Abstract
Background
Tibia fracture in rats evokes chronic hindpaw warmth, edema, allodynia, and regional osteopenia, a syndrome resembling complex regional pain syndrome (CRPS). Previous studies suggest that the pathogenesis of some of these changes involves an exaggerated regional inflammatory response to injury and we postulated that inflammatory cytokines contribute to the development of CRPS-like changes after fracture.
Methods
The distal tibia was fractured and the hindlimb casted for 4 weeks. The rats were given drinking water with or without the cytokine inhibitor pentoxifylline (PTX) starting the day before fracture and continuing for 4 weeks, after which time the cast was removed and multiple assays were performed in the hindpaw. PCR and immunoassays were used to evaluate changes in cytokine expression. Bilateral hindpaw thickness, temperature, and nociceptive thresholds were determined, and bone microarchitecture was measured by microcomputed tomography (μCT).
Results
Tibia fracture chronically upregulated TNFα, IL-1β and IL-6 mRNA and protein levels in hindpaw skin and PTX treatment significantly reduced the mRNA expression and cytokine protein levels for all these cytokines. PTX inhibited the nociceptive sensitization and some vascular changes, but had insignificant effects on most of the bone-related parameters measured in these studies. Immunostaining of hindpaw skin was negative for immunocyte infiltration at 4 weeks post-fracture.
Conclusions
These results suggest that proinflammatory cytokines contribute to the nociceptive and vascular sequelae of fracture and that PTX treatment can reverse these CRPS-like changes.
Keywords: tibia fracture, neuropathic pain, cytokines, microCT scanning, pentoxifylline, complex regional pain syndrome
Introduction
Previously proposed animal models poorly represent the inciting trauma and the complex nociceptive, vascular and bone changes observed in the CRPS patient. Recently we described a rat tibia fracture model of CRPS type I (Guo et al. 2004). CRPS is a frequent consequence of distal tibia (Sarangi et al. 1993) and distal radius fractures (Atkins et al. 1990; Bickerstaff and Kanis 1994). The affected limb displays increased skin temperature, increased cutaneous protein extravasation, distal limb edema, osteoporosis, postural changes and allodynia (Guo et al. 2004). The constellation of these post-fracture features closely resembles those observed in CRPS patients (Bickerstaff et al. 1993; Oyen et al. 1993; Veldman et al. 1993;Blumberg and Janig 1994; Wasner et al. 2001).
The pathogenesis of CRPS is complex and involves a broad set of interlinked processes. Experimental evidence suggests that neurogenic inflammatory responses are enhanced in the CRPS limb(Oyen et al. 1993; Waston 1995; Weber et al. 2001; Leis et al. 2003). Neurogenic inflammation is mediated by the peripheral release of the sensory neuropeptides substance P (SP) and calcitonin gene-related peptide (CGRP). Peripherally released SP is able to elicit vasodilatation and protein extravasation via activation of the SP receptors (NK1)(McDonald 1988; Bowden et al. 1994). Not only does SP have inflammatory effects, it also acts as a central neurotransmitter facilitating sensitization of second order spinal neurons via activation of the NK1 receptors on ascending spinal neurons resulting in spontaneous pain and hyperalgesia (Nichols et al. 1999). Previously we observed that an NK1 receptor antagonist partially reversed the development of hindpaw warmth, edema, spontaneous extravasation, and allodynia in the CRPS fracture model (Guo et al. 2004). Furthermore, intravenous SP-evoked extravasation and edema responses are chronically enhanced after fracture in rats (Guo et al. 2006) and SP-evoked extravasation responses in CRPS patients are also facilitated (Leis et al. 2003). Collectively, these data suggest that SP-signaling is enhanced in the CRPS extremity.
Peripheral SP release also stimulates the production of pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and TNFα in the skin of rats(Saade et al. 2002; Massaad et al. 2004). Several studies have demonstrated that suction-induced skin blisters on the affected but not the contralateral extremity of CRPS patients contain elevated levels of cytokines IL-6 and TNFα(Huygen et al. 2002; Huygen et al. 2004b). In addition, the cerebrospinal fluid (CSF) of CRPS patients contains increased levels of IL-1β and IL-6.(Alexander et al. 2005) and serum expression of TNFα is increased in CRPS (Uceyler et al. 2007). Moreover, the anti-TNFα agent infliximab was observed to reduce spontaneous pain and articular tenderness in CRPS patients(Huygen et al. 2004a). Facilitated cytokine signaling appears critical to the development of inflammatory pain behavior in several animal models (Woolf et al. 1997; Tonussi and Ferreira 1999; Cunha et al. 2005; Inglis et al. 2005) and cytokine inhibitors have proven to be selective and highly effective therapeutics for rheumatoid arthritis and a variety of chronic inflammatory diseases(Feldmann 2002; Smolen et al. 2007).
In light of these results we hypothesized that cytokine production would be enhanced in our fracture rat model of CRPS, contributing to the development of nociceptive sensitization, vascular changes and bone loss. To elaborate this hypothesis, we utilized the rat tibia fracture model seeking for changes in hindpaw cytokine expression after fracture and tested the therapeutic efficacy of chronic and acutely administered pentoxifylline (PTX), a phosphodiesterase inhibitor, which also has a broad-spectrum inhibitory effect on cytokine production(Strieter et al. 1988; Zabel et al. 1993; Dorazil-Dudzik et al. 2004; Ji et al. 2004; Lu et al. 2004; Vale et al. 2004; Liu et al. 2007).
Materials and methods
These experiments were approved by our institute’s Subcommittee on Animal Studies. Adult (10-month-old) male Sprague Dawley rats (Harlan, Indianapolis, IN) were used in all experiments. The animals were housed individually in isolator cages with solid floors covered with 3 cm of soft bedding and were fed Lab Diet 5012 (PMI Nutrition Institute, Richmond, IN), which contains 1.0% calcium, 0.5% phosphorus, and 3.3 IU/g of vitamin D3.
Surgery
Tibia fracture was performed under isoflurane anesthesia as we have previously described (Guo et al. 2004). The right hindlimb was wrapped in stockinet and the distal tibia was fractured using pliers with an adjustable stop that had been modified with a 3-point jaw. The hindlimb was wrapped in casting tape so the hip, knee and ankle were flexed. The cast extended from the metatarsals of the hindpaw up to a spica formed around the abdomen. At 4 weeks the cast was removed.
RNA isolation, reverse transcription and real-time PCR
Hindpaw dorsal skin was collected by dissection under isoflurane anesthesia and homogenized in lysis buffer. Samples were then centrifuged for 10 min at 12,000g at 4°C. The supernatants were subsequently processed using RNeasy Mini Kit (Qiagen, Valencia, CA). Complementary DNA (cDNA) was synthesized using a reverse transcriptase iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA): 1 μg of RNA in a total volume of 20 μl was incubated at 25°C for 5 min followed by 42°C for 30 min and then heat inactivation at 85°C for 5 min. After incubation, cDNA reactions were diluted 1:10 in RNase-free water prior to quantitative PCR (qPCR). Real-time qPCR was performed using ABI 7900HT sequencing detection system (Applied Biosystems, Foster City, CA). We utilized Assay-on-Demand gene expression products for TNFα (Rn00562055 m1), IL-1β (Rn00580432 m1) and IL-6 (Rn00561420 m1) (TaqMan MGB probes, FAM dye-labeled) and TaqMan 2X Universal PCR Master Mix (Applied Biosystems; Foster City, CA). Ribosomal 18s mRNA (Hs99999901 s1), was used as an internal control. Amplification kinetics for these products were similar. The data from real-time PCR experiments were analyzed by the comparative Ct method as described in the manual for the ABI prism 7900HT real-time system. All samples were analyzed in triplicate or quadruplicate.
