Abstract
The majority of lymphomas arising in the thyroid gland are MALT lymphomas and diffuse large B cell lymphomas, which arise from a background of chronic lymphocytic thyroiditis. Follicular lymphoma may also present in the thyroid gland, but its clinicopathological features at this site are not well characterised, leading to difficulties in diagnosis and clinical management. We have addressed this problem by studying the clinical, morphological, immunophenotypic and genetic features of 22 such cases. All cases showed morphology characteristic of follicular lymphoma, However, in many the interfollicular neoplastic infiltrate was particularly prominent and all lymphomas contained readily identifiable and often striking lymphoepithelial lesions, features heretofore considered indicative of MALT lymphoma at this site. Furthermore, 13 of 18 cases for whom sufficient evidence was available had clinical and/or histological evidence of chronic lymphocytic thyroiditis. Analysis of genetic and immunohistochemical features identified two distinct groups. In one group, similar to typical adult follicular lymphoma, cases carried a t(14;18)/IGH-BCL2 and/or expressed Bcl2, and were mostly CD10-positive and of WHO grade 1-2. Follicular lymphomas in the other group lacked IGH-BCL2 and Bcl-2 expression, were often of WHO grade 3 and were often CD10-negative, similar to the minority of follicular lymphomas previously described that are Bcl-2-negative and are often encountered at other extranodal sites. The two groups differed in clinical stage at presentation, 11 patients in the former group but none in the latter group having disease beyond the thyroid gland. Appreciation of the spectrum of morphological, immunophenotypic and genetic characteristics of follicular lymphoma presenting in the thyroid gland should aid both diagnosis and clinical management.
Keywords: Thyroid gland, follicular lymphoma, extranodal lymphoma
INTRODUCTION
Lymphoma involves the thyroid gland uncommonly, and may do so as primary, localised disease, as the first clinical presentation of disseminated lymphoma, or rarely as secondary involvement in patients with known lymphoma at other sites. Primary lymphoma of the thyroid gland represents 2-5% of extranodal non-Hodgkin lymphomas, occurs approximately three times more frequently in women than in men, and typically affects those over 50 years of age (median age, 60-65 years) (2,9,10,13,20,50). The majority of these lymphomas arises on a background of chronic lymphocytic / Hashimoto thyroiditis, the acquired auto-reactive lymphoid infiltrate of which is thought to provide the substrate for lymphoma development (9,13,29,50). Many early investigators, using previous classification schemes, considered most primary thyroid lymphomas to be of follicle centre cell origin (1,3,26,45,46). However, following the identification of mucosa associated lymphoid tissue (MALT) lymphoma (extranodal marginal zone lymphoma of MALT type) as a discrete clinicopathological entity (30,33), it is now recognised that most primary thyroid lymphomas are in fact MALT lymphomas with (approximately 35% of thyroid lymphomas) or without (approximately 20%) a diffuse large B cell lymphoma (DLBCL) component (13,44,50,58,63). Most of the remaining primary thyroid lymphomas (approximately 40% of cases) are DLBCL without evidence of MALT lymphoma (13,44,50,58,63). In six large contemporary studies of the pathology of primary thyroid lymphomas, follicular lymphomas represented only 3-5% of all cases (13,25,44,50,58,63).
The histological appearances of MALT lymphoma of the thyroid gland have been well described (13,29,33,34). As at other mucosal sites, reactive lymphoid follicles are surrounded by an expanse of neoplastic marginal zone cells, which show frequent and often striking plasma cell differentiation. The lymphoma cells typically form prominent lymphoepithelial lesions with the thyroid acini and in some cases colonise reactive germinal centres producing a nodular architecture which may be mistaken morphologically for follicular lymphoma (13,31,34). Four main recurrent chromosomal translocations have been identified in MALT lymphomas: t(11;18)(q21;q21)(API2-MALT1) (14,70), t(1;14)(p22;q32)(BCL10-IGH) (68,70,69), t(14;18)(q32;q21)(IGH-MALT1) (59,69), and t(3;14)(p14;q32)(FOXP1-IGH) (62). These are found with varying frequencies in MALT lymphomas at different sites but, with the possible exception of t(3;14)(p14;q32), occur very rarely or not at all in the thyroid gland (51,61,62,69). MALT lymphomas of the thyroid tend to present with localised disease, and usually respond favourably to therapy with complete remission in almost all cases and only rare relapses (13,64,67,72,73). In contrast, follicular lymphoma is a neoplasm of germinal centre B cells which typically presents with nodal or disseminated disease (48,53). Most patients experience a protracted clinical course characterised by transient responses to therapy and multiple relapses, often ending in death from resistant disease or transformation to DLBCL (36,53). Histologically, follicular lymphomas usually grow with a predominantly follicular pattern and a variable, but smaller, interfollicular component (17,48). In more than 90% of nodal cases neoplastic follicle centre cells show strong aberrant expression of the anti-apoptotic protein Bcl-2, in most instances as a result of a t(14;18)(q32;q21) which juxtaposes BCL2 and the immunoglobulin heavy chain gene (IGH) (28,43,48). Given the different biology and natural history of these two entities, accurate distinction between them at diagnosis is important for optimal patient management. However, our experience suggests that this distinction is frequently difficult for general surgical pathologists and specialist haematopathologists alike, in part because the immunohistological and molecular genetic features of follicular lymphoma in the thyroid gland have not been well described.
Although primary extranodal presentation of follicular lymphoma is relatively uncommon, it is now recognised that follicular lymphomas arising at some extranodal sites have distinct clinicopathological characteristics. For example, follicular lymphomas arising within the skin or the testis more often remain localised, more often lack t(14;18)(IGH-BCL2) rearrangements and Bcl-2 protein expression, and have a better clinical outcome than nodal follicular lymphomas (5,40). Recent studies have suggested that these properties may also extend to follicular lymphomas arising in other extranodal tissues (23,22), but whether cases of primary follicular lymphoma of the thyroid gland might share similar features is unknown. In order to address this issue, and to identify features which may facilitate the recognition of follicular lymphoma presenting in the thyroid gland in diagnostic practice, we have studied the clinical, histological, immunophenotypic, and molecular features of 22 such cases.
MATERIALS AND METHODS
Case material
Twenty-two cases of follicular lymphoma presenting in the thyroid gland were identified from the surgical pathology archives of University College London Hospitals, London, UK; The Royal Marsden Hospital, London, UK; and University of British Columbia / British Columbia Cancer Agency, Vancouver, British Columbia, Canada. Representative sections from thyroidectomy or diagnostic biopsy specimens were examined in each case by three or four haematopathologists (CB, AD, PI, +/- RG or AW). Follicular lymphoma and DLBCL were diagnosed, and follicular lymphoma was graded, according to established WHO criteria (48). Disease stage was determined according to Musshoff's modification of the Ann Arbor staging system (7).
Immunohistochemistry
Immunohistochemical staining was performed on paraffin-embedded tissue sections following heat-mediated antigen retrieval or (for CD21 only) chymotrypsin pre-treatment using antibodies as follows: CD20 (L26), CD3 (polyclonal), CD21 (1F8), CD138 (MI15), Bcl-6 (PG-B6p), Bcl-2 (124), IgD (polyclonal), IgM (polyclonal), IgA (polyclonal), IgG (polyclonal), Ki-67 (MIB-1), kappa light chain (polyclonal), lambda light chain (polyclonal) (all from Dako Ltd., Ely, UK), CD10 (56C6), CD43 (MT1) and CD5 (4C7) (all from Novocastra/Leica Biosystems Newcastle Ltd., Newcastle, UK). Signal was detected by the streptavidin-immunoperoxidase method with diaminobenzidine (DAB) chromogen (ChemMate, Dako Ltd.).
