitochondrial protein required for degradation of unstable forms of cytochrome c |
| YNL305C | YNL305C | 5.38 | Hypothetical protein |
| YKL163W | PIR3 | 5.35 | Cell wall protein required for cell wall stability; expression is regulated by cell cycle and the cell integrity pathway |
| YML089C | YML089C | 5.34 | Hypothetical protein |
| YER037W | PHM8 | 5.27 | Protein of unknown function, expression is induced by low phosphate levels and by inactivation of Pho85p |
| YKL066W | YKL066W | 5.26 | Dubious open reading frame, unlikely to encode a protein; not conserved in closely related Saccharomyces species; |
| YLR149C | YLR149C | 5.20 | Putative protein of unknown function; YLR149C is not an essential gene |
| YMR271C | URA10 | 5.17 | One of two isozymes that catalyze the fifth enzymatic step in the de novo biosynthesis of pyrimidines |
| YIL087C | YIL087C | 5.15 | Hypothetical protein |
| YKL150W | MCR1 | 5.04 | Mitochondrial NADH-cytochrome b5 reductase, involved in ergosterol biosynthesis |
| YNL274C | YNL274C | 5.03 | Putative hydroxyisocaproate dehydrogenase |
| YER121W | YER121W | 5.01 | Hypothetical protein |
| YOR382W | FIT2 | 5.01 | Cell wall mannoprotein involved in the retention of siderophore-iron in the cell wall |
| YPL281C | ERR2 | 5.01 | Protein of unknown function, has similarity to enolases |
| YHR006W | STP2 | 1.90 | Transcription factor that activates transcription of amino acid permease genes |
| YDR423C | CAD1 | 1.80 | Transcriptional activator involved in stress responses, iron metabolism, drug resistance and protein stabilization |
| YML007W | YAP1 | 1.74 | Transcription factor required for oxidative stress tolerance; mediates pleiotropic drug and metal resistance |
| YOL089C | HAL9 | 1.74 | Putative transcription factor; salt tolerance through increased expression of the ENA1 (Na+/Li+ extrusion pump) gene |
| YDL020C | RPN4 | 1.73 | Transcription factor that stimulates proteasome gene expression; regulated by various stress responses |
| YHR124W | NDT80 | 1.69 | Meiosis-specific transcription factor required for full meiotic recombination; activates sporulation genes |
| YPR199C | ARR1 | 1.50 | Transcriptional activator required for transcription of genes involved in resistance to arsenic compounds |
| YNL103W | MET4 | 1.21 | Transcriptional activator responsible for the regulation of the sulfur amino acid pathway |
| YMR016C | SOK2 | −1.19 | Regulatory role in the cyclic AMP (cAMP)-dependent protein kinase (PKA) signal transduction pathway |
| YML076C | WAR1 | −1.22 | Transcription factor; induces transcription of PDR12 (acid transporter) and FUN34 (putative ammonia transporter) |
| YGL209W | MIG2 | −1.81 | Protein involved in repression, along with Mig1p, of SUC2 (invertase) expression by high levels of glucose |
| YHR144C | DCD1 | −5.00 | Deoxycytidine monophosphate (dCMP) deaminase required for dCTP and dTTP synthesis |
| YLR048W | RPS0B | −5.06 | Protein component of the small (40S) ribosomal subunit; required for maturation of 18S rRNA |
| YDR345C | HXT3 | −5.31 | Low affinity glucose transporter; expression is induced in low or high glucose conditions |
| YMR305C | SCW10 | −5.34 | Cell wall protein with similarity to glucanases; may play a role in conjugation during mating |
| YMR321C | YMR321C | −5.47 | Hypothetical protein |
| YPL079W | RPL21B | −5.48 | Protein component of the large (60S) ribosomal subunit, nearly identical to Rpl21Ap |
| YFL056C | AAD6 | −5.55 | Putative aryl-alcohol dehydrogenase; involved in the oxidative stress response |
| YLR061W | RPL22A | −6.02 | Protein component of the large (60S) ribosomal subunit, has similarity to Rpl22Bp |
| YML026C | RPS18B | −6.03 | Protein component of the small (40S) ribosomal subunit; nearly identical to Rps18Ap |
| YEL071W | DLD3 | −6.48 | D-lactate dehydrogenase; retrograde regulon (genes stimulated by damage to mitochondria…) |
| YAR073W | IMD1 | −6.60 | Nonfunctional protein with homology to IMP dehydrogenase; probable pseudogene, located close to the telomere |
| YFL057C | AAD16 | −6.74 | Putative aryl-alcohol dehydrogenase; mutational analysis has not yet revealed a physiological role |
| YML116W | ATR1 | −7.41 | Multidrug efflux pump; required for resistance to aminotriazole and 4-nitroquinoline-N-oxide |
| YMR318C | ADH6 | −7.67 | NADPH-dependent cinnamyl alcohol dehydrogenase; possible role in fusel alcohol synthesis or aldehyde tolerance |
| YLR460C | YLR460C | −8.32 | Hypothetical protein |
| YER055C | HIS1 | −8.99 | ATP phosphoribosyltransferase, a hexameric enzyme, catalyzes the first step in histidine biosynthesis |
| YDR399W | HPT1 | −9.53 | Dimeric hypoxanthine-guanine phosphoribosyltransferase, catalyzes the formation of both IMP and GMP |
| YMR177W | MMT1 | −10.16 | Putative metal transporter involved in mitochondrial iron accumulation; closely related to Mmt2p |
| YKL071W | YKL071W | −14.01 | Putative protein of unknown function; green fluorescent protein (GFP)-fusion protein localizes to the cytoplasm |
| YAR075W | YAR075W | −19.31 | Nonfunctional protein with homology IMP dehydrogenase |
| YGR138C | TPO2 | −19.36 | Polyamine transport protein specific for spermine; localizes to the plasma membrane; regulated by Haa1p |
aEach of these genes were significantly differentially expressed with a P-value of ≤10−4, determined using Rosetta Resolver (see Materials and Methods section).