Homogenization procedure and ELISA for TNFα, IL-1β and IL-6 cytokines
The hindpaw dorsal skin was collected under isoflurane anesthesia and cut into fine pieces in ice-cold phosphate buffered saline, pH 7.4, containing protease inhibitors (aprotinin (2 μg/ml), leupeptin (5 μg/ml), pepstatin (0.7 μg/ml), and PMSF (100 μg/ml); Sigma, St. Louis, MO, USA), followed by homogenization. Homogenates were centrifuged for 5 min at 14,000g, then diluted with Triton X-100 to a final concentration of 0.01 %, and then centrifuged again for 5 min at 14,000g. The supernatants were aliquoted, stored at −80 °C and assayed in duplicate (after dilution in the standard buffer supplied) using ELISA kits for TNF (Biosource Europe, Nivelle, Belgium), IL-1β, and IL-6 (Bender MedSystems, Burlingame, CA) according to the manufacturer’s instructions. These assay systems detect rat TNFα, IL-1β and IL-6 with a sensitivity of 4 pg/ml, 4.4 pg/ml and 12 pg/ml, respectively. Positive and negative controls were included in each assay. TNFα, IL-1β and IL-6 cytokines concentrations were expressed as pg/mg protein. Protein content was determined by the bicinchoninic acid protein assay reagent (Pierce, KMF Laborchemie, St. Augustin, Germany).
Hindpaw nociception
To measure mechanical allodynia in the rats an up-down von Frey testing paradigm was used as we have previously described (Kingery et al. 2003). Rats were placed in a clear plastic cylinder (20 cm in diameter) with a wire mesh bottom and allowed to acclimate for 15 minutes. A series of 8 von Frey hairs ranging in stiffness from 0.41 g to 15.14 g were employed. The von Frey hair was applied against the hindpaw plantar skin at approximately midsole, taking care to avoid the tori pads. The initial fiber presentation was 2.1 g and the fibers were presented according to the up-down method of Dixon to generate 6 withdrawal responses in the immediate vicinity of the 50% threshold.
An incapacitance device (IITC Inc. Life Science. Woodland, CA) was used to measure hindpaw unweighting, a postural effect of hind limb nociception. The rats were manually held in a vertical position over the apparatus with the hindpaws resting on separate metal scale plates and the entire weight of the rat was supported on the hindpaws. The duration of each measurement was 6 seconds and 10 consecutive measurements were taken at 60-second intervals. Eight readings (excluding the highest and lowest ones) were averaged to calculate the bilateral hindpaw weight bearing values.
Tail-flick testing was performed as previously described(Guo et al. 1999). Using a heating blanket, tail temperatures were maintained at 30 °C. Tail-flick latencies were determined from the mean of two consecutive latencies using a tail-flick apparatus (Columbus Instruments, Columbus, OH). The light stimulus intensity was pre-set at an intensity that elicited a mean latency of 3.7 s with a cut-off set at 10 s to avoid tissue injury.
Hindpaw thickness
A laser sensor technique was used to determine the dorsal-ventral thickness of the hindpaw, as we have previously described (Kingery et al. 2003). For laser measurements each rat was briefly anesthetized with isoflurane and then held vertically so the hindpaw rested on a table top below the laser. The paw was gently held flat on the table with a small metal rod applied to the top of the ankle joint. Using optical triangulation, a laser (4381 Precicura, Limab, Goteborg, Sweden) with a distance measuring sensor was used to determine the distance to the table top and to the top of the hindpaw and the difference was used to calculate the dorsal-ventral paw thickness.
Hindpaw temperature
The room temperature was maintained at 23°C and humidity ranged between 25–45%. The temperature of the hindpaw was measured using a fine wire thermocouple (Omega, Stamford, CT) applied to the paw skin in awake, unanesthetized rats, as previously described (Kingery et al. 2003). The investigator held the thermistor wire using an insulating Styrofoam block. Three sites were tested over the dorsum of the hindpaw; the space between the first and second metatarsals (medial), the second and third metatarsals (central), and the fourth and fifth metatarsals (lateral). After a site was tested in one hindpaw the same site was immediately tested in the contralateral hindpaw. Six measurements for each hindpaw were averaged for the mean temperature.
Microcomputed tomography (μCT)
Ex vivo scanning was performed for assessment of trabecular and cortical bone architecture using μCT (VivaCT 40, Scanco Medical AG, Basserdorf Switzerland). Specifically, trabecular bone architecture was evaluated at the distal femur and fourth lumbar vertebra and cortical bone morphology was evaluated at the femur midshaft. CT images were reconstructed in 1024 × 1024-pixel matrices for vertebral, distal femur, and midfemur samples and stored in 3-D arrays. The resulting grayscale images were segmented using a constrained Gaussian filter to remove noise, and a fixed threshold (25.5% of the maximal grayscale value for vertebrae and distal femur and 35% for midfemur cortical bone) was used to extract the structure of the mineralized tissue. The μCT parameters set at threshold = 255, σ = 0.8, support = 1 for vertebral samples, threshold = 255, σ = 0.8, support = 1 for distal femur, and threshold = 350, σ = 1.2, and support = 2 for midfemur evaluation analysis. A single operator outlined the trabecular bone region within distal femur and vertebral body, and cortical bone region in midfemur shaft.
Each vertebral body was scanned using 223 transversely oriented 21μm thick slices (21-μm isotropic voxel size) encompassing a length of 4.68mm. In the distal femur 150 transverse slices of 21μm thickness (21-μm isotropic voxel size) encompassing a length of 3.15mm were acquired, but only 100 slices encompassing 2.1mm of the distal femur were evaluated, starting where the growth plate bridge across the middle of the metaphysis ends. At the femur midshaft, 10 transverse CT slices were obtained, each 21 μm thick totaling 0.21 mm in length (21 μm isotropic voxel size) and these were used to compute the cortical thickness (CtTh, μm), total area (TAr, mm2), bone area (BAr, mm2), and bone perimeter (BPm, mm).
Histological analysis, immunohistochemistry
After transcardiac perfusion with 200 ml normal saline the dorsal hindpaw skin was removed and post-fixed in 10% buffered formalin overnight before embedding in paraffin. Following embedding 8μM slices were cut, mounted onto slides, deparaffinization in xylene, hydrated through graded alcohols to distilled water, and stained with hematoxylin and eosin (H&E). Other sections were immunolabeled for OX-42 (macrophages, Serotec, Oxford, UK, diluted 1:400), CD-43 (T-lymphocytes, Serotec, diluted 1:250), and HIS48 (neutrophils, Santa Cruz Biotechnology, Santa Cruz, CA, diluted 1:20) protein expression. After incubated with the primary antibodies the immunostained sections were incubated with a biotinylated secondary antibody against mouse IgG (Vector Laboratories, Burlingame, CA, diluted 1:200), followed byincubation with avidin-FITC (Vector Laboratories). Sections were viewed using an Olympus BH-2 microscope and digital imaging equipment (Diagnostic Instruments, Sterling Heights, MI). Fluorescent images were visualized by a confocal microscope (Zeiss LSM/510 META).
Study design
Pentoxifylline (Sigma, St. Louis, MO), was given in water, in a dosage of 200mg/kg/day for 30 days. The first 7 days the drug was added in water and administered by gavage while the next 23 days it was given in drinking water (200 mg/kg in 5 ml water) between the hours of 9am and 5pm. If all the water was not consumed each day the residual was given by gavage. The dosage and administration protocol for PTX was based on previous studies using PTX intraperitoneal (i.p.) administration in rat models of neuropathic and inflammatory pain (Wordliczek et al. 2000; Liu et al. 2007). The rats were given either PTX in water or drinking water for 30 days, starting the day before the fracture, over the 28 day post-fracture interval.
