Abstract
The field of biomaterial design has begun to focus upon methods by which materials can modulate immune response. While certain approaches appear promising, they are limited to isolated facets of inflammation. It is well documented that both bacteria and viruses have highly developed methods for evading the immune system, providing impetus for a more biomimetic approach to material design. This review presents the immune evasive tactics employed by viruses and bacteria and offers suggestions for future directions in applying these principles to biomaterial design.
Keywords: inflammation, wound healing, immunomodulation, bacteria, biomimetic material
1. Introduction
1.1. Innate and Adaptive Immunity
A major clinical concern with medical implants is the threat of exacerbated inflammation in the surrounding tissue. Whether acute or chronic in nature, such inflammation compromises both patient well being as well as the effective life of the implant [1]. These inflammatory events are coordinated by the immune system, whose role it is to recognize self from non-self and dispose of non-self foreign bodies in a controlled and multi-faceted manner [2]. Once the body begins to interrogate the foreign object, the immune system will seek to isolate the object through a series of layered defenses. An excellent review by James Anderson provides more in depth assessment of the immune response to biomaterials [1].
Briefly, immune defenses are classified into innate immunity and adaptive immunity. Innate immunity is considered the first line of defense from a foreign object as it reacts to the object in a non-specific manner that is comprised of four distinctive types of non-specific barriers [2].
Anatomic Barriers: Skin and mucous membranes provide a mechanical barrier to the influx of microbes and pathogens, as well as environmental barriers that can inhibit growth of pathogens.
Physiologic Barriers: Physiologic conditions like the average body temperature or low pH in the digestive tract kill most foreign microorganisms.
Phagocytic Barriers: Specialized cells such as neutrophils, macrophages and dendritic cells engulf whole cells and foreign macromolecules.
Inflammatory Barriers: Tissue damage induces leakage of exudate from engorged vasculature into the damaged region, leading to the directed migration of inflammatory cells to promote phagocytosis of foreign material.
If a foreign object or pathogen cannot be neutralized by the innate immune system, the adaptive immune system will be triggered to combat the object. As the name implies, the adaptive immune system reacts to pathogens in a more specific manner than the generic response of the innate immune system. The major process governing adaptive immunity is the interaction between lymphocytes and antigen-presenting cells. Lymphocytes are generated in bone marrow during hematopoiesis, and circulate both the blood and lymphatic systems. Two major classes of lymphocytes involved in adaptive immunity are B cells and T cells.
B lymphocytes express membrane-bound antigen-binding motifs that are unique to a specific antigen. When a naïve B lymphocyte encounters an antigen that matches its surface-bound antibody, the lymphocyte will divide rapidly into either memory B lymphocytes or effector B lymphocytes. Memory B lymphocytes have a longer lifespan than naïve B lymphocytes and possess the same surface-bound antibody to bind to an antigen with high specificity. Effector B lymphocytes produce the antibody in a form that may be secreted. This antibody can then bind to an antigen, facilitating its clearance from the body.
Similar to B lymphocytes, T lymphocytes arise in bone marrow; however they migrate to the thymus gland to mature. Mature T lymphocytes express a unique, surface-bound antigenic binding protein called a T-cell receptor. The role of a T lymphocyte will vary based upon its T-cell receptor. Those T lymphocytes expressing CD4 receptors generally function as T helper cells (CD+4) and interact with anti-presenting cells, while those expressing CD8 receptors are T cytotoxic cells (CD+8) and interact with all cell types. Unlike B cells, T lymphocytes cannot recognize free antigen. Instead, T-cell receptors can only recognize antigen that is bound to cell membrane proteins called major histocompatibility complex (MHC) molecules. CD4-bearing T-lymphocytes interact with class II MHC molecules, which are expressed only by antigen-presenting cells. Antigen-presenting cells, such as macrophages, dendritic cells, and B cells, will present processed antigen in the form of antigenic peptides and, once bound to a T-cell receptor, will provide a costimulatory signal to activate the T cell. Once a naïve T lymphocyte encounters an antigen/MHC complex on a cell surface, the T cell will proliferate and its progeny will differentiate into memory T cells and effector T cells. Activated T-helper cells will secrete factors that coordinate activation of B cells, macrophages and other cells involved in inflammation. T-cytotoxic cells will differentiate into cytotoxic T lymphocytes that will exhibit cytotoxic activity [2].
The process by which naïve lymphocytes will rapidly multiply once bound to a specific antigen is called clonal selection. An effect of clonal selection is immunologic memory since many daughter lymphocytes, such as plasma and memory cells, have a longer life span than their naïve parents. With more daughter lymphocytes in the body, a later contact of a lymphocyte with an antigen will produce a more rapid and heightened secondary response relative to the primary response of the naïve lymphocytes. This specificity thus provides for stronger and quicker responses each time lymphocytes are exposed to those pathogens [2].
Innate and adaptive immunity however do not operate independently of one another. Innate immune cells, like macrophages, release signaling molecules that can stimulate initiation of the adaptive immune response. There is also feedback from the adaptive to the innate immune system in the way of cytokine secretion that may activate differentiation of monocytes into macrophages for an increased phagocytic presence at the site of inflammation [2].
1.2. Overview of Cyotkines
The inflammatory and phagocytic barriers of the innate and adaptive immune systems are coordinated by the secretion of cytokines, which are “molecular traffic lights,” that regulate the pace and extent of the immune reaction [3]. Cytokines are short-lived, small (10–30kDa) glycoproteins produced de novo in response to an immune stimulus to mediate and regulate immunity, inflammation, cell growth and differentiation, and hematopoiesis. They are secreted predominantly by lymphocytes, monocytes and macrophages, but act on a broad array of cells by binding to specific membrane receptors. Binding to receptors on cell membranes triggers second messenger complexes that carry the signal to the nucleus to alter gene expression. Cellular responses to cytokine binding include up- or down-regulation of membrane protein expression, cell growth/proliferation/differentiation, and secretion of effector molecules. Cytokines as a class are potent (work at femto-nanomolar concentrations), redundant (multiple cytokines elicit same response), pleiotropic (single cytokine elicits multiple responses), and act locally between neighboring cells. These factors can also act synergistically (to amplify) or antagonistically (to attenuate) a biological response. A more definitive reference on cytokines has been published by Thomson and Lotze [4].
Briefly, several cytokines are characterized by their predominantly pro-inflammatory or anti-inflammatory functions [5, 6]. IL-1 and TNF-α are the prototypical “alarm” cytokines because of their ubiquity and early and intense response to the invading agents such as bacterium or toxin. TNF- α and IL-1 can work separately or synergistically to drive biological activities in a wide number of cell types (e.g., B and T lymphocytes, monocytes, macrophages, fibroblasts, endothelial cells, smooth muscle cells, astrocytes, microglia), including induction of cell mediator expression (e.g., cytokines, chemokines, immunoglobulins, lytic enzymes, prostaglandins, antigen presentation) that chemoattract leukocytes and monocytes, promote B and T cell proliferation, increase vascular permeability and angiogenesis, promote thrombus formation, increase cell adhesion, and induce cell lysis. IL-10 and the isoforms of TGF-β on the other hand are commonly identified as anti-inflammatory and/or immuno-suppressive cytokines. IL-10 inhibits the synthesis of IL-1, IL-2, IL-6, IL-12, IFN-γ, TNF-α and immunoglobulins, deactivates macrophages, and down regulates cellular immunity and the presentation of class II major histocompatibility complex antigens. The isoform TGF-β inhibits the growth and proliferation of a number of cell types (e.g., endothelial cells, fibroblasts, neuronal cells, epithelial cells, smooth muscle cells, chrondrocytes), deactivates macrophages, antagonizes growth factors (e.g., EGF, PDGF, α/βFGF), down regulates immunoglobulin synthesis, and inhibits proliferation of lymphocytes.
1.3. Overview of Macrophages
When a biomaterial is implanted in the body, the inflammatory response is initiated by injury of the vascularized tissue proximal to the site of implantation. This injury allows fluid, proteins and cells, such as neutrophils and monocytes, from the vasculature to escape to the site of implantation through a process called exudation. The surrounding chemical composition of the exudate will induce differentiation of blood borne monocytes into macrophages of different phenotypes that may attempt to phagocytose the foreign body as well as release chemokines, reactive oxygen species, growth factors, and cytokines [1]. The released factors in turn recruit a number of cell types such as monocytes, macrophages, fibroblasts and epithelial cells to the site of injury [1].
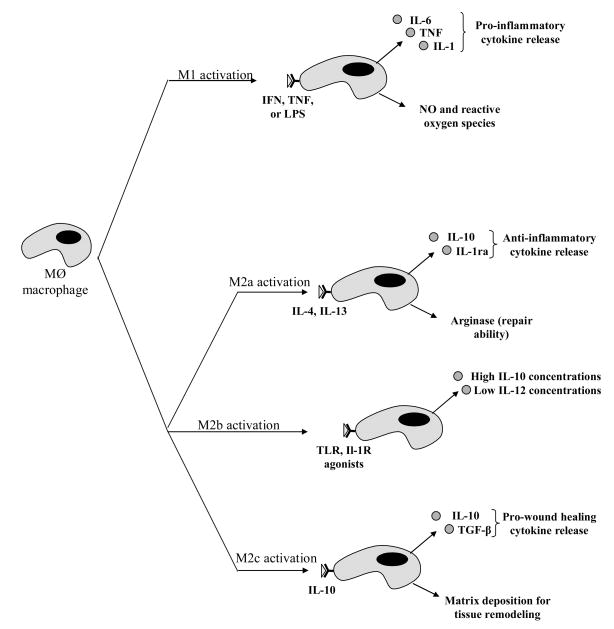
The classic phagocytic activated macrophage, the M1 phenotype is elicited by cytokines including interferon-gamma (IFN-γ) and tumor necrosis factor (TNF) as well as by Gram-negative bacterial cell wall lipopolysaccharides (LPS) (Figure 1). M1 macrophages generate large amounts of reactive oxygen species and inflammatory cytokines, which all serve to augment killing of phagocytosed intracellular parasites and local cell-mediated immune response (the Th1 response). While M1 macrophages are critical for the early response to injury and infection, prolonged or aberrant activation of M1 macrophages can also result in undesired tissue destruction and chronic inflammation [7].
Figure 1.
Diagram of differentiation of macrophage phenotypes. M1 activation represents classical activation and production of pro-inflammatory cytokines. M2 activation may take on three different forms. All three share the properties of being either immunosuppressive or immunoregulatory in nature. Figure adapted from [8].
The M2 macrophage phenotype describes macrophages in a broad category that encompasses macrophages activated in a manner different than the classic M1 macrophages, and includes macrophages activated by IL-4, IL-13, IL-10, immune complexes, and glucocorticoids [7–9]. M2 macrophages promote angiogenesis, tissue remodeling, parasite encapsulation, and can act as immunoregulators by suppressing the inflammatory response (Figure 1). While M2 macrophages can be activated via different mechanisms, they are generally characterized by low levels of IL-12 and IL-23 secretion along with high levels of IL-10 secretion. M1 macrophages can be distinguished from M2 macrophages by the expression of distinct sets of chemokines and chemokine receptors [9]. It has been proposed that polarization of macrophages towards the M2 form can create an environment that is favorable towards wound healing, tissue regeneration and implantable biomaterials [10]. Consequently, the identification of pathways that can be modified to regulate macrophages polarization will be extremely beneficial for harnessing inflammation in pathologic states.
If the recruited M1 macrophages are unable to engulf an implant, they may release factors that will induce them to coalesce to form foreign body giant cells (FBGC) in an attempt to better engulf the implant [1]. Research by McNally et al., has shown that FBGC will form in the presence of interleukin-4 (IL-4) and may be enhanced with the addition of granulocyte macrophage colony stimulating factor (GM-CSF) [11]. The aggregation of FBGCs as well as the collagen synthesized by fibroblasts in the area will result in the formation of a fibrous encapsulation around the implant. The resulting capsule will surround the implant and isolate it from the adjacent tissue environment, thereby reducing their positive effects. Implant fibrous encapsulation has been shown to occur in virtually all tissues [12–14], except the brain and central nervous system, where glial scars will form in order to maintain the blood brain barrier and prevent lymphocyte infiltration [15]. In glucose sensors, this consequence nullifies the ability of the probe to acquire analyte measurements by inhibiting analyte transport to the sensor. In dynamic joints like the hip, a fibrous capsule limits joint range of motion, as well as integration into boney tissue.