PCR-based analysis of B cell clonality and detection of t(14;18)(IGH-BCL2)
DNA was extracted from paraffin-embedded tissue sections as previously described (49) and subjected to PCR-based analysis of B cell clonality using BIOMED-2 primer sets against rearranged immunoglobulin heavy and light chain genes (66). Samples were assessed for the presence of t(14;18)(IGH-BCL2) by PCR using BIOMED-2 primer sets A-C (covering breakpoints in the major breakpoint region (MBR), the 3' MBR, the minor cluster region (mcr), and the intermediate cluster region (icr) / 5' mcr). This assay detects greater than 80% of IGH-BCL2 translocations in cases with adequate DNA quality (66).
Fluorescence in situ hybridisation
Interphase fluorescence in situ hybridisation (FISH) for t(14;18)(q32;q21)(IGH-BCL2), translocations involving IGH, and translocations involving BCL6 was performed using LSI IGH-BCL2 dual colour dual fusion translocation probe, LSI IGH dual colour break-apart rearrangement probe, and LSI BCL6 dual colour break-apart rearrangement probe (Vysis Inc. / Abbott Laboratories, Maidenhead, UK) respectively. FISH for translocations involving FOXP1 was performed using an “in-house” dual colour break-apart rearrangement probe. FISH was performed as previously described (70).
Statistical Analysis
Analysis of associations between categorical variables was performed using Fisher's Exact Probability Test. Unsupervised hierarchical clustering of cases in Figure 4 was performed using Cluster 3.0 and TreeView 1.6 software (from Michael Eisen, University of California, Berkeley, CA).
RESULTS
Clinical Features
The clinical features of the patients are summarised in Table 1. There were 18 women and four men (M:F, 4.5:1), aged between 26 and 72 years (median, 50 years). The patients typically presented with single nodular masses in the thyroid gland or with a multinodular goitre. In each case the thyroid gland was both the presenting site and the site of the largest lymphomatous mass. In eight patients, lymphoma was restricted to the thyroid gland (Ann Arbor stage 1E), 11 patients had stage 2E to 4E disease (sites of extrathyroidal disease are given in Table 1), and staging information was not available for three. Six of the 17 patients for whom information was available had serological and/or clinical evidence of pre-existing auto-immune thyroiditis. Twenty-one patients underwent total, subtotal or hemi-thyroidectomy, with or without involved-field radiotherapy and/or chemotherapy as detailed in Table 1. Of these, follow-up information was available on 15 patients. After a median follow-up of 44 months (range, 10 - 204 months), 11 patients were alive with no lymphoma. Ten of these achieved a complete remission (CR) following initial therapy and did not relapse; the other relapsed with DLBCL following an initial partial response, but achieved a complete response to salvage therapy. Four patients, three of whom achieved only a partial response (PR), had died of progressive or relapsed transformed disease. One patient had a diagnostic needle core biopsy followed only by observation and remained alive with lymphoma four years from diagnosis.
Table 1.
Clinical characteristics
| Case No. |
Sex/ Age |
Presentation | Clinical Evidence of Thyroiditis |
Referral Diagnosis |
Ann-Arbor Stage (Other Sites) |
Initial Treatment |
Subsequent Course & Outcome |
|---|---|---|---|---|---|---|---|
| 1 | F 66 | Mass in thyroid gland | Yes | MALT L | 1E | Surgery (TT), RT, AnChT |
CR, ANL (3y) |
| 2 | M 69 | Transient thyrotoxicosis, palpable “cold” nodule |
No | MALT L vs FL | 1E | Surgery (HT), RT |
CR, ANL (18 mo) |
| 3 | F 61 | Mass in thyroid gland | Yes | MALT L | 1E | Surgery (CT)1 | CR, ANL (44 mo) |
| 4 | F 49 | Mass in thyroid gland | No | MALT L | Not staged | Surgery (ST) | CR, ANL (41 mo) |
| 5 | F 47 | Mass in thyroid gland | No | FL & DLBCL | 1E | Surgery (HT), RT, AnChT |
CR, ANL (14 mo) |
| 6 | M 56 | N/A | Yes | MALT L | N/A | N/A | N/A |
| 7 | F 26 | Hypothyroidism | Yes | None given | 1E | Surgery (TT), RT |
CR, ANL (24 mo) |
| 8 | F 47 | Mass in thyroid gland | No | MALT L | 1E | Surgery (HT), AnChT |
CR, ANL (15 mo) |
| 9 | F 62 | Longstanding multinodular goitre |
No | MALT L vs FL | 1E | Surgery (TT), RT, ChT |
CR, ANL (10 y) |
| 10 | F 74 | N/A | N/A | MALT L & DLBCL |
2E (Cervical LN) |
N/A | N/A |
| 11 | F 50 | Multinodular goitre | N/A | Reactive vs FL vs MALT L |
N/A | N/A | N/A |
| 12 | F 39 | N/A | N/A | FL | 2E (Cervical LN) |
N/A | N/A |
| 13 | F ?age | N/A | No | FL vs MALT L | 2E (Cervical LN) |
N/A | N/A |
| 14 | F 56 | Mass in thyroid gland | No | FL | 2E (Cervical LN) |
Surgery (HT), RT, AnChT |
CR Multiple relapses with disseminated FL and DLBCL (1-8y) Several further AnChT & PBSCT DOL (8y) |
| 15 | F 61 | N/A | Yes | MALT L | 1E | Surgery (TT), RT |
CR, ANL (6 y) |
| 16 | F ?age | N/A | N/A | DLBC L | 2E (Cervical LN) |
Surgery, RT, AnChT |
CR, ANL (17y) |
| 17 | M 51 | N/A | No | FL | 3E (Multiple LNs) |
Observation | FL in sigmoid colon (1y) No treatment, AWL (4y) |
| 18 | F 47 | N/A | No | FL | 3E (Multiple LNs) |
Surgery, RT, AnChT |
PR Relapsed with nodal FL (4y) Further ChT Progression to gastric & nodal DLBCL (10y) Further ChT, DOL (11 y) |
| 19 | F 49 | Multinodular goitre | No | MALT L | 3E (Abdominal LNs) |
Surgery (ST), RT, AnChT |
PR Progressive disseminated FL and DLBCL (1-3y) Several further ChT & Rituximab DOL (4y) |
| 20 | M 69 | Mass in neck, mesenteric lymphadenopathy |
N/A | MALT L & DLBCL | ≥3E (Mesenteric LNs) |
N/A | N/A |
| 21 | F 42 | N/A | No | FL & DLBCL | 4E (Multiple LNs, bone marrow) |
Surgery, AnChT |
PR Relapsed with cervical DLBCL (3y) Further ChT, Rituximab & allo-BMT ANL (4y) |
| 22 | F 37 | Mass in thyroid gland | Yes | MALT L | 4E (Cervical LN; bone marrow) |
Surgery (TT), RT |
PR Progressive disseminated FL & DLBCL Several further AnChT & Rituximab DOL (11y) |
N/A, not available; MALT L, extranodal marginal zone lymphoma of MALT type (MALT lymphoma); FL, follicular lymphoma; DLBCL, diffuse large B cell lymphoma; LN, lymph node; HT, hemithyroidectomy; TT, total thyroidectomy; CT, completion thyroidectomy; ST, subtotal thyroidectomy; RT, radiotherapy; ChT, non-anthracycline-containing chemotherapy; AnChT, anthracycline-containing chemotherapy; PBSCT, peripheral blood stem cell transplant; CR, complete response; ANL, alive no lymphoma; DOL, died of lymphoma; AWL, alive with lymphoma.
Completion thyroidectomy after hemithyroidectomy for Hashimoto thyroiditis 9 years previously.