Examining these genes, we noted the up-regulation of genes associated with mitochondrial function, gluconeogenesis, the TCA cycle, the β-oxidation of fatty acids, transport (including the uptake of amino acids), cell wall stability, copper and iron utilization (both required as prosthetic groups in the cytochromes in the electron transport chain) and glycerol and lactate utilization. Conversely, we observed down-regulation of genes associated with the accumulation of iron in the mitochondrion (required for heme and cytochrome biosyntheses), ribosomal subunit biosynthesis, and cellular growth—a response to glucose starvation.
The increased mitochondrial activity associated with oxidative phosphorylation, required for respiratory growth on nonfermentable carbon sources including ethanol and glycerol, summarized by Maris et al. (8), is accompanied by increased production of reactive oxygen species. In response, we found that numerous genes associated with the response to oxidative stress were up-regulated in our diauxic-shifted cells (Figure 3; Table 2), most notably CTA1 (catalase A, present in the peroxisomal and mitochondrial matrices; 40.2-fold up-regulated), HSP12 (plasma membrane heat-shock protein;18.4-fold), CTT1 (cytosolic catalase T; 7.2-fold), PRX1 (mitochondrial peroxiredoxin; 5.5-fold), MCR1 (mitochondrial NADH-cytochrome b5 reductase; 5.0-fold) and GPX1 (phospholipid hydroperoxide glutathione peroxidase; 4.6-fold). The superoxide dismutases encoded by SOD1 (cytosol; mitochondrial intermembrane space) and SOD2 (mitochondrial matrix) were modestly up-regulated (2.1 and 1.9-fold, respectively), indicating that the burden of the response to increased reactive oxygen species in this strain under these conditions is shared by the other antioxidant defense mechanisms (Cta1, Ctt1, etc.). Interestingly, many genes associated with the response to oxidative stress were down-regulated (Figure 3; Table 2), most notably GPX2 (cytoplasmic phospholipid hydroperoxide glutathione peroxidase; 5.7-fold), TRR1 (cytoplasmic thioredoxin reductase; 4.0-fold) and GSH1 (glutathione biosynthesis; 3.0-fold).
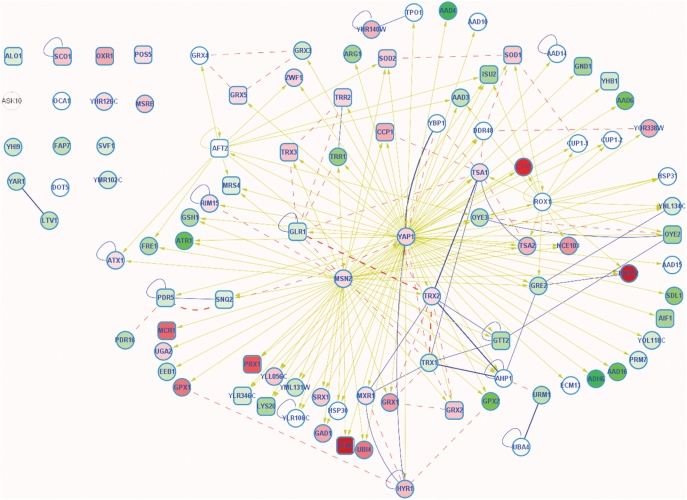
Figure 3.
