Abstract
Although pain was previously not considered an important element of multiple sclerosis (MS), recent evidence indicates that over 50% of MS patients suffer from chronic pain. In the present study, we utilized the Theiler’s murine encephalomyelitis virus (TMEV) model of MS to examine whether changes in nociception occur during disease progression and to investigate whether sex influences the development of nociception or disease-associated neurological symptoms. Using the rotarod assay, TMEV infected male mice displayed increased neurological deficits when compared to TMEV infected female mice, which mimics what is observed in human MS. While both male and female TMEV infected mice exhibited thermal hyperalgesia and mechanical allodynia, female mice developed mechanical allodynia at a faster rate and displayed significantly more mechanical allodynia than male mice. Since neuropathic symptoms have been described in MS patients, we quantified sensory nerve fibers in the epidermis of TMEV-infected and non-infected mice to determine if there were alterations in epidermal nerve density. There was a significantly higher density of PGP9.5 and CGRP immunoreactive axons in the epidermis of TMEV-infected mice versus controls. Collectively these results indicate that the TMEV model is well suited to study the mechanisms of MS-induced nociception and suggest that alterations in peripheral nerve innervation may contribute to MS pain.
Keywords: Multiple Sclerosis, TMEV, thermal hyperalgesia, mechanical allodynia, epidermal nerve density, CGRP
1. Introduction
MS is an autoimmune inflammatory disease affecting 2.5 million people world wide and is characterized by immune mediated myelin loss and variable degrees of axonal degeneration. Classical symptoms of MS include trunk and limb paresthesias, limb weakness and gait ataxia. While MS patients are debilitated by lesions affecting motor areas of the CNS, they also suffer from sensory deficits and pain, symptoms, which until recently, have typically gone unnoticed. Recent studies have demonstrated that pain is an important component of MS with a prevalence of 50–80% among MS patients (Vermote et al., 1986; Kassirer and Osterberg, 1987; Moulin, 1989; Stenager et al., 1991; Archibald et al., 1994; Indaco et al., 1994; Stenager et al., 1995; Ehde et al., 2003; 2006; Svendsen et al., 2003). The pain severity in MS can be severely debilitating (Ehde et al., 2006) and MS patients exhibit cold allodynia and mechanical hyperalgesia (Svendsen et al., 2003), symptoms typically found in non-MS patients suffering from chronic pain conditions.
MS shows significant gender differences in incidence and disease progression with women having a 2:1 higher incidence of MS (Vein et al., 1995; Ford et al., 1998; McDonnell and Hawkins, 1998; Moreau et al., 2000; Totaro et al., 2000; Nicoletti et al., 2001), but with males developing more severe clinical symptoms and deteriorating faster (Wynn et al., 1990; Bronnum-Hansen et al., 1994; Vein et al., 1995; Cottrell et al., 1999). Gender differences are not only evident in MS prevalence, but also in MS pain. Moulin et al. (1989) found that while their overall patient population had a female to male ratio of 2.1/1, this ratio was 3.0/1 in the group of MS patients reporting pain versus 1.4/1 in MS patients without pain. Unfortunately, most studies examining MS pain prevalence and severity have ignored gender differences, leaving this an open question.
Most investigations examining MS pain have been survey studies in human patients and thus the actual cause of MS pain remains obscure. To date there has been only one published study that has employed an animal model (experimental allergic encephalomyelitis, EAE) to study MS-induced pain. This study reported decreased forepaw and tail withdrawal latencies to radiant heat (Aicher et al., 2004). While several of the EAE models mimic the relapsing/remitting quality of MS, the Theiler’s Murine Encephalomyelitis Virus (TMEV) model is perhaps a better model of the chronic progressive quality of MS and it may better model the contribution that CD8+ and CD4+ T lymphocytes make to the pathology observed in the human disease (Oleszak et al., 2004; Sriram and Steiner, 2005; Steinman and Zamvil, 2005). Intracerebral inoculation of mice with TMEV induces a biphasic CNS disease characterized by acute encephalitis during the first 2 weeks, followed by chronic demyelination (Lipton, 1975; Rodriguez et al., 1987). Both TMEV and MS are characterized by inflammatory infiltration, demyelination, remyelination and axonal damage that correlate with neurological disability (Oleszak et al., 2004). The TMEV mouse model was used in the present study to examine nociceptive responses and sex differences during disease progression.
2. Methods
2.1 Virus
The Daniel’s (DA) strain of Theilers Murine Encephalomyelitis Virus (TMEV) was used in all experiments. The virus was grown in BHK-21 cells and the titer was determined by plaque assay in L2 cells as described previously (Rodriguez et al., 1983).
2.2 Animals
One hundred and thirty-seven aged-matched male and female SJL/J mice (4–6 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME) and used for these experiments. Mice were housed in an animal facility at the University of Minnesota in accordance with institution guidelines and were given food and water ad lib. Six-week-old mice were inoculated intracerebrally with 4 × 106 PFU of TMEV in a 20-μl volume. To prevent fighting, male SJL/J mice were housed in individual cages.
2.3 Rotarod Analysis
A total of 70 mice were used for this experiment (n=20 TMEV female and 20 TMEV male mice, 15 uninfected control female and 15 uninfected control male mice). The accelerating rotarod test was performed as described previously (McGavern et al., 1999) with minor modifications. The Rotamex Rotarod device (Columbus Instruments; Columbus, OH) is equipped with a 4-cm variable speed, motor-driven rotating rod and was used to analyze balance and motor coordination in all of the mice. All mice were trained on the Rotarod for three consecutive days beginning at 5 revolutions per minute (rpm) on day 1, 6 rpm on day 2 and 7 rpm on day three. Each training period lasted for 5 min and mice that fell during training were placed back on the rod. For performance testing on day 4, mice were placed on the rod between dividers spaced 6 cm apart. The accelerated speed was started and allowed to run until each mouse fell and disrupted the infrared beam located at the bottom of the Rotarod. The Rotarod automatically recorded the length of time that each mouse was able to stay on the rotating rod. Three trials were performed for each mouse at each time point. The Rotarod test for each trial was carried out at an accelerating speed of between 5 and 40 rpm for 20 min with at least 30 min rest between trials for each mouse. In all cases, animals were selected in a random order for Rotarod testing and observations were performed in a blinded manner to the experimental status of the mouse. Since all measures of activity are sensitive to the circadian clock, rotarod measurements were started in the late morning. Statistical comparison of the latency to fall of the rod between infected and uninfected age-matched mice of the same gender and between infected male and female mice was performed using Statistical comparison between male and female mice was performed using a two-way repeated measures ANOVA with time as the repeated measures factor. When the F value was significant post hoc analysis was performed using Bonferroni’s t test. P < 0.05 was considered to be statistically significant.
2.4 Thermal Tail-Immersion Assay
The 70 mice used for rotarod analysis plus an additional 67 mice were used for analysis of thermal and mechanical nociception. Thus a total of 137 mice were used for analysis of the development of thermal hyperalgesia and mechanical allodynia. The thermal tail-immersion assay was performed as described by Shimizu et al, (2005). Animals were selected in random order for thermal tail immersion testing and the investigator was blinded to the experimental condition of the animals. Briefly, the animal was gently restrained and the distal two-thirds of the tail was immersed in a water bath set at 50° C +/− 1°C, and the latency to tail flick was recorded. The cut off time was 15 s, after which the tail was removed from the bath regardless of response. The test was repeated three times and averaged at each time point tested. In initial studies, no significant differences were found in nociceptive responses of TMEV or control female mice at different phases of the estrous cycle and thus the data of all females were pooled for analysis of thermal hyperalgesia.