1.4 Biomimetic immune evasive biomaterials
A great deal of research has addressed the design of immune modulating materials in an effort to reduce long-term inflammatory effects that may compromise the useful life of an implant. A common mode for the modulation of the immune response has been to mediate the release of pro-inflammatory cytokines or promote the release of pro-wound healing cytokines like IL-10, TGF-β and IL-1ra, thereby decreasing the severity and length of inflammatory response of the body to an implant while encouraging the healing of the area of interest. Current strategies for the mediation of inflammatory immune responses are listed in Table 1 and are still in the early stages of development. While these methods show promise, they are relatively unsophisticated approaches towards modulating inflammation.
Table 1.
Current Methods for Inflammatory Immune Evasion in Material Science
| Method | Description | Example Immune Evasive Agent | Ref |
|---|---|---|---|
| Coating material surface with non-fouling substrates | |||
| Coating with hydrogels | Hydrogels are known to produce a hydrophilic barrier between the bulk material and cellular medium. | PHEMA PEG |
[60] |
| Immobilization of non-biofouling substances to material surface | Non-biofouling compounds like PEG have been conjugated with peptide mimics of mussel adhesive proteins to facilitate immobilization to a material surface. | PEG-DOPA | [64, 65] |
| PEO-like tetraglyme is known to inhibit the deposition of fibrinogen, a mediator of phagocytic response, when adsorbed to the surface of a material. | Tetraglyme | [70, 120, 121] | |
| Coating with microgels | Coating of microgel beads crosslinked with PEG on a PET surface induces a 4-fold decrease in leukocyte adhesion as well as decreases in fibrinogen deposition and MCP-1 concentration over controls. | PEG-pNIPAm | [66–69] |
| Release of anti-inflammatory drugs | |||
| Release of glucocorticoids | Dexamethasone is a known anti-inflammatory that has been used in steroid-eluting stents and pacemaker leads. Has been shown to reduce implant related inflammation. | Dexamethasone | [71–75, 77, 79, 80] |
| Delivery of pro-wound healing or anti-inflammatory factors | |||
| Release of pro-angiogenic factors | VEGF has been released from hydrogels coating glucose sensors to promote angiogenesis. | VEGF | [73, 77, 79, 80] |
| bFGF has been delivered via a biodegradable hydrogel to improve limb ischemia via angiogenesis. | bFGF | [81] | |
| Release of pro-inflammatory receptor antagonists | The receptor antagonist for the IL-1 family of cytokines has been delivered for the treatment of rheumatoid arthritis and osteoarthritis. Delivery of both the receptor and the gene encoding the receptor has been studied. | IL-1ra ELP-IL-1ra |
[82, 83, 122, 123] |
| Release of nitric oxide | Activated macrophages produce reactive oxygen and nitrogen intermediates like nitric oxide, which kills bacteria in the surrounding environment. By loading a hydrogel surface with species like nitric oxide, the environment will be free of bacteria, reducing the effect of inflammation. | Nitric oxide | [86–88] |
| Release of anti-inflammatory cytokines | IL-10 has been delivered for the treatment of a number of inflammatory diseases and is known to down-regulate the synthesis of pro-inflammatory cytokines. Delivery of both the cytokine and the gene encoding the cytokine has been studied. | IL-10 | [89–93, 124, 125] |
| Immobilization of pro-wound healing or anti-inflammatory factors onto material surfaces | |||
| Immobilization of erythropoietin | Erythropoietin was immobilized and patterned onto a substrate and its bioactivity was verified by examining the proliferation of a erythropoietin-dependent cell line. Provided impetus for later immobilization studies. | Erythropoietin | [95, 96, 105] |
| Immobilization of pro-inflammatory receptor antagonists | The receptor antagonist for the IL-1 family of cytokines was immobilized onto a SAM. The modified surface exhibited attenuated inflammatory cytokine production relative to a control. | ELP-IL-1ra | [97] |
| Immobilization of EGF | EGF has been immobilized in gradients on material surfaces to encourage keratinocyte migration and increased wound healing response. | EGF | [98–101, 104] |
| Immobilization of bFGF | bFGF has been immobilized onto hydrogel scaffolds and was able to maintain mitogenic and chemotactic activity. Both gradient immobilization and co-immobilization with EGF have been considered. | bFGF | [103, 104] |
| Immobilization of VEGF | VEGF gradients were immobilized on SAMs to investigate cell migration and potential for inducing angiogenesis. VEGF gradients were found to increase directional migration 2-fold compared to controls. | VEGF | [102] |
| Cell-based surface modifications | |||
| Attachment of adipose-derived stem cells to the surface of an implant | When implanted into rat fat pads, implants elicit a much different immune response than if they were implanted into subcutaneous tissue (more neovascularization, smaller fibrous capsule). Attachment of adipose derived stem cells harvested from fat pads to the surface of an implant promotes neovascularization and small fibrous capsules. | ASCs | [106] |
Nature, on the other hand, has evolved immune evasive strategies over millennia that may point us in a new, biomimetic direction for combating inflammation. Specifically, both viruses and bacteria have evolved strategies for evading the immune system [16–19] This review examines aspects of these bodies that effectively elude the immune system and proposes a novel paradigm for the design of inflammation-evading biomaterials that can mimick these strategies.
2. Viral Immune Evasion
Viruses are biological vehicles that can infect both prokaryotic and eukaryotic cells by inserting either DNA or RNA into the hosts, thereby inducing the host’s cellular machinery to replicate the viral genetic material. In order to infect cells whilst minimizing the prospect of an immune response, viruses have evolved elegant methods of circumventing the numerous mechanisms of immune reaction.
2.1 Surface Interactions between Virus and Host
2.1.1. Viral Camouflage
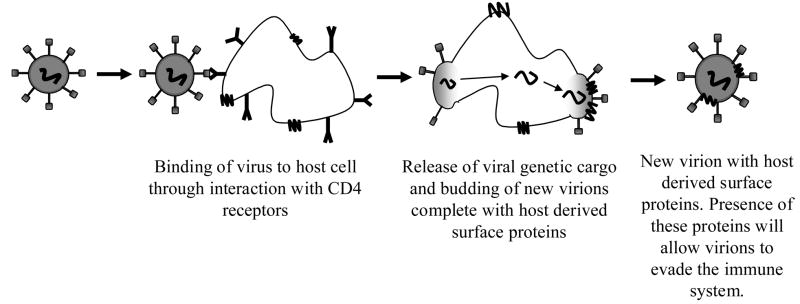
The first event that occurs between viral particles and host cells is contact of the virus with the cell surface. Initial recognition of non-self by an immune cell would typically induce inflammation at the site of identification [1]. Unlike most foreign implants that stimulate inflammatory immune responses, viruses use the opportunity for surface interaction with the host to stealthfully dodge immune response through a variety of mechanisms. Individual virus particles, known as virions, often can display host-derived proteins on their surfaces, thus confusing the host into identifying the viral particle as a host cell [20]. This technique of viral surface camouflage has been most documented in relation to the Human Immunodeficiency Virus Type-1 (HIV-1) [20, 21]. HIV-1 is particularly adept at incorporating host surface-derived molecules onto its surface. Molecules like Human Leukocyte Antigens I and II (HLA I–II), Intracellular Adhesion Molecule I and Lymphocyte-Function Antigen I are expressed on the surface of the HIV molecule as a result of previous interactions with a host cell (Figure 2) [21]. Expression of these transmembrane proteins provides not only a decreased immune response, but also increased specificity and affinity for binding between the virion and its target cell.
Figure 2.
Schematic of how HIV may both interrogate a host cell and incorporate host-derived proteins onto its surface. The gp120 receptor on the HIV virion binds preferentially to CD4 receptors on the host and the viral cargo is transmitted to the host through the gp120/CD4 complex. During the budding process, the virion can incorporate host-derived proteins on the cell surface, thereby providing itself with camouflage to disguise itself against immune response. Figure modified from [137].
2.1.2. Viral Surface Secretions
Another method for viruses to elude the immune system is to modulate the immune response via the secretion of immunomodulatory proteins at either the infected cell surface or the viral surface [19]. Invasion of the immune system by foreign objects or implants induces the secretion of cytokines to direct the extent and pace of the immune response. The same responses may be seen upon viral invasion of a host organism. Cytokine classes like interferons (IFN) and tumor necrosis factor (TNF) can induce anti-viral states in infected regions as well as apoptosis in infected cells [22]. T-lymphocytes may also be activated by cytokine secretion to combat viral infection via the killing of infected cells [22]. Because cytokine and chemokine gradients are necessary in the activation of numerous signaling pathways associated with immune response, the viral control of inflammatory cytokine production is a key method for viral immune evasion [23, 24]. This immunomodulatory effect is achieved via the secretion of four different classes of modulating proteins from an infected host.
2.1.2.1. Cytokine Inhibitors
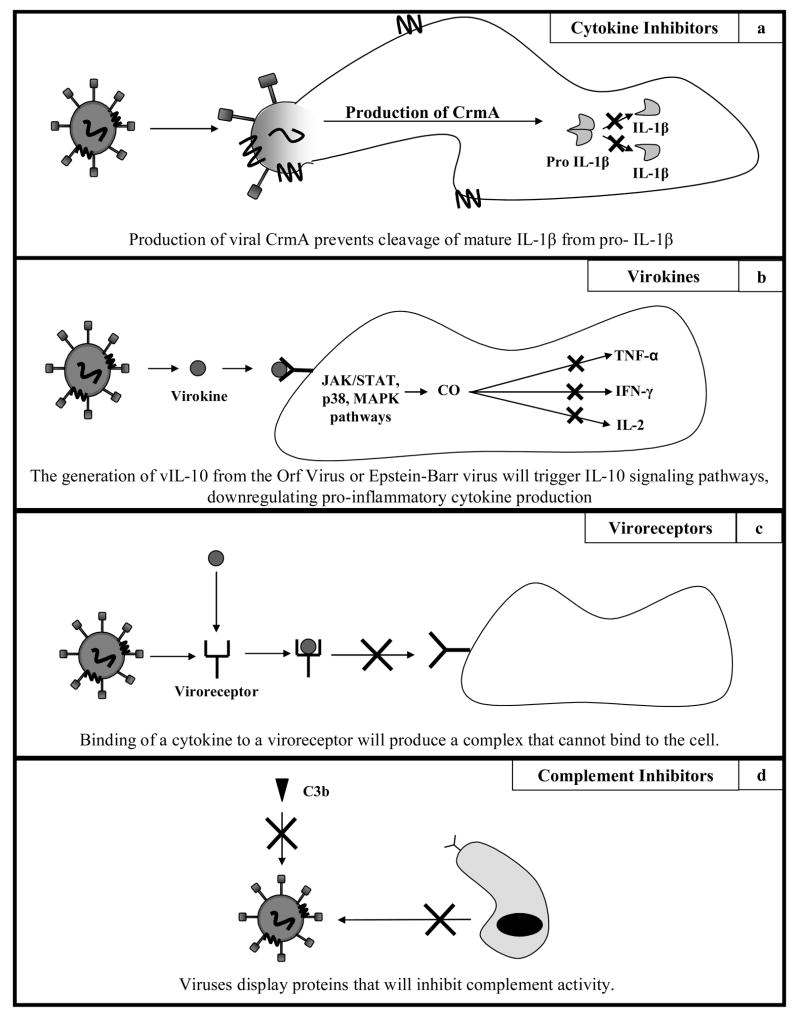
Viruses can affect the release of proteins that can inhibit the formation of pro-inflammatory cytokines within host cells. Cytokine inhibitors expressed by either viruses or virus-infected cells inhibit cytokine function by either attenuating cytokine production or reducing cytokine effector function [22]. One example of the former occurs in the cowpox virus. It encodes for a cytokine response modifier protein (CrmA), which prevents the cleavage of pro-IL-1β to mature IL-1β via attenuation of the production of the enzyme caspase-1 (Figure 3a) [22]. Similarly, African swine fever virus encodes for an inhibiting homologue of nuclear factor κB (NF-κB). When this viral protein binds to sites that normally bind NF-κB, the expression of all cytokines mediated by the NF-κB pathway is inhibited [22]. Modification of cytokine effector functions is found in mechanisms that can block apoptosis triggered by IFN or TNF. Epstein-Barr virus (EBV) encodes for a cytokine effector modifier called latent membrane protein 1 (LMP1). This protein modifies the effector functions of TNF cytokines by recruiting the TNF receptor signal transduction pathway to induce virus replication instead of apoptosis [22].
Figure 3.
Schematic of viral immune evasive secretions. a: Schematic of cytokine inhibition by viral proteins. Cowpox virus encodes for CrmA, preventing cleavage of pro IL-1β to IL-1β. b: The generation of vIL-10 from viruses activates JAK/STAT, p38 and MAP kinase pathways, producing carbon monoxide. It is believed that carbon monoxide is the agent that blocks transcription of pro-inflammatory cytokines. c: The complexation of a pro-inflammatory cytokine with a virally derived receptor homologue will prevent cytokine binding and activation of pro-inflammatory pathways in surrounding cells. d: Viruses incorporate host derived proteins like CD59 that will inhibit complementary response.