A schematic displaying genes associated with the response to oxidative stress, in cells shifted to growth on glycerol. For clarity, interactions associated with transcription factor Msn4—that virtually mirror those from Msn2—were removed from this figure. For convenience, these genes are also summarized in Table.
Table 2.
Expression levels of genes associated with the response to oxidative stress in cells shifted to growth on glycerol
| Systematic name | Common name | Fold-changea | Saccharomyces Genome Database gene description (abbreviated) |
|---|---|---|---|
| YDR256C | CTA1 | 40.22 | Catalase A, breaks down hydrogen peroxide in the peroxisome formed during fatty acid beta-oxidation |
| YFL014W | HSP12 | 18.36 | Plasma membrane protein; induced by heat shock, oxidative stress, glucose depletion |
| YGR088W | CTT1 | 7.18 | Cytosolic catalase T, has a role in protection from oxidative damage by hydrogen peroxide |
| YBL064C | PRX1 | 5.47 | Mitochondrial peroxiredoxin; induced during respiratory growth and under conditions of oxidative stress |
| YKL150W | MCR1 | 5.04 | Mitochondrial NADH-cytochrome b5 reductase, involved in ergosterol biosynthesis |
| YKL026C | GPX1 | 4.57 | Phospholipid glutathione peroxidase; induced by glucose starvation; protection from oxidative stress |
| YLL039C | UBI4 | 4.50 | Ubiquitin; marks proteins for selective degradation; essential for the cellular stress response |
| YNL036W | NCE103 | 3.62 | Carbonic anhydrase; poorly transcribed under aerobic conditions |
| YCL035C | GRX1 | 3.38 | Hydroperoxide and superoxide-radical responsive oxidoreductase; protection from oxidative damage |
| YMR250W | GAD1 | 3.11 | Glutamate decarboxylase (glutamate to gamma-aminobutyric acid); response to oxidative stress |
| YPL196W | OXR1 | 2.97 | Protein of unknown function required for resistance to oxidative damage |
| YOR338W | YOR338W | 2.94 | Hypothetical protein |
| YKR066C | CCP1 | 2.89 | Mitochondrial cytochrome-c peroxidase; degrades reactive oxygen species; response to oxidative stress |
| YHR140W | YHR140W | 2.71 | Putative integral membrane protein of unknown function |
| YDR453C | TSA2 | 2.61 | Inducible cytoplasmic thioredoxin peroxidase; removal of reactive oxygen, nitrogen and sulfur species |
| YIR037W | HYR1 | 2.43 | Thiol peroxidase; senses intracellular hydroperoxide levels, transduces a redox signal to Yap1p |
| YCR083W | TRX3 | 2.24 | Mitochondrial thioredoxin; maintains cellular redox homeostasis with Trr2p and Glr1p |
| YBR037C | SCO1 | 2.20 | Mitochondrial inner membrane copper-binding protein required for cyt c oxidase activity and respiration |
| YCL033C | MSRB | 2.19 | Putative protein-methionine-R-oxide reductase; involved in response to oxidative stress |
| YDR513W | GRX2 | 2.18 | Cytoplasmic glutaredoxin; involved in maintaining redox state of target proteins; induced by stress |
| YLL056C | YLL056C | 2.11 | Putative protein of unknown function; activated along with genes involved in pleiotropic drug resistance |
| YJR104C | SOD1 | 2.06 | Copper, Zinc-containing superoxide dismutase |
| YKL086W | SRX1 | 2.02 | Sulfiredoxin, contributes to oxidative stress resistance by reducing peroxiredoxins Tsa1p and Ahp1p |
| YBR006W | UGA2 | 1.95 | Succinate semialdehyde dehydrogenase; utilization of gamma-aminobutyrate as a nitrogen source |
| YHR008C | SOD2 | 1.93 | Manganese-containing superoxide dismutase; protects cells against oxygen toxicity |
| YNL241C | ZWF1 | 1.75 | Glucose-6-phosphate dehydrogenase (pentose phosphate pathway); adaption to oxidative stress |
| YML007W | YAP1 | 1.74 | Transcription factor required for oxidative stress tolerance; mediates pleiotropic drug, metal resistance |
| YHR106W | TRR2 | 1.63 | Mitochondrial thioredoxin reductase; oxidative stress protection; with Glr1p maintains Trx3p redox status |
| YHR126C | YHR126C | 1.47 | Hypothetical protein |
| YML028W | TSA1 | 1.46 | Ubiquitous housekeeping thioredoxin peroxidase, reduces reactive oxygen, nitrogen and sulfur species |
| YMR037C | MSN2 | 1.46 | Transcriptional activator related to Msn4p; activated in stress conditions |