In the context of the present study the experiments included 74 female and 63 male mice, randomly assigned to the control or to the TMEV groups. Each mouse was measured between one and five times, with 102 of the mice measured five times, between day 0 and day 180 after allocation of treatments. We fit a linear regression of thermal withdrawal latency (as a measure of thermal hyperalgesia) on day number, and considered separate slopes and intercepts for each of the four groups [sex (male vs female) by treatment (TMEV vs control)]. Since at day zero we expect the same response for males in either the control or TMEV condition, the same intercept for females in either control or TMEV, we constrained the intercepts to be equal within sex. We used the methodology outlined in S. Weisberg (2005), Applied Linear Regression, to simplify this model further by looking for differences between the sexes in intercepts, and differences in slope between the four sex by treatment combinations. We fit using a random coefficients model (Littell et al., 2006) which allows each mouse to have its own intercept and slope, using SAS proc mixed and the package nlme in R (Pinhero and Bates, 2000). The random coefficients model represents one of the major tools for the analysis of repeated measures. In the context of repeated measures studies, this model is based on the idea that the process of change is defined for each individual, yet also is related to the population mean trajectory.
2.5 von Frey Test for Mechanical Allodynia
The 137 mice used for testing thermal tail immersion assay were also used for evaluation of mechanical allodynia. Withdrawal responses to punctate mechanical stimulation were determined by using calibrated von Frey filaments as previously described (Wacnik et al., 2001), but with some modification. Male and female mice were initially tested with a range of filaments (0.196 mN to 9.80mN) to determine a baseline filament that produced 0–1 responses out of 10 trials. Male mice were found to be more sensitive than females and thus mechanical hypersensitivity was assessed using a 5.88 mN von Frey monofilament in female mice and a 1.57 mN monofilament for male mice. Mice were selected in random order for von Frey testing and the investigator performing the testing was blinded to the experimental condition of the animals. Mice were allowed to acclimate to the testing environment for at least 30 min before testing. The monofilament was applied ten times on the plantar surface of each hindpaw. The number of withdrawal responses to the monofilament was counted and expressed as percent of stimuli that evoke a response (withdrawal response frequency). The monofilament was applied for 1–2 s with an interstimulus interval of 5–10 s. Baseline values for mechanical sensitivity were determined for each animal prior to TMEV infection. In initial studies, no differences were found in nociceptive responses of TMEV or control female mice at different phases of the estrous cycle and thus the data of all females were pooled for analysis of mechanical allodynia.
Each mouse was measured between one and five times, with 102 of the mice measured five times, between day 0 and day 180 after allocation of treatments. We fit a linear regression of percent response to von Frey stimulation (as a measure of mechanical allodynia) on day number, and considered separate slopes and intercepts for each of the four groups [sex (male vs female) by treatment (TMEV vs control)]. Since at day zero we expect the same response for males in either the control or TMEV condition, the same intercept for females in either control or TMEV, we constrained the intercepts to be equal within sex. We fit using a random coefficients model (Littell et al., 2006), which allows each mouse to have its own intercept and slope, using SAS proc mixed and the package nlme in R (Pinhero and Bates, 2000). Use of a mixed-effects statistical model has been used previously to analyze mechanical allodynia in a spared nerve injury model of neuropathic pain (Bourquin et al., 2007). The main advantage of using a random coefficients model over ANOVA methods is that it allows an integrated treatment of the data, which takes into account the different sources of variation – between time, individuals, treatment and sexes – resulting in more appropriate assessment of uncertainty, and thus in tests and confidence intervals that are better adapted to the experimental set-up.
2.6 Immunohistochemical Procedure for Staining Peripheral Nerves
Painful neuropathies have been characterized by changes in epidermal nerve fiber innervation and skin biopsy represents an effective test for diagnosis of peripheral nerve disorders. The most commonly reported indication of abnormality in a skin biopsy from patients with peripheral neuropathy is a reduction of epidermal nerve density (Wendelschafer-Crabb et al., 2006). Since sensory-motor neuropathic symptoms have been described in MS patients (Sarova-Pinhas et al., 1995), we examined nerves innervating the epidermis of the skin in TMEV-infected mice to determine if there were changes in epidermal nerve density. Mouse back skin was examined in this study because back pain is a common complaint among MS patients (Al-Smadi et al., 2003; Solaro et al., 2004) and because larger areas of skin could be obtained from the back region. Mouse back skins were immersed in Zamboni’s fixative (0.03(w/v)% picric acid and 2 (w/v)% paraformaldehyde) for 48 hours at 4°C and transferred to a 20% sucrose solution with 0.05% sodium azide in PBS for storage. Processing and staining of the mouse skin sections were carried out according to our previously published procedure (Wacnik et al., 2005). Excised back skin was mounted in optimal cutting temperature (OCT) compound, frozen, and cut into 50 micron-thick sections. Histopathological quantification of cutaneous nerves innervating the epidermis was performed using a PGP9.5 (protein gene-product 9.5) antibody, because this antibody is generally used for the evaluation of clinical and experimental cutaneous innervation and is well characterized as a pan-neuronal marker. PGP 9·5 is a cytoplasmic constituent present in all parts of the neuron, and immunocytochemically antibodies against PGP9.5 label the majority of neurons in the peripheral and the central nervous system (Wilson et al., 1988) although some neurons are PGP9.5 negative (Weiler and Benali, 2005). From a functional standpoint, PGP9.5 is an ubiquitin carboxyl hydrolase (UCHL1), an important regulatory enzyme that cleaves terminal ubiquitin products from proteins (Wilkinson et al., 1989), and recent evidence indicates that it is required for normal synaptic function (Gong et al., 2006). The PGP9.5 antibody used in this study was obtained from Biogenesis (Poole, UK, Cat No 7863-0504) and stains a single band at 27 kD on Western blot. The PGP9.5 antibody has been used previously to study the innervation of mouse back skin (Botohkarev et al., 1998) and innervation in the foot of a murine hindpaw cancer model (Wacnik et al., 2006). We also quantified the number of cutaneous nerve fibers that expressed CGRP immunoreactivity, since almost all nerve fibers in the epidermis are unmyelinated and are either CGRP-positive or nonpeptidergic free nerve endings (Zylka et al., 2005). Moreover, CGRP is considered to be a marker of sensory innervation and demonstrates thin sensory C-fibers that transmit nociception (Wallengren et al., 1999). The CGRP antibody used in this study was raised in sheep (Biohit, Finland), cross-reacts with all known forms of rat and human CGRP and was used previously by our laboratories (Wacnik et al., 2005). Sections were incubated in (a) polyclonal CGRP (1:500; gift from Biohit, Finland) and anti-sheep IgG conjugated to cyanine 2 (Cy2) (1:1000) (Jackson ImmunoResearch, West Grove, PA), (b) polyclonal PGP9.5 (PGP) (1:500) made in rabbit raised against native brain PGP9.5 and anti-rabbit IgG conjugated to Cy3 (1:1000) (Jackson ImmunoResearch) and (c) polyclonal Collagen Type IV (CollIV) (1:500) made in goat (Southern Biotechnology Assoc., Birmingham, AL) and anti-goat IgG conjugated to cyanine 5 (Cy5) (1:1000) (Jackson ImmunoResearch). Samples were mounted in agar on slide covers, dehydrated in ethanol, cleared with methyl salicylate, and mounted in DEPEX (Electron Microscopy Science, Poole, UK).
2.7 Image Capture and Morphometric Analysis of Epidermal Nerves
To quantify PGP- and CGRP-immunoreactivity in the epidermis of the skin we used a morphometric analysis approach that was based on the methodologies described by Christmas et al. (Christmas et al., 1990), Engeland et al. (Engeland et al., 1997), Mihm et al. (Mihm et al., 2001) and Pan et al. (Pan et al., 2004). Single-photon laser scanning confocal microscopy (LSCM) was used to image mouse back skin in the green channel (CGRP-ir), red channel (PGP9.5-ir), and far-red channel (Collagen Type-IV-ir, allowing us to delineate the epidermal/dermal boundary). Imaging and image capture was performed using an MRC 1024 Confocal/Multiphoton Imaging System (Bio-Rad, Hercules, CA).