Baseline determinations were made of bilateral hindpaw temperature, thickness, mechanical nociceptive withdrawal thresholds, and weighting bearing. After baseline tests the rats underwent a right distal tibia fracture with casting. The casts were removed after 28 days and repeat bilateral testing of hindpaw temperature, thickness, mechanical nociceptive withdrawal thresholds, and weighting bearing was performed. Overall, there were two cohorts of fracture rats treated with PTX. One was given PTX chronically for 30 days, whereas another cohort of fracture rats underwent baseline behavioral testing on the day after cast removal and were then given a single dose of PTX (200mg/kg) by gavage. Behavioral tests were repeated within 1–2 hours after drug administration in the fracture rats given a single dose of PTX. In addition, tail-flick latency testing was performed in control rats that were given either a single dose of PTX (200mg/kg) or water by gavage 1–2 hours after drug administration in order to determine an analgesic efficacy of the drug for acute nociceptive stimuli in intact animals. Hindpaw temperature, thickness, and mechanical allodynia data were analyzed as the difference between the treatment side and the contralateral untreated side. Weight bearing data were analyzed as the ratio between right and left hindpaw weight bearing values ((2R/(R+L)) ×100%). Ex vivo μCT scanning was used to determine vertebral and femoral bone parameters for control rats (no fracture) and fracture rats that were given with either PTX or water. Hindpaw skin mRNA and protein expression for TNFα, IL-1β and IL-6 were determined at 4 weeks post-fracture.
Statistical analysis
Statistical analysis was accomplished using a one-way analysis of variance (ANOVA) followed by post hoc Newman-Keuls multiple comparison testing to compare between three cohorts: control rats, fracture rats treated with water, and fracture rats treated chronically with PTX for 30 days. In addition, unpaired Student t-testing was performed in two fracture groups treated either with a single PTX dose or water on the day after cast removal. An unpaired Student t-test was also used to compare tail-flick latencies in control rats treated either with a single dose of PTX or with water. All data are presented as the mean ± SEM, and differences were considered significant at a p value less than 0.05.
Results
Increased expression of TNFα, IL-1β and IL-6 mRNA in hindpaw skin after fracture
We used real-time PCR to detect changes in expression of TNFα, IL-1β and IL-6 mRNA in control, fracture and PTX treated fracture groups. Overall, we observed a consistent increase in the mRNA levels for all three cytokines in the ipsilateral hindpaw skin at 4 weeks after fracture (Fig. 1).
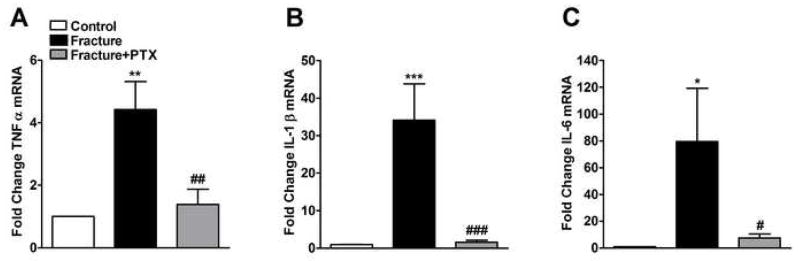
Figure 1.
Cytokine mRNA levels in hindpaw skin ipsilateral to tibia fracture. The mRNA levels of TNFα (panel A), IL-1β (panel B) and IL-6 (panel C) were measured using real-time PCR. Groups include control rats, fracture rats given regular water, or fracture rats given 200mg/kg/day pentoxiphylline (PTX) in drinking water. At 4 weeks after fracture the levels of mRNA expression for all cytokines were increased in ipsilateral hindpaw skin relative to the control animals. Treatment with PTX reduced mRNA levels of these cytokines relative to tibia fracture rats receiving regular drinking water (n = 8–10 per cohort). *P < 0.05, **P < 0.01 and ***P < 0.001 for fracture vs. control groups, #P < 0.05, ##P < 0.01 and ###P < 0.001 for fracture/PTX treated vs. fracture/regular water groups.
Cytokine mRNA expression levels were examined by one-way ANOVA with a significant effect for all 3 cytokines indicating overall differences: F2,16 = 10.65, p = 0.001 for TNFα (panel 1A); F2,19 = 12.35, p = 0.0004 for IL-1β (panel 1B); and F2,19 = 3.89, p = 0.038 for IL-6 (panel 1C). TNFα, IL-1β and IL-6 mRNA levels were significantly increased by 4, 33, and 79-fold, respectively, in the fracture cohort (post-hoc testing vs control rats; p < 0.01 for TNFα, p < 0.001 for IL-1β and p < 0.05 for IL-6). PTX treatment in fracture rats (200mg/kg/day for 4 weeks), starting the day before fracture, prevented any increase in cytokine expression (post-hoc testing vs untreated fracture rats; p < 0.01 for TNFα, p < 0.001 for IL-1β and p < 0.05 for IL-6).
Increased TNFα, IL-1β and IL-6 protein levels in hindpaw skin after fracture
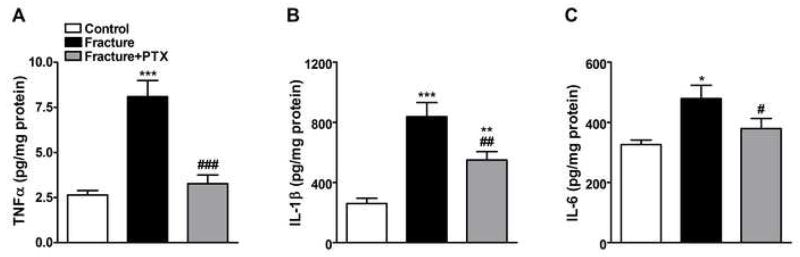
TNFα, IL-1β and IL-6 protein levels in hindpaw skin were determined using rat ELISA cytokine assays (Fig. 2) at 4 weeks post-fracture. Protein levels among 3 rat cohorts were examined by an analysis of variance with a significant effect for all three cytokines indicating overall differences; F2,26 = 16.68, p < 0.0001 for TNFα (panel 2A), F2,23 = 18.21, p < 0.0001 for IL-1β (panel 2B), and F2,23 = 4.86, p = 0.017 for IL-6 (panel 2C). After fracture all three cytokine levels were significantly increased (post-hoc testing vs controls; p < 0.001 for TNFα, p < 0.001 for IL-1β and p < 0.05 for IL-6). When fracture rats were treated with PTX there was a significant inhibition of fracture-induced elevation in skin cytokine levels (post-hoc testing vs untreated fracture rats; p < 0.001 for TNFα, p < 0.01 for IL-1β and p < 0.05 for IL-6).
Figure 2.
Skin cytokine protein levels in hindpaw skin ipsilateral to tibia fracture. The protein levels of TNFα (panel A), IL-1β (panel B) and IL-6 (panel C) were measured by ELISA assay at 4 weeks after fracture. Tibia fracture induced a significant increase in the levels of all cytokines at this time point. PTX treatment completely blocked the fracture induced increase in TNFα and IL-6 levels, and partially blocked the increase in IL-1β protein (n =8–10 per cohort). *P < 0.05, **P < 0.01 and ***P < 0.001 for fracture vs. control groups, #P < 0.05, ##P < 0.01 and ###P < 0.001 for fracture/PTX treated vs. fracture/regular water groups.