| YPL059W | GRX5 | 1.42 | Mitochondrial hydroperoxide, superoxide-radical responsive oxidoreductase; iron-sulfur center synthesis |
| YGR209C | TRX2 | 1.39 | Cytoplasmic thioredoxin isoenzyme; protects cells against both oxidative and reductive stress |
| YNL259C | ATX1 | 1.35 | Cytosolic copper metallochaperone; copper eventually inserted into Fet3p (high-affinity iron uptake) |
| YPL188W | POS5 | 1.34 | Mitochondrial NAD(H) kinase; required for the response to oxidative stress |
| YER042W | MXR1 | 1.33 | Reverses oxidation of methionine residues; involved in repair and resistance to oxidative stress |
| YFL033C | RIM15 | 1.28 | Glucose-repressible protein kinase; signal transduction during cell proliferation in response to nutrients |
| YBL043W | ECM13 | 1.21 | Nonessential protein of unknown function |
| YER174C | GRX4 | 1.19 | Hydroperoxide, superoxide-radical responsive oxidoreductase; protection from oxidative damage |
| YBR216C | YBP1 | 1.17 | Oxidation of transcription factor Yap1p, resulting in nuclear localization of Yap1p in response to stress |
| YNL331C | AAD14 | 1.15 | Putative aryl-alcohol dehydrogenase; mutational analysis has not yet revealed a physiological role |
| YDR533C | HSP31 | 1.12 | Possible chaperone and cysteine protease with similarity to Hsp32p, Hsp33p, and Sno4p |
| YPR065W | ROX1 | 1.11 | Heme-dependent repressor of hypoxic genes |
| YGR097W | ASK10 | 1.11 | Component of the RNA polymerase II holoenzyme, phosphorylated in response to oxidative stress |
| YLR108C | YLR108C | 1.03 | Protein of unknown function; green fluorescent-fusion protein localizes to the nucleus; non-essential |
| YNL099C | OCA1 | 1.03 | Putative protein tyrosine phosphatase; induces cell cycle arrest in response to oxidative DNA damage |
| YLL028W | TPO1 | −1.04 | Polyamine transporter; catalyzes uptake of polyamines at alkaline pH and excretion at acidic pH |
| YIL010W | DOT5 | −1.08 | Nuclear thiol peroxidase; functions as an alkyl-hydroperoxide reductase during post-diauxic growth |
| YCR021C | HSP30 | −1.12 | Plasma membrane stress response protein; negatively regulates H(+)-ATPase Pma1p |
| YOL165C | AAD15 | −1.15 | Putative aryl-alcohol dehydrogenase; mutational analysis has not yet revealed a physiological role |
| YHR111W | UBA4 | −1.16 | Urmylates thioredoxin peroxidase Ahp1p, suggesting a role of urmylation in oxidative stress response |
| YPL202C | AFT2 | −1.16 | Iron-regulated transcriptional activator, required for iron homeostasis and resistance to oxidative stress |
| YLR109W | AHP1 | −1.21 | Thiol-specific peroxiredoxin, reduces hydroperoxides to protect against oxidative damage |
| YJR155W | AAD10 | −1.22 | Putative aryl-alcohol dehydrogenase; mutational analysis has not yet revealed a physiological role |
| YHR053C | CUP1-1 | −1.23 | Metallothionein, binds copper and mediates resistance to high concentrations of copper and cadmium |
| YHR055C | CUP1-2 | −1.26 | Metallothionein, binds copper and mediates resistance to high concentrations of copper and cadmium |
| YMR173W | DDR48 | −1.26 | DNA damage-responsive protein, induced in response to heat-shock stress or DNA lesions |
| YDR011W | SNQ2 | −1.29 | ABC transporter protein involved in multidrug resistance and resistance to singlet oxygen species |
| YKR052C | MRS4 | −1.31 | Mitochondrial iron transporter; functions under low-iron conditions; may transport other cations |
| YDL039C | PRM7 | −1.38 | Pheromone-regulated protein, predicted to have a transmembrane segment; regulated by Gcn4p |
| YLR043C | TRX1 | −1.40 | Cytoplasmic thioredoxin isoenzyme; protects cells against both oxidative and reductive stress |
| YML086C | ALO1 | −1.44 | Catalyzes the final step of erythroascorbic acid biosynthesis, protective against oxidative stress |
| YNL134C | YNL134C | −1.48 | Uncharacterized ORF; alcohol dehydrogenase (NADP+) activity; biological process unknown |
| YMR102C | YMR102C | −1.51 | Protein of unknown function; activated along with genes involved in multidrug resistance |
| YDR346C | SVF1 | −1.53 | Protein with a potential role in cell survival pathways, required for the diauxic growth shift |