Histological Appearances
The morphological appearance of each case is summarised in Table 2. All cases contained an extensive, dense, lymphoid infiltrate which effaced the thyroid parenchyma and comprised numerous lymphoid follicles amongst a variably prominent interfollicular or diffuse component. In some cases / areas, the infiltrate obliterated the underlying thyroid follicles, while in others acini remained, surrounding individual lymphoid follicles. In each case the lymphoid follicles showed morphological features typical of follicular lymphoma such as an absence of polarisation, attenuation of mantle zones and an absence of tingible body macrophages. The germinal centres contained characteristic centrocytes and centroblasts in variable proportions: five cases were WHO grade 1, nine were grade 2, seven were grade 3a and one was grade 3b. Reactive follicles, or follicles only partially infiltrated by neoplastic cells, were not seen within the lymphomatous infiltrate in any case.
Table 2.
Morphological, Immunophenotypic and Molecular Characteristics
| Case No. |
Morphology |
Immunohistochemistry |
Molecular Biology |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO Grade |
Prominent IF Areas |
LELs | DLBCL (%Area) |
Presence of Lymphocytic Thyroiditis |
CD10 |
Bcl-6 |
Bcl-2 |
IGH-BCL2 |
BCL6 translocation |
|||
| FCCs | IF Cells | FCCs | IF Cells | (FCCs) | ||||||||
| 1 | 3b | N | Present | N | Y | - | - | + | - (↓) | - | - | - |
| 2 | 3a | Y | Prominent | Y (50%) | Y | - | - | + | - (↓) | - | - | - |
| 3 | 3a | Y | Prominent | N | N | - | - | + | - (↓) | - | - | nd |
| 4 | 3a | N | Prominent | N | N | - | - | + | Weak (↓) | - | - | nd |
| 5 | 3a | Y | Present | Y (40%) | Y | + | - (↓) | + | Strong | - | - | nd |
| 6 | 3a | Y | Present | N | N | + | - (↓) | + | Weak (↓) | - | - | - |
| 7 | 2 | Y | Present | N | N | + | Weak (↓) | + | Strong | - | - | - |
| 8 | 1 | Y | Prominent | N | Y | + | - (↓) | + | - (↓) | - | - | - (3 signals) |
| 9 | 2 | N | Present | N | Y | + | - (↓) | Failed | Failed | - | nd | nd |
| 10 | 3a | Y | Prominent | Y (<10%) | N | + | Weak (↓) | + | Weak (↓) | Weak | - | + |
| 11 | 2 | Y | Prominent | N | N | + | - (↓) | + | - (↓) | Weak | - | - |
| 12 | 1 | N | Present | N | Y | + | Strong | + | Weak (↓) | - | + | - |
| 13 | 1 | N | Prominent | N | Y | - | - | + | Weak (↓) | + | + | + |
| 14 | 3a | N | Present | Y (80%) | Y | + | Strong | + | Weak (↓) | + | + | - (3 signals) |
| 15 | 2 | Y | Prominent | N | Y | + | Weak (↓) | + | Strong | + | + | - |
| 16 | 2 | Y | Prominent | N | N | + | Weak (↓) | + | Weak (↓) | + | + | - (3 signals) |
| 17 | 2 | Y | Present | N | N | + | Weak (↓) | + | - (↓) | + | + | nd |
| 18 | 1 | Y | Prominent | N | N | + | Strong | + | Strong | + | + | nd |
| 19 | 1 | Y | Prominent | N | N | + | Weak (↓) | + | - (↓) | + | + | nd |
| 20 | 2 | N | Prominent | Y (80%) | N | + | Strong | + | Strong | + | nd | nd |
| 21 | 2 | Y | Present | Y | N | + | Weak (↓) | + | - (↓) | + | + | nd |
| 22 | 2 | Y | Present | N | Y | + | Strong | + | Weak (↓) | + | + | nd |
FCCs, follicle centre cells; IF, interfollicular; IgH, immunoglobulin heavy chain; nd, not done; (↓) staining in IF cells reduced compared to FCC; IGH-BCL2 translocation status was examined by FISH & PCR (cases 1, 2, 6, 8, 10-13, 15, & 16), FISH (cases 7, 14, & 21), or PCR (cases 3-5, 18, 19, & 22).
The neoplastic follicles were separated by an extrafollicular population of small lymphocytes together with numerous slightly larger “centrocyte-like” cells with slightly irregular euchromatic nuclei and scant cytoplasm as described in the interfollicular region of nodal follicular lymphomas (17). In all cases there were areas in which neoplastic follicles were closely packed, as seen in nodal follicular lymphomas, but in 15 cases there were areas in which the interfollicular infiltrate was more extensive, sometimes forming diffuse areas and representing more than 25% of the total area of the lymphoma. The interfollicular cells not only permeated between residual thyroid acini but formed unequivocal lymphoepithelial lesions, present in each case and striking in 12. The lymphoepithelial lesions occurred in two overlapping forms: those in which aggregates of lymphoma cells filled and distended the acinar lumina, and those in which atypical cells were found in clusters within the acinar epithelium (which was typically hyperplastic and sometimes showed squamous metaplasia). In the latter type, lymphoma cells seemed eventually to overrun the underlying epithelium altogether. Plasma cell differentiation was identified in only one case (case 21, which carried a t(14;18)(IGH-BCL2)). Two cases (cases 3 and 6) showed subtle marginal zone differentiation. In six cases, areas of DLBCL, characterised by extrafollicular sheets of large centroblastic cells, were present accounting for between less than 10% and approximately 80% of the total cellularity.
In ten cases, including three of the six cases with clinical evidence of auto-immune thyroiditis, there were areas of lymphocytic thyroiditis separate from the lymphoma, in which reactive lymphoid follicles accompanied by a non-destructive T and B cell infiltrate were present amongst small thyroid acini often showing oncocytic change. Thus, in total, 13 cases had clinical and/or histological evidence of lymphocytic thyroiditis. In three other cases, thyroid acini amongst the lymphoma showed oncocytic change, but areas of thyroiditis were not present in the material examined.
Immunohistochemistry
The immunophenotype of the neoplastic cells in each case is summarised in Table 2. In each case the neoplastic cells expressed CD20, confirming the B cell phenotype and highlighting both the extensive interfollicular infiltrate and the lymphoepithelial lesions. The latter were also accentuated by staining for cytokeratin. In each case the neoplastic follicle centre cells showed a germinal centre immunophenotype. In 16 cases these expressed both Bcl-6 and CD10, and in one CD10-positive case staining for Bcl-6 failed. In five cases (four of which were grade 3) there was expression of Bcl-6 but aberrant loss of CD10, as is well recognised in a proportion of follicular lymphomas (24). In most cases the interfollicular component expressed CD10 and/or Bcl-6, but as described in nodal follicular lymphomas (17), the intensity of staining for Bcl-6 and/or CD10 in the interfollicular cells was frequently reduced compared to the intrafollicular cells, and was sometimes negative. The neoplastic follicle centre cells expressed Bcl-2 strongly in 10 cases, did so weakly in two, and were negative in the remaining 10 cases. In 11 of 18 cases assessed, immunoglobulin heavy chain expression was not detected by immunohistochemistry. The remainder expressed IgM (4 cases), IgD/IgM (2 cases) or IgG (1 case). Expression of CD5 and CD43 was assessed in 15 and eight cases respectively, and was negative in each. In each of the six cases with areas of DLBCL, this had an immunophenotype identical to that of the follicular lymphoma component.
Molecular Biology
The results of the molecular investigations are presented for each case in Table 2. The presence of a t(14;18)(q32;q21)/IGH-BCL2 was sought by FISH and/or by PCR depending upon the material available. IGH-BCL2 was detected in 10 of 20 cases studied. The two cases with weak expression of Bcl-2 were negative for IGH-BCL2 by FISH, and in one IGH-BCL2-positive case Bcl-2 could not be detected in neoplastic cells, possibly as a result of a mutation in the Bcl-2 epitope recognised by the antibody used (55). FISH for BCL6 translocations was performed in 12 cases, including seven of the 10 cases negative for IGH-BCL2. A split signal for BCL6 indicative of a translocation at this locus was detected in two cases: one IGH-BCL2 negative case (case 10) also showed an IGH translocation suggesting the presence of an IGH-BCL6 translocation; one IGH-BCL2 positive case (case 13) showed only one split IGH signal consistent with either a non-IGH partner gene or a complex translocation. Three cases (two of which carried an IGH-BCL2 translocation) showed an extra copy of BCL6 in the majority of neoplastic cells. FISH for a translocation involving FOXP1 was performed in five of the cases without IGH-BCL2 (cases 1, 2, 6, 8 and 11), and was negative in each case. PCR-based analysis of B cell clonality gave a clonal result in 12 of the 15 cases in which DNA of sufficient quality for analysis was obtained.