LSCM serial optical section data sets were then collected using identical parameters: 5-micron thick z stacks were collected at 1200x magnification of dorsal skin epidermis. Projections of these 3D data sets were then generated and the individual projections (green, red, and far-red) were merged into a single (green, red, blue) image using Confocal Assistant. Using Adobe PhotoShop 6.0 softwarethe epidermis was selected with boundaries at the top edge of the epidermis, the epidermal/dermal boundary at the lower edge as delineated by Collagen Type-IV-immunoreactivity and the dimensions of the viewing field on either side were selected as side boundaries. Total area pixel count of the entire selected epidermis was obtained. The area pixel count of the CGRP-ir (green channel) and PGP-ir (red channel) nerve fibers in the selected epidermal area was calculated by first thresholding the respective grayscale images to reduce the contribution of non-specific background signal. The immuno-positive pixels were selected with the wand tool and a thresholded pixel count was acquired for each channel. Data is presented as the percentage of the total area of the selected epidermal region occupied by CGRP- or PGP-immunoreactive fibers. For each mouse, two non-overlapping fields of view from each of four sections were counted, i.e. eight fields of view from each mouse. Results were analyzed using a one-way ANOVA test with Fisher’s protected least significant difference (PLSD) post hoc test using p<0.05.
3. Results
3.1 TMEV-Infected Male Mice Show Increased Disease Progression as Measured by Neurological Deficits
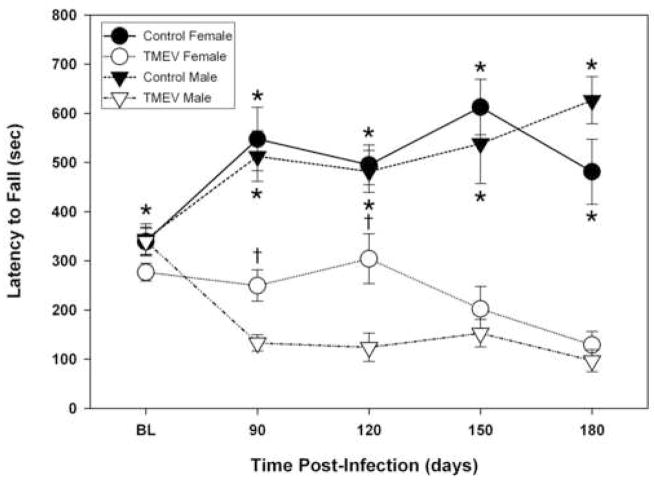
Motor balance and coordination were assessed using the rotarod assay. Several previous studies have demonstrated a significant correlation among disease progression, spinal cord pathology and decreased ability to perform on the rotarod using female animals (McGavern et al., 1999; McGavern et al., 2000). The purpose of this portion of the study was to examine the time-course of neurological deficits in male and female mice as measured using the rotarod test. This was done in order to determine whether male mice have increased disease progression as compared to females, which typically occurs in human MS patients. We tested the performance of uninfected control and TMEV infected SJL male and female mice at days 90, 120, 150 and 180 post-infection to assess the progression of the demyelinating disease. These time points were chosen based on literature and previous experiments in our lab. The day 90 time-point has consistently been shown to be one of the earliest time-points to consistently measure neurological deficits using the rotarod test in TMEV infected SJL mice. In order to measure the progression of the disease we evaluated neurological deficits every 30 days from day 90 until day 180. All mice were euthanized at day 180 post-infection since they typically display very severe neurological deficits at this time-point and beyond this time-point they have difficulty both with mobility and with feeding.
TMEV infected male and female mice started to display posterior limb paralysis by day 90 PI. All of the paralyzed females showed unilateral, hind limb paralysis, but were able to walk at all time points. In contrast, 20% of male mice at days 90 and 120 PI, and 30% of male mice at days 150 and 180 PI displayed bilateral hind limb paralysis and had difficulties walking. All of the remaining male mice showed unilateral hindlimb paralysis. These visual observations of an increased disease progression in TMEV infected male mice were correlated with significant decreases in rotarod scores in male mice compared to female mice at days 90 and 120 post-infection (see Figure 1). Both TMEV-infected male and female mice had neurological deficits at all time points measured as indicated by significantly decreased scores in the rotarod test compared uninfected controls (Figure 1). Since neurological deficits as measured by rotarod in TMEV mice correlates with the loss of medium to large myelinated axons in the spinal cord and with associated electrophysiological abnormalities (McGavern et al., 2000), it seems likely that the shorter run times observed in TMEV infected mice are a reflection of these neurological deficits rather than an inability to learn. Analysis of rotarod scores across time points revealed a significant difference between TMEV female and TMEV male mice (p≤0.0025). Specifically, TMEV infected males had significantly decreased rotarod scores at days 90 (p<0.0001) and 120 (p≤0.0044) versus TMEV infected females indicating increased disease progression in TMEV infected male mice (Figure 1). This finding correlates with our previous report using activity box measurements (Alley et al., 2003) in which TMEV male mice performed up to threefold fewer spontaneous horizontal and vertical movements (activity box test) compared to infected age-matched females, which would imply that motor impairment rather than motor learning contributed to the reduced motor performance observed between TMEV mice and controls as well as between male and female TMEV mice at days 90 and 120.
Figure 1. Assessment of motor coordination and balance (Rotarod test) in TMEV-infected and uninfected male and female mice.
An accelerating Rotamex Rotorod (Columbus Instruments, Columbus OH) was used to test the effects of TMEV-induced demyelination on motor coordination. Mice were tested on the rotarod at an accelerating speed between 5 and 40 rpm over a 20 minute test period and the run time was recorded in seconds. TMEV infected mice (n=20) had significantly shorter run times when compared to uninfected controls (n=15; *p<0.0001). TMEV infected-female mice performed at a significantly higher level than TMEV-infected male mice (†p≤0.0025). Control male and female mice were not significantly different from each other (p=0.921).
3.2 Mechanical Allodynia and Thermal Hyperalgesia in TMEV infected mice
Prior to TMEV infection baseline testing was done to determine the appropriate threshold filament size that produced 0 or 1 withdrawal response out of 10 trials (see (Wacnik et al., 2001)). Naïve male SJL mice were found to be much more sensitive to von Frey stimuli than naïve female mice and thus a 5.88 mN monofilament was found to produce 0–1/10 responses in female mice, while a 1.57 monofilament produced 0–1/10 responses in male mice. Since male and female mice had different baseline thresholds to mechanical stimulation, the 1.57mN von Frey filament was used to test the development of mechanical allodynia in males, while the 5.88 mN monofilament was used to test females throughout the course of this study. While it might be argued that paw withdrawal thresholds should have also been assessed throughout the study, recent work indicates that there is a strong negative correlation between withdrawal threshold force and paw withdrawal frequency (Hamamoto et al., 2007), and thus either measure should suffice for measuring allodynia/hyperalgesia. Moreover, if we had performed the entire experiment using thresholds rather than percent response we would still have the issue of different baselines for the sexes. If we had used one von Frey filament with a force in between 1.57 and 5.88 mN, the males would have had higher withdrawal frequencies than the females at baseline. Conversely if we had used the 1.57 mN monofilament for both, we would have missed the lower levels of allodynia in the females, thus we believe the use of different monofilaments for males and females was appropriate in this study.