| YPL091W | GLR1 | −1.58 | Cytosolic and mitochondrial glutathione oxidoreductase; reduces oxidized glutathione |
| YLR346C | YLR346C | −1.61 | Putative mitochondrial protein of unknown function; regulated by drug resistance transcription factors |
| YOL118C | YOL118C | −1.64 | Hypothetical protein |
| YGR234W | YHB1 | −1.75 | Nitric oxide oxidoreductase; flavohemoglobin; role in the oxidative and nitrosative stress responses |
| YOR153W | PDR5 | −1.82 | Membrane ATP-binding cassette transporter; involved in transport and cellular detoxification |
| YDR098C | GRX3 | −1.83 | Hydroperoxide and superoxide-radical responsive oxidoreductase; protection from oxidative damage |
| YHR029C | YHI9 | −1.95 | Protein of unknown function possibly involved in a membrane regulation metabolic pathway |
| YPL239W | YAR1 | −2.09 | Cytoplasmic protein; proposed to link 40S ribosomal subunit biogenesis to adaption to oxidative stress |
| YML131W | YML131W | −2.13 | Putative protein of unknown function; increased after osmotic shock; non-essential gene |
| YOL151W | GRE2 | −2.15 | NADPH-dependent methylglyoxal reductase; stress induced (osmotic, ionic, oxidative, heat, metals) |
| YPL095C | EEB1 | −2.17 | Acyltransferase; major part of short-chain fatty acid ethyl ester production during fermentation |
| YPL171C | OYE3 | −2.38 | Widely conserved NADPH oxidoreductase; may be involved in sterol metabolism |
| YKL143W | LTV1 | −2.39 | Protein required for growth at low temperature |
| YCR107W | AAD3 | −2.40 | Putative aryl-alcohol dehydrogenase; mutational analysis has not yet revealed a physiological role |
| YDL166C | FAP7 | −2.51 | Essential NTPase required for small ribosome subunit synthesis |
| YIL008W | URM1 | −2.57 | Ubiquitin-like protein; molecular function of Urm1p pathway is unknown; required for normal growth |
| YNL231C | PDR16 | −2.71 | Phosphatidylinositol transfer protein; controls levels of various lipids, may regulate lipid synthesis |
| YLL060C | GTT2 | −2.75 | Glutathione S-transferase capable of homodimerization; functional overlap with Gtt2p, Grx1p, Grx2p |
| YJL101C | GSH1 | −2.95 | Catalyzes the first step of glutathione biosynthesis; induced by oxidants, cadmium, and mercury |
| YLR214W | FRE1 | −2.98 | Ferric and cupric reductase; reduces iron and copper prior to uptake; induced by low copper, iron levels |
| YDL182W | LYS20 | −3.20 | Homocitrate synthase isozyme; catalyzes first step in the lysine biosynthesis pathway |
| YOR226C | ISU2 | −3.29 | Conserved mitochondrial matrix protein; synthesis of mitochondrial and cytosolic iron-sulfur proteins |
| YHR179W | OYE2 | −3.39 | Widely conserved NADPH oxidoreductase; may be involved in sterol metabolism |
| YNR074C | AIF1 | −3.42 | Mitochondrial cell death effector that translocates to the nucleus in response to apoptotic stimuli |
| YOL058W | ARG1 | −3.44 | Catalyzes the formation of L-argininosuccinate in the arginine biosynthesis pathway |
| YHR183W | GND1 | −3.47 | Regenerates NADPH in the pentose phosphate pathway; required for adaptation to oxidative stress |
| YDR353W | TRR1 | −4.05 | Cytoplasmic thioredoxin reductase; protects cells against both oxidative and reductive stress |
| YIL167W | SDL1 | −4.47 | Open reading frame, unlikely to produce a functional protein in S288C |
| YFL056C | AAD6 | −5.55 | Putative aryl-alcohol dehydrogenase; involved in the oxidative stress response |
| YBR244W | GPX2 | −5.68 | Glutathione peroxidase; protects cells from hydroperoxides and peroxides during oxidative stress |
| YFL057C | AAD16 | −6.74 | Putative aryl-alcohol dehydrogenase; mutational analysis has not yet revealed a physiological role |
| YML116W | ATR1 | −7.41 | Multidrug efflux pump; required for resistance to aminotriazole, 4-nitroquinoline-N-oxide |
| YMR318C | ADH6 | −7.67 | NADPH-dependent alcohol dehydrogenase; possibly involved in fusel alcohol synthesis or aldehyde tolerance |
| YDL243C | AAD4 | −7.91 | Putative aryl-alcohol dehydrogenase; involved in the oxidative stress response |
aEach of these genes were significantly differentially expressed with a P-value of ≤10−4, determined using Rosetta Resolver (see Materials and Methods section).