Clinicopathological Correlations
Many of the clinical, immunohistological and molecular genetic features described above were not randomly distributed amongst the cases. Instead there were strong associations between them such that many cases shared common constellations of features. There were significant associations between IGH-BCL2 positivity and Bcl-2 protein expression (p=0.03), IGH-BCL2 negativity and grade 3 histology (p=0.01), Bcl-2 immunonegativity and grade 3 histology (p=0.04), CD10 negativity and grade 3 histology (p=0.04), Bcl-2 immunonegativity, and stage 1 disease (p=0.001), and IGH-BCL2 negativity and stage 1 disease p=0.003). Both visual inspection of the data set and use of unsupervised clustering (with Cluster and TreeView programs) to objectively identify related cases, revealed two clearly distinct clinicopathological groups of follicular lymphoma presenting in the thyroid gland (Figures 2, 3 and 4.). These groups showed significant differences in Bcl-2 protein expression (p=0.00002), presence of IGH-BCL2 (p=0.0003), and WHO grade (p=0.02). One group (cases 1-9) was characterised by negative immunostaining for Bcl-2 (9/9 cases), absence of the IGH-BCL2 translocation (8/8 cases), more frequent grade 3 histology (6/9 cases), and more frequent negativity for CD10 (4/8 cases). In contrast, the other group (cases 10-22) was characterised by positive staining for Bcl-2 (12/13 cases), presence of the IGH-BCL2 translocation (10/12 cases), infrequent grade 3 histology (2/13 cases), and CD10 positivity (12/13 cases).
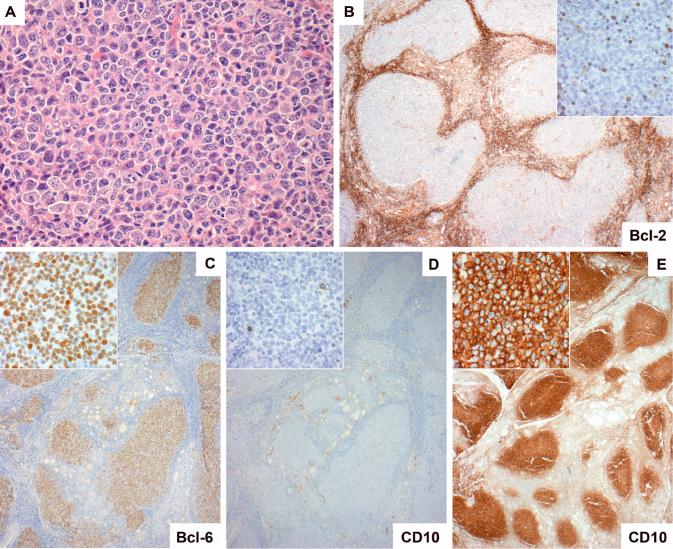
Figure 2.
Follicular lymphoma of the thyroid gland lacking both Bcl-2 expression and IGH-BCL2. Many of these cases were composed of neoplastic follicles of WHO grade 3 (A, case 10). In each case the germinal centre cells were negative for Bcl-2 (B, case 1). The germinal centre cells were positive for Bcl-6 in all cases (C, case 1), while staining for CD10 was negative in four cases (D, case 1) and strongly positive in five (E, case 7).
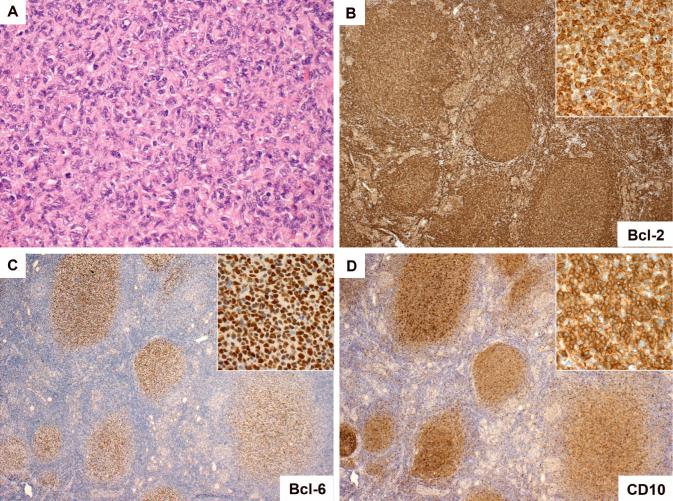
Figure 3.
Follicular lymphoma of the thyroid gland with features Bcl-2 expression/IGH-BCL2. Most of these lymphomas were composed of neoplastic follicles of WHO grade 1 or 2 (A, case 13). Germinal centre cells typically expressed Bcl-2 (B), Bcl-6 (C) and CD10 (D) (all case 17). As evident in panels C and D, in many cases there was downregulation of Bcl-6 and CD10 expression in the extrafollicular lymphoma cells.
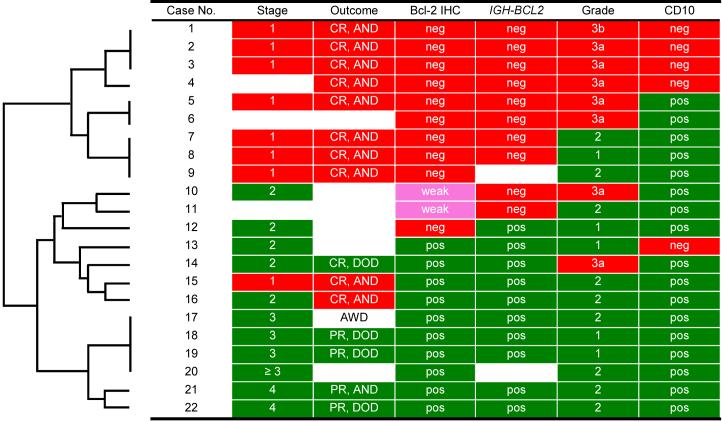
Figure 4.
Two subsets of follicular lymphoma arising in the thyroid gland. Visual inspection and unsupervised clustering (Cluster 3.0 and TreeView 1.6) revealed two clinicopathologically distinct groups of cases (cases 1-9 and cases 10-22) which differed significantly with respect to Bcl2 protein expression (p=0.00002), the presence of IGH-BCL2 (p=0.0003), WHO grade (p=0.02), stage at presentation (p=0.0002) and clinical outcome (p=0.007) (Fisher's exact test). CR, complete response; PR, partial response; AND, alive with no disease; AWD, alive with disease (without treatment); DOD, died of disease; IHC, immunohistochemistry; neg, negative; pos, positive.
There was clinical and/or histological evidence of thyroiditis in eight of the nine cases in the first group and in five of the nine cases in the second group for whom sufficient information was available (p=0.15). There was a significant association between evidence of thyroiditis and stage 1 disease (p=0.04), but not any other variable. The two groups were significantly different in clinical stage at presentation (p=0.0002); all patients in the first group for which information was available presented with stage 1 disease (7/7 cases) while 11/12 patients in the second group presented with stage 2-4 disease. The differences in biology and stage between the groups may be associated with differences in clinical outcome. All of the eight patients in the first group for whom information is available achieved a complete response and were alive without evidence of disease relapse at last follow-up. In contrast, of the seven treated patients from the second group for whom information is available, four did not achieve a complete response, five experienced progressive disease or relapse, and four died of lymphoma. The two groups did not differ with respect to age, presence of DLBCL or other histological or molecular genetic features.