PTX effects on hindpaw vascular and nociceptive parameters after fracture
The effect of prolonged PTX treatment on fracture-induced hindpaw warmth, edema, mechanical sensitivity and weight bearing was evaluated (Fig. 3). An analysis of variance showed overall differences among three rat cohorts for behavioral vascular indices, with F2,23 = 8.87, p = 0.001 for edema (panel 3A), and F2,23 = 17.78, p < 0.0001 for temperature (panel 3B). At 4 weeks post-fracture the right hindpaw thickness (Fig. 3A) and temperature (Fig. 3B) were increased in the ipsilateral hindpaws of untreated fracture rats (post-hoc testing vs control rats; p < 0.001 for edema, and p < 0.001 for temperature). Four weeks of PTX treatment in fracture rats inhibited the expected increase in hindpaw temperature (post-hoc testing vs untreated fracture rats; p < 0.05, panel 3B), but had no effect on edema (post-hoc testing vs untreated fracture rats; p > 0.05, panel 3A).
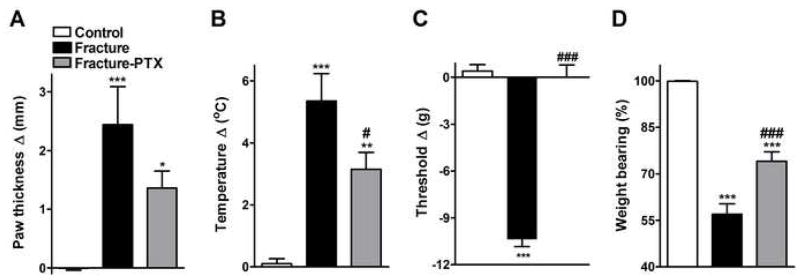
Figure 3.
Vascular and nociceptive changes after tibia fracture with and without prolonged PTX treatment. Treatment with PTX appeared to diminish hindpaw edema (but did not reach significance on ANOVA test, panel A), decreased warmth (panel B) and reversed the hindpaw mechanical allodynia (panel C) that developed after fracture. It also partially restored normally distributed hindlimb weight bearing (panel D). Measurements presented in panels A, B, and C represent the difference between the fracture side and the contralateral paw. The data presented in panel D represent the percentage weight bearing on the fracture hindlimb versus normal (100%) weight bearing on that limb if the animal’s weight were evenly distributed. A value of 100%, therefore, represents equally distributed weight (n = 8 per cohort). *P < 0.05, **P < 0.01 and ***P < 0.001 fracture vs. control groups, and #P < 0.05 and ###P<0.001 fracture/PTX treated vs fracture/plain water groups.
An analysis of variance showed overall differences among three cohorts for mechanical allodynia (F2,23 = 92.42, p < 0.0001, panel 3C) and weight bearing (F2,23 = 57.22, p < 0.0001, panel 3D). Figure 3C illustrates that von Frey nociceptive thresholds in the right hindpaw were reduced after fracture (post-hoc testing vs control rats; p < 0.001) and chronic PTX treatment completely reversed fracture-induced mechanical allodynia (post-hoc testing vs untreated fracture rats; p < 0.001). Untreated fracture rats unweighted the ipsilateral hindpaw by 43% at 4 weeks post-fracture (post-hoc testing vs control rats; p < 0.001) and PTX treatment partially restored weight bearing in the fracture hindlimb (post-hoc testing vs untreated controls; p < 0.001, Fig. 3D). Neither fracture nor PTX treatment had any effect on nociceptive thresholds in the contralateral hindpaw vs. control rats (data not shown).
Treatment with a single dose of PTX (200 mg/kg, p.o.) at 4 weeks post fracture had no effect on warmth and edema parameters. However, the drug completely reversed mechanical allodynia (unpaired t-Test = 5.5, df = 17, p < 0.001 vs week 4 fracture rats treated with water) and weight bearing was partially restored (unpaired t-Test = 4.23, df = 16, p < 0.001 vs week 4 fracture rats treated with water). The magnitude of the drug-induced increases in nociceptive thresholds and weight bearing after a single dose of PTX were similar to the effects of prolonged PTX administration (data not shown). Treatment with a single dose of PTX (200 mg/kg, p.o.) in control rats had no effect on tail-flick latencies (vs control rats administered just water, unpaired t-test, data not shown), indicating a lack of analgesic efficacy for acute nociceptive stimuli.
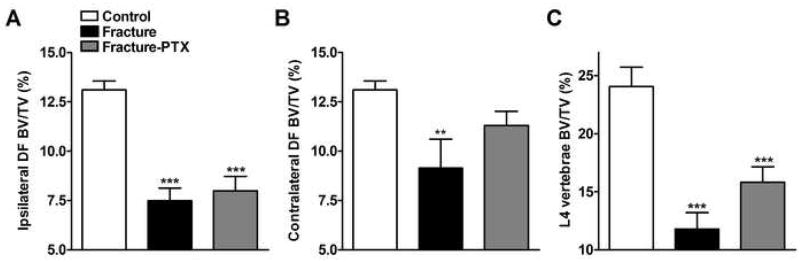
PTX effect on trabecular and cortical bone loss after fracture
Representative μCT scans in figure 4 illustrate fracture induced trabecular bone loss in the distal femur and L4 vertebrae. Figure 5 shows quantitative data for the effects of fracture and PTX treatment on trabecular bone. Trabecular bone BV/TV% changes among three cohorts were examined by one-way ANOVA with a significant effect for three trabecular sites indicating overall differences: F2,31 = 31.16, p < 0.0001 for ipsilateral distal femur (panel 5A), F2,30 = 6.41, p = 0.005 for contralateral distal femur (panel 5B), and F2,23 = 17.18, p < 0.0001 for L4 vertebrae (panel 5C). After fracture there was a 43% reduction in the ipsilateral distal femur BV/TV% (post-hoc testing vs control rats; p < 0.001), a 30% reduction in the contralateral distal femur BV/TV% (post-hoc testing vs control rats; p < 0.01) and a 51% reduction in the L4 vertebral BV/TV% (post-hoc testing vs control rats; p < 0.001). Chronic PTX treatment had no significant effect on fracture induced bone loss in any of these skeletal sites.
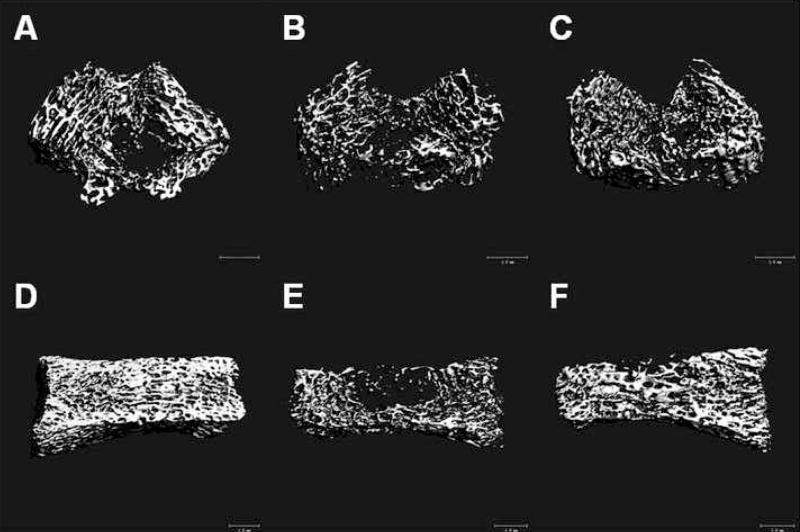
Figure 4.
Three dimensional μCT analysis of skeletal components. The images of the ipsilateral distal femur (panels A, B, C) and L4 vertebra (panels D, E, F) illustrate changes in the trabecular bone compartment at 4 weeks after tibia fracture. Scans A and D are from a control rat, B and E are from a fracture rat, and C and F are from a fracture rat treated with PTX. There was marked trabecular bone loss after tibia fracture. Overall, PTX treatment had little effect on trabecular bone loss. The white bar represents 1 mm.