The numbers of genes differentially expressed in these cells, the modest changes in expression of SOD1 and SOD2, and the down-regulation of oxidative stress-related genes GPX2, TRR1 and GSH1 suggests that the regulation of gene expression in these cells is rather complex. Examining our yeast interactome, it is readily apparent that the regulation of gene expression in yeast is extraordinarily complex, with most genes simultaneously regulated by two or more transcription factors, as indicated in Figures 1–3. Additionally, the 168 transcription factors downloaded from YEASTRACT interact among one another in an extraordinarily complex way while directly regulating the expression of at least 5902 target genes (our yeast interactome: data not shown).
DISCUSSION
Our laboratory has a long-standing interest in exploring mitochondrial function and maintenance, including the stability and replication of the mitochondrial genome, and mutations and naturally occurring nucleotide polymorphisms associated with mitochondrial disease (13–15). Among the tools that we employ for these studies is the model organism, S. cerevisiae (2,3,13,16).
Energy demands in the facultative anaerobe S. cerevisiae are met under different physiological states when cells are grown on fermentable carbon sources such as glucose versus non-fermentable carbon sources such as ethanol or glycerol (8,17,18). Fermentation and glycolysis supply the cell with energy through the breakdown of glucose and other simple fermentable sugars; however, when the glucose concentration drops below ∼0.2%, the cells stop growing for a few hours as they undergo the diauxic shift, accompanied by transcriptional and translational changes including the mitochondrial biosynthesis. The cells then resume slower growth for a few generations by oxidative phosphorylation using the ethanol, glycerol and other byproducts accumulated during the fermentive stage of growth, before entering the stationary phase of growth.
Oxidative phosphorylation, which is dependent on mitochondrial activity and oxygen metabolism, provides the most efficient means of energy production (19,20). However, a deleterious consequence of oxidative phosphorylation is the production of reactive oxygen species in the mitochondrion—including the superoxide anion, hydrogen peroxide (H2O2) and the hydroxyl radical—that must be detoxified to minimize damage to nucleic acids, proteins, carbohydrates and lipids (21). The superoxide anion radical is reduced to H2O2 by superoxide dismutases (SOD), and further reduced to water by the antioxidant glutathione (GSH) and the enzymatic activity of catalases and peroxidases (21,22).
Cells switched to growth dependent on mitochondrial activity—the utilization of glycerol via oxidative phosphorylation—experience elevated levels of reactive oxygen species, evidenced by the increased nuclear mutational rates in cells grown in YPG versus YPD (2), a 28-fold increase in oxidative damage to mitochondrial proteins (2), and the changes in gene expression observed in this study, facilitated using Cytoscape and our yeast interactome. The genes that were most significantly affected by the switch from growth on glucose to growth on glycerol were identified using our interactome and the Cytoscape jActiveModules plugin (Figure 2; Table 1). As shown in Figure 2, transcription factors directly associated with the regulation of these genes include the stress response transcription factors Yap1 (required for oxidative stress tolerance), Rpn4 (stimulates expression of proteasome genes), Cad1 (multiple stress responses), Arr1 (resistance to arsenic compounds), Cat8 (derepression of genes following the diauxic shift), Sok2 (signal transduction), Stp2 (external amino acid permease) and Met4 (sulfur amino acid pathway). The involvement of several of these transcription factors in regulating the cellular response to the diauxic shift (e.g. Yap1) is not surprising given the increases in oxidative phosphorylation and reactive oxygen species as a consequence of increased mitochondrial function. Transcription factor Arr1 (Yap8), normally associated with the transcription of genes involved in resistance to arsenic compounds, appeared to directly coregulate the expression of 39 of the 95 genes shown in Figure 2, including up-regulated genes associated with the β-oxidation of fatty acids, carbohydrate metabolism and the TCA cycle, and the response to diauxic shift, suggesting a substantial role for this transcription factor in diauxic-shifted cells.
The complex regulatory nature of gene expression in S. cerevisiae is readily apparent from the interaction data displayed in Figures 1–3. It appears that transcription factors in S. cerevisiae form a highly interconnected self-regulatory subnetwork, while additionally regulating at least 5734 additional genes (our interactome: data not shown), indicating that substantial redundancy exists among the regulation and utilization of metabolic pathways. These cells may thus be able to respond quickly to changes in their external (e.g. adverse growth conditions) or internal (e.g. nonlethal mutations) environment by adjusting the regulation of their cellular metabolism via modest changes in gene expression involving hundreds or thousands of genes.