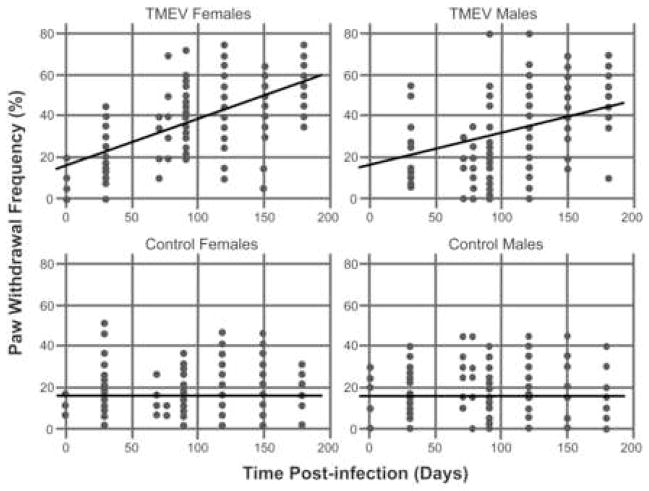
TMEV infection induced mechanical allodynia bilaterally in both hind paws of male and female mice as determined using a normally non-noxious von Frey monofilament. Results are summarized in Figure 1, which show the estimated average response curves over days. We find that a linear mixed model in which the intercept is the same for each sex (t=−1.58, df=135, p=.11) and allowing each sex/treatment combination to have its own linear slope for the response on time. The best fitting model has slope equal to zero for both male and female controls (overall test, t=0.66, df=469, p=.50). In the TMEV condition, separate, nonzero slopes were required for males and females. For females, the estimated slope was .0225 units per day (for testing the slope equal to zero, t=17.21, df=469, p<.0001), while for males the slope was estimated to be 0.0163 (for testing the equality of slopes for males and females, t=−4.01, df=469, p=.0001). Thus both the male and female TMEV mice developed mechanical allodynia over time compared to the non-infected control mice. Importantly the slope of the regression line is significantly different for males and females and indicates that the female TMEV mice develop mechanical allodynia faster than the male TMEV mice.
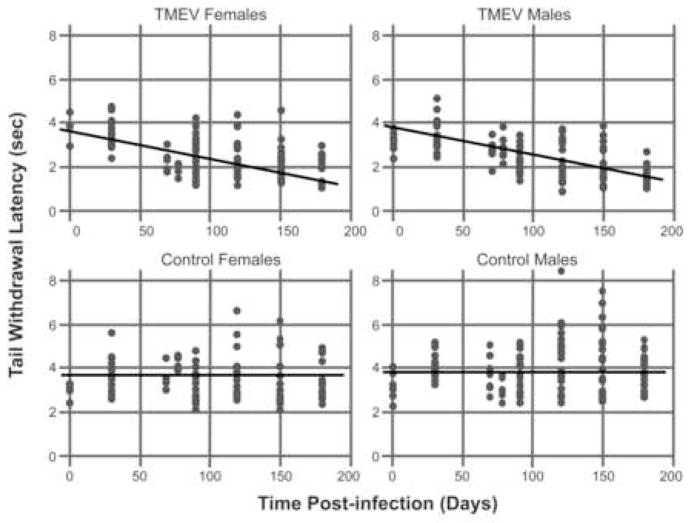
With respect to thermal testing, we used the same modeling strategy and found that TMEV-infected mice developed significant thermal hyperalgesia over time compared to control uninfected mice. Similar to what we observed with mechanical von Frey testing, we found that thermal latencies did not vary over time in the control condition (t=−1.35, df=510, p=.18). The slope in the TMEV condition is significantly different from zero (t=−12.72, df=510, p <.0001) indicating that TMEV mice develop thermal hyperalgesia over time. However, with respect to sex, we reached a somewhat different conclusion as summarized in Figure 2 compared to that found for mechanical allodynia (Fig. 1). Here we found that the males and females required different intercepts (t=2.05, df=135, p=.04) indicating that there were significant sex differences in thermal latencies at baseline. The slope in the TMEV condition is significantly different from zero (t=−12.72, df=510, p <.0001), but the slopes do not differ by Sex (t=1.40, df=510, p=.16) indicating that male and female TMEV mice develop thermal hyperalgesia at a similar rate. In the thermal condition, we estimate a decrease of 0.013 seconds per day in tail withdrawal latency over the course of disease progression.
Figure 2. Development of mechanical allodynia in TMEV-infected male and female mice.
Graphs illustrating the mean paw withdrawal score (paw withdrawal frequency as a percent) for each mouse (individual dots) at various time points. The results for TMEV-infected mice are shown in the top two plots, while the data for the non-infected controls are shown in the bottom two plots. The straight lines in each plot represent fit of a mixed model assuming linear time effects. The intercept is the same for each sex (t=−1.58, df=135, p=.11). The slope is equal to zero for both male and female controls (overall test, t=0.66, df=469, p=.50). In the TMEV condition, separate, nonzero slopes were required for males and females. For females, the estimated slope was .0225 units per day (for testing the slope equal to zero, t=17.21, df=469, p<.0001), while for males the slope was estimated to be 0.0163 (for testing the equality of slopes for males and females, t=−4.01, df=469, p=.0001). The data indicate that TMEV-infected mice developed significant mechanical allodynia over time compared to control uninfected mice and that TMEV-infected female mice develop mechanical allodynia at a faster rate than male TMEV-infected mice.
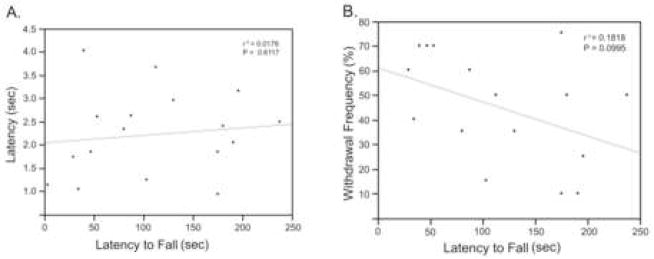
Surveys of human MS have shown that pain is not associated with the degree of a patient’s disability. To this end we examined possible correlations between withdrawal frequency, which measures pain severity, and the rotarod score, which measures neurological disability. We found that there was no correlation between pain severity as measured by thermal hyperalgesia or mechanical allodynia and the rotarod score at any of the time points measured (only day 180 is shown in figure 3).
Figure 3. Development of thermal hyperalgesia in TMEV-infected male and female mice.
Graphs illustrating the mean tail withdrawal latency for each mouse (individual dots) at various time points. The results for TMEV-infected mice are shown in the top two plots, while the data for the non-infected controls are shown in the bottom two plots. The straight lines in each plot represent the fit of a mixed model assuming linear time effects. We used the same modeling strategy that we utilized for evaluating the mechanical-induced paw withdrawal scores illustrated in figure 1. We found that TMEV-infected mice developed significant thermal hyperalgesia over time compared to control uninfected mice. Similar to what we observed with mechanical von Frey testing, we found that thermal latencies did not vary over time in the control condition (t=−1.35, df=510, p=.18). The slope in the TMEV condition is significantly different from zero (t=−12.72, df=510, p <.0001) indicating that TMEV mice develop thermal hyperalgesia over time.
3.3 Increased innervation
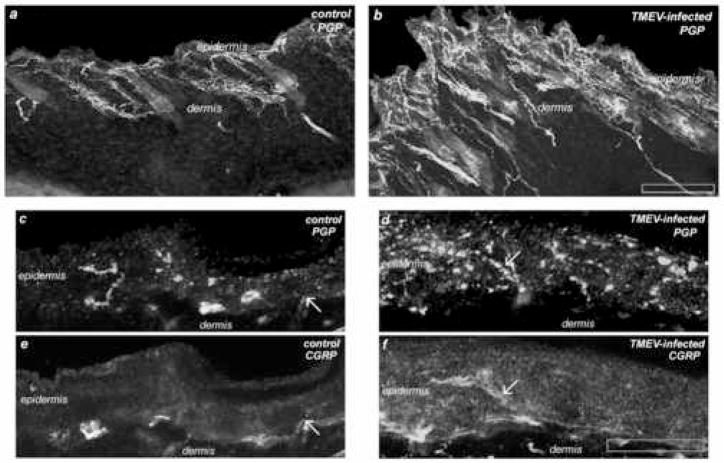
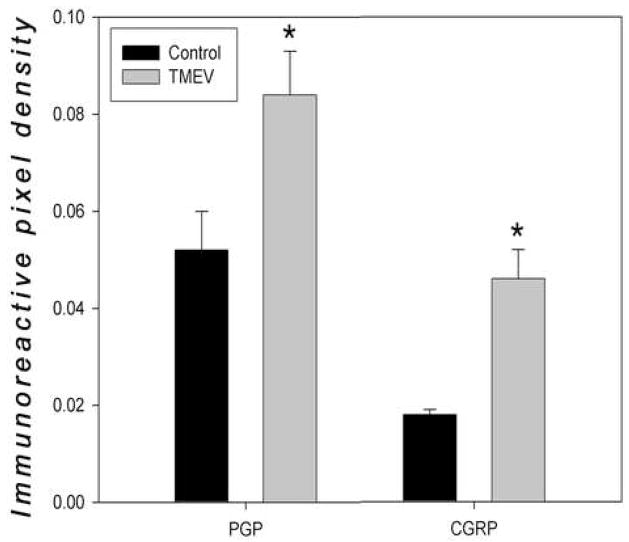
Immunoreactive sensory nerve fibers in the epidermis were quantified in TMEV infected and non-infected mice of both genders, sacrificed at Day 180 post-infection. We found a significantly higher density of both PGP9.5-ir nerve fibers and CGRP-ir immunoreactive nerves in the epidermis of TMEV-infected mice versus non-infected control mice (Figures 4 and 5). In addition to significantly higher density of PGP9.5- and CGRP-immunoreactive fibers in the epidermis, qualitative observations revealed increased nerve fiber immunoreactivity in the dermis of both male and female TMEV mice as well. Of interest CGRP-immunoreactivity was also enhanced in epidermal keratinocytes in the TMEV-infected skin samples compared to controls. Though we did not stain our sections with a nuclear-specific stain, close examination of individual optical sections revealed that this CGRP immmunoreactivity was cytoplasmic. Finally, negative control slides, in which no primary CGRP or PGP9.5 antibodies were used, revealed a complete lack of immunostaining (data not shown).