The data thus reveal two distinct groups of follicular lymphoma presenting in the thyroid gland: one group (cases 10-22) with features typical of systemic follicular lymphoma (including positivity for Bcl-2 and/or IGH-BCL2) and a tendency to disseminated disease, and another group (cases 1-9) with localised disease and features (including a lack of both Bcl-2 expression and IGH-BCL2) not characteristic of systemic follicular lymphoma but instead similar to the features of follicular lymphoma occurring at some other extranodal sites.
DISCUSSION
The morphological features of follicular lymphoma presenting in the thyroid gland are not well characterised, leading to difficulties in its recognition by histopathologists and to uncertainties in patient management. In the present study, regardless of t(14;18)/IGH-BCL2 status, all 22 cases showed a destructive atypical lymphoid infiltrate which contained areas with, or was predominantly composed of, follicles showing typical morphological characteristics of follicular lymphoma. Recognition of these was aided by immunohistochemistry which showed a germinal centre phenotype (Bcl-6 +/- CD10 positive) together with immunophenotypic features supportive of malignancy (Bcl-2 expression, immunoglobulin light chain restriction, altered MIB1 staining, and/or loss of CD10 expression). However, many cases also contained an expansive extrafollicular neoplastic component. This feature was seen more frequently in the present series than in the large series of (predominantly nodal) follicular lymphomas that we have previously examined (4), and has also been recognised in follicular lymphomas at other extranodal sites (23). In most cases CD10 and/or Bcl-6 were expressed by the interfollicular B cells, but as in other follicular lymphomas (17), these antigens were often downregulated and staining was sometimes negative. Importantly in all of the present cases, the interfollicular infiltrate formed readily identified, and often striking, lymphoepithelial lesions.
Appreciation of the above features afforded ready distinction from reactive infiltrates. Additional support was provided in many cases by molecular demonstration of clonal immunoglobulin gene rearrangement and/or by the detection of a chromosomal translocation involving BCL2 or BCL6. Potentially more difficult is the distinction of follicular lymphoma in the thyroid gland from MALT lymphoma, particularly those cases showing prominent follicular colonisation (31). Indeed, in this study, MALT lymphoma was the diagnosis or differential diagnosis in 13 of the 17 cases that were received as referrals for second opinion. In those study cases lacking t(14;18)/IGH-BCL2, the presence of typical follicular lymphoma architecture in at least one area, the cytomorphology of the neoplastic cells, and the expression of CD10 and/or Bcl-6 in the neoplastic follicles and often in the extrafollicular component, confirmed the diagnosis of follicular lymphoma. A lack of CD10 staining (seen in four cases) is well recognised in follicular lymphomas showing grade 3 morphology or t(14;18)/IGH-BCL2 negativity (22,24,35,38), while expression of Bcl-6, even in colonised follicles, is not expected in marginal zone lymphomas (16,42). Additional features which were helpful in the differential diagnosis from MALT lymphoma include a lack of plasma cell differentiation, a lack of any reactive or partially infiltrated follicles, and weak or absent immunoglobulin heavy chain staining. The presence in some of our cases of features overlapping with MALT lymphomas is similar to reports of CD10+ Bcl-2+, t(14;18)+ follicular lymphomas mimicking MALT lymphomas in the stomach and lung (37,65). Together these studies highlight that the presence of an expansive extrafollicular infiltrate forming lymphoepithelial lesions, even those with lumina expanded by lymphoid cells (coined “MALT ball” lymphoepithelial lesions (13)), is not diagnostic of a MALT lymphoma.
MALT lymphoma and DLBCL of the thyroid gland arise in almost all cases from pre-existing chronic lymphocytic / Hashimoto thyroiditis, and patients with Hashimoto thyroiditis have a 60-80-fold increased risk of thyroid lymphoma (13,27). By analogy to gastric MALT lymphoma, it is likely that MALT lymphoma of the thyroid gland arises from an auto-reactive post-germinal centre B cell in the context of immunological help from auto-reactive T cells (32). It is therefore pertinent that evidence of chronic lymphocytic thyroiditis was present in 13 of our 22 cases of follicular lymphoma, suggesting that follicular lymphoma of the thyroid gland may also arise from the organised lymphoid tissue of thyroiditis. Since the clinical data available were, in some cases, incomplete, and thyroid tissue surrounding the lymphomas was not always available for review, the incidence of thyroiditis in our series may be underestimated. Examination of immunoglobulin heavy chain gene somatic mutation patterns has shown that follicular lymphoma arises from an antigen-selected germinal centre B cell, and has suggested that, at least early in the evolution of the lymphoma, antigen stimulation may provide an important stimulus for clonal expansion (6,71). One study suggested that immunoglobulins derived from some follicular lymphomas may bind auto-antigens (15). It is also clear that additional, perhaps non-cognate, signals from other cells in the germinal centre microenvironment can provide important stimuli to the neoplastic cells in at least a subset of follicular lymphomas (12,54). It can be hypothesised, therefore, that follicular lymphoma of the thyroid gland may also be supported by (auto)antigen stimulation and/or by immunological stimuli generated in the context of thyroiditis.
The combined analysis of clinical, morphological, immunophenotypic and genetic data revealed the presence of two clearly distinct groups among our study cases. One group shared pathological features with typical adult follicular lymphoma: the presence of a t(14;18)/IGH-BCL2, the expression of Bcl-2 and CD10, and a predominance of WHO grade 1-2 lymphomas (48). These features are not only shared with the majority of primary nodal follicular lymphomas, but can also be seen in follicular lymphomas with characteristic clinicopathological features arising at some other extranodal sites including the gastro-intestinal tract (11,57) and the ocular adnexa (18), as well as in a proportion of primary follicular lymphomas of the skin (40,47,60) and salivary gland (41). For example, primary follicular lymphoma of the gastrointestinal tract has a predilection for duodenal involvement, is usually of low grade and CD10-positive, and typically expresses Bcl-2 as a result of a t(14;18)/IGH-BCL2 (11,57). Interestingly, all but one of the IGH-BCL2 and/or Bcl-2-positive cases in the present study presented with stage 2-4 disease. However, follicular lymphoma rarely involves the thyroid gland secondarily (44,50,52) and several features suggest that at least the majority of these cases arose within the thyroid gland itself: in all cases the thyroid gland was the presenting and largest single site of disease; 5 cases had only stage 2 disease (small volume cervical lymph node involvement); and there was evidence of thyroiditis in five cases (including a history of autoimmune thyroiditis in a patient with stage 4 disease). Nevertheless, the possibility that this group includes some cases in which the thyroid gland is the site of presentation of disease originating in nearby lymph nodes cannot entirely be excluded.
The other group of cases lacked both IGH-BCL2 translocations and Bcl-2 expression, were often WHO grade 3, and included several CD10-negative cases. This latter constellation of features is thus similar to that of a heterogeneous minority of follicular lymphomas lacking IGH-BCL2 that has been recognised in several studies of follicular lymphomas arising at other sites (22,24,35,39,56). Interestingly, although most such cases occur in lymph nodes, they may be over-represented at extranodal sites (23,22). For example, in several studies of primary cutaneous follicular lymphoma, IGH-BCL2 was absent in 60-100% of cases, while Bcl-2 staining was negative in more than approximately 40% of cases (8,40,47,60). We and others have identified similar features in follicular lymphomas of the testes of both adults and children, Kojima et al found the majority of primary follicular lymphomas of the salivary gland to lack IGH-BCL2, and Goodlad et al reported similar findings in non-cutaneous extranodal follicular lymphomas from a range of sites (5,19,23,41).