The transcription factors present in our yeast interactome appear to regulate most, but not all of the genes present in this interactome. Subtracting the 168 transcription factors and their regulated genes from the yeast interactome reveals that 286 of the 6188 genes present in the interactome (4.6%) are currently not associated with any transcription factor (data not shown). These genes, ranging from 3.1-fold up-regulated (TAM41) to 6.2-fold down-regulated (PAM18), passed through the SGD GO Slim Mapper, are variously associated with unknown biological processes (58 of 286 genes; 20.3%), transport (53/286; 18.5%), transcription (16/286; 5.6%), the cell cycle (11/286 genes; 3.8%), signal transduction (10/286; 3.5%) and amino acid metabolism (3/286 genes; 1.0%). Sixty-four of these genes (64/286; 22.4%) are annotated by SGD as being associated with the mitochondrion.
There is increasing interest regarding the application of bioinformatics and systems biology to the study of organisms and their regulatory mechanisms and metabolic profiles (23–28). The data provided in this study suggest that most genes are regulated in a highly complex manner by more than one transcription factor, and that bioinformatic tools such as Cytoscape—in conjunction with a robust interactome—may provide a useful framework for additional avenues of investigation. For example, by noting the transcription factors associated with specific groups of genes that are differentially expressed, the effect of deleting these transcription factors may be determined, at least partly. Finally, by applying methods similar to those used in the construction of the interactome described in this article, additional types of interaction data—for example those associated with protein kinases and their targets—can be readily incorporated.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Institutes of Health, National Institute of Environmental Health Sciences (Z01-ES065078 to W.C.C.); US Army Research Office (W991NF-04-D-0001 and DAAG55-98-D-0002 to G.R.S.); National Research Council Research Associateship Award. Funding for open access charge: Intramural Research Program, National Institutes of Health, National Institute of Environmental Health Sciences.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Astrid Haugen (NIEHS) and Leroy Worth (NIEHS) for critical reading of the article, and Astrid Haugen for discussions regarding the use of Cytoscape.
REFERENCES
- 1.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strand MK, Stuart GR, Longley MJ, Graziewicz MA, Dominick OC, Copeland WC. POS5 gene of Saccharomyces cerevisiae encodes a mitochondrial NADH kinase required for stability of mitochondrial DNA. Eukaryot. Cell. 2003;2:809–820. doi: 10.1128/EC.2.4.809-820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stuart GA, Humble MM, Strand MK, Copeland WC. Transcriptional response to mitochondrial NADH kinase deficiency in Saccharomyces cerevisiae. Mitochondrion. 2009 doi: 10.1016/j.mito.2009.02.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spencer F, Hugerat Y, Simchen G, Hurko O, Connelly C, Hieter P. Yeast kar1 mutants provide an effective method for YAC transfer to new hosts. Genomics. 1994;22:118–126. doi: 10.1006/geno.1994.1352. [DOI] [PubMed] [Google Scholar]
- 5.Teixeira MC, Monteiro P, Jain P, Tenreiro S, Fernandes AR, Mira NP, Alenquer M, Freitas AT, Oliveira AL, Sá-Correia I. The YEASTRACT database: a tool for the analysis of transcription regulatory associations in Saccharomyces cerevisiae. Nucleic Acids Res. 2006;34:D446–D451. doi: 10.1093/nar/gkj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonander N, Ferndahl C, Mostad P, Wilks MDB, Chang C, Showe L, Gustafsson L, Larsson C, Bill RM. Transcriptome analysis of a respiratory Saccharomyces cerevisiae strain suggests the expression of its phenotype is glucose insensitive and predominantly controlled by Hap4, Cat8 and Mig1. BMC Genomics. 2008;9:365. doi: 10.1186/1471-2164-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeRisi JL, Iyer VR, Brown PO. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997;278:680–686. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- 8.Maris AF, Assumpção ALK, Bonatto D, Brendel M, Henriques JAP. Diauxic shift-induced stress resistance against hydroperoxides in Saccharomyces cerevisiae is not an adaptive stress response and does not depend on functional mitochondria. Curr. Genet. 2001;39:137–149. doi: 10.1007/s002940100194. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn KM, DeRisi JL, Brown PO, Sarnow P. Global and specific translational regulation in the genomic response of Saccharomyces cerevisiae to a rapid transfer from a fermentable to a nonfermentable carbon source. Mol. Cell Biol. 2001;21:916–927. doi: 10.1128/MCB.21.3.916-927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young ET, Dombek KM, Tachibana C, Ideker T. Multiple pathways are co-regulated by the protein kinase Snf1 and the transcription factors Adr1 and Cat8. J. Biol. Chem. 2003;278:26146–26158. doi: 10.1074/jbc.M301981200. [DOI] [PubMed] [Google Scholar]