Figure 4. Graphs demonstrating a lack of correlation between nociception (thermal hyperalgesia and mechanical allodynia) and motor deficits (rotarod).
(A) There was a lack of correlation between thermal hyperalgesia as measured by tail withdrawal latency (y axis) and rotarod mechanical deficits in TMEV infected mice at day 180 PI. (B) There was also a lack of correlation between mechanical allodynia as determined with von Frey filaments and rotarod at day 180 PI.
Figure 5. Photomicrographs of Intra-epidermal PGP9.5 and CGRP Immunoreactivity.
Representative photomicrographs at low power magnification (100Xmag, upper panels) and high power magnification (1200Xmag, four lower panels) of dorsal skin from SJL/J mice, infected and non-infected. Skin was collected at day 180 post-infection and double-immunostained with anti-PGP9.5 and anti-CGRP. (a) Control, non-infected mouse epidermis and dermis exhibit lower levels of PGP9.5-immunoreactivity as compared to (b) TMEV-infected mice. At higher magnification (c and e) control, non-infected mouse epidermis exhibits lower levels of PGP9.5- and CGRP-immunoreactivity as compared to (d and f) TMEV-infected mice. (Arrows designate the co-localization of CGRP and PGP9.5-immunoreactive nerve fibers in the epidermis both in the control, c and e, and in the TMEV-infected mice, d and f.) Interestingly CGRP-immunoreactivity was enhanced in epidermal keratinocytes in the TMEV-infected skin samples (f) compared to controls (e). Scale bar for lower magnification (Olympus UPlanFl 10X/0.30) image is 500 microns and for higher magnification (Olympus UPlanApo_60X/1.4 oil) scale bar is 10 microns.
4. Discussion
The EAE and TMEV models represent the two most common animal models of MS and their use has led to a greater understanding of MS and the development of clinical therapies (Nelson et al., 2004). While EAE serves as a better model of the relapsing/remitting quality of MS, the TMEV model may be a better model of the progressive quality of the disease (Fuller et al., 2004; Oleszak et al., 2004) and may be particularly valuable because of increasing epidemiologic evidence suggesting a possible viral etiology of this disease (Love, 2006; Olson et al., 2005). Since nociception has not been studied in TMEV mice and since the TMEV model mimics human MS both pathologically and immunologically, it serves as an ideal model for studying MS pain and progression.
4.1 Males and Female Both Exhibit Mechanical Allodynia and Thermal Hyperalgesia
Pain is an important and frequent symptom accompanying multiple sclerosis (Svendsen et al., 2005; Kumpfel et al., 2007). MS pain develops in 50–80% of MS patients, with 30% of those patients describing pain as their worst symptom (Beard et al., 2003). Unfortunately only recently has pain been systematically studied in MS patients (Svendsen et al., 2005). In the only study using an EAE animal model of MS, thermal hyperalgesia was found to develop in EAE mice (Aicher et al., 2004). We found that both TMEV infected male and female mice exhibited significant thermal hyperalgesia and mechanical allodynia compared to controls. This is consistent with reports that most MS patients display sensory abnormalities, dominated by mechanical and thermal hyperalgesia (Osterberg et al., 2005; Svendsen et al., 2005). The fact that TMEV-induced nociception preceded motor deficits in many of the mice mimics what is observed in human MS, where 12% of patients are diagnosed with MS based on chronic pain symptoms rather than motor deficits (Kalia and O’Connor, 2005). Moreover, surveys of human MS have shown that pain is not associated with the degree of a patient’s motor disability. This was replicated in TMEV infected mice, in which the magnitude of thermal hyperalgesia and mechanical allodynia were not correlated with the severity of clinical symptoms as measured by rotarod.
TMEV infected mice experience motor impairment that could impact their ability to produce a withdrawal response. However, it is unlikely that motor impairment affected our results, since there was no correlation between motor impairment and pain severity. In this regard, some of the most severely paralyzed TMEV infected mice displayed the most severe thermal hyperalgesia and mechanical allodynia, Importantly, nociceptive responses (shorter latencies to withdrawal) developed in TMEV infected animals prior to or at the same time that motor deficits began.
4.2 Sex Differences in Neurological Deficits and Nociception
Our results indicated that TMEV-infected male SJL/J mice developed more severe neurological deficits than infected female mice. This agrees with the results of human studies, which show that males develop more severe neurological deficits and deteriorate faster than females (Wynn et al., 1990; Bronnum-Hansen et al., 1994; Vein et al., 1995;Cottrell et al., 1999). While TMEV-infected female mice developed unilateral, hind limb paralysis, 20–30% of infected male mice developed bilateral hind limb paralysis and had difficulties walking. TMEV-infected males also experienced more rapid disease progression similar to what is observed in male patients with MS.
Considerable evidence exists for sex differences in human pain sensitivity with women exhibiting reduced tolerance for experimentally induced pain (LaCroix-Fralish et al., 2005; Wiesenfeld-Hallin, 2005; Berkley et al., 2006) and an increased frequency of chronic pain conditions including fibromyalgia, arthritis, migraine and temperomandibular disorders (Fillingim and Maixner, 1995; Fillingim and Ness, 2000). With respect to MS most studies of pain in human MS patients have ignored possible gender differences. However, Moulin and colleagues (1989) found that while their patient population had a female to male ratio of 2.1/1, this ratio was 3.0/1 in the group of MS patients reporting pain. Further support for a female prevalence of pain in MS patients comes from the recent study demonstrating that women reported increased pain severity (Hadjimichael et al., 2006). The EAE model of MS is the only animal model that has been employed to investigate possible sex differences in nociception. Aicher et al. (2004) found that both male and female SJL mice showed decreased tail withdrawal latencies during the chronic phase of the disease and further reported that this thermal hyperalgesia appeared to be somewhat more sustained in actively immunized females. In the present study we not only observed that male and female TMEV mice exhibited thermal hyperalgesia, but also mechanical allodynia. Importantly, mechanical allodynia developed more rapidly in TMEV-infected female mice compared to TMEV-infected males (figure 2). The earlier onset and increased severity in TMEV infected female mice mimics what is seen in the MS population (Buchanan et al., 2003; Kalia and O’Connor, 2005).
4.3 Mechanisms of TMEV-Infection Induced Pain
Pain in multiple sclerosis is heterogeneous with symptoms varying from patient to patient (Marchettini et al, 2006). Due to its heterogeneity, MS pain probably has multiple causes, as does TMEV-induced pain. The present results would suggest that increased peripheral innervation may be a factor that contributes to increased nociception in TMEV mice.