2.1.2.2. Cytokine Homologues (Virokines)
Viruses generate viral homologues within hosts of pro-wound healing cytokines, anti-inflammatory cytokines, and chemokines in order to modulate immune response. Many viruses such as herpesvirus and poxvirus avoid the immune system through the secretion of a number of evasive factors. These viruses promote the expression of molecules that will mimic cytokine function (virokines) as well as cytokine receptor function (viroreceptors) [19, 22, 23]. Such proteins are found almost exclusively in herpesvirus and poxvirus strains, as only these types of viruses have a genome large enough to support the sequence encoding for the proteins (100–200 genes). Herpesvirus immunomodulatory proteins are cytokine homologues while poxvirus immunomodulatory proteins are cytokine receptor homologues [22]. Epstein-Barr virus (EBV) as well as the Orf virus are known to induce the production of a homologue to IL-10, known as vIL-10 (Figure 3b) [25, 26]. This homologue is able to downregulate immune response through the decreased production of pro-inflammatory cytokines. Similar IL-10 homologues have been reported in cytomegaloviruses, where the homolog has been shown to bind competitively to IL-10 receptors despite sharing only 27% homology with cellular IL-10 [27, 28]. The Orf virus also encodes for a viral homologue of vascular endothelial growth factor (VEGF), known as vVEGF [29]. vVEGF is able to bind to the VEGF receptor VEGFR2 and induce pro-wound healing effects like angiogenesis in the surrounding tissue. Another example of viral proteins that act as cytokine homologues is in the human myxoma virus. This virus secretes a chemokine homologue called MT-7 that has similar binding specificity as monocyte chemotactic protein 1 (MCP-1) to chemokine-specific receptors. This protein can then inhibit chemokine effects by interacting with the conserved extracellular glycosaminoglycan binding domains of chemokines, thereby interfering with the formation of extracellular ligand gradients necessary for directed cell chemotaxis [16, 30, 31].
2.1.2.3. Cytokine Receptor Homologues (Viroreceptors)
Viruses generate proteins within hosts that will bind competitively to binding motifs on a cytokine. Cytokine receptor homologues act on the principal that if the receptors can be competitively bound before reaching the receptor region, the conformational change caused from binding to the receptor will render the new cytokine/homologue complex unable to bind to the cell surface receptors, thereby inhibiting downstream signaling pathways involved in the process of inflammation (Figure 3c). An example of this behavior can be also seen from the myxoma virus, which can secrete proteins from an infected cell to bind to the receptor binding domain on the surface of the chemokine, thus forming a complex with a conformation that is unsuited for binding at the chemokine surface receptor [16, 30, 31]. Soluble cytokine receptors have been documented for the receptors of the TNF family, IL-1β, and IFN-γ [32–34]. By binding with high specificity to cytokines, these virus-based soluble cytokine receptors provide an additional avenue for viral immune evasion, allowing for immune latency in the target area that will allow the viruses to infect surrounding cells. A very useful table listing a number of viral cytokines and chemokines as well as viral cytokine and chemokine binding proteins may be found in a review by Alcami in Nature Immunology [22].
2.1.2.4. Complement Inhibitors
Viruses create homologues of complementary regulatory proteins on the surfaces of infected cells to block complement activation and elimination of infected cells. Briefly, the complement system is a non-specific host defense mechanism that is the major effector of the humoral branch of the immune system. The system consists of small proteins in the blood that remain inactive until activated by a stimulus. Complement can be activated by either the innate immune system’s recognition of pathogen-associated molecular patterns (PAMPs) or the antibodies in the adaptive system binding to foreign antigens. Once activated, the complement system can perform a host of functions, including lysis of cells, bacteria and virus, opsonization to promote phagocytosis, triggering cell function through secretion of cytokines, and clearance of circulating immune complexes by cells in the liver or spleen [2]. Viruses like human cytomegalovirus (HCMV) and vaccinia virus (VV) incorporate host derived cellular surface factors to protect against complement-mediated lysis (Figure 3d) [23], [35]. Each of these viruses display a host protein called CD59 that will protect host cells from complement lysis, and therefore protect the virus from complement lysis.
Co-evolution of viruses and host organisms has brought about both improvements in the host system for battling the onset of various viruses as well as improvements in the ability of viruses to evade the immune system. Through both the stealth tactics of camouflage along with homologue secretion, viruses have become the ultimate saboteurs of the immune system, often using the immune system’s own machinery against itself.
3. Bacterial Immune Evasion
Once bacteria have developed a parasitic relationship with a host, they are classified as pathogens. As causative agents of disease like tuberculosis, tetanus, meningitis and typhoid fever, bacteria have also become efficient pathogenic vehicles. Unlike viruses, bacteria have their own secretory pathways, allowing for evasive schemes that are not necessarily reliant on the machinery of host cells [19]. The evasion and resistance of bacteria to outside forces has been well documented in the case of bacteria evolving antibiotic resistance after successive generations of treatment by the same antibiotic strains [36, 37]. Much like viruses, the majority of bacterial immune evasion occurs due to surface interactions with a host. More complete reviews of bacterial immune evasion have been published by Foster [38] and Costerton and Stewart [39].
3.1 Suspended bacterial evasive maneuvers
The primary defense against infection is the innate immunity provided by neutrophils, macrophages, and dendritic cells. However, Staphylococcus aureus has the ability to thwart neutrophils and macrophages by (a) inhibiting chemotaxis (by blocking formylated peptide recognition, blocking C5a binding, secreting leukotoxins, and blocking LFA1-ICAM1 mediated extravasation), (b) negating opsonization (via protein G reverse IgG binding, plasmin degradation of bound IgG and C3b, and blocking C3b binding), and (c) thwarting phagocytosis (cell wall modifications to resist low endosomal pH, enzymatic degradation of endosomes) [40], [38]. Staphylococcus epidermidis, another Gram-positive bacterium and the main species isolated in the majority of nosocomial infections, avoids the immune system also through protein G and A reverse binding of IgGs and through adhesion and biofilm formation [41]. Otto and coworkers have documented that Gram-positive bacteria actually sense antimicrobial peptides released by neutrophils and macrophage and can coordinate a directed defensive response [42]. They discovered an antimicrobial peptide sensor system that controls major specific resistance mechanisms; the sensor contains a classical two-component signal transducer and an unusual third protein, all of which are indispensable for signal transduction and antimicrobial peptide resistance.
Complement evasion by many Gram-positive bacteria involves incorrect binding of complement recognition factors by bacterial cell wall proteins (Protein A, G, M) [43]. Gram-negative bacteria have evolved secretory proteins that can degrade complement factors or their binding components, or that prove anti-chemotactic or toxic to immune cells.
3.2. Formation of a Capsule or Biofilm
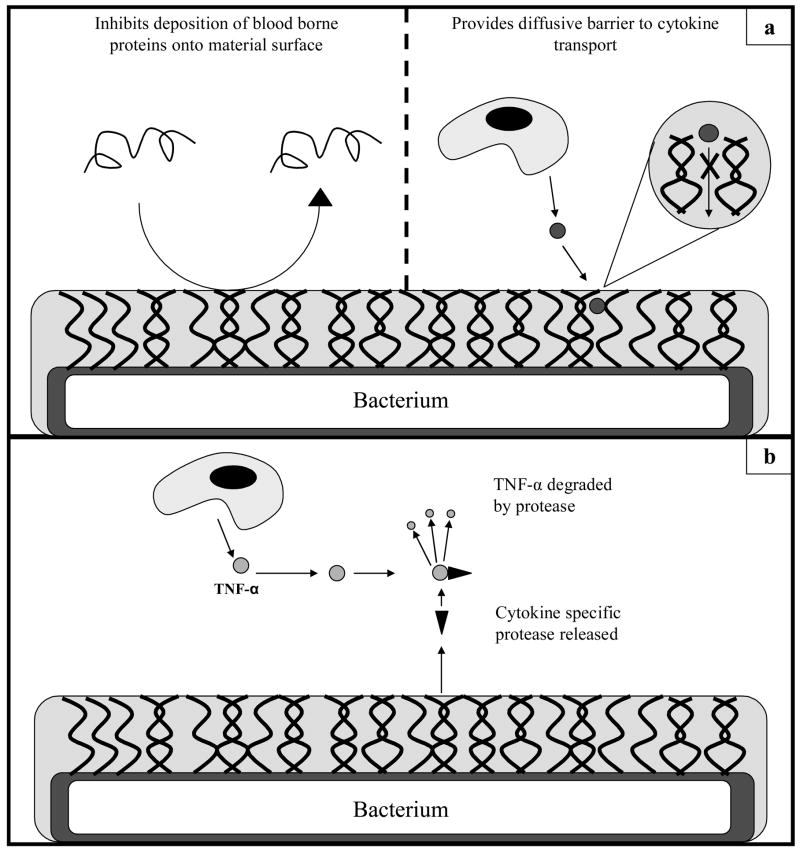
Bacterial surfaces are complex, multi-faceted structures that contain many different sites of recognition for the immune system [19, 38]. A common mechanism for masking proteins at the bacterium surface is the expression of a carbohydrate capsule or biofilm formation (Figures 4a and 4b) [44]. While complex in composition, it is known that the capsule is composed of a number of different polymers collectively called exopolysaccharides [45]. Besides serving as an effective camouflage, this carbohydrate capsule has functional properties that are immune evasive as well. Numerous bacteria, like S. pneumoniae as well as S. aureus, exude capsules with both anti-opsonic and anti-complement functions (Figures 4a, 5a) [38]. Formation of the capsule prevents antibody and complement deposition on the surface of the bacterium, thus inhibiting opsonization and phagocytosis. Phagocytosis assays have shown that even with high opsonin concentrations, the presence of a capsule on S. aureus will inhibit bacterial uptake by neutrophils in vitro [38, 46].
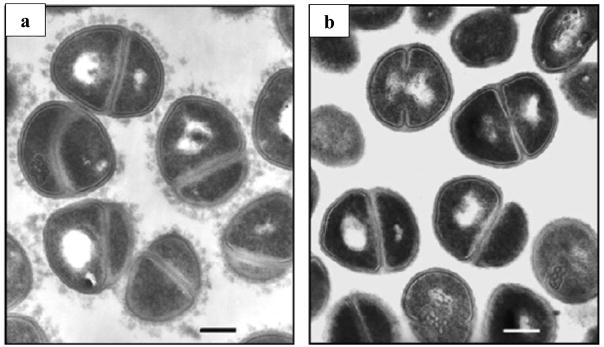
Figure 4.
Transmission electron micrographs of Staphylococcus haemolyticus. a: Visualization of the bacterial capsule formed around the bacterium body. b: Images of bacteria that have yet to form capsules. Borrowed from [42].
Figure 5.
a: Means by which the bacterial capsule will attenuate inflammation. b: Bacterially secreted proteases will attenuate immune response by degrading pro-inflammatory cytokines.
Other bacteria, like S. epidermidis and Pseudomonas aeruginosa, are unique in their formation of a protective capsule, as they exude a biofilm upon contact with a biomaterial surface [39]. Biofilms provide a protective structure for survival in harsh conditions and behave as a biologically active selectively permeable membrane. The polymer matrix secreted to form biofilms contains conduits for nutrient transport while impeding the transport of antimicrobial agents and proinflammatory cytokines to the surface of the bacterium for opsonization and phagocytosis [39]. Mathematical models have been developed using reaction-diffusion theory to characterize how the biofilm diffusion barriers inhibit the effectiveness of antimicrobial agents [47]. These diffusion limitations were verified in an experimental artificial biofilm system composed of beads in an alginate gel, further showing how biofilms limit transport of anti-microbicides and pro-inflammatory cytokines [48]. In Gram-positive bacteria, the adhesion of bacterial cells is mediated by surface proteins like autolysin, fibronectin receptors, and fibrinogen binding protein [38]. The bridge between the biofilm and bacteria is formed by a number of molecules such as polysaccharide intracellular adhesin, PIA, a charged homopolymer comprised of a β-1,6-linked N-acetylglucosamine [41, 45]. As the adhesive agent between the cell and the biofilm, PIA is essential in the evasion of innate immunity. Vuong, et al, have demonstrated that when de-acetylated with the surface protein IcaB, PIA was unable to complex with the cell and biofilm, thus reducing the amount of biofilm and increasing the amount of phagocytosis from neutrophils [41]. This occurrence is primarily due to the loss of PIA’s cationic character, thus not allowing it to covalently interact with the negatively charged surface of the bacterium.