In contrast to the cases showing Bcl-2 expression and/or IGH-BCL2, all of those lacking both these features remained localised to the thyroid gland (stage 1). This is in keeping with the results of other studies which suggest that IGH-BCL2/Bcl-2-negative follicular lymphomas more often present with low stage disease than do typical follicular lymphomas (21,35). The reason for this strict localisation is unclear, but it is possible that follicular lymphomas lacking Bcl-2 expression might remain dependent upon antigen or other stimuli within the thyroid microenvironment for survival, while the expression of Bcl-2 in other cases may allow lymphoma cells to survive in lymphoid tissue away from the thyroid gland. The question of primary site notwithstanding, the analysis of the Bcl-2 / IGH-BCL2 status of follicular lymphomas presenting in the thyroid gland is of potential clinical importance in distinguishing cases in which disease is likely to be localised from those in which there may be extra-glandular disease, as different treatment approaches may be appropriate in these two groups. Furthermore, this study suggests that the differences in biology and stage between the two groups identified may be reflected in differences in clinical outcome, although extended follow-up and study of additional cases are required before conclusions can be drawn.
In summary, follicular lymphoma of the thyroid gland includes cases with typical genetic and immunophenotypic features of follicular lymphoma, as well as a group lacking both Bcl-2 expression and IGH-BCL2 translocation and often having a higher grade morphology, in keeping with a recognised subset of follicular lymphomas over-represented at several other extranodal sites. These groups differ in their tendency to spread beyond the thyroid gland, but both show similar morphological features including frequent expansive extra-follicular infiltrates with prominent lymphoepithelial lesions which have previously been regarded as indicative of MALT lymphoma.
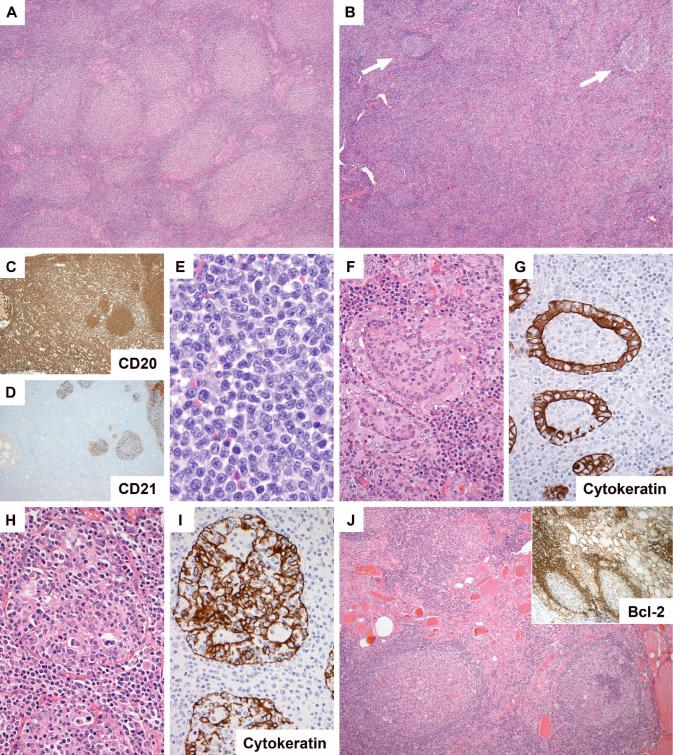
Figure 1.
Morphology of follicular lymphoma in the thyroid gland. In some areas/cases the neoplastic follicles were closely packed (A: H&E, case 2), while in several cases there were areas in which follicles (arrowed) were separated by expansive interfollicular neoplastic B cell infiltrates (B, case 16; C (CD20) and D (CD21), same area of case 10). The interfollicular cells were small centrocytic cells similar to those seen in the interfollicular region of nodal follicular lymphomas (E, case 18). Lymphoepithelial lesions were seen in all cases. These were of two overlapping types: those with intraluminal aggregates of neoplastic B cells (F and G (CD21), same area of case 13) and those with clusters of lymphocytes amongst hyperplastic epithelium (H and I (CD21), same area of case 16). Several cases contained foci of lymphocytic thyroiditis separate from the lymphoma (J, case 13).
ACKNOWLEDGEMENTS
The authors are grateful to the pathologists and physicians who provided case material and clinical information, and thank UCL Advanced Diagnostics for immunohistochemistry.
Support: The Health Foundation / The Royal College of Pathologists / The Pathological Society of Great Britain and Ireland Senior Clinician Scientist Fellowship (CB); The Leukaemia Research Fund (HY, RH, MQD); The Wellcome Trust PhD Studentship in Infection and Immunity (AG); National Institutes of Health (AD).
REFERENCES
- 1.Anscombe AM, Wright DH. Primary malignant lymphoma of the thyroid--a tumour of mucosa-associated lymphoid tissue: review of seventy-six cases. Histopathology. 1985;9:81–97. doi: 10.1111/j.1365-2559.1985.tb02972.x. [DOI] [PubMed] [Google Scholar]
- 2.Ansell SM, Grant CS, Habermann TM. Primary thyroid lymphoma. Semin Oncol. 1999;26:316–23. [PubMed] [Google Scholar]
- 3.Aozasa K, Inoue A, Tajima K, et al. Malignant lymphomas of the thyroid gland. Analysis of 79 patients with emphasis on histologic prognostic factors. Cancer. 1986;58:100–4. doi: 10.1002/1097-0142(19860701)58:1<100::aid-cncr2820580118>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Bacon CM, Avivi I, Obermann EC, et al. Histological and clinical prognostic factors in follicular lymphoma. Journal of Pathology. 2003;201:3A. [Google Scholar]
- 5.Bacon CM, Ye H, Diss TC, et al. Primary follicular lymphoma of the testis and epididymis in adults. Am J Surg Pathol. 2007;31:1050–8. doi: 10.1097/PAS.0b013e31802ee4ab. [DOI] [PubMed] [Google Scholar]
- 6.Bahler DW, Levy R. Clonal evolution of a follicular lymphoma: evidence for antigen selection. Proc Natl Acad Sci U S A. 1992;89:6770–4. doi: 10.1073/pnas.89.15.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971;31:1860–1. [PubMed] [Google Scholar]
- 8.Cerroni L, Arzberger E, Putz B, et al. Primary cutaneous follicle center cell lymphoma with follicular growth pattern. Blood. 2000;95:3922–8. [PubMed] [Google Scholar]
- 9.Compagno J, Oertel JE. Malignant lymphoma and other lymphoproliferative disorders of the thyroid gland. A clinicopathologic study of 245 cases. Am J Clin Pathol. 1980;74:1–11. doi: 10.1093/ajcp/74.1.1. [DOI] [PubMed] [Google Scholar]
- 10.d'Amore F, Christensen BE, Brincker H, et al. Clinicopathological features and prognostic factors in extranodal non-Hodgkin lymphomas. Danish LYFO Study Group. Eur J Cancer. 1991;27:1201–8. doi: 10.1016/0277-5379(91)90081-n. [DOI] [PubMed] [Google Scholar]
- 11.Damaj G, Verkarre V, Delmer A, et al. Primary follicular lymphoma of the gastrointestinal tract: a study of 25 cases and a literature review. Ann Oncol. 2003;14:623–9. doi: 10.1093/annonc/mdg168. [DOI] [PubMed] [Google Scholar]
- 12.de Jong D. Molecular pathogenesis of follicular lymphoma: a cross talk of genetic and immunologic factors. J Clin Oncol. 2005;23:6358–63. doi: 10.1200/JCO.2005.26.856. [DOI] [PubMed] [Google Scholar]
- 13.Derringer GA, Thompson LD, Frommelt RA, et al. Malignant lymphoma of the thyroid gland: a clinicopathologic study of 108 cases. Am J Surg Pathol. 2000;24:623–39. doi: 10.1097/00000478-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Dierlamm J, Baens M, Wlodarska I, et al. The apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomas. Blood. 1999;93:3601–9. [PubMed] [Google Scholar]
- 15.Dighiero G, Hart S, Lim A, et al. Autoantibody activity of immunoglobulins isolated from B-cell follicular lymphomas. Blood. 1991;78:581–5. [PubMed] [Google Scholar]