- 11.Ohlmeier S, Kastaniotis AJ, Hiltunen JK, Bergmann U. The yeast mitochondrial proteome, a study of fermentative and respiratory growth. J. Biol. Chem. 2004;279:3956–3979. doi: 10.1074/jbc.M310160200. [DOI] [PubMed] [Google Scholar]
- 12.Xie X, Wilkinson HH, Correa A, Lewis ZA, Bell-Pedersen D, Ebbole DJ. Transcriptional response to glucose starvation and functional analysis of a glucose transporter of Neurospora crassa. Fungal Genet. Biol. 2004;41:1104–1119. doi: 10.1016/j.fgb.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Stuart GR, Santos JH, Strand MK, Van Houten B, Copeland WC. Mitochondrial and nuclear DNA defects in Saccharomyces cerevisiae with mutations in DNA polymerase γ associated with progressive external ophthalmoplegia. Hum. Mol. Genet. 2006;15:363–374. doi: 10.1093/hmg/ddi454. [DOI] [PubMed] [Google Scholar]
- 14.Graziewicz MA, Longley MJ, Copeland WC. DNA polymerase γ in mitochondrial DNA replication and repair. Chem. Rev. 2006;106:383–405. doi: 10.1021/cr040463d. [DOI] [PubMed] [Google Scholar]
- 15.Chan SSL, Copeland WC. DNA polymerase gamma and mitochondrial disease: Understanding the consequence of POLG mutations. Biochimica et Biophysica Acta. 2008 doi: 10.1016/j.bbabio.2008.10.007. In press [29 October, 2008, Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strand MK, Copeland WC. Measuring mtDNA mutation rates in Saccharomyces cerevisiae using the mtArg8 assay. Methods Mol. Biol. 197:151–157. doi: 10.1385/1-59259-284-8:151. [DOI] [PubMed] [Google Scholar]
- 17.Lagunas R. Energy metabolism of Saccharomyces cerevisiae discrepancy between ATP balance and known metabolic functions. Biochim. Biophys. Acta. 1976;440:661–674. doi: 10.1016/0005-2728(76)90049-9. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld E, Beauvoit B. Role of the non-respiratory pathways in the utilization of molecular oxygen by Saccharomyces cerevisiae. Yeast. 2003;20:1115–1144. doi: 10.1002/yea.1026. [DOI] [PubMed] [Google Scholar]
- 19.Lin S-J, Kaeberlein M, Andalis AA, Sturtz LA, Defossez P-A, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 20.Johnston M, Kim J-H. Glucose as a hormone: receptor-mediated glucose sensing in the yeast Saccharomyces cerevisiae. Biochem. Soc. Trans. 2005;33:247–252. doi: 10.1042/BST0330247. [DOI] [PubMed] [Google Scholar]
- 21.Jackson MJ, Papa S, Bolaños J, Bruckdorfer R, Carlsen H, Elliott RM, Flier J, Griffiths HR, Heales S, Holst B, et al. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol. Aspects Med. 2002;23:209–285. doi: 10.1016/s0098-2997(02)00018-3. [DOI] [PubMed] [Google Scholar]
- 22.Grant CM, Perrone G, Dawes IW. Glutathione and catalase provide overlapping defenses for protection against hydrogen peroxide in the yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 1998;253:893–898. doi: 10.1006/bbrc.1998.9864. [DOI] [PubMed] [Google Scholar]
- 23.Futcher B. Transcriptional regulatory networks and the yeast cell cycle. Curr. Opin. Cell Biol. 2002;14:676–683. doi: 10.1016/s0955-0674(02)00391-5. [DOI] [PubMed] [Google Scholar]
- 24.Lee TI, Rinaldi NJ, Robert F, Odom DT, Bar-Joseph Z, Gerber GK, Hannett NM, Harbison CT, Thompson CM, Simon I, et al. Transcriptional regulatory networks in Saccharomyces cerevisiae. Science. 2002;298:799–804. doi: 10.1126/science.1075090. [DOI] [PubMed] [Google Scholar]
- 25.Begley TJ, Samson LD. Network responses to DNA damaging agents. DNA Repair. 2004;3:1123–1132. doi: 10.1016/j.dnarep.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Tong AHY, Lesage G, Bader GD, Ding H, Xu H, Xin X, Young J, Berriz GF, Brost RL, Chang M, et al. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–813. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- 27.Wu W-S, Li W-H, Chen B-S. Computational reconstruction of transcriptional regulatory modules of the yeast cell cycle. BMC Bioinformatics. 2006;7:421. doi: 10.1186/1471-2105-7-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boone C, Bussey H, Andrews BJ. Exploring genetic interactions and networks with yeast. Nat. Rev. Genet. 2007;8:437–449. doi: 10.1038/nrg2085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.