Research has recently intensified to quantify the relationship between immune cell presence and biofilm formation. Research published by Wu, et al., has shown that interactions between bacterium surface proteins and pro-inflammatory cytokines may induce anti-inflammatory reactions by the bacterium P. aeruginosa [49]. Using the gene expression of type- I P. aeruginosa (PA-I) lectin, an adhesin of P. aeruginosa, as a representative output of virulence, the response of the bacterium to different inflammatory cytokines was examined. It had been previously established that the lethality of P. aeruginosa was dependent on expression of PA-I lectin. Overall, IFN-γ was found to induce overexpression of PA-I lectin, suggesting that P. aeruginosa may combat the immune system by monitoring host immune response and triggering virulence factor production in response to it [49, 50]. Walker et al., reported that human neutrophils serve to enhance the initial development of P. aeruginosa biofilms [51]. The mechanism of biofilm enhancement by neutrophils was attributed to neutrophil-generated polymers comprised of actin and DNA. The bacteria bind to F-actin and the free DNA promotes biofilm matrix stability. Disruption of the polymers with DNase results in dispersion of the biofilm bacteria and a reduction in biofilm development. The presence of these actin-DNA polymers, with co-localization of P. aeruginosa, was confirmed in both neutrophil lysates and cystic fibrosis sputum. Introduction of additional neutrophils after 24 and 48 h further enhanced P. aeruginosa biofilm development, while exposure to fewer neutrophils resulted in a lesser degree of biofilm enhancement. Similarly, Chandra et al. showed that peripheral blood mononuclear cells (PBMCs) enhanced the ability of the yeast, Candida albicans, to form biofilms and that the majority of PBMCs were localized to the basal and middle layers of the biofilm [52]. They also found that only viable PBMCs enhanced C. albicans biofilm formation and that PBMCs cell surface components did not contribute to this biofilm enhancement; the biofilm-enhancing effect is mediated by a soluble factor released into the medium, and that the supernatant collected from cocultures contained differential levels of pro- and anti-inflammatory cytokines. This research presents the bacterial biofilm as more than just a passive partition between a bacterium and the surrounding environment, showing instead that biofilim formation as well as inflammatory response may be a result of the bacterium to sense the environment around it.
Through the formation of capsules and biofilms, bacteria present unique methods for immune evasion through not only their anti-opsonic properties, such as limited capability of antibody diffusion, but also through the evolution of systems that may monitor the behavior of the immune system and create specified responses.
3.3. Bacterial Induction of Cytokine Secretion
In addition to evading the immune system through capsule formation, classes of bacteria have displayed the ability to attenuate immune response through modulation of signaling molecules. Similar to viruses, bacteria may induce cellular production of pro-wound healing and anti-inflammatory cytokines to limit immune response (Figure 5b). Mycobacteria have been known to infect macrophages by binding to cell-specific receptors on the cell surface [27]. Once infected with the species Myobacterium tuberculosis, monocytes have been shown to upregulate production of IL-10 compared to uninfected samples [53, 54]. Human monocyte cell lines treated with M. tuberculosis purified protein derivative (PPD) along with TGF-β expressed 70 times more IL-10 than unstimulated monocytes. Additionally, the coculture of PPD-infected cells with uninfected cells inhibited production of IFN-γ in the uninfected cells, demonstrating the ability to attenuate release of pro-inflammatory factors [55]. Such behavior is also known to occur in Legionella pneumophila, Salmonella typhimurium, and Legionella monocytogenes [27, 56]. P. gingivalis expresses unique immunosuppressive lipopolysaccharides (LPS) that will stimulate dendritic cells to secrete IL-10 in vitro [57] and in vivo [58]. Cytokine secretion is also limited through the specific inactivation of C-type lectin receptors, thereby downregulating the signaling necessary for cytokine secretion as well as maturation of dendritic cells [59].
Recent work by Gantner et al. suggests that there may be recognition signaling through C-type lectin receptors that may cooperate with toll-like receptor (TLR) signaling in defining inflammatory responses [60]. They examined how dectin-1, a lectin family receptor for β-glucans, cooperates with TLRs in recognizing microbes. Dectin-1 expression enhances TLR-mediated activation of NF-κB by β-glucan–containing particles. In both macrophages and dendritic cells, dectin-1 and toll-like receptors are synergistic in mediating production IL-12 and TNF-α. Data demonstrate that collaborative recognition of distinct microbial components by different classes of innate immune receptors is crucial in orchestrating inflammatory responses.
Moreover, certain bacteria have been shown to completely inactivate pro-inflammatory cytokine production. Porphyromonas gingivalis, an oral Gram-negative pathogen associated with periodontitis, expresses a host of cytokine-specific proteases. Calkins et al., examined the ability of Arg-specific hydroxyproline-rich glycoprotein (HRGP) and reversibly glycosylated polypeptide 2 (RGP-2) and Lys-specific gingipain (KGP) to degrade TNF-α, a well-documented pro-inflammatory cytokine [61]. All three proteases were found by immunoblot analysis to rapidly degrade TNF-α, thereby eliminating one of major mediators of inflammation.
4. Applications to Biomaterial Science
4.1 Current Techniques
Table 1 summarizes a number of different techniques that have been utilized to evade implant-related inflammation. As with viral and bacterial methods of immune evasion, these existing techniques seek to modify the interactions that occur between the surface of the foreign object (ie: virus, bacteria, implant) and the native milieu of the body.
4.1.1. Coating of material surfaces with non-biofouling substrates
Early studies involved in the attenuation of inflammatory response from material implants involved treating implant surfaces with membranes that resist the deposition and adsorption of proteins and cells, known as biofouling, thereby reducing inflammatory processes like opsonization and phagocytosis [62–64]. The goal of these non-biofouling surfaces was to resist protein and cell deposition without hindering implant performance. Phospholipids, naturally derived materials like chitosan, and synthetic hydrogels have all been shown to demonstrate anti-biofouling properties [62, 65]. Hydrogels in particular have been attractive options for non-biofouling substrates as they produce a hydrophilic barrier at the surface of the bulk material that resists protein adsorption and mammalian cell adhesion [62]. Additionally, physical properties of these gels are tunable and may be altered by changing water content within the polymer[62]. Poly(hydroxyethyl methacrylate) (PHEMA) and poly(ethylene glycol) (PEG) have been used as the crosslinking polymers within such hydrogels since they allow for water soluble solutes to diffuse through the hydrogel [62, 63, 66, 67]. In addition to being incorporated into hydrogels, compounds like PEG have begun to be immobilized onto surfaces by conjugating PEG with peptide mimics of mussel adhesive proteins [66, 67]. Dalsin et al., described a particularly attractive approach of combining PEG segments with peptides containing residues of 3,4 dihydroxyphenylalanine (DOPA), a major constituent of mussel adhesive proteins, to create a composite molecule that could be immobilized to the surface of a material and ensure anti-biofouling characteristics [66, 67].
New technology in the realm of non-biofouling coatings has involved the deposition of microgels onto the surfaces of materials [68]. Poly(N-alkylacrylamides), pNIPAm, are hydrogels that when thermally triggered will hydrophobically collapse on themselves, expelling their water soluble contents into the surrounding area. While these hydrogels present a large promise for the field of drug delivery, their biocompatibility has yet to be well characterized. Nolan et al. crosslinked microgel particles of pNIPAm with PEG chains and spin coated the result onto a cationic glass substrate [69]. These substrates showed non-biofouling behavior when compared to control samples. From this work, microgel films have been created by the same group that have been shown to modulate acute inflammation by creating a non-biofouling surface [70, 71].
Recently, Mayorga et al., have demonstrated that deposition of PEG-like tetraethylene glycol dimethyl ether (tetraglyme) onto fluorinated ethylene propylene (FEP) discs attenuated protein adsorption and therefore monocyte adhesion that would lead to acute phase inflammation [72]. Since fibrinogen has been shown to mediate the adhesion of phagocytic bodies to materials through binding to the phagocytic Mac-1 integrin, it was posited that a surface with ultra-low fibrinogen deposition would significantly attenuate immune response.
4.1.2. Release of anti-inflammatory drugs
Another strategy employed to reduce inflammation at the site of implantation is the release of anti-inflammatory drugs. Glucocorticalsteroids, or glucocorticoids, are generally considered the most potent substances for the control of chronic inflammatory diseases like asthma[73]. In particular, glucocorticoids have been shown to have strong inhibitory effects on cytokine-related inflammation by downregulating transcription of IL-1, TNF-α, GM-CSF, IL-3, IL-4, IL-5, IL-6 and IL-8 [73]. For these cytokines, once the steroid and its receptor have bound into a complex, this complex will bind to negative glucorticoid response elements that exist close to promoter sequences on cytokine-encoding genes, inhibiting transcription of the gene [73]. Glucocorticoids may also act to reduce cytokine effects by downregulating the synthesis of cytokine receptors.
A frequently used anti-inflammatory glucocorticoid for material research is dexamethasone, which has been used in steroid-eluting stents and pacemaker leads to reduce inflammation [74, 75]. Administration of dexamethasone microspheres was shown by Hickey et al to reduce implant-related inflammation and has been documented to prevent restenosis when release from coronary stents [76–78]. Additionally, dexamethasone release technology has been merged with non-biofouling hydrogel technology. Research by a number of groups has incoporated dexamethasone into non-biofouling hydrogel coatings and found both favorable release characteristics as well as attenuated immune response when examined in vivo [79, 80].
4.1.3. Release of pro-wound healing or anti-inflammatory factors
Work by Norton et al and well as Patil et al has investigated the release of pro-wound healing cytokines as a means of attenuating immune response [75, 79, 81, 82]. In order to improve the life of probe coatings of glucose sensors, probes were coated with hydrogels loaded with both vascular endothelial growth factor (VEGF), a well characterized pro-angiogenic and pro-wound healing cytokine, and dexamethasone [75]. Coating the surface of a sensor with a hydrogel containing proangiogenic growth factors and anti-inflammatory drugs has been shown to increase vascularization while decreasing inflammatory immune response [79]. Komeda et al, have demonstrated pro-angiogneic effects upon delivery of basic fibroblast growth factor (bFGF) via a biodegradable hydrogel in an effort to improve limb ischemia [83]. Additionally, hydrogels composed of poly(hydroxyethylmethacrylate) (PHEMA) or poly(ethylene glycol) (PEG) resist biofouling by inhibiting the accumulation of blood borne proteins onto the surface, thereby mitigating one cause of implant-related failure [62].
The type I receptor of the IL-1 family of molecules is biologically active while the type II receptor is a dummy receptor that sequesters IL-1 at the plasma membrane or as a soluble receptor. When IL-1 binds to the functional type I receptor in the cell membrane it recruits a second accessory protein to join the complex (IL-1R AcP) that activates intracellular cascades leading to NF-κB and c-JUN transcription of several pro-inflammatory genes in a broad array of cell types. The antagonist IL-1ra binds with comparable avidity to both types of receptors, but blocks the activating step of IL-1R AcP association with type I receptor. Bresnihan et al., first examined the effect that administration of the anti-inflammatory IL-1ra could have upon patients with rheumatoid arthritis [84]. At 24 weeks into the daily drug treatment, a significant decrease in swollen, tender and stiff joints was observed over placebo. Work by Shamji et al., reports on the release of IL-1ra, a receptor antagonist for the pro-inflammatory IL-1 family of cytokines, in order to treat osteoarthritis-related instances of inflammation [85]. By fusing the IL-1ra protein to an elastin like polypeptide (ELP), the complex was able to thermosensitively trigger and release, allowing for controlled and sustained release to the body. Additionally, administration of IL-1ra in vitro to fibrochondrocytes resulted in decreased proinflammatory cytokine expression relative to control values.
TNF-α neutralization using an anti-TNF-α antibody or a soluble TNF-α receptor is now routine clinical practice in cases of chronic and severe rheumatoid arthritis [86]. An example of a commonly used therapeutic utilizing this approach is Etanercept (Enbrel), which is a fusion protein of a soluble TNF-α receptor and the Fc component of human immunoglobulin G1 (IgG1). This drug is able to mimic the function of TNF receptor by binding the cytokine, creating a cytokine that will decrease the effect of the pro-inflammatory cytokine. And by virtue of being fused to the Fc region of IgG1, the drug will persist in the bloodstream much longer than native TNF-α receptor [87].
Current research has also investigated the release of factors like nitric oxide from hydrogel coatings as a means of attenuating immune response [88–90]. Activated macrophages produce reactive oxygen and nitrogen intermediates like nitric oxide, which kills bacteria in the surrounding environment. By loading a hydrogel surface with species like nitric oxide, it is the hope that the environment will be free of bacteria, reducing the potential for inflammation.