- 16.Dogan A, Bagdi E, Munson P, et al. CD10 and BCL-6 expression in paraffin sections of normal lymphoid tissue and B-cell lymphomas. Am J Surg Pathol. 2000;24:846–52. doi: 10.1097/00000478-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Dogan A, Du MQ, Aiello A, et al. Follicular lymphomas contain a clonally linked but phenotypically distinct neoplastic B-cell population in the interfollicular zone. Blood. 1998;91:4708–14. [PubMed] [Google Scholar]
- 18.Ferry JA, Fung CY, Zukerberg L, et al. Lymphoma of the ocular adnexa: A study of 353 cases. Am J Surg Pathol. 2007;31:170–84. doi: 10.1097/01.pas.0000213350.49767.46. [DOI] [PubMed] [Google Scholar]
- 19.Finn LS, Viswanatha DS, Belasco JB, et al. Primary follicular lymphoma of the testis in childhood. Cancer. 1999;85:1626–35. doi: 10.1002/(sici)1097-0142(19990401)85:7<1626::aid-cncr27>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–60. doi: 10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Goodlad JR, Batstone PJ, Hamilton D, et al. Grade, BCL2 gene abnormality and expression, and clinical relevance in follicular lymphoma. Journal of Pathology. 2005;207:10. [Google Scholar]
- 22.Goodlad JR, Batstone PJ, Hamilton DA, et al. BCL2 gene abnormalities define distinct clinical subsets of follicular lymphoma. Histopathology. 2006;49:229–41. doi: 10.1111/j.1365-2559.2006.02501.x. [DOI] [PubMed] [Google Scholar]
- 23.Goodlad JR, MacPherson S, Jackson R, et al. Extranodal follicular lymphoma: a clinicopathological and genetic analysis of 15 cases arising at non-cutaneous extranodal sites. Histopathology. 2004;44:268–76. doi: 10.1111/j.1365-2559.2004.01804.x. [DOI] [PubMed] [Google Scholar]
- 24.Guo Y, Karube K, Kawano R, et al. Low-grade follicular lymphoma with t(14;18) presents a homogeneous disease entity otherwise the rest comprises minor groups of heterogeneous disease entities with Bcl2 amplification, Bcl6 translocation or other gene aberrances. Leukemia. 2005;19:1058–63. doi: 10.1038/sj.leu.2403738. [DOI] [PubMed] [Google Scholar]
- 25.Ha CS, Shadle KM, Medeiros LJ, et al. Localized non-Hodgkin lymphoma involving the thyroid gland. Cancer. 2001;91:629–35. [PubMed] [Google Scholar]
- 26.Heimann R, Vannineuse A, De Sloover C, et al. Malignant lymphomas and undifferentiated small cell carcinoma of the thyroid: a clinicopathological review in the light of the Kiel classification for malignant lymphomas. Histopathology. 1978;2:201–13. doi: 10.1111/j.1365-2559.1978.tb01710.x. [DOI] [PubMed] [Google Scholar]
- 27.Holm LE, Blomgren H, Lowhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med. 1985;312:601–4. doi: 10.1056/NEJM198503073121001. [DOI] [PubMed] [Google Scholar]
- 28.Horsman DE, Gascoyne RD, Coupland RW, et al. Comparison of cytogenetic analysis, southern analysis, and polymerase chain reaction for the detection of t(14;18) in follicular lymphoma. Am J Clin Pathol. 1995;103:472–8. doi: 10.1093/ajcp/103.4.472. [DOI] [PubMed] [Google Scholar]
- 29.Hyjek E, Isaacson PG. Primary B cell lymphoma of the thyroid and its relationship to Hashimoto's thyroiditis. Hum Pathol. 1988;19:1315–26. doi: 10.1016/s0046-8177(88)80287-9. [DOI] [PubMed] [Google Scholar]
- 30.Isaacson P, Wright DH. Extranodal malignant lymphoma arising from mucosa-associated lymphoid tissue. Cancer. 1984;53:2515–24. doi: 10.1002/1097-0142(19840601)53:11<2515::aid-cncr2820531125>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 31.Isaacson PG, Androulakis-Papachristou A, Diss TC, et al. Follicular colonization in thyroid lymphoma. Am J Pathol. 1992;141:43–52. [PMC free article] [PubMed] [Google Scholar]
- 32.Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4:644–53. doi: 10.1038/nrc1409. [DOI] [PubMed] [Google Scholar]
- 33.Isaacson PG, Muller-Hermelink HK, Piris M, et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organisation Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon: 2001. pp. 157–60. [Google Scholar]
- 34.Isaacson PG, Norton A. Extranodal Lymphomas. Churchill Livingstone; London: 1994. [Google Scholar]
- 35.Jardin F, Gaulard P, Buchonnet G, et al. Follicular lymphoma without t(14;18) and with BCL-6 rearrangement: a lymphoma subtype with distinct pathological, molecular and clinical characteristics. Leukemia. 2002;16:2309–17. doi: 10.1038/sj.leu.2402707. [DOI] [PubMed] [Google Scholar]
- 36.Johnson PW, Rohatiner AZ, Whelan JS, et al. Patterns of survival in patients with recurrent follicular lymphoma: a 20-year study from a single center. J Clin Oncol. 1995;13:140–7. doi: 10.1200/JCO.1995.13.1.140. [DOI] [PubMed] [Google Scholar]
- 37.Jourdan F, Molina TJ, Le Tourneau A, et al. Florid marginal zone differentiation in follicular lymphoma mimicking marginal zone lymphoma of MALT type in the lung. Histopathology. 2006;49:426–9. doi: 10.1111/j.1365-2559.2006.02462.x. [DOI] [PubMed] [Google Scholar]
- 38.Karube K, Guo Y, Suzumiya J, et al. CD10-MUM1+ follicular lymphoma lacks BCL2 gene translocation and shows characteristic biologic and clinical features. Blood. 2007;109:3076–9. doi: 10.1182/blood-2006-09-045989. [DOI] [PubMed] [Google Scholar]
- 39.Keller C, Subramaniyam S, Murty V, et al. Clinicopathologic characteristics of nodal follicular lymphomas lacking IgH/BCL2 translocations. Modern Pathology. 2007;20:247A–8A. [Google Scholar]
- 40.Kim BK, Surti U, Pandya A, et al. Clinicopathologic, immunophenotypic, and molecular cytogenetic fluorescence in situ hybridization analysis of primary and secondary cutaneous follicular lymphomas. Am J Surg Pathol. 2005;29:69–82. doi: 10.1097/01.pas.0000146015.22624.c7. [DOI] [PubMed] [Google Scholar]
- 41.Kojima M, Nakamura S, Ichimura K, et al. Follicular lymphoma of the salivary gland: a clinicopathological and molecular study of six cases. Int J Surg Pathol. 2001;9:287–93. doi: 10.1177/106689690100900405. [DOI] [PubMed] [Google Scholar]
- 42.Kojima M, Nakamura S, Murase T, et al. Follicular colonization of nodal marginal-zone B-cell lymphoma resembling follicular lymphoma: report of 6 cases. Int J Surg Pathol. 2005;13:73–8. doi: 10.1177/106689690501300110. [DOI] [PubMed] [Google Scholar]
- 43.Lai R, Arber DA, Chang KL, et al. Frequency of bcl-2 expression in non-Hodgkin's lymphoma: a study of 778 cases with comparison of marginal zone lymphoma and monocytoid B-cell hyperplasia. Mod Pathol. 1998;11:864–9. [PubMed] [Google Scholar]
- 44.Lam KY, Lo CY, Kwong DL, et al. Malignant lymphoma of the thyroid. A 30-year clinicopathologic experience and an evaluation of the presence of Epstein-Barr virus. Am J Clin Pathol. 1999;112:263–70. doi: 10.1093/ajcp/112.2.263. [DOI] [PubMed] [Google Scholar]