Although MS has classically been considered a CNS disease, many patients have been identified with changes in their peripheral nervous system (Hasson et al., 1958; Pollock et al., 1977; Riggs et al., 1986; Ro et al., 1983; Sarova-Pinhas et al., 1995). Some MS patients exhibit decreased motor conduction (Hopf, 1963) and prolonged proximal peripheral nerve segment conduction time (Mills and Murray, 1986). While several chronic pain conditions, such as peripheral neuropathy, show alterations in peripheral innervation density, peripheral innervation has not been examined in MS patients to date. We identified a significantly higher density of both PGP9.5-ir and CGRP-ir epidermal nerve fibers in TMEV infected mice compared to controls. The increase in nerve fibers expressing PGP9.5 was unexpected, since a previous study of human skin biopsies from neuropathic patients showed significant reductions in intraepidermal PGP9.5 immunostaining (McCarthy et al., 1995). However, recent work has shown increased CGRP nerve fibers in rat skin following partial peripheral nerve injury (Yen et al., 2006) similar to what we observe. These increased numbers of nerve fibers may represent intraepidermal nerve fiber sprouting (Kinkelin et al., 2000). The increase in both CGRP- and PGP9.5-immunoreactivity is important since a recent study has shown increased intraepidermal PGP9.5 with no change in CGRP in GDNF family receptor 2 knockout mice that show a deficit in inflammatory pain response (Lindfors et al., 2006), but do not show thermal hyperalgesia or mechanical allodynia. It is feasible that the increased number of epidermal CGRP fibers may contribute to the thermal hyperalgesia and mechanical allodynia present in TMEV mice. In this regard a previous study by our laboratories has shown a positive correlation between the innervation density of CGRP-positive fibers in a bone tumor and the level of tumor-induced hyperalgesia (Wacnik et al., 2005). It is unknown whether this increased CGRP innervation is also present in MS patients, but if it does occur it may represent a novel peripheral mechanism that may contribute to MS-induced pain and may also serve as a potential diagnostic marker. The increased immunoreactivity in the epidermal keratinocytes that we observe in the TMEV-infected mouse skin may be indicative of a sensory role in this model system. Epidermal keratinocytes are known to express TRPV1 (Bodo 2005 and Inoue 2002) and a recent report describes CGRP-like immunoreactivity in epidermal keratinocytes (Hou et al., 2006).
Finally, MS pain may also have a central origin, which is thought to result from lesion or dysfunction in the brain or spinal cord (Osterberg et al., 2005). In this regard, spinal cord injury (SCI) frequently results in dysesthesias that have remained refractory to clinical treatments (Christensen and Hulsebosch, 1997). Spinal cord injury triggers sensitization of wide dynamic range dorsal horn neurons (Zhang and Oppenheim, 2005) and recent findings demonstrate that injury-induced abnormal expression of the Nav1.3 Na(+) channel in spinal cord dorsal horn neurons contributes to their hyperexcitability (Waxman and Hains, 2006). Since most MS patients display sensory abnormalities, dominated by mechanical and thermal hyperalgesia (Osterberg et al., 2005; Svendsen et al., 2005), it is likely that central sensitization occurs in MS patients similar to that observed following spinal cord injury or that associated with other chronic pain conditions (Curatolo et al., 2006). The fact that both mechanical and thermal hyperalgesia were present in TMEV mice suggest that the nociception observed in this animal model may truly reflect that found in MS patients.
It is probable that MS pain represents a heterogeneous etiology and that different mechanisms, both central and peripheral, contribute to the variability of MS pain both within a single patient at different phases of their disease, and between different MS patients. In the present study, we have discovered a novel increased intraepidermal innervation in TMEV infected mice, which likely contributes to the increased nociception observed during the disease course. Further research is required to determine if similar alterations also occur in human MS patients.
Figure 6. Quantification of Intra-epidermal Nerve Density.
The immunoreactivity density counts for PGP9.5 and CGRP were calculated by determining immunopositive pixel density area per total intra-epidermal pixel area at 120Xmag. Data are presented as mean and SEM, analyzed using ANOVA and Fisher’s post-hoc test (*p<.05).
Acknowledgments
This study was supported by the National Multiple Sclerosis Society (PP1125), T32 DA07097 (NIH/NIDA), and the Stark Award from the Department of Neuroscience at the University of Minnesota. We would like to thank Dr. M.K. Njenga for providing the virus and Jeremy Alley for his help with behavioral testing. We would also like to thank Dr. Sandy Weisberg from the School of Statistics at the University of Minnesota for performing the statistical analysis on the mechanical paw withdrawal test and thermal tail withdrawal test data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aicher SA, Silverman MB, Winkler CW, Bebo BF., Jr Hyperalgesia in an animal model of multiple sclerosis. Pain. 2004;110:560–570. doi: 10.1016/j.pain.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Alley J, Khasabov S, Simone D, Beitz A, Rodriguez M, Njenga MK. More severe neurologic deficits in SJL/J male than female mice following Theiler’s virus-induced CNS demyelination. Exp Neurol. 2003;180:14–24. doi: 10.1016/s0014-4886(02)00054-7. [DOI] [PubMed] [Google Scholar]
- Al-Smadi J, Warke K, Wilson I, Cramp AF, Noble G, Walsh DM, Lowe-Strong AS. A pilot investigation of the hypoalgesic effects of transcutaneous electrical nerve stimulation upon low back pain in people with multiple sclerosis. Clin Rehabil. 2003;17:742–749. doi: 10.1191/0269215503cr672oa. [DOI] [PubMed] [Google Scholar]
- Archibald CJ, McGrath PJ, Ritvo PG, Fisk JD, Bhan V, Maxner CE, Murray TJ. Pain prevalence, severity and impact in a clinic sample of multiple sclerosis patients. Pain. 1994;58:89–93. doi: 10.1016/0304-3959(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Beard S, Hunn A, Wight J. Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technology Assessment. 2003;7:1–124. doi: 10.3310/hta7400. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Zalcman SS, Simon VR. Sex and Gender Differences in Pain and Inflammation: A Rapidly Maturing Field. Am J Physiol Regul Integr Comp Physiol. 2006 doi: 10.1152/ajpregu.00287.2006. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Lommatzsch M, Peters EM, Lewin GR, Subramaniam A, Braun A, Renz H, Paus R. BDNF overexpression induces differential increases among subsets of sympathetic innervation in murine back skin. Eur J Neurosci. 1998;10:3276–3283. doi: 10.1046/j.1460-9568.1998.00365.x. [DOI] [PubMed] [Google Scholar]
- Bourquin AF, Süveges M, Pertin M, Gilliard N, Sardy S, Davison AC, Spahn DR, Decosterd I. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain. 2006;122:14.e1–14.e14. doi: 10.1016/j.pain.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Bronnum-Hansen H, Koch-Henriksen N, Hyllested K. Survival of patients with multiple sclerosis in Denmark: a nationwide, long-term epidemiologic survey. Neurology. 1994;44:1901–1907. doi: 10.1212/wnl.44.10.1901. [DOI] [PubMed] [Google Scholar]