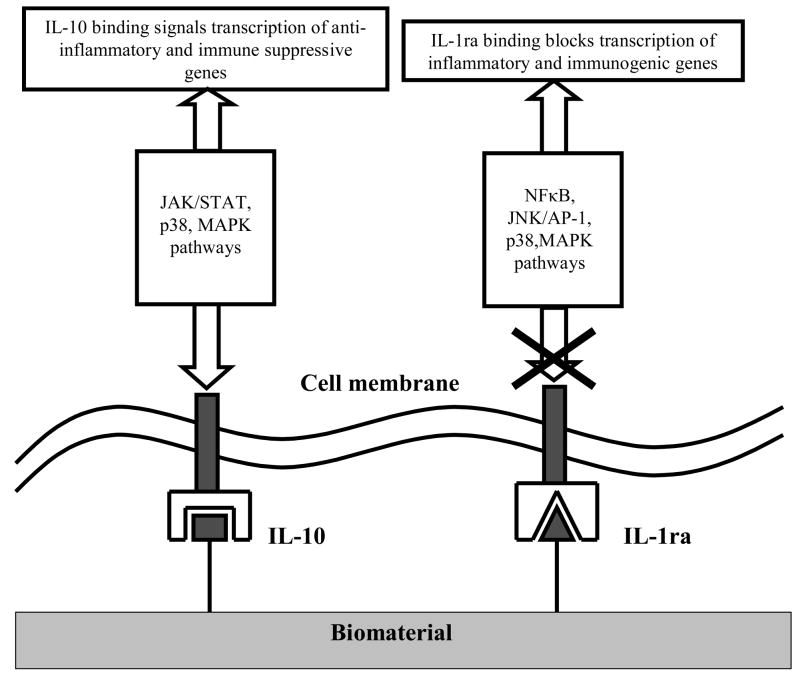
Delivery of the anti-inflammatory cytokine IL-10 has been widely studied for the treatment of colitis [91], rheumatoid arthritis [92–94], Crohn’s disease [95] and multiple sclerosis [95]. IL-10 not only down-regulates Th1 cellular immunity, but also up-regulates Th2 humoral immunity by stimulating the growth and proliferation of B cells [96]. IL-10 acts on monocytes/macrophages by down-regulating the stimulated synthesis of pro-inflammatory TNF-α, IL-1, IL-6, IL-8, and GM-CSF, as well as suppressing the synthesis of reactive oxygen intermediates, nitric oxide, collagenase and gelatinase. In macrophages, IL-10 binding to its transmembrane receptor co-activates the JAK/STAT, p38 and MAP kinase pathways that lead to the transcription of heme oxygenase genes for the production of bilirubin and carbon monoxide. It is believed that carbon monoxide is the agent that blocks transcription of pro-inflammatory cytokines. Figure 6 illustrates the means by which both IL-1ra and IL-10 are able to attenuate immune response.
Figure 6.
Schematic of signaling pathways involved in attenuating signaling pathways upon exposure to IL-10 (left) and IL-1ra (right)
4.1.4. Immobilization of pro-wound healing or anti-inflammatory cytokines onto implant surfaces
Research conducted by Ito et al., established a precedent for the immobilization of cytokines and growth factors onto material surfaces [97, 98]. By immobilizing erythropoietin onto culture plates, Ito’s group was able to create a substrate dependent means for growth of a erythropoietin-dependent leukemia cell line, thus showing the bioactivity of immobilized factors on surfaces [98]. Furthering the work by Ito et al., work by Kim et al., have demonstrated in vitro the effect that immobilizing a surface with an anti-inflammatory factor may have in attenuating immune response [99]. ELP-IL-1ra fusion proteins were immobilized onto the surface of a self assembled monolayer. When exposed to the fusion protein modified monolayer, lipopolysaccharide (LPS)-stimulated monocytes exhibited attenuated inflammatory cytokine production relative to control surfaces.
Nakaji-Hirabayshi et al., have also achieved the immobilization of epidermal growth factor (EGF) onto surfaces [100]. EGF was fused with a hexahistidine tag and this complex was immobilized onto the surface of a self-assembled monolayer (SAM) through coordination chemistry between surface immobilized Ni2+ ions and the hexahistidine tag. Subsequent research found that EGF immobilized surfaces promoted the proliferation of neural stem cells seeded onto the surface [101]. Moreover, when exposed to a micropatterned surface containing islands of immobilized EGF surrounded by fibronectin, neural stem cells cultured on the EGF island expressed a significantly higher amount of nestin, a marker for undifferentiated neural stem cells, than those on the fibronectin surface, where glial differentiation was more prominent [102]. Stefonek et al., demonstrated that immobilized gradients of EGF on the surface of a biomaterial could induce keratinocyte migration in the direction of increasing growth factor concentration [103].
Liu et al., have recently immobilized VEGF on self assembled monolayers to investigate the effect of an immobilized cytokine gradient on cell migration [104]. While the slope of the gradient formation was found to be statistically insignificant, an immobilized VEGF gradient was found to induce directional migration towards the increasing slope from bovine aortic endothelial cells (BAECs) that was two fold greater than on a surface of uniform VEGF concentration. From a wound healing standpoint, this finding is significant, as rapid migration of cells is essential for effective wound healing and angiogenesis.
DeLong et al., reported the immobilization of bFGF onto hydrogel scaffolds through functionalization of the growth factor with acryloyl-PEG-NHS chemistry [105]. The growth factor maintained mitogenic and chemotactic activity despite being immobilized and was found to increase smooth muscle cell (SMC) proliferation by 41% and migration by 15%. When exposed to a gradient of immobilized bFGF, the SMCs were found to align and migrate along the gradient. Stefonek et al applied the findings of this study with their previous work to investigate possible synergistic effects of gradients of both EGF and bFGF on the same surface [106]. A review paper recently published by Ito provides a more thorough evaluation of biosignal immobilization to biomaterials [107].
4.1.5. Cell-based implant surface modifications
Research by Prichard et al., has demonstrated how immune response may be affected when the surface of an implant is attached to adipose stem cells before implantation [108],[109]. When implanted into rat fat pads, implants elicit a much different immune response than if they were implanted into subcutaneous tissue (more neovascularization, smaller fibrous capsule). As a result, it has been posited and shown that attachment of adipose derived stem cells harvested from these fat pads to the surface of an implant will also promote neovascularization and small fibrous capsules.
4.2. Biomimetic Techniques
An understanding of the methods by which viruses and bacteria evade the immune system provides an exciting new paradigm by which biomedical engineers can design material implants. While some have investigated isolated effects of these biological agents in attenuating immune response, these effects have yet to be applied in a salient, directly translatable way to material science. This new biomimetic approach to material design could serve to use well established and evolved cellular machinery for the evasion of the immune system. This section will serve to outline a few potential approaches to applying immune evasive capabilities of biological organisms to material design.
4.2.1. Application of Viral Techniques
4.2.1.1. Precedent Technologies
Research by Boomker et al., has provided precedent for the use of viral proteins in combating inflammation [110]. The administration of a plasmid containing the gene encoding for the MT-7 protein from the human myxoma virus to mice that had been implanted with discs of cross linked collagen was shown to decrease leukocyte recruitment by competitively binding to GAG domains in the extracellular domain surrounding the site of injury. Similar findings have been shown with the vaccina virus complement control protein (VCP) that occurs within poxviruses [111]. Work by Smith et al., shows that VCP, through its ability to bind to glycosaminoglycans on the surface of human endothelial cells, is able to block antibody binding to surface major histocompatibility complex class I molecules [111]. Structurally, VCP is very similar to the human C4b-BP and the other complement control proteins. Functionally it is most similar to the CR1 protein. VCP blocks both major pathways of complement activation. In addition to binding complement, VCP also binds to heparin. These two binding abilities can take place simultaneously and contribute to its many functions and to its potential use in several inflammatory diseases or in reducing inflammation at an implant site. In fact, researchers have isolated specific binding motifs within the protein itself that allow it to bind preferentially to glycosaminoglycans like heparin, thereby reducing chemokine binding to the extracellular matrix. Using these findings as a proof of concept that this specific protein may attenuate immune response by limiting the formation of chemokine gradients, one could seek to design a more feasible material construct utilizing this technology.
4.2.1.2. Future Directions
One potential design would include the controlled release of the protein to the surrounding milieu of the extracellular space. Much like in the research performed by Boomker et al., cells could be transfected with a plasmid containing the gene encoding for MT-7 protein. These cells could then be encapsulated in a biodegradable hydrogel coating that could be adhered to the surface of a material implant. By encapsulating the cells in a biocompatible and biodegradable hydrogel, one could be able to allow the cells to use their own machinery to express the MT-7 protein, thereby creating a perpetually constant dose source within the material construct. The encapsulation of cells and the release of cell-derived proteins within hydrogels has been sufficiently studied and reported, allowing for the modification of those current technologies for cell-mediated protein release [112–114]. This coating would create an anti-fouling surface during the early stages of inflammation, thereby further decreasing the inflammatory effects of the implant [62]. Table 2 gives a listing of possible hydrogels that could be used for the release of these virally derived bioactive elements. This design is also highly tunable in that the release profile as well as cell type used can be altered for maximal release characteristics. Additionally, one could transfect cells with a number of different virally-derived proteins, such as virokines mimicking wound healing cytokines like IL-10, promoting a pro-wound healing environment while encouraging the M2 phenotype of macrophage. Table 3 gives examples of other viral based bioactive agents that could be incorporated. Precedent for use of IL-10 exists, as the gene encoding for viral IL-10 in Epstein-Barr virus has also been isolated and delivered to mice subjected to rheumatoid arthritis [93]. Despite its short half life, vIL-10 lacks the immunostimulatory properties of human IL-10, making it a possibly more potent immunomodulator. Administration of vIL-10 was found to decrease incidence and severity of rheumatoid arthritis, thereby showing demonstrating the capabilities of vIL-10 as an anti-inflammatory.
Table 2.
Possible biomaterials to be used for the controlled release of bioactive agents in Table 3.
| Hydrogel | Synthetic/Naturally Derived | Advantages | Ref |
|---|---|---|---|
| Dextran | Naturally derived | Ubiquitous in medical applications: blood plasma substitute, drug delivery vehicle. Dextran-HEMA hydrogels degrade in a controlled manner at physiologic temperature and pH. |
[126, 127] |
| Poly (ethylene glycol) (PEG) | Synthetic | Non-cytotoxic, biodegradable. Does not interact with blood and cellular proteins. Numerous methods of polymerization. Responsive to a number of stimuli: temperature, pH, cellular milieu. |
[126, 128] |
| Alginate | Naturally derived | Fast kinetics of gel formation. Tunable porosity allows for controlled drug release. |
[126, 127] |
| Chitosan | Naturally derived | Has natural wound healing properties. Used in wound dressings. Easily complexes with other chemicals (ie: alginate, gellan). |
[127] |
| Gelatin | Naturally derived | Extensively used in pharmaceutical and medical applications. Electrical properties of gelatin matrix may be altered by collagen extraction method. |
[129] |
| Hyaluronic Acid | Naturally derived | Well established in drug delivery. Negative charge allows for complexing with cationic compounds. Biocompatible; major component of extracellular matrix. |
[126, 130] |
| Poly(acrylic acid) | Synthetic | Used for controlled release of insulin, lysozyme, albumin and fibrinogen. Has tunable porous and mucoadhesive properties. |
[126] |
Table 3.
Possible viral or bacterial agents for biomimetic immune evasive design
| Bioactive Element | Sources | Effects | Ref |
|---|---|---|---|
| Viral Agents | |||
| Cytokine response modifier A protein (CrmA) | Cowpox virus (CPV) | Inhibits the production of caspase-1. Prevents formation of mature IL-1β from pro-IL-1β. |
[22, 24] |
| vIL-10 | Epstein Barr virus (EBV) Human cytomegalovirus (HCMV) Orf virus (OV) |
Produces homologue to pro-wound healing and anti-inflammatory IL-10. | [22, 26, 28] |
| vVEGF | OV | Binds to VEGFR2. Induces pro-wound healing and pro-inflammatory effects of VEGF. | [22, 29] |
| vTNFR | CPV HCMV |
Secreted to bind to TNF class of cytokines. Inhibits effect of TNF. |
[22, 131] |
| vIL-18BP | EBV OV Molluscum contagiosum virus (MCV) |
Binds to IL-18. Inhibits IL-18-dependent IFN-γ production. | [22, 132] |
| vGM-CSFBP | EBV | Binds to granulocyte macrophage colony stimulating factor. Modulates colony formation of macrophages. | [22, 133] |
| vMIP2 | Kaposi’s sarcoma- associated virus (KSHV) | Binds MIP2. Inhibits chemokine effect for immune evasion. | [22, 134] |
| vCKBP2 | Vaccinia virus | Interacts with receptor binding domain of monocyte chemoattractant protein-1 (MCP-1). Blocks chemokine-receptor interactions. | [22, 135] |
| MT-7 | Human myxoma virus (HMV) | Binds to chemokine binding motifs on GAGs in extracellular matrix. Disables the chemokine from binding. No chemokine gradient may be formed and maintained. | [16, 22, 108, 136] |
| Bacterial Agents | |||
| Cytokine-specific proteases | Porphyromonas gingivalis | Arg-specific HRGP and RGP2 and Lys- specific KGP proteases degrade TNF-α, eliminating one of major mediators of inflammation. | [59] |
| Protein A | Staphylococcus aureus | Protein A binds to the Fc region of IgG antibodies. Once bound, the IgG molecules are in the incorrect conformation for recognition from neutrophils or macrophages | [116] |
| Extracellular fibrinogen-binding protein (Efb) | Staphylococcus aureus | Efb, is a virulence factor that is able to bind to complement components, C3b. Is able to inhibit complementary opsonization. | [117, 118] |
| Clumping factor A (ClfA) | Staphylococcus aureus | ClfA is the main fibrinogen-binding protein found in S. aureus during the stationary phase of growth. Through surface deposition of fibrinogen, the material becomes anti-opsonic. | [119] |
This design concept presents a marked increase in the efficiency MT-7 release compared to the work by Boomker since we would be able to create protein using cellular machinery, thereby circumventing the low transfection efficiencies that would be seen by administration of plasmid to the area of inflammation. Figure 7b demonstrates a possible design where cells transfected with the gene encoding for a virally evasive protein like MT-7 are encapsulated in a non-fouling hydrogel coating to allow for synthesis of MT-7 and diffusion into the surrounding cellular space. Of course, one caveat of this design is how the body will respond to virally-derived proteins. Current methods of cytokine based treatment involve the modification of human cytokines, so investigation would have to go into the immunogenicity of the proteins themselves.