Figure 5.
Quantitative analysis of three dimensional μCT data for trabecular bone. Analysis of the images collected as described for Figure 4 revealed extensive trabecular bone loss in the ipsilateral (panel A) and contralateral (panel B) distal femur as well as in the L4 vertebra (panel C) compared to control rats. PTX treatment had no effect on trabecular bone loss (panels A through C). (n = 8–16 per cohort). **P < 0.01 and ***P < 0.001 fracture vs. control groups.
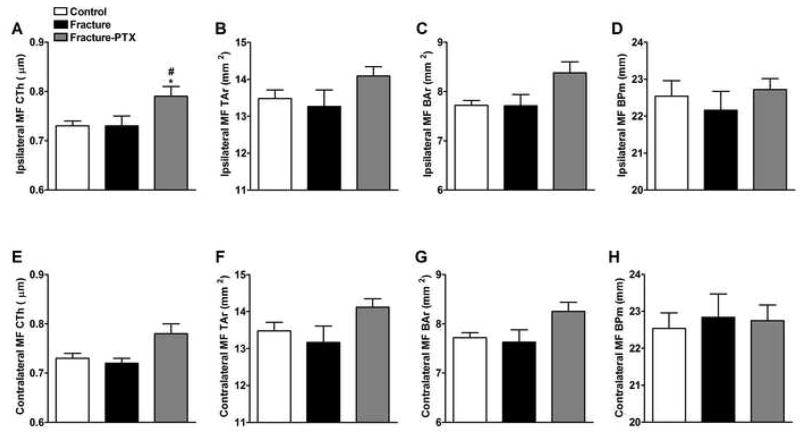
In cortical bone an analysis of variance showed overall differences among the three cohorts for ipsilateral cortical thickness (F2,31 = 4.89, p = 0.014, panel 6A). Interestingly, there were no significant changes in the cortical bone parameters in the fracture group (Fig. 6), whereas PTX treatment increased the ipsilateral cortical bone thickness at 4 weeks post-fracture relative to control and fracture cohorts (Fig. 6A, post-hoc p < 0.05).
Figure 6.
Quantitative analysis of three dimensional μCT data for cortical bone. Femurs were scanned bilaterally by μCT in the midfemur (MF) region for cortical bone parametersat 4 weeks post-fracture. Panels A, B, C and D represent the right MF (ipsilateral to the fracture side), whereas panels E, F, G and H represent the left MF (contralateral to the fracture side) regions. Fracture did not have an effect on cortical thickness (CtTh), total area (TAr), bone area (BAr), or bone perimeter (BPm) on either side. PTX treatment increased CtTh ipsilaterally (panel A) (n = 8–16 per cohort). *P < 0.05 fracture vs. control group, and #P < 0.05 fracture/PTX treated vs. fracture/regular water groups.
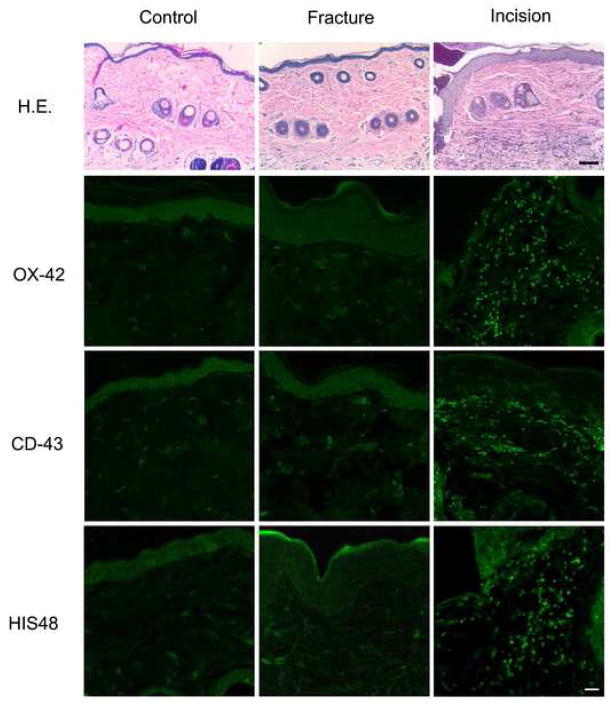
Immunocyte infiltration into hindpaw skin after fracture
Immunohistochemistry was used to detect the infiltration of immunocytes into hindpaw skin. In Figure 7 the top row shows representative micrographs of H&E stained skin. Inspection of these sections did not reveal the existence of an immunocyte infiltration in the fractured hindpaw skin. Skin harvested 3 days after hindpaw incision in positive control experiments did, however, show a dense cellular infiltrate. We also tested antibodies selective for macrophages (OX-42, second row), T-lymphocytes (CD-43, third row), and neutrophils (HIS48, bottom row). This staining was negative for any type of immunocytes in hindpaw tissue from control and fracture rats, while robust infiltrations of macrophages (day 3), T-lymphocytes (day 7), and neutrophils (day 3) were observed after hindpaw incision (positive controls).
Figure 7.
H&E and immunohistochemical staining of rat hind paw tissue from rats with and without fracture and positive control rats with hindpaw incision. The dorsal hind paw skin from rats 4 weeks after fracture, 3 and 7 days after incision were harvested for these studies. In the top row are representative H&E stained sections. Cellular infiltration was observed only in skin from rats incised 3 days earlier. The top row for fluorescent microscopy contains micrographs from sections exposed to antibodies for OX-42, a macrophage marker. No specific staining was noted in the fractured hind paw skin, while a robust response is observed in the skin incised 3 days earlier. The middle row for fluorescent microscopy shows sections exposed to antibodies for CD-43, a T-lymphocyte marker. Again, no specific staining was noted in the fracture group, though abundant T-lymphocytes were identified in skin from rats incised 7 days earlier. The bottom row shows no specific staining observed in the fracture hindpaw for HIS48, a neutrophil marker, but robust staining in the rats incised 3 days earlier. Scale bar: top panel - 100μm, middle and bottom panels -25μm.
PTX had no effect on body mass after fracture
At four weeks after tibia fracture both the untreated and PTX treated rats lost body weight (p < 0.001, data not shown). The untreated fracture rats lost 22% of their body mass (458 ± 9.8 g baseline vs 358 ± 14.4 g at week 4), while the drug treated fracture rats lost 21% of their body weight (455 ± 8.3 g baseline vs 358 ± 7.1 g at week 4).
Discussion
At 4 weeks after fracture there was an increase in TNFα, IL-1β and IL-6 mRNA (Fig. 1) and protein expression (Fig. 2) in the ipsilateral hindpaw skin, similar to the increases seen in the CRPS affected limbs of humans. Over-expression of pro-inflammatory cytokines can cause inflammatory pain and bone loss in animal models and clinical studies. The pharmacological manipulation of cytokine levels might be expected, therefore, to alter nociceptive sensitization and perhaps other facets of CRPS.
PTX, a nonspecific phosphodiesterase inhibitor, has several actions that improve blood rheology and tissue perfusion and it is used clinically as a treatment for intermittent claudication,(Accetto 1982) the most common symptom of mild-to-moderate peripheral arterial disease. Laboratory investigations, however, generally employ the drug for its anti-cytokine effects and previous reports demonstrate that PTX administration virtually eliminates the up-regulation of cytokines in various pain models (Strieter et al. 1988; Zabel et al. 1993; Dorazil-Dudzik et al. 2004; Ji et al. 2004; Lu et al. 2004; Vale et al. 2004; Liu et al. 2007). In this study we showed that chronic PTX treatment completely blocked fracture induced increases in hindpaw TNFα, IL-1β and IL-6 mRNA (Fig. 1) and protein (Fig. 2) levels, except for the increase in IL-1β protein, which was only partially inhibited by PTX treatment (Fig. 2B).