- Buchanan RJ, Wang S, Ju H. Gender analyses of nursing home residents with multiple sclerosis. J Gend Specif Med. 2003;6:35–46. [PubMed] [Google Scholar]
- Christensen MD, Hulsebosch CE. Chronic central pain after spinal cord injury. J Neurotrauma. 1997;14:517–537. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- Christmas TJ, Rode J, Chapple CR, Milroy EJ, Turner-Warwick RT. Nerve fibre proliferation in interstitial cystitis. Virchows Arch A Pathol Anat Histopathol. 1990;416:447–451. doi: 10.1007/BF01605152. [DOI] [PubMed] [Google Scholar]
- Cottrell DA, Kremenchutzky M, Rice GP, Koopman WJ, Hader W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain. 1999;122 ( Pt 4):625–639. doi: 10.1093/brain/122.4.625. [DOI] [PubMed] [Google Scholar]
- Curatolo M, Arendt-Nielsen L, Petersen-Felix S. Central hypersensitivity in chronic pain: mechanisms and clinical implications. Phys Med Rehabil Clin N Am. 2006;17:287–302. doi: 10.1016/j.pmr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Ehde DM, Gibbons LE, Chwastiak L, Bombardier CH, Sullivan MD, Kraft GH. Chronic pain in a large community sample of persons with multiple sclerosis. Mult Scler. 2003;9:605–611. doi: 10.1191/1352458503ms939oa. [DOI] [PubMed] [Google Scholar]
- Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Multiple Sclerosis. 2006;12:629–638. doi: 10.1177/1352458506071346. [DOI] [PubMed] [Google Scholar]
- Engeland WC, Levay-Young BK, Rogers LM, Fitzgerald D. Differential gene expression of cytochrome P450 11beta-hydroxylase in rat adrenal cortex after in vivo activation. Endocrinology. 1997;138:2338–2346. doi: 10.1210/endo.138.6.5157. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Maixner W. differences in the responses to noxious stimuli. Pain Forum. 1995;4:209–211. [Google Scholar]
- Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24:485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- Ford HL, Gerry E, Airey CM, Vail A, Johnson MH, Williams DR. The prevalence of multiple sclerosis in the Leeds Health Authority. J Neurol Neurosurg Psychiatry. 1998;64:605–610. doi: 10.1136/jnnp.64.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller KG, Olson JK, Howard LM, Croxford JL, Miller SD. Mouse models of multiple sclerosis: experimental autoimmune encephalomyelitis and Theiler’s virus-induced demyelinating disease. Methods Mol Med. 2004;102:339–361. doi: 10.1385/1-59259-805-6:339. [DOI] [PubMed] [Google Scholar]
- Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126:775–788. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Hadjimichael O, Kerns RD, Rizzo MA, Cutter G, Vollmer T. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain. 2006 doi: 10.1016/j.pain.2006.07.015. (In Press) [DOI] [PubMed] [Google Scholar]
- Hamamoto DT, Giridharagopalan S, Simone DA. Acute and chronic administration of the cannabinoid receptor agonist CP 55,940 attenuates tumor-evoked hyperalgesia. Eur J Pharmacol. 2007;558:73–87. doi: 10.1016/j.ejphar.2006.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson J, Terry RD, Zimmerman HM. Peripheral neuropathy in multiple sclerosis. Neurology. 1958;8:503–510. doi: 10.1212/wnl.8.7.503. [DOI] [PubMed] [Google Scholar]
- Hopf HC. Electromyographic study on so-called mononeuritis. Arch Neurol. 1963;9:307–312. doi: 10.1001/archneur.1963.00460090113014. [DOI] [PubMed] [Google Scholar]
- Indaco A, Iachetta C, Nappi C, Socci L, Carrieri PB. Chronic and acute pain syndromes in patients with multiple sclerosis. Acta Neurol (Napoli) 1994;16:97–102. [PubMed] [Google Scholar]
- Kalia LV, O’Connor PW. Severity of chronic pain and its relationship to quality of life in multiple sclerosis. Multiple Sclerosis. 2005;11:322–327. doi: 10.1191/1352458505ms1168oa. [DOI] [PubMed] [Google Scholar]
- Kassirer MR, Osterberg DH. Pain in chronic multiple sclerosis. J Pain Symptom Manage. 1987;2:95–97. doi: 10.1016/s0885-3924(87)80022-2. [DOI] [PubMed] [Google Scholar]
- Kinkelin I, Motzing S, Koltenzenburg M, Brocker EB. Increase in NGF content and nerve fiber sprouting in human allergic contact eczema. Cell Tissue Res. 2000;302:31–37. doi: 10.1007/s004410000202. [DOI] [PubMed] [Google Scholar]
- Kumpfel T, Hoffmann LA, Pollmann W, Riechmann P, Zettl UK, Kuhbach R, Borasio GD, Voltz R. Palliative care in patients with severe multiple sclerosis: two case reports and a survey among German MS neurologists. Palliat Med. 2007;21:109–114. doi: 10.1177/0269216306075112. [DOI] [PubMed] [Google Scholar]
- LaCroix-Fralish ML, Tawfik VL, DeLeo JA. The organizational and activational effects of sex hormones on tactile and thermal hypersensitivity following lumbar nerve root injury in male and female rats. Pain. 2005;114:71–80. doi: 10.1016/j.pain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindfors PH, Voikar V, Rossi J, Airaksinen MS. Deficient nonpeptidergic epidermis innervation and reduced inflammatory pain in glial cell line-derived neurotrophic factor family receptor alpha2 knock-out mice. J Neurosci. 2006;26:1953–60. doi: 10.1523/JNEUROSCI.4065-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton HL. Theiler’s virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell R, Milliken G, Stroup W, Wolfinger R, Schabenberger O. SAS for Mixed Models. 2. Cary NC: SAS Press; 2006. [Google Scholar]
- Love S. Demyelinating diseases. J Clin Pathol. 2006;59:1151–1159. doi: 10.1136/jcp.2005.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchettini P, Formaglio F, Lacerenza M. Pain as heralding symptom in multiple sclerosis. Neurol Sci. 2006;27(supplement 4):s294–s296. [Google Scholar]
- McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- McDonnell GV, Hawkins SA. An epidemiologic study of multiple sclerosis in Northern Ireland. Neurology. 1998;50:423–428. doi: 10.1212/wnl.50.2.423. [DOI] [PubMed] [Google Scholar]
- McGavern DB, Murray PD, Rivera-Quinones C, Schmelzer JD, Low PA, Rodriguez M. Axonal loss results in spinal cord atrophy, electrophysiological abnormalities and neurological deficits following demyelination in a chronic inflammatory model of multiple sclerosis. Brain 123 Pt. 2000;3:519–531. doi: 10.1093/brain/123.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern DB, Zoecklein L, Drescher KM, Rodriguez M. Quantitative assessment of neurologic deficits in a chronic progressive murine model of CNS demyelination. Exp Neurol. 1999;158:171–181. doi: 10.1006/exnr.1999.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihm MJ, Schanbacher BL, Wallace BL, Wallace LJ, Uretsky NJ, Bauer JA. Free 3-nitrotyrosine causes striatal neurodegeneration in vivo. J Neurosci. 2001;21:RC149. doi: 10.1523/JNEUROSCI.21-11-j0003.2001. (1–5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KR, Murray NM. Neurophysiological evaluation of associated demyelinating peripheral neuropathy and multiple sclerosis: a case report. J Neurol Neurosurg Psychiatry. 1986;49:320–323. doi: 10.1136/jnnp.49.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau T, Manceau E, Lucas B, Lemesle M, Urbinelli R, Giroud M. Incidence of multiple sclerosis in Dijon, France: a population-based ascertainment. Neurol Res. 2000;22:156–159. doi: 10.1080/01616412.2000.11741053. [DOI] [PubMed] [Google Scholar]
- Moulin DE. Pain in multiple sclerosis. Neurol Clin. 1989;7:321–331. [PubMed] [Google Scholar]
- Nelson AL, Bieber AJ, Rodriguez M. Contrasting murine models of MS. Int MS J. 2004;11:95–99. [PubMed] [Google Scholar]