Figure 7.
Outline of applications of different immune evasive tactics from virology and bacteriology to biomaterials. a: Outline of inflammation when immune system is allowed to function unfettered. b: Possible viral design. Virally derived proteins like MT-7 can be released from a coating around the biomaterial to occupy chemokine binding domains and limit formation of a chemokine gradient. c: Possible bacterial design: By coating the material in an artificial derivative of a capsular polysaccharide, deposition of blood borne proteins will decrease, thereby limiting opsonization and inflammatory response. A similar approach would be coating the material in bacterially derived proteins that will inhibit complement deposition. d: A possible combination of viral and bacterial mechanisms. By coating the material in an artificial derivative of a capsular polysaccharide, the surface will become anti-biofouling. Additionally, the presence of viroreceptors will bind cytokines with high specificity, inhibiting the cytokine’s ability to bind to cellular receptors.
4.2.2. Application of Bacterial Techniques
4.2.2.1. Precedent Technologies
Material implants are decorated with a number of features on their surfaces that may be easily recognized as foreign bodies by the body’s innate immune system. Therefore, it may be advantageous to employ similar techniques to those used by bacteria for immune attenuation in implants. The most obvious technique that may be utilized is the masking of surface features by a carbohydrate capsule. Research by Griesser et al., has provided the impetus for this approach by assessing protein absorption to nanometer thick immobilized layers of polysaccharides [115]. Additional work has been done by Bumgardner et al., on to show that chitosan, a derivative of the polysaccharide chitin, may bind to the surface of a titanium disc [65]. While the deposition of a generic polysaccharide like chitosan has been shown to discourage bio-fouling on the material surface, it could possibly be extended to induce anti-opsonic and anti-complement properties.
4.2.2.2. Future Directions
The capsules formed by the bacterium Staphylococcus aureus have been reported to have both anti-opsonic as well as anti-complement functions when cultured on solid media [46]. Of the eleven different serotypes of capsules that may be formed, most S. aureus strains exhibit serotype 5 capsular polysaccharides [46, 116]. This capsular polysaccharide has been isolated and its chemical composition and structure has been found [117]. It consists of D-glucose, D-galactose, 2-acteamido-2-deoxy-D-glucose, and sialic acid in a molar rato of 3:2:1:1. With this known structure and composition, it may be possible to synthesize this polysaccharide and to immobilize it onto the surface of a biomaterial. This would represent an improvement in the surface modification of materials with polysaccharides, as it would discourage opsonization and complement activity towards the implant. Such a design could be modified by the immobilization of bacterium-derived pro-inflammatory cytokine proteases to the material, thereby limiting further the presence of pro-inflammatory factors. One limitation of this method is the specificity with which the proteases act on proteins in the surrounding area. A schematic of the above designs is given in Figure 7c.
Bacterially derived factors may also be immobilized to the surfaces of materials to attenuate immune response. Protein A is a wall-anchored protein with either four or five domains that each bind to the Fc region of IgG antibodies [118]. The purpose of the interaction between protein A and IgG in the bacterium, S. aureus, is to coat the surface of the cell with IgG molecules that are in the incorrect orientation to be recognized by neutrophils or MØ Fc receptors. If a material surface could be immobilized with protein A, then the material could behave similar to S. aureus, negating the effects of IgG molecules. The S. aureus-secreted extracellular fibrinogen-binding protein, Efb, is a virulence factor that is able to bind to complement components, C3b [119, 120]. Lee et al., have shown that opsonophagocytosis may be inhibited by C3 binding to Efb in a dose-dependent manner [120]. A surface modified with a recombinant form of Efb will decrease incidents of biofouling by limiting opsonin-driven inflammatory responses. Similar anti-opsonic behavior could be achieved through the immobilization of clumping factor A to the surface of a material. Clumping factor A (ClfA) is the main fibrinogen-binding protein found in S. aureus during the stationary phase of growth[38]. Since fibrinogen may bind to ClfA on the bacterium surface, the cell itself has become anti-opsonic by virtue of the fact that opsonin cannot deposit on the surface [38]. Palmqvist et al., have utilized the ability of ClfA to induce an anti-opsonic state by showing that ClfA protected S. aureus from phagocytosis from both murine macrophages and neutrophils [121]. Table 3 lists possible bacterially derived factors that could be used to attenuate inflammation.
In addition, a possible strategy that would incorporate both viral and bacterial approaches could involve the complexation of viral cytokine receptor homologues with immobilized bacterially derived proteins. Figure 7d demonstrates possible strategies for the complexation of viral and bacterial techniques. Much like the complexation of the biofilm coating with pro-inflammatory cytokine proteinases, the complexation of bacterially derived proteins with these homologues would create a multi-tiered approach to immune attenuation. First, the bacterially derived proteins would limit opsonization and complement activity. Secondly, the viroreceptors would be able to bind preferentially to pro-inflammatory cytokines in the site of implantation, with the resulting complex inactivating the deleterious effects of the pro-inflammatory cytokines. This design could also be expanded to include virally derived proteins such as CrmA as well, limiting the formation of mature IL-1β, thereby decreasing the signals necessary for a pro-inflammatory response.
5. Conclusions and Perspectives
Currently, a number of different types of surface modifications have been employed to attenuate both acute phase and chronic immune responses as a result of material implantation. However, as new mechanisms for immune evasion are devised, it is important to note how biological bodies such viruses and bacteria have evolved immune evasive mechanisms over millennia. As the scientific community’s awareness of different viral and bacterial immune evasive properties comes to light, we as biomedical engineers may be able to mimic their machinery for novel approaches to improving material biocompatibility. It is therefore our hope that unique biomimetic paradigms for biocompatible design based upon long standing knowledge of the machinery at work within different biological bodies will advance the life of material implants, thereby increasing implant efficacy and patient well-being.
Acknowledgments
This work was supported by an NIH Biotechnology Predoctoral Fellowship, GM 8555, as well as NIH Grant DK 54932 (MTN and WMR). The authors would also like to thank the input of Dr. David J. Pickup, Dr. Anthony R. Geonnotti, Dr. Charles C. Anamelechi, and Robert D. Kirkton during the writing process.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson JM. Biological Responses to Materials. Annual review of materials science. 2001;31(1):81. [Google Scholar]
- 2.Kuby J, Kindt T, Osborne B, Goldsby R. Immunology. New York, New York: W.H. Freeman and Co; 2006. [Google Scholar]
- 3.Li Y, Schutte RJ, Abu-Shakra A, Reichert WM. Protein array method for assessing in vitro biomaterial-induced cytokine expression. Biomaterials. 2005;26(10):1081–1085. doi: 10.1016/j.biomaterials.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Thomson AW, Lotze MT. The cytokine handbook. 4. San Diego, CA: Academic Press; 2003. [Google Scholar]
- 5.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117(4):1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 6.Hanada T. Regulation of cytokine signaling and inflammation. Cytokine & growth factor reviews. 2002;13(4–5):413. doi: 10.1016/s1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 7.Gordon S. Alternative activation of macrophages. Nature reviews Immunology. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Mosser D. The many faces of macrophage activation. Journal of leukocyte biology. 2003;73(2):209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 10.Deonarine K, Panelli MC, Stashower ME, Jin P, Smith K, Slade HB, et al. Gene expression profiling of cutaneous wound healing. Journal of translational medicine. 2007;5(1):11. doi: 10.1186/1479-5876-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNally AK, Anderson JM. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. The American journal of pathology. 1995;147(5):1487–1499. [PMC free article] [PubMed] [Google Scholar]
- 12.Szmukler-Moncler S, Salama H, Reingewirtz Y, Dubruille JH. Timing of loading and effect of micromotion on bone-dental implant interface: review of experimental literature. Journal of biomedical materials research. 1998;43(2):192–203. doi: 10.1002/(sici)1097-4636(199822)43:2<192::aid-jbm14>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Koschwanez HE, Reichert WM. In vitro, in vivo and post explantation testing of glucose-detecting biosensors: current methods and recommendations. Biomaterials. 2007;28(25):3687–3703. doi: 10.1016/j.biomaterials.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konttinen Y, Zhao D, Beklen A, Ma G, Takagi M, Kivela-Rajamaki M, et al. The Microenvironment around Total Hip Replacement Protheses. Clinical Orthopaedics and Related Research. 2005;430:28–38. doi: 10.1097/01.blo.0000150451.50452.da. [DOI] [PubMed] [Google Scholar]
- 15.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. Journal of Neuroscience Methods. 2005;148(1):1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Boomker JM. Viral chemokine-modulatory proteins: tools and targets. Cytokine & growth factor reviews. 2005;16(1):91. doi: 10.1016/j.cytogfr.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Benedict CA. Viruses and the TNF-related cytokines, an evolving battle. Cytokine & growth factor reviews. 2003;14(3–4):349–357. doi: 10.1016/s1359-6101(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 18.Brodsky FM. Stealth, Sabotage, and Exploitation. Immunological Reviews. 1999;168:5–11. doi: 10.1111/j.1600-065x.1999.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 19.Finlay B. Anti-Immunology: Evasion of the Host Immune System by Bacterial and Viral Pathogens. Cell. 2006;124(4):767. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 20.Cantin R, Méthot S, Tremblay MJ. Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. Journal of virology. 2005;79(11):6577–6587. doi: 10.1128/JVI.79.11.6577-6587.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosma A, Blanc D, Braun J, Quillent C, Barassi C, Moog C, et al. Enhanced HIV infectivity and changes in GP120 conformation associated with viral incorporation of human leucocyte antigen class I molecules. AIDS. 1999;13(15):2033–2042. doi: 10.1097/00002030-199910220-00005. [DOI] [PubMed] [Google Scholar]
- 22.Alcami A. Viral mimicry of cytokines, chemokines and their receptors. Nature reviews Immunology. 2003;3(1):36–50. doi: 10.1038/nri980. [DOI] [PubMed] [Google Scholar]
- 23.Alcami A. Viral mechanisms of immune evasion. Molecular Medicine Today. 2000;6(9):365. doi: 10.1016/S1357-4310(00)01775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tortorella D. Viral Subversion of the Immune System. Annual review of immunology. 2000;18(1):861. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 25.Fleming SB, McCaughan CA, Andrews AE, Nash AD, Mercer AA. A homolog of interleukin-10 is encoded by the poxvirus orf virus. Journal of virology. 1997;71(6):4857–4861. doi: 10.1128/jvi.71.6.4857-4861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imlach W, McCaughan CA, Mercer AA, Haig D, Fleming SB. Orf virus-encoded interleukin-10 stimulates the proliferation of murine mast cells and inhibits cytokine synthesis in murine peritoneal macrophages. The Journal of general virology. 2002;83(Pt 5):1049–1058. doi: 10.1099/0022-1317-83-5-1049. [DOI] [PubMed] [Google Scholar]
- 27.Redpath S, Ghazal P, Gascoigne NRJ. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends in Microbiology. 2001;9(2):86–92. doi: 10.1016/s0966-842x(00)01919-3. [DOI] [PubMed] [Google Scholar]