It is notable that changes in mRNA levels and changes in cytokine protein were not always proportional in this CRPS model. For example, the relative changes in both IL-1β and IL-6 mRNA after fracture were far in excess of the protein level changes (Figures 1 and 2). This is not an uncommon type of observation, and may be attributable to translational control. For example, Ferlito et al. reported enhanced TNFα protein production in human promonocytic cells after LPS stimulation due to regulation at the translational and post-translational levels. No changes in transcription or mRNA stability were found.(Ferlito and De Maio 2005). Gornikiewicz et al. demonstrated that increased IL-6 mRNA caused by transcriptional up-regulation can occur without large changes in protein production.(Gornikiewicz et al. 2000) Also, it has been shown that terminal complement component mRNA levels could be increased in synovial cells and keratinocytes stimulated with various cytokines, whereas changes in complement protein levels could not be detected. (Guc et al. 1993; Timar et al. 2007) With a host of regulatory mechanisms controlling cytokine expression at the transcriptional, splicing, mRNA stability and translational levels, it is perhaps not surprising that fracture does not affect the levels of all cytokines in the same way. Thus a strength of our study is the measurement of both mRNA and cytokine protein levels.
Previous reports have demonstrated that PTX suppresses phosphodiesterase activity, leading to increased intracellular cAMP levels (for review see (Ward and Clissold 1987)). The increased cAMP has been reported to stimulate a protein kinase A (PKA), resulting in the phosphorylation of a number of regulatory proteins,(Remold-O’Donnell 1974) including cAMP-responsive element binding protein (CREB), which can inhibit nuclear factor kappa B (NF-κB) mediated transcription of cytokines (Parry and Mackman 1997). Thus, sustained elevated levels of cAMP due to PTX treatment can reduce different inflammatory processes through the stimulation of PKA-CREB pathway shutting down NF-κB-induced transcription(Biswas et al. 1994; Blackwell and Christman 1997; Ji et al. 2004). In the current study we showed that PTX down-regulated TNFα, IL-1β and IL-6 mRNA levels, perhaps due to this cAMP-PKA-CREB-NF-κB pathway.
An additional aim of this study was to identify PTX effects on different clinically relevant manifestations of CRPS, including nociceptive and vascular abnormalities. Numerous clinical and translational studies have demonstrated cytokine involvement in the development of inflammatory nociception, and cytokine levels have been shown to be elevated in the skin of patients with CRPS. We hypothesized that PTX inhibition of cytokine overexpression after fracture might prevent the development of hindlimb nociceptive sensitization, edema and warmth. Mechanical allodynia was completely reversed and weight bearing partially restored after a 4-week course of PTX treatment (200 mg/kg/d, p.o.), started the day before tibia fracture. Furthermore, a single dose of PTX (200 mg/kg, p.o.) had similar antinociceptive efficacy when given at 4 weeks post-fracture, suggesting that PTX treatment is not just effective when administered preemptively but can also relieve pain in the fully developed CRPS model. Prolonged PTX administration also reduced the hindpaw warmth characteristic of the fracture model (inhibition of edema did not quite reach significance). Though not previously studied in CRPS patients, PTX has been demonstrated to reduce nociceptive sensitization and cytokine production in animal models of inflammatory and neuropathic pain (Wordliczek et al. 2000; Dorazil-Dudzik et al. 2004; Vale et al. 2004; Liu et al. 2007; Mika et al. 2007). Others have administered PTX to surgical patients and observed lower postoperative pain scores, opioid requirements and serum cytokine levels(Wordliczek et al. 2000; Lu et al. 2004).
Another distinct component of CRPS is regional trabecular bone loss(Bickerstaff et al. 1993; Sarangi et al. 1993). We observed trabecular bone loss in distal femur ipsilateral and contralateral to the fracture site, as well as in the 4th lumbar vertebra. However, there was no cortical bone loss in these animals. When the normal interaction between the immune and skeletal systems is disrupted by chronic inflammation the balance favors net bone loss (Rauner et al. 2006). TNFα, IL-1β and IL-6 regulate bone resorption by stimulating expression of receptor activator of NF-κB ligand (RANKL) in osteoblasts(Thomson et al. 1986; Thomson et al. 1987; Kishimoto 1989; Rauner et al. 2006), which is necessary for osteoclast differentiation(Yasuda et al. 1998). The bone loss observed after tibia fracture, in the setting of elevated levels of multiple cytokines, is consistent with our current state of understanding of interaction between the immune and skeletal systems. PTX treatment can induce osteoblastic differentiation, stimulate bone formation and increase bone mass in mice(Kinoshita et al. 2000; Rawadi et al. 2001; Tsutsumimoto et al. 2002; Horiuchi et al. 2004), and other phosophodiesterase inhibitors have been shown to exert therapeutic effect in different osteopenia models(Robin and Ambrus 1983; Waki et al. 1999). In the present study, we observed that chronic PTX treatment had no significant effect on trabecular bone loss after fracture, but there was a significant increase in cortical bone thickness after PTX treatment (Fig 6A), perhaps attributable to its inhibitory effects on phosphodiesterases and the NF-κB pathway.
Because the paws of the tibia fracture animals exhibited signs of inflammation (warmth, edema, nociceptive sensitization and enhanced cytokine production), we looked for immunocytes in the fracture hindpaw. We failed to observe any infiltration of macrophages, T-cells, or neutrophils in the hindpaw skin at 4 weeks post-fracture (Fig. 7). Our control experiments using previously incised skin confirmed that the immunohistochemical techniques selected would have allowed us to detect the infiltration of these cell types. We postulate that the overexpressed cytokines observed in the hindpaw skin after fracture were produced by resident cells such as keratinocytes. These cells can produce TNFα, IL-6 and other cytokines in the settings of acute and chronic disease-related inflammation, traumatic injury, and after exposure to irritants(Allen et al. 2000; Johansen et al. 2006; Eming et al. 2007).
Collectively, our data support the idea that CRPS is a syndrome involving a host of different end organ pathophysiological mechanisms resulting in a diverse range of manifestations. At 4 weeks after tibia fracture there was a dramatic up-regulation of cytokines in the injured limb and we postulated that this inflammatory response could contribute to the CRPS-like consequences fracture. The antinociceptive effects of PTX were profound, but vascular and bone abnormalities were less responsive to PTX treatment. It does not appear that strategies directed only at modifying cytokine levels will effectively treat all signs and symptoms of CRPS. On the other hand, changes in the abundance of inflammatory cytokines like TNFα seem to be associated with antinociceptive effects and a reasonable path forward would be to delineate the role of individual cytokines in peripheral and neural tissues as they function to support the development of this post-fracture syndrome.
Acknowledgments
This study was supported by National Institutes of Health Grants GM65345 and DK67197.