- Nicoletti A, Lo Bartolo ML, Lo Fermo S, Cocuzza V, Panetta MR, Marletta C, Ciancio MR, Cataldi ML, Patti F, Reggio A. Prevalence and incidence of multiple sclerosis in Catania, Sicily. Neurology. 2001;56:62–66. doi: 10.1212/wnl.56.1.62. [DOI] [PubMed] [Google Scholar]
- Oleszak EL, Chang JR, Friedman H, Katsetos CD, Platsoucas CD. Theiler’s virus infection: a model for multiple sclerosis. Clin Microbiol Rev. 2004;17:174–207. doi: 10.1128/CMR.17.1.174-207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Ercolini AM, Miller SD. A virus-induced molecular mimicry model of multiple sclerosis. Curr Top Microbiol Immunol. 2005;296:39–53. doi: 10.1007/3-540-30791-5_3. [DOI] [PubMed] [Google Scholar]
- Osterberg A, Boivie J, Thuomas KA. Central pain in multiple sclerosis--prevalence and clinical characteristics. Eur J Pain. 2005;9:531–542. doi: 10.1016/j.ejpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Pan J, Yeger H, Cutz E. Innervation of pulmonary neuroendocrine cells and neuroepithelial bodies in developing rabbit lung. J Histochem Cytochem. 2004;52:379–389. doi: 10.1177/002215540405200309. [DOI] [PubMed] [Google Scholar]
- Pinhero J, Bates D. Mixed Effects Models in S and S-Plus. New York: Springer; 2000. [Google Scholar]
- Pollock M, Calder C, Allpress S. Peripheral nerve abnormality in multiple sclerosis. Ann Neurol. 1977;2:41–48. doi: 10.1002/ana.410020107. [DOI] [PubMed] [Google Scholar]
- Riggs JE, Schochet SS, Jr, Kopitnik TA, Gutmann L. Target fibers in multiple sclerosis: implications for pathogenesis. Neurology. 1986;36:297–298. doi: 10.1212/wnl.36.2.297. [DOI] [PubMed] [Google Scholar]
- Ro YI, Alexander CB, Oh SJ. Multiple sclerosis and hypertrophic demyelinating peripheral neuropathy. Muscle Nerve. 1983;6:312–316. doi: 10.1002/mus.880060411. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Leibowitz JL, Lampert PW. Persistent infection of oligodendrocytes in Theiler’s virus-induced encephalomyelitis. Ann Neurol. 1983;3(4):426–33. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Oleszak E, Leibowitz J. Theiler’s murine encephalomyelitis: a model of demyelination and persistence of virus. Crit Rev Immunol. 1987;7:325–365. [PubMed] [Google Scholar]
- Sarova-Pinhas I, Achiron A, Gilad R, Lampl Y. Peripheral neuropathy in multiple sclerosis: a clinical and electrophysiologic study. Acta Neurol Scand. 1995;91:234–238. doi: 10.1111/j.1600-0404.1995.tb06996.x. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Iida T, Guan Y, Zhao C, Raja SN, Jarvis MF, Cockayne DA, Caterina MJ. Enhanced thermal avoidance in mice lacking the ATP receptor P2X3. Pain. 2005;116:96–108. doi: 10.1016/j.pain.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Solaro C, Brichetto G, Amato MP, Cocco E, Colombo B, D’Aleo G, Gasperini C, Ghezzi A, Martinelli V, Milanese C, Patti F, Trojano M, Verdun E, Mancardi GL PaIMS Study Group. The prevalence of pain in multiple sclerosis: a multicenter cross-sectional study. Neurology. 2004;63:919–921. doi: 10.1212/01.wnl.0000137047.85868.d6. [DOI] [PubMed] [Google Scholar]
- Sriram S, Steiner I. Experimental allergic encephalomyelitis: a misleading model of multiple sclerosis. Ann Neurol. 2005;58:939–945. doi: 10.1002/ana.20743. [DOI] [PubMed] [Google Scholar]
- Steinman L, Zamvil SS. Virtues and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends Immunol. 2005;26:565–571. doi: 10.1016/j.it.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Stenager E, Knudsen L, Jensen K. Acute and chronic pain syndromes in multiple sclerosis. Acta Neurol Scand. 1991;84:197–200. doi: 10.1111/j.1600-0404.1991.tb04937.x. [DOI] [PubMed] [Google Scholar]
- Stenager E, Knudsen L, Jensen K. Acute and chronic pain syndromes in multiple sclerosis. A 5-year follow-up study. Ital J Neurol Sci. 1995;16:629–632. doi: 10.1007/BF02230913. [DOI] [PubMed] [Google Scholar]
- Svendsen KB, Jensen TS, Overvad K, Hansen HJ, Koch-Henriksen N, Bach FW. Pain in patients with multiple sclerosis: a population-based study. Arch Neurol. 2003;60:1089–1094. doi: 10.1001/archneur.60.8.1089. [DOI] [PubMed] [Google Scholar]
- Svendsen KB, Jensen TS, Hansen HJ, Bach FW. Sensory function and quality of life in patients with multiple sclerosis and pain. Pain. 2005;114:473–481. doi: 10.1016/j.pain.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Totaro R, Marini C, Cialfi A, Giunta M, Carolei A. Prevalence of multiple sclerosis in the L’Aquila district, central Italy. J Neurol Neurosurg Psychiatry. 2000;68:349–352. doi: 10.1136/jnnp.68.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vein AM, Voznesenskaia TG, Khromova LG. The course of disseminated sclerosis in men and women. Zh Nevropatol Psikhiatr Im S S Korsakova. 1995;95:43–44. [PubMed] [Google Scholar]
- Vermote R, Ketelaer P, Carton H. Pain in multiple sclerosis patients. A prospective study using the Mc Gill Pain Questionnaire. Clin Neurol Neurosurg. 1986;88:87–93. doi: 10.1016/s0303-8467(86)80002-6. [DOI] [PubMed] [Google Scholar]
- Wacnik PW, Eikmeier LJ, Ruggles TR, Ramnaraine ML, Walcheck BK, Beitz AJ, Wilcox GL. Functional interactions between tumor and peripheral nerve: morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J Neurosci. 2001;21:9355–9366. doi: 10.1523/JNEUROSCI.21-23-09355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacnik PW, Baker CM, Herron MJ, Kren BT, Blazar BR, Wilcox GL, Hordinsky MK, Beitz AJ, Ericson ME. Tumor-induced mechanical hyperalgesia involves CGRP receptors and altered innervation and vascularization of DsRed2 fluorescent hindpaw tumors. Pain. 2005;115:95–106. doi: 10.1016/j.pain.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Wallengren J, Chen D, Sundler F. Neuropeptide-containing C-fibres and wound healing in rat skin. Neither capsaicin nor peripheral neurotomy affect the rate of healing. Br J Dermatol. 1999;140:400–408. doi: 10.1046/j.1365-2133.1999.02699.x. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Hains BC. Fire and phantoms after spinal cord injury: Na+ channels and central pain. Trends Neurosci. 2006;29:207–215. doi: 10.1016/j.tins.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Weiler E, Benali A. Olfactory epithelia differentially express neuronal markers. J Neurocytol. 2005;4:217–240. doi: 10.1007/s11068-005-8355-z. [DOI] [PubMed] [Google Scholar]
- Weisberg S. Applied Linear Regression. 3. New York: Wiley; 2005. Section 6.2.2. [Google Scholar]
- Wendelschafer-Crabb G, Kennedy WR, Walk D. Morphological features of nerves in skin biopsies. J Neurol Sci. 2006;242:15–21. doi: 10.1016/j.jns.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2:137–145. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- Wilson PO, Barber PC, Hamid QA, Power BF, Dhillon AP, Rode J, Day IN, Thompson RJ, Polak JM. The immunolocalization of protein gene product 9.5 using rabbit polyclonal and mouse monoclonal antibodies. Brit J Exp Path. 1988;69:91–104. [PMC free article] [PubMed] [Google Scholar]
- Wynn DR, Rodriguez M, O’Fallon WM, Kurland LT. A reappraisal of the epidemiology of multiple sclerosis in Olmsted County, Minnesota. Neurology. 1990;40:780–786. doi: 10.1212/wnl.40.5.780. [DOI] [PubMed] [Google Scholar]
- Yen LD, Bennett GJ, Ribeiro-da-Silva A. Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury. J Comp Neurol. 2006;495:679–690. doi: 10.1002/cne.20899. [DOI] [PubMed] [Google Scholar]
- Zhang N, Oppenheim JJ. Crosstalk between chemokines and neuronal receptors bridges immune and nervous systems. J Leukoc Biol. 2005;78:1210–1214. doi: 10.1189/jlb.0405224. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]