- 28.Kotenko SV, Saccani S, Izotova LS, Mirochnitchenko OV, Pestka S. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10) Proceedings of the National Academy of Sciences of the United States of America. 2000;97(4):1695–1700. doi: 10.1073/pnas.97.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wise LM, Veikkola T, Mercer AA, Savory LJ, Fleming SB, Caesar C, et al. Vascular endothelial growth factor (VEGF)-like protein from orf virus NZ2 binds to VEGFR2 and neuropilin-1. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(6):3071–3076. doi: 10.1073/pnas.96.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenkilde MM, Waldhoer M, Lüttichau HR, Schwartz TW. Virally encoded 7TM receptors. Oncogene. 2001;20(13):1582–1593. doi: 10.1038/sj.onc.1204191. [DOI] [PubMed] [Google Scholar]
- 31.Webb LM, Alcami A. Virally encoded chemokine binding proteins. Mini reviews in medicinal chemistry. 2005;5(9):833–848. doi: 10.2174/1389557054867110. [DOI] [PubMed] [Google Scholar]
- 32.Smith CA, Davis T, Wignall JM, Din WS, Farrah T, Upton C, et al. T2 open reading frame from the Shope fibroma virus encodes a soluble form of the TNF receptor. Biochemical and biophysical research communications. 1991;176(1):335–342. doi: 10.1016/0006-291x(91)90929-2. [DOI] [PubMed] [Google Scholar]
- 33.Spriggs MK, Hruby DE, Maliszewski CR, Pickup DJ, Sims JE, Buller RM, et al. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell. 1992;71(1):145–152. doi: 10.1016/0092-8674(92)90273-f. [DOI] [PubMed] [Google Scholar]
- 34.Alcami A. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71(1):153. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- 35.Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, et al. Enterobacter bacteremia- clinical features and emergence of antibiotic-resistance during therapy. Annals of internal medicine. 1991;115(8):585–590. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 36.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264(5157):375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 37.Foster TJ. Immune evasion by staphylococci. Nature reviews Microbiology. 2005;3(12):948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 38.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 39.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, et al. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cellular microbiology. 2004;6(3):269–275. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 40.Li M, Lai Y, Villaruz AE, Cha DJ, Sturdevant DE, Otto M. Gram-positive three-component antimicrobial peptide-sensing system. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(22):9469. doi: 10.1073/pnas.0702159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rooijakkers SHM, van Strijp JAG. Bacterial complement evasion. Molecular immunology. 2007;44(1–3):23–32. doi: 10.1016/j.molimm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Flahaut S, Vinogradov E, Kelley KA, Brennan S, Hiramatsu K, Lee JC. Structural and biological characterization of a capsular polysaccharide produced by Staphylococcus haemolyticus. Journal of bacteriology. 2007;190(5):1649–1657. doi: 10.1128/JB.01648-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vuong C. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. The Journal of biological chemistry. 2004;279(52):54881–54886. doi: 10.1074/jbc.M411374200. [DOI] [PubMed] [Google Scholar]
- 44.Thakker M. Staphylococcus aureus Serotype 5 Capsular Polysaccharide Is Antiphagocytic and Enhances Bacterial Virulence in a Murine Bacteremia Model. Infection and immunity. 1998;66(11):5183. doi: 10.1128/iai.66.11.5183-5189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart PS. Implications of reaction-diffusion theory for the disinfection of microbial biofilms by reactive antimicrobial agents. Chemical engineering science. 1995;50(19):3099. [Google Scholar]
- 46.Stewart PS, Grab L, Diemer JA. Analysis of biocide transport limitation in an artificial biofilm system. Journal of applied microbiology. 1998;85(3):495–500. doi: 10.1046/j.1365-2672.1998.853529.x. [DOI] [PubMed] [Google Scholar]
- 47.Wu L, Estrada O, Zaborina O, Bains M, Shen L, Kohler JE, et al. Recognition of host immune activation by Pseudomonas aeruginosa. Science. 2005;309(5735):774–777. doi: 10.1126/science.1112422. [DOI] [PubMed] [Google Scholar]
- 48.Wagner VE, Frelinger JG, Barth RK, Iglewski BH. Quorum sensing: dynamic response of Pseudomonas aeruginosa to external signals. Trends in Microbiology. 2006;14(2):55–58. doi: 10.1016/j.tim.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Walker TS, Tomlin KL, Worthen GS, Poch KR, Lieber JG, Saavedra MT, et al. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infection and immunity. 2005;73(6):3693–3701. doi: 10.1128/IAI.73.6.3693-3701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandra J, McCormick TS, Imamura Y, Mukherjee PK, Ghannoum MA. Interaction of Candida albicans with adherent human peripheral blood mononuclear cells increases C-albicans biofilm formation and results in differential expression of pro- and anti-inflammatory cytokines. Infection and immunity. 2007;75(5):2612–2620. doi: 10.1128/IAI.01841-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang M, Gong JH, Yang ZH, Samten B, Cave MD, Barnes PF. Enhanced capacity of a widespread strain of Mycobacterium tuberculosis to grow in human macrophages. The journal of infectious diseases. 1999;179(5):1213–1217. doi: 10.1086/314738. [DOI] [PubMed] [Google Scholar]
- 52.Manca C, Tsenova L, Barry CE, Bergtold A, Freeman S, Haslett PA, et al. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. The journal of immunology. 1999;162(11):6740–6746. [PubMed] [Google Scholar]
- 53.Othieno C, Hirsch CS, Hamilton BD, Wilkinson K, Ellner JJ, Toossi Z. Interaction of Mycobacterium tuberculosis-induced transforming growth factor beta 1 and interleukin-10. Infection and immunity. 1999;67(11):5730–5735. doi: 10.1128/iai.67.11.5730-5735.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenberger C, Finlay B. Phagocyte sabotage: Disruption of macrophage signalling by bacterial pathogens. Nature reviews Molecular cell biology. 2003;4(5):385–396. doi: 10.1038/nrm1104. [DOI] [PubMed] [Google Scholar]
- 55.Jotwani R, Pulendran B, Agrawal S, Cutler CW. Human dendritic cells respond to Porphyromonas gingivalis LPS by promoting a Th2 effector response in vitro. European Journal of Immunology. 2003;33(11):2980–2986. doi: 10.1002/eji.200324392. [DOI] [PubMed] [Google Scholar]
- 56.Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, van Dyke T, Bancherau J. The journal of immunology. 9. Vol. 167. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo; pp. 5067–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Kooyk Y, Geijtenbeek TBH. DC-sign: Escape mechanism for pathogens. Nature reviews Immunology. 2003;3(9):697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 58.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and toll-like receptor 2. The Journal of experimental medicine. 2003;197(9):1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calkins CC, Platt K, Potempa J, Travis J. Inactivation of tumor necrosis factor-alpha by proteinases (gingipains) from the periodontal pathogen, Porphyromonas gingivalis - Implications of immune evasion. The Journal of biological chemistry. 1998;273(12):6611–6614. doi: 10.1074/jbc.273.12.6611. [DOI] [PubMed] [Google Scholar]
- 60.Wisniewski N. Methods for reducing biosensor membrane biofouling. Colloids and surfaces B, Biointerfaces. 2000;18(3–4):197–219. doi: 10.1016/s0927-7765(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 61.Lewis A. Phosphorylcholine-based polymers and their use in the prevention of biofouling. Colloids and surfaces B, Biointerfaces. 2000;18(3–4):261–275. doi: 10.1016/s0927-7765(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 62.Wisniewski N, Moussy F, Reichert WM. Characterization of implantable biosensor membrane biofouling. Fresenius’ journal of analytical chemistry. 2000;366(6–7):611–621. doi: 10.1007/s002160051556. [DOI] [PubMed] [Google Scholar]
- 63.Bumgardner JD, Wiser R, Elder SH, Jouett R, Yang Y, Ong JL. Contact angle, protein adsorption and osteoblast precursor cell attachment to chitosan coatings bonded to titanium. Journal of biomaterials science Polymer edition. 2003;14(12):1401–1409. doi: 10.1163/156856203322599734. [DOI] [PubMed] [Google Scholar]
- 64.Dalsin J, Lin L, Tosatti S, Voros J, Textor M, Messersmith P. Protein resistance of titanium oxide surfaces modified by biologically inspired mPEG-DOPA. Langmuir. 2005;21(2):640–646. doi: 10.1021/la048626g. [DOI] [PubMed] [Google Scholar]
- 65.Statz A, Meagher RJ, Barron AE, Messersmith P. New peptidomimetic polymers for antifouling surfaces. Journal of the American Chemical Society. 2005;127(22):7972–7973. doi: 10.1021/ja0522534. [DOI] [PubMed] [Google Scholar]
- 66.Das M. Microgels: old materials with new applications. Annual review of materials science. 2006;36(1):117. [Google Scholar]
- 67.Nolan C, Reyes CD, Debord JD, Garcia AJ, Lyon LA. Phase transition behavior, protein adsorption, and cell adhesion resistance of poly(ethylene glycol) cross-linked microgel particles. Biomacromolecules. 2005;6(4):2032–2039. doi: 10.1021/bm0500087. [DOI] [PubMed] [Google Scholar]
- 68.Bridges AW, Singh N, Burns K, Babensee JE, Lyon LA, Garcia AJ. Non-fouling microgel coatings reduce inflammatory responses to implanted biomaterials. 8th World Biomaterials Congress; Amsterdam, Netherlands. 2008. [Google Scholar]
- 69.Singh N, Bridges AW, Garcia AJ, Lyon LA. Covalent tethering of functional microgel films onto poly(ethylene terephthalate) surfaces. Biomacromolecules. 2007;8(10):3271–3275. doi: 10.1021/bm700516v. [DOI] [PubMed] [Google Scholar]
- 70.Mayorga L, Ratner BD, Horbett TA. The role of complement adsorption and activation in monocyte adhesion to ultralow protein adsorption surfaces made by RF plasma deposition of PEO-like tetraethylene glycol dimethyl ether (tetraglyme). 8th World Biomaterials Congress; Amsterdam, Netherlands. 2008. [Google Scholar]
- 71.Barnes P, Adcock I, Spedding M, Vanhoutte PM. Antiinflammatory actions of steroids-molecular mechanisms. Trends in Pharmacological Sciences. 1993;14(12):436–441. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- 72.Indolfi C, Mongiardo A, Curcio A, Torella D. Molecular mechanisms of in-stent restenosis and approach to therapy with eluting stents. Trends in Cardiovascular Medicine. 2003;13(4):142–148. doi: 10.1016/s1050-1738(03)00038-0. [DOI] [PubMed] [Google Scholar]
- 73.Norton LW, Tegnell E, Toporek SS, Reichert WM. In vitro characterization of vascular endothelial growth factor and dexamethasone releasing hydrogels for implantable probe coatings. Biomaterials. 2005;26(16):3285–3297. doi: 10.1016/j.biomaterials.2004.07.069. [DOI] [PubMed] [Google Scholar]
- 74.Hickey T, Kreutzer D, Burgess DJ, Moussy F. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials. 2002;23(7):1649–1656. doi: 10.1016/s0142-9612(01)00291-5. [DOI] [PubMed] [Google Scholar]
- 75.Hickey T, Kreutzer D, Burgess DJ, Moussy F. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. Journal of biomedical materials research. 2002;61(2):180–187. doi: 10.1002/jbm.10016. [DOI] [PubMed] [Google Scholar]
- 76.Lincoff AM, Furst JG, Ellis SG, Tuch RJ, Topol EJ. Sustained local delivery of dexamethasone by a novel intravascular eluting stent to prevent restenosis in the porcine coronary injury model. Journal of the American College of Cardiology. 1997;29(4):808–816. doi: 10.1016/s0735-1097(96)00584-0. [DOI] [PubMed] [Google Scholar]
- 77.Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. Journal of biomedical materials research Part A. 2007;81(4):858–869. doi: 10.1002/jbm.a.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moussy Y, Hersh L, Dungel P. Distribution of [H-3]dexamethasone in rat subcutaneous tissue after delivery from osmotic pumps. Biotechnology progress. 2006;22(3):819–824. doi: 10.1021/bp060031j. [DOI] [PubMed] [Google Scholar]
- 79.Patil SD, Papadimitrakopoulos F, Burgess DJ. Dexamethasone-loaded poly(lactic-co-glycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes technology & therapeutics. 2004;6(6):887–897. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- 80.Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. Journal of Controlled Release. 2007;117(1):68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 81.Komeda M, Tabata Y. Biomaterials technology of growth factor release realizes clinical angiogenic therapy. 8th World Biomaterials Congress; Amsterdam, Netherlands. 2008. [Google Scholar]