Footnotes
Statement Summary: Pentoxifylline treatment inhibited fracture induced up-regulation of inflammatory cytokines in the rat hindpaw and reversed nociceptive sensitization and vascular abnormalities in this chronic pain model. These results suggest that proinflammatory cytokines contribute to the nociceptive and vascular sequelae of fracture and that pentoxifylline treatment can reverse these CRPS-like changes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accetto B. Beneficial hemorheologic therapy of chronic peripheral arterial disorders with pentoxifylline: results of double-blind study versus vasodilator-nylidrin. Am Heart J. 1982;103(5):864–869. doi: 10.1016/0002-8703(82)90401-x. [DOI] [PubMed] [Google Scholar]
- Alexander GM, van Rijn MA, van Hilten JJ, Perreault MJ, Schwartzman RJ. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain. 2005;116(3):213–219. doi: 10.1016/j.pain.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Allen MH, Wakelin SH, Holloway D, Lisby S, Baadsgaard O, Barker JN, McFadden JP. Association of TNFA gene polymorphism at position -308 with susceptibility to irritant contact dermatitis. Immunogenetics. 2000;51(3):201–205. doi: 10.1007/s002510050032. [DOI] [PubMed] [Google Scholar]
- Atkins RM, Duckworth T, Kanis JA. Features of algodystrophy after colles’ fracture. J Bone Joint Surg. 1990;72 B:105–110. doi: 10.1302/0301-620X.72B1.2298766. [DOI] [PubMed] [Google Scholar]
- Bickerstaff DR, Charlesworth D, Kanis JA. Changes in cortical and trabecular bone in algodystrophy. Br J Rheumatol. 1993;32(1):46–51. doi: 10.1093/rheumatology/32.1.46. [DOI] [PubMed] [Google Scholar]
- Bickerstaff DR, Kanis JA. Algodystrophy: an under-recongnized complication of minor trauma. Br J Rheumatol. 1994;33:240–248. doi: 10.1093/rheumatology/33.3.240. [DOI] [PubMed] [Google Scholar]
- Biswas DK, Ahlers CM, Dezube BJ, Pardee AB. Pentoxifylline and other protein kinase C inhibitors down-regulate HIV-LTR NF-kappa B induced gene expression. Mol Med. 1994;1(1):31–43. [PMC free article] [PubMed] [Google Scholar]
- Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17(1):3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Janig W. In: Clinical manifestations of reflex sympathetic dystrophy and sympathetically maintained pain. Wall PD, Melzack R, editors. |. Book Title|, Vol. Volume|. City|: Publisher|, Year|. p.^pp. Pages|. [Google Scholar]
- Bowden JJ, Garland AM, Baluk P, Lefevre P, Grady EF, Vigna SR, Bunnett NW, McDonald DM. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci USA. 1994;91:8964–8968. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 2005;102(5):1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorazil-Dudzik M, Mika J, Schafer MK, Li Y, Obara I, Wordliczek J, Przewlocka B. The effects of local pentoxifylline and propentofylline treatment on formalin-induced pain and tumor necrosis factor-alpha messenger RNA levels in the inflamed tissue of the rat paw. Anesth Analg. 2004;98(6):1566–1573. doi: 10.1213/01.ANE.0000113235.88534.48. table of contents. [DOI] [PubMed] [Google Scholar]
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2(5):364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- Ferlito M, De Maio A. Enhanced LPS-induced TNF alpha production in heat-shocked human promonocytic cells: regulation at the translational/post-translational level. Biochim Biophys Acta. 2005;1743(1–2):20–28. doi: 10.1016/j.bbamcr.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Gornikiewicz A, Sautner T, Brostjan C, Schmierer B, Fugger R, Roth E, Muhlbacher F, Bergmann M. Catecholamines up-regulate lipopolysaccharide-induced IL-6 production in human microvascular endothelial cells. Faseb J. 2000;14(9):1093–1100. doi: 10.1096/fasebj.14.9.1093. [DOI] [PubMed] [Google Scholar]
- Guc D, Gulati P, Lemercier C, Lappin D, Birnie GD, Whaley K. Expression of the components and regulatory proteins of the alternative complement pathway and the membrane attack complex in normal and diseased synovium. Rheumatol Int. 1993;13(4):139–146. doi: 10.1007/BF00301260. [DOI] [PubMed] [Google Scholar]
- Guo TZ, Davies MF, Kingery WS, Patterson AJ, Limbird LE, Maze M. Nitrous oxide produces antinociceptive response via alpha2B and/or alpha2C adrenoceptor subtypes in mice. Anesthesiology. 1999;90:470–476. doi: 10.1097/00000542-199902000-00022. [DOI] [PubMed] [Google Scholar]
- Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108(1–2):95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Guo TZ, Wei T, Kingery WS. Glucocorticoid inhibition of vascular abnormalities in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2006;121(1–2):158–167. doi: 10.1016/j.pain.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Saito N, Kinoshita T, Wakabayashi S, Tsutsumimoto T, Otsuru S, Takaoka K. Enhancement of recombinant human bone morphogenetic protein-2 (rhBMP-2)-induced new bone formation by concurrent treatment with parathyroid hormone and a phosphodiesterase inhibitor, pentoxifylline. J Bone Miner Metab. 2004;22(4):329–334. doi: 10.1007/s00774-003-0490-y. [DOI] [PubMed] [Google Scholar]
- Huygen FJ, De Bruijn AG, De Bruin MT, Groeneweg JG, Klein J, Zijistra FJ. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators Inflamm. 2002;11(1):47–51. doi: 10.1080/09629350210307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen FJ, Niehof S, Zijlstra FJ, van Hagen PM, van Daele PL. Successful treatment of CRPS 1 with anti-TNF. J Pain Symptom Manage. 2004a;27(2):101–103. doi: 10.1016/j.jpainsymman.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Huygen FJ, Ramdhani N, van Toorenenbergen A, Klein J, Zijlstra FJ. Mast cells are involved in inflammatory reactions during Complex Regional Pain Syndrome type 1. Immunol Lett. 2004b;91(2–3):147–154. doi: 10.1016/j.imlet.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Inglis JJ, Nissim A, Lees DM, Hunt SP, Chernajovsky Y, Kidd BL. The differential contribution of tumour necrosis factor to thermal and mechanical hyperalgesia during chronic inflammation. Arthritis Res Ther. 2005;7(4):R807–816. doi: 10.1186/ar1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q, Zhang L, Jia H, Xu J. Pentoxifylline inhibits endotoxin-induced NF-kappa B activation and associated production of proinflammatory cytokines. Ann Clin Lab Sci. 2004;34(4):427–436. [PubMed] [Google Scholar]
- Johansen C, Funding AT, Otkjaer K, Kragballe K, Jensen UB, Madsen M, Binderup L, Skak-Nielsen T, Fjording MS, Iversen L. Protein expression of TNF-alpha in psoriatic skin is regulated at a posttranscriptional level by MAPK-activated protein kinase 2. J Immunol. 2006;176(3):1431–1438. doi: 10.4049/jimmunol.176.3.1431. [DOI] [PubMed] [Google Scholar]
- Kingery WS, Davies MF, Clark JD. A substance P receptor (NK1) antagonist can reverse vascular and nociceptive abnormalities in a rat model of complex regional pain syndrome type II. Pain. 2003;104(1–2):75–84. doi: 10.1016/s0304-3959(02)00467-0. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Kobayashi S, Ebara S, Yoshimura Y, Horiuchi H, Tsutsumimoto T, Wakabayashi S, Takaoka K. Phosphodiesterase inhibitors, pentoxifylline and rolipram, increase bone mass mainly by promoting bone formation in normal mice. Bone. 2000;27(6):811–817. doi: 10.1016/s8756-3282(00)00395-1. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989;74(1):1–10. [PubMed] [Google Scholar]