- 45.Logue JP, Hale RJ, Stewart AL, et al. Primary malignant lymphoma of the thyroid: a clinicopathological analysis. Int J Radiat Oncol Biol Phys. 1992;22:929–33. doi: 10.1016/0360-3016(92)90790-o. [DOI] [PubMed] [Google Scholar]
- 46.Maurer R, Taylor CR, Terry R, et al. Non-Hodgkin lymphomas of the thyroid. A clinicopathological review of 29 cases applying the Lukes-Collins classification and an immunoperoxidase method. Virchows Arch A Pathol Anat Histol. 1979;383:293–317. doi: 10.1007/BF00430248. [DOI] [PubMed] [Google Scholar]
- 47.Mirza I, Macpherson N, Paproski S, et al. Primary cutaneous follicular lymphoma: an assessment of clinical, histopathologic, immunophenotypic, and molecular features. J Clin Oncol. 2002;20:647–55. doi: 10.1200/JCO.2002.20.3.647. [DOI] [PubMed] [Google Scholar]
- 48.Nathwani BN, Harris NL, Weisenberger D, et al. Follicular Lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organisation Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon: 2001. pp. 162–7. [Google Scholar]
- 49.Pan LX, Diss TC, Peng HZ, et al. Clonality analysis of defined B-cell populations in archival tissue sections using microdissection and the polymerase chain reaction. Histopathology. 1994;24:323–7. doi: 10.1111/j.1365-2559.1994.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen RK, Pedersen NT. Primary non-Hodgkin's lymphoma of the thyroid gland: a population based study. Histopathology. 1996;28:25–32. doi: 10.1046/j.1365-2559.1996.268311.x. [DOI] [PubMed] [Google Scholar]
- 51.Remstein ED, Dogan A, Einerson RR, et al. The incidence and anatomic site specificity of chromosomal translocations in primary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in North America. Am J Surg Pathol. 2006;30:1546–53. doi: 10.1097/01.pas.0000213275.60962.2a. [DOI] [PubMed] [Google Scholar]
- 52.Risdall R, Hoppe RT, Warnke R. Non-Hodgkin's lymphoma: a study of the evolution of the disease based upon 92 autopsied cases. Cancer. 1979;44:529–42. doi: 10.1002/1097-0142(197908)44:2<529::aid-cncr2820440222>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 53.Rohatiner AZ, Lister TA. The clinical course of follicular lymphoma. Best Pract Res Clin Haematol. 2005;18:1–10. doi: 10.1016/j.beha.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Roulland S, Suarez F, Hermine O, et al. Pathophysiological aspects of memory B-cell development. Trends Immunol. 2008;29:25–33. doi: 10.1016/j.it.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Schraders M, de Jong D, Kluin P, et al. Lack of Bcl-2 expression in follicular lymphoma may be caused by mutations in the BCL2 gene or by absence of the t(14;18) translocation. J Pathol. 2005;205:329–35. doi: 10.1002/path.1689. [DOI] [PubMed] [Google Scholar]
- 56.Shi XL, Dave BJ, d'Amore F, et al. T(14;18)-negative non-cutaneous follicular lymphoma (FL): A clinicopathological study of 59 cases. Modern Pathology. 2007;20:260A. [Google Scholar]
- 57.Shia J, Teruya-Feldstein J, Pan D, et al. Primary follicular lymphoma of the gastrointestinal tract: a clinical and pathologic study of 26 cases. Am J Surg Pathol. 2002;26:216–24. doi: 10.1097/00000478-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Skacel M, Ross CW, Hsi ED. A reassessment of primary thyroid lymphoma: high-grade MALT-type lymphoma as a distinct subtype of diffuse large B-cell lymphoma. Histopathology. 2000;37:10–8. doi: 10.1046/j.1365-2559.2000.00941.x. [DOI] [PubMed] [Google Scholar]
- 59.Streubel B, Lamprecht A, Dierlamm J, et al. T(14;18)(q32;q21) involving IGH and MALT1 is a frequent chromosomal aberration in MALT lymphoma. Blood. 2003;101:2335–9. doi: 10.1182/blood-2002-09-2963. [DOI] [PubMed] [Google Scholar]
- 60.Streubel B, Scheucher B, Valencak J, et al. Molecular cytogenetic evidence of t(14;18)(IGH;BCL2) in a substantial proportion of primary cutaneous follicle center lymphomas. Am J Surg Pathol. 2006;30:529–36. doi: 10.1097/00000478-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 61.Streubel B, Simonitsch-Klupp I, Mullauer L, et al. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia. 2004;18:1722–6. doi: 10.1038/sj.leu.2403501. [DOI] [PubMed] [Google Scholar]
- 62.Streubel B, Vinatzer U, Lamprecht A, et al. T(3;14)(p14.1;q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma. Leukemia. 2005;19:652–8. doi: 10.1038/sj.leu.2403644. [DOI] [PubMed] [Google Scholar]
- 63.Thieblemont C, Mayer A, Dumontet C, et al. Primary thyroid lymphoma is a heterogeneous disease. J Clin Endocrinol Metab. 2002;87:105–11. doi: 10.1210/jcem.87.1.8156. [DOI] [PubMed] [Google Scholar]
- 64.Tsang RW, Gospodarowicz MK, Pintilie M, et al. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol. 2003;21:4157–64. doi: 10.1200/JCO.2003.06.085. [DOI] [PubMed] [Google Scholar]
- 65.Tzankov A, Hittmair A, Muller-Hermelink HK, et al. Primary gastric follicular lymphoma with parafollicular monocytoid B-cells and lymphoepithelial lesions, mimicking extranodal marginal zone lymphoma of MALT. Virchows Arch. 2002;441:614–7. doi: 10.1007/s00428-002-0670-5. [DOI] [PubMed] [Google Scholar]
- 66.van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 67.Widder S, Pasieka JL. Primary thyroid lymphomas. Curr Treat Options Oncol. 2004;5:307–13. doi: 10.1007/s11864-004-0021-7. [DOI] [PubMed] [Google Scholar]
- 68.Willis TG, Jadayel DM, Du MQ, et al. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell. 1999;96:35–45. doi: 10.1016/s0092-8674(00)80957-5. [DOI] [PubMed] [Google Scholar]
- 69.Ye H, Gong L, Liu H, et al. MALT lymphoma with t(14;18)(q32;q21)/IGH-MALT1 is characterized by strong cytoplasmic MALT1 and BCL10 expression. J Pathol. 2005;205:293–301. doi: 10.1002/path.1715. [DOI] [PubMed] [Google Scholar]
- 70.Ye H, Liu H, Attygalle A, et al. Variable frequencies of t(11;18)(q21;q21) in MALT lymphomas of different sites: significant association with CagA strains of H pylori in gastric MALT lymphoma. Blood. 2003;102:1012–8. doi: 10.1182/blood-2002-11-3502. [DOI] [PubMed] [Google Scholar]
- 71.Zelenetz AD, Chen TT, Levy R. Clonal expansion in follicular lymphoma occurs subsequent to antigenic selection. J Exp Med. 1992;176:1137–48. doi: 10.1084/jem.176.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zinzani PL, Magagnoli M, Galieni P, et al. Nongastrointestinal low-grade mucosa-associated lymphoid tissue lymphoma: analysis of 75 patients. J Clin Oncol. 1999;17:1254. doi: 10.1200/JCO.1999.17.4.1254. [DOI] [PubMed] [Google Scholar]
- 73.Zucca E, Conconi A, Pedrinis E, et al. Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood. 2003;101:2489–95. doi: 10.1182/blood-2002-04-1279. [DOI] [PubMed] [Google Scholar]