- 82.Bresnihan B. Treatment of rheumatoid arthritis with interleukin 1 receptor antagonist. Arthritis and rheumatism. 1998;41(12):2196. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 83.Shamji MF, Betre H, Kraus VB, Chen J, Chilkoti A, Pichika R, et al. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: sustained release of a local antiinflammatory therapeutic. Arthritis and rheumatism. 2007;56(11):3650–3661. doi: 10.1002/art.22952. [DOI] [PubMed] [Google Scholar]
- 84.Feldmann M, Maini RN. Anti-TNFα therapy of rheumatoid arthritis: What Have We Learned? Annual review of immunology. 2001;19(1):163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 85.Madhusudan S, Muthuramalingam S, Braybrooke J, Wilner S, Kaur K, Han C, et al. Study of Etanercept, a Tumor Necrosis Factor-Alpha Inhibitor, in Recurrent Ovarian Cancer. Journal of clinical oncology. 2005;23(25):5950. doi: 10.1200/JCO.2005.04.127. [DOI] [PubMed] [Google Scholar]
- 86.Hetrick EM. Reduced foreign body response at nitric oxide-releasing subcutaneous implants. Biomaterials. 2007;28(31):4571. doi: 10.1016/j.biomaterials.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chemical Society reviews. 2006;35(9):780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 88.Nablo BJ, Prichard HL, Butler RD, Klitzman B, Schoenfisch MH. Inhibition of implant-associated infections via nitric oxide release. Biomaterials. 2005;26(34):6984–6990. doi: 10.1016/j.biomaterials.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 89.Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289(5483):1352–1355. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 90.Jorgensen C, Apparailly F, Canovas F, Verwaerde C, Auriault C, Jacquet C, et al. Systemic viral interleukin-10 gene delivery prevents cartilage invasion by human rheumatoid synovial tissue engrafted in SCID mice. Arthritis and rheumatism. 1999;42(4):678–685. doi: 10.1002/1529-0131(199904)42:4<678::AID-ANR10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 91.Ma Y, Thornton S, Duwel LE, Boivin GP, Giannini EH, Leiden JM, et al. Inhibition of collagen-induced arthritis in mice by viral IL-10 gene transfer. The journal of immunology. 1998;161(3):1516–1524. [PubMed] [Google Scholar]
- 92.Whalen J, Lechman EL, Carlos CA, Weiss K, Kovesdi I, Glorioso JC, et al. Adenoviral transfer of the viral IL-10 gene periarticularly to mouse paws suppresses development of collagen-induced arthritis in both injected and uninjected paws. The journal of immunology. 1999;162(6):3625–3632. [PubMed] [Google Scholar]
- 93.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy--review of a new approach. Pharmacological reviews. 2003;55(2):241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 94.Akdis CA. Mechanisms of interleukin-10-mediated immune suppression. Immunology. 2001;103(2):131. doi: 10.1046/j.1365-2567.2001.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ito Y, Liu SQ, Imanishi Y. Enhancement of cell growth on growth factor-immobilized polymer film. Biomaterials. 1991;12(5):449–453. doi: 10.1016/0142-9612(91)90141-v. [DOI] [PubMed] [Google Scholar]
- 96.Ito Y, Hasuda H, Yamauchi T, Komatsu N, Ikebuchi K. Immobilization of erythropoietin to culture erythropoietin-dependent human leukemia cell line. Biomaterials. 2004;25(12):2293–2298. doi: 10.1016/j.biomaterials.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 97.Kim DH, Smith JT, Chilkoti A, Reichert WM. The effect of covalently immobilized rhIL-1ra-ELP fusion protein on the inflammatory profile of LPS-stimulated human monocytes. Biomaterials. 2007;28(23):3369–3377. doi: 10.1016/j.biomaterials.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kato K, Sato H, Iwata H. Immobilization of histidine-tagged recombinant proteins onto micropatterned surfaces for cell-based functional assays. Langmuir. 2005;21(16):7071–7075. doi: 10.1021/la050893e. [DOI] [PubMed] [Google Scholar]
- 99.Nakaji-Hirabayashi T, Kato K, Arima Y, Iwata H. Oriented immobilization of epidermal growth factor onto culture substrates for the selective expansion of neural stem cells. Biomaterials. 2007;28(24):3517–3529. doi: 10.1016/j.biomaterials.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 100.Nakaji-Hirabayshi T, Kato K, Arima Y, Iwata H. Epidermal growth factor signaling at cell-substrate interfaces. 8th World Biomaterials Congress; Amsterdam, Netherlands. 2008. [Google Scholar]
- 101.Stefonek T, Masters KS. Immobilized gradients of epidermal growth factor promote accelerated and directed keratinocyte migration. Wound repair and regeneration. 2007;15(6):847–855. doi: 10.1111/j.1524-475X.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 102.Liu L, Ratner BD, Sage EH, Jiang S. Endothelial cell migration on surface-density gradients of fibronectin, VEGF, or both proteins. Langmuir. 2007;23(22):11168–11173. doi: 10.1021/la701435x. [DOI] [PubMed] [Google Scholar]
- 103.DeLong S, Moon JL, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26(16):3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 104.Stefonek TJ, Masters KS. Immobilized growth factor gradients to direct keratinocyte migration. 8th World Biomaterials Congress; Amsterdam, Netherlands. 2008. [Google Scholar]
- 105.Ito Y. Covalently immobilized biosignal molecule materials for tissue engineering. Soft matter. 2008;4(1):46–56. doi: 10.1039/b708359a. [DOI] [PubMed] [Google Scholar]
- 106.Prichard HL, Reichert WM, Klitzman B. Adult adipose-derived stem cell attachment to biomaterials. Biomaterials. 2007;28(6):936–946. doi: 10.1016/j.biomaterials.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prichard HL, Reichert W, Klitzman B. IFATS Series: Adipose-Derived Stromal Cells Improve The Foreign Body Response. Stem Cells. 2008 April 24; doi: 10.1634/stemcells.2008-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boomker JM. The modulation of angiogenesis in the foreign body response by the poxviral protein M-T 7. Biomaterials. 2005;26(23):4874. doi: 10.1016/j.biomaterials.2004.11.059. [DOI] [PubMed] [Google Scholar]
- 109.Smith S. Conserved surface-exposed K/R-X-K/R motifs and net positive charge on poxvirus complement control proteins serve as putative heparin binding sites and contribute to inhibition of molecular interactions with human endothelial cells: a novel mechanism for evasion of host defense. Journal of virology. 2000;74(12):5659–5666. doi: 10.1128/jvi.74.12.5659-5666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rowley J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 111.Park H. Delivery of TGF-beta 1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials. 2005;26(34):7095–7103. doi: 10.1016/j.biomaterials.2005.05.083. [DOI] [PubMed] [Google Scholar]
- 112.Gombotz W. Protein release from alginate matrices. Advanced Drug Delivery Reviews. 1998;31(3):267–285. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 113.Griesser HJ. Interfacial properties and protein resistance of nano-scale polysaccharide coatings. Smart materials & structures. 2002;11(5):652. [Google Scholar]
- 114.Arbeit RD, Karakawa WW, Vann WF, Robbins JB. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagnostic Microbiology and Infectious Disease. 1984;2(2):85–91. doi: 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- 115.Wessels MR, DiFabio JL, Benedì VJ, Kasper DL, Michon F, Brisson JR, et al. Structural determination and immunochemical characterization of the type V group B Streptococcus capsular polysaccharide. The Journal of biological chemistry. 1991;266(11):6714–6719. [PubMed] [Google Scholar]
- 116.Kessler SW. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. The journal of immunology. 1975;115(6):1617–1624. [PubMed] [Google Scholar]
- 117.Lee LYL, Liang X, Hook M, Brown EL. Identification and characterization of the C3 binding domain of the Staphylococcus aureus extracellular fibrinogen-binding protein (Efb) The Journal of biological chemistry. 2004;279(49):50710–50716. doi: 10.1074/jbc.M408570200. [DOI] [PubMed] [Google Scholar]
- 118.Lee LYL, Hook M, Haviland D, Wetsel RA, Yoster EO, Syribeys P, et al. Inhibition of complement activation by a secreted Staphylococcus aureus protein. The journal of infectious diseases. 2004;190(3):571–579. doi: 10.1086/422259. [DOI] [PubMed] [Google Scholar]
- 119.Palmqvist N, Patti JM, Tarkowski A, Josefsson E. Expression of staphylococcal clumping factor A impedes macrophage phagocytosis. Microbes and infection. 2004;6(2):188–195. doi: 10.1016/j.micinf.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 120.Jeon SI, Lee JH, Andrade JD, De Gennes PG. Protein-surface interactions in the presence of polyethylene oxide. Journal of colloid and interface science. 1991;142(1):149. [Google Scholar]
- 121.López GP, Ratner BD, Tidwell CD, Haycox CL, Rapoza RJ, Horbett TA. Glow discharge plasma deposition of tetraethylene glycol dimethyl ether for fouling-resistant biomaterial surfaces. Journal of biomedical materials research. 1992;26(4):415–439. doi: 10.1002/jbm.820260402. [DOI] [PubMed] [Google Scholar]
- 122.Sud S, Yang SY, Evans CH, Robbins PD, Wooley PH. Effects of cytokine gene therapy on particulate-induced inflammation in the murine air pouch. Inflammation. 2001;25(6):361–372. doi: 10.1023/a:1012898513512. [DOI] [PubMed] [Google Scholar]
- 123.Wooley PH, Schwarz EM. Aseptic loosening. Gene therapy. 2004;11(4):402–407. doi: 10.1038/sj.gt.3302202. [DOI] [PubMed] [Google Scholar]
- 124.Higuchi N, Maruyama H, Kuroda T, Kameda S, Iino N, Kawachi H, et al. Hydrodynamics-based delivery of the viral interleukin-10 gene suppresses experimental crescentic glomerulonephritis in Wistar-Kyoto rats. Gene therapy. 2003;10(16):1297–1310. doi: 10.1038/sj.gt.3301988. [DOI] [PubMed] [Google Scholar]
- 125.Woods AM, Thompson SJ, Wooley PH, Panayi G, Klavinskis LS. Immune modulation of collagen-induced arthritis by intranasal cytokine gene delivery: a model for the therapy of rheumatoid arthritis. Arthritis and rheumatism. 2005;52(12):3761–3771. doi: 10.1002/art.21473. [DOI] [PubMed] [Google Scholar]
- 126.Lee KY, Yuk SH. Polymeric protein delivery systems. Progress in polymer science. 2007;32(7):669–697. [Google Scholar]
- 127.Coviello T, Matricardi P, Marianecci C, Alhaique F. Polysaccharide hydrogels for modified release formulations. Journal of Controlled Release. 2007;119(1):5–24. doi: 10.1016/j.jconrel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 128.Van Thienen T, Demeester J, De Smedt SC. Screening poly(ethyleneglycol) micro- and nanogels for drug delivery purposes. International Journal of Pharmaceutics. 2008;351(1–2):174–185. doi: 10.1016/j.ijpharm.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 129.Tabata Y, Ikada Y. Protein release from gelatin matrices. Advanced Drug Delivery Reviews. 1998;31(3):287–301. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 130.Zhao X. Synthesis and characterization of a novel hyaluronic acid hydrogel. Journal of biomaterials science Polymer edition. 2006;17(4):419–433. doi: 10.1163/156856206776374115. [DOI] [PubMed] [Google Scholar]
- 131.Saraiva M, Alcami A. CrmE, a novel soluble tumor necrosis factor receptor encoded by poxviruses. Journal of virology. 2001;75(1):226–233. doi: 10.1128/JVI.75.1.226-233.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiang Y, Moss B. IL-18 binding and inhibition of interferon gamma induction by human poxvirus-encoded proteins. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(20):11537–11542. doi: 10.1073/pnas.96.20.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deane D, McInnes CJ, Percival A, Wood A, Thomson J, Lear A, et al. Orf virus encodes a novel secreted protein inhibitor of granulocyte-macrophage colony-stimulating factor and interleukin-2. Journal of virology. 2000;74(3):1313–1320. doi: 10.1128/jvi.74.3.1313-1320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kledal TN, Rosenkilde M, Coulin F, Simmons G, Johnsen AH, Alouani S, et al. A broad-spectrum chemokine antagonist encoded by Kaposi’s sarcoma-associated herpesvirus. Science. 1997;277(5332):1656–1659. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 135.Alcami A, Symons JA, Collins PD, Williams TJ, Smith GL. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. The journal of immunology. 1998;160(2):624–633. [PubMed] [Google Scholar]
- 136.Lalani AS, Graham K, Mossman K, Rajarathnam K, Clark-Lewis I, Kelvin D, et al. The purified myxoma virus gamma interferon receptor homolog M-T7 interacts with the heparin-binding domains of chemokines. Journal of virology. 1997;71(6):4356–4363. doi: 10.1128/jvi.71.6.4356-4363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Walker BD, Burton DR. Toward an AIDS Vaccine. Science. 2008 May 9;320(5877):760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]