- Leis S, Weber M, Isselmann A, Schmelz M, Birklein F. Substance-P-induced protein extravasation is bilaterally increased in complex regional pain syndrome. Exp Neurol. 2003;183(1):197–204. doi: 10.1016/s0014-4886(03)00163-8. [DOI] [PubMed] [Google Scholar]
- Liu J, Feng X, Yu M, Xie W, Zhao X, Li W, Guan R, Xu J. Pentoxifylline attenuates the development of hyperalgesia in a rat model of neuropathic pain. Neurosci Lett. 2007;412(3):268–272. doi: 10.1016/j.neulet.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Lu CH, Chao PC, Borel CO, Yang CP, Yeh CC, Wong CS, Wu CT. Preincisional intravenous pentoxifylline attenuating perioperative cytokine response, reducing morphine consumption, and improving recovery of bowel function in patients undergoing colorectal cancer surgery. Anesth Analg. 2004;99(5):1465–1471. doi: 10.1213/01.ANE.0000132974.32249.C8. table of contents. [DOI] [PubMed] [Google Scholar]
- Massaad CA, Safieh-Garabedian B, Poole S, Atweh SF, Jabbur SJ, Saade NE. Involvement of substance P, CGRP and histamine in the hyperalgesia and cytokine upregulation induced by intraplantar injection of capsaicin in rats. J Neuroimmunol. 2004;153(1–2):171–182. doi: 10.1016/j.jneuroim.2004.05.007. [DOI] [PubMed] [Google Scholar]
- McDonald DM. Neurogenic inflammation in the rat trachea. I. Changes in venules, leucocytes and epithelial cells. J Neurocytol. 1988;17(5):583–603. doi: 10.1007/BF01260988. [DOI] [PubMed] [Google Scholar]
- Mika J, Osikowicz M, Makuch W, Przewlocka B. Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur J Pharmacol. 2007;560(2–3):142–149. doi: 10.1016/j.ejphar.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Oyen WJ, Arntz IE, Claessens RM, Van der Meer JW, Corstens FH, Goris RJ. Reflex sympathetic dystrophy of the hand: an excessive inflammatory response? Pain. 1993;55(2):151–157. doi: 10.1016/0304-3959(93)90144-E. [DOI] [PubMed] [Google Scholar]
- Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol. 1997;159(11):5450–5456. [PubMed] [Google Scholar]
- Rauner M, Sipos W, Pietschmann P. Osteoimmunology. Int Arch Allergy Immunol. 2006;143(1):31–48. doi: 10.1159/000098223. [DOI] [PubMed] [Google Scholar]
- Rawadi G, Ferrer C, Spinella-Jaegle S, Roman-Roman S, Bouali Y, Baron R. 1-(5-oxohexyl)-3,7-Dimethylxanthine, a phosphodiesterase inhibitor, activates MAPK cascades and promotes osteoblast differentiation by a mechanism independent of PKA activation (pentoxifylline promotes osteoblast differentiation) Endocrinology. 2001;142(11):4673–4682. doi: 10.1210/endo.142.11.8499. [DOI] [PubMed] [Google Scholar]
- Remold-O’Donnell E. Stimulation and desensitization of macrophage adenylate cyclase by prostaglandins and catecholamines. J Biol Chem. 1974;249(11):361–321. [PubMed] [Google Scholar]
- Robin JC, Ambrus JL. Studies on osteoporoses. XI. Effects of a methylxanthine derivative. A preliminary report. J Med. 1983;14(2):137–145. [PubMed] [Google Scholar]
- Saade NE, Massaad CA, Ochoa-Chaar CI, Jabbur SJ, Safieh-Garabedian B, Atweh SF. Upregulation of proinflammatory cytokines and nerve growth factor by intraplantar injection of capsaicin in rats. J Physiol. 2002;545(Pt 1):241–253. doi: 10.1113/jphysiol.2002.028233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangi PP, Ward AJ, Smith EJ, Staddon GE, Atkins RM. Algodystrophy and osteoporosis after tibial fractures. J Bone Joint Surg Br. 1993;75(3):450–452. doi: 10.1302/0301-620X.75B3.8496220. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007 doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Remick DG, Ward PA, Spengler RN, Lynch JP, 3rd, Larrick J, Kunkel SL. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988;155(3):1230–1236. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- Thomson BM, Mundy GR, Chambers TJ. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J Immunol. 1987;138(3):775–779. [PubMed] [Google Scholar]
- Thomson BM, Saklatvala J, Chambers TJ. Osteoblasts mediate interleukin 1 stimulation of bone resorption by rat osteoclasts. J Exp Med. 1986;164(1):104–112. doi: 10.1084/jem.164.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timar KK, Dallos A, Kiss M, Husz S, Bos JD, Asghar SS. Expression of terminal complement components by human keratinocytes. Mol Immunol. 2007;44(10):2578–2586. doi: 10.1016/j.molimm.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Tonussi CR, Ferreira SH. Tumour necrosis factor-alpha mediates carrageenin-induced knee-joint incapacitation and also triggers overt nociception in previously inflamed rat knee-joints. Pain. 1999;82(1):81–87. doi: 10.1016/S0304-3959(99)00035-4. [DOI] [PubMed] [Google Scholar]
- Tsutsumimoto T, Wakabayashi S, Kinoshita T, Horiuchi H, Takaoka K. A phosphodiesterase inhibitor, pentoxifylline, enhances the bone morphogenetic protein-4 (BMP-4)-dependent differentiation of osteoprogenitor cells. Bone. 2002;31(3):396–401. doi: 10.1016/s8756-3282(02)00839-6. [DOI] [PubMed] [Google Scholar]
- Uceyler N, Tscharke A, Sommer C. Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav Immun. 2007 doi: 10.1016/j.bbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Vale ML, Benevides VM, Sachs D, Brito GA, da Rocha FA, Poole S, Ferreira SH, Cunha FQ, Ribeiro RA. Antihyperalgesic effect of pentoxifylline on experimental inflammatory pain. Br J Pharmacol. 2004;143(7):833–844. doi: 10.1038/sj.bjp.0705999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman PHJM, Reynen HM, Arntz IE, Goris RJA. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342:1012–1016. doi: 10.1016/0140-6736(93)92877-v. [DOI] [PubMed] [Google Scholar]
- Waki Y, Horita T, Miyamoto K, Ohya K, Kasugai S. Effects of XT-44, a phosphodiesterase 4 inhibitor, in osteoblastgenesis and osteoclastgenesis in culture and its therapeutic effects in rat osteopenia models. Jpn J Pharmacol. 1999;79(4):477–483. doi: 10.1254/jjp.79.477. [DOI] [PubMed] [Google Scholar]
- Ward A, Clissold SP. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987;34(1):50–97. doi: 10.2165/00003495-198734010-00003. [DOI] [PubMed] [Google Scholar]
- Wasner G, Schattschneider J, Heckmann K, Maier C, Baron R. Vascular abnormalities in reflex sympathetic dystrophy (CRPS I): mechanisms and diagnostic value. Brain. 2001;124:587–599. doi: 10.1093/brain/124.3.587. [DOI] [PubMed] [Google Scholar]
- Waston CP. The treatment of postherpetic neuralgia. Neurology. 1995;45 (suppl 8):S58–S60. doi: 10.1212/wnl.45.12_suppl_8.s58. [DOI] [PubMed] [Google Scholar]
- Weber M, Birklein F, Neundorfer B, Schmelz M. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain. 2001;91(3):251–257. doi: 10.1016/S0304-3959(00)00445-0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121(3):417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordliczek J, Szczepanik AM, Banach M, Turchan J, Zembala M, Siedlar M, Przewlocki R, Serednicki W, Przewlocka B. The effect of pentoxifiline on post-injury hyperalgesia in rats and postoperative pain in patients. Life Sci. 2000;66(12):1155–1164. doi: 10.1016/s0024-3205(00)00419-7. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95(7):3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P, Schade FU, Schlaak M. Inhibition of endogenous TNF formation by pentoxifylline. Immunobiology. 1993;187(3–5):447–463. doi: 10.1016/S0171-2985(11)80356-6. [DOI] [PubMed] [Google Scholar]