Abstract
Butyrate, a short chain fatty acid (SCFA) produced by bacterial fermentation of undigested carbohydrates in the colon, constitutes the major fuel for colonocytes. We have earlier shown the role of apically localized monocarboxylate transporter isoform 1 (MCT1) in transport of butyrate into human colonic Caco-2 cells. In an effort to study the regulation of MCT1 gene, we and others have cloned the promoter region of the MCT1 gene and identified cis elements for key transcription factors. A previous study has shown up-regulation of MCT1 expression, and activity by butyrate in AA/C1 human colonic epithelial cells, however, the detailed mechanisms of this up-regulation are not known. In this study, we demonstrate that butyrate, a substrate for MCT1, stimulates MCT1 promoter activity in Caco-2 cells. This effect was dose dependent and specific to butyrate as other predominant SCFAs, acetate, and propionate, were ineffective. Utilizing progressive deletion constructs of the MCT1 promoter, we showed that the putative butyrate responsive elements are in the −229/+91 region of the promoter. Butyrate stimulation of the MCT1 promoter was found to be independent of PKC, PKA, and tyrosine kinases. However, specific inhibitors of the NF-κB pathway, lactacystein (LC), and caffeic acid phenyl ester (CAPE) significantly reduced the MCT1 promoter stimulation by butyrate. Also, butyrate directly stimulated NF-κB-dependent luciferase reporter activity. Histone deacetylase (HDAC) inhibitor trichostatin A (TSA) also stimulated MCT1 promoter activity, however, unlike butyrate, this stimulation was unaltered by the NF-κB inhibitors. Further, the combined effect of butyrate, and TSA on MCT1 promoter activity was additive, indicating that their mechanisms of action were independent. Our results demonstrate the involvement of NF-κB pathway in the regulation of MCT1 promoter activity by butyrate.
Keywords: short chain fattyacids, Caco-2, HDAC, TSA
MCT1 is a member of the proton-linked monocarboxylate transporter (MCT) family, which is known to comprise at least 14 isoforms in mammals [Halestrap and Meredith, 2004]. Of these, four isoforms (MCT1-MCT4) have been structurally and functionally well characterized [Halestrap and Meredith, 2004]. We have recently demonstrated the presence of various MCT isoforms along the length of the human intestinal epithelium [Gill et al., 2005]. The MCTs have been shown to catalyze the proton-linked transport of short-chain substituted monocarboxylates such as lactate, and pyruvate, ketone bodies, and also the short chain fatty acids (SCFA) [Ritzhaupt et al., 1998; Halestrap and Meredith, 2004]. The major SCFAs produced during bacterial fermentation of dietary fiber in the gut are acetate, propionate and butyrate. At the colonic luminal pH, these SCFAs exist almost entirely as anions, and require a carrier protein for cellular entry. Studies from our laboratory and others have clearly demonstrated that butyrate is transported across the human colonocyte luminal membrane via (MCT1) [Ritzhaupt et al., 1998; Hadjiagapiou et al., 2000; Stein et al., 2000]. The involvement of MCT1 in SCFA transport in bovine caecum and caprine rumen has been recently reported [Kirat and Kato, 2006; Kirat et al., 2006]. Although controversies exist regarding precise membrane localization of MCT1 in the intestine of human, and other species, its role as a major route of SCFA transport is widely recognized. Our recent report showing that inhibition of luminal butyrate uptake following enteropathogenic Eschericia coli (EPEC) infection was associated with a decreased level of surface MCT1 in Caco-2 cells also emphasizes importance of MCT1 in luminal SCFA transport [Borthakur et al., 2006].
SCFAs, particularly butyrate are excellent energy substrates for colonic epithelial cells and possess trophic effects on the colon [Cook and Sellin, 1998]. SCFAs are also known to stimulate water and NaCl absorption [Binder and Mehta, 1989] and blood flow [Mortensen et al., 1990] in the colon. They play an important role in homeostasis of colonic mucosa by inducing pathways of cell maturation, including cell cycle arrest, differentiation, and apoptosis [Barnard and Warwick, 1993; Heerdt et al., 1997]. Reduced luminal availability and impaired intracellular oxidation of butyrate have been implicated in the pathogenesis of colonic disorders such as ulcerative colitis [Cook and Sellin, 1998]. Butyrate is known to modulate expression of an array of genes including the cell cycle inhibitor p21 [Archer et al., 1998], cyclins [Siavoshian et al., 2000], and the BCL2 family [Hague et al., 1997]. Butyrate has been shown to stimulate NHE3 gene promoter [Kiela et al., 2001], and to up-regulate MCT1 mRNA, and protein levels [Cuff et al., 2002].
Because of the pivotal role of butyrate in cellular metabolism, and colonic tissue homeostasis, an understanding of the regulation of its transport into colonic tissues is important. However, very little information is available on the transcriptional regulation of MCT1. We and others have recently reported cloning and characterization of the promoter region of MCT1 gene [Cuff and Shirazi-Beechey, 2002; Hadjiagapiou et al., 2002; Hadjiagapiou et al., 2005]. We have also shown that USF proteins, particularly USF2, act as repressor of the basal promoter activity of MCT1 gene [Hadjiagapiou et al., 2005]. A recent report showed up-regulation of MCT1 and MCT4 mRNA by testosterone in rat skeletal muscle [Enoki et al., 2006]. In another study, it has been suggested that MCT1 expression, and activity are up-regulated by butyrate via involvement of both transcriptional and post-transcriptional mechanisms [Cuff et al., 2002]. However, to date, detailed mechanisms of transcriptional regulation of MCT1 expression by butyrate are not known.
The ability of butyrate to modulate gene expression is often attributed to histone hyperacetylation through inhibition of histone deacetylases [Riggs et al., 1977; Sealy and Chalkley, 1978; Grunstein, 1997]. Histone acetylation causes a relaxed state of the chromosome(s) rendering it more accessible to transcription factors [Grunstein, 1997]. Despite the extensive knowledge about histone acetylation, the relationship between butyrate and transcriptional activation remains relatively unclear. The multiple effects of butyrate are implicated in modulation of gene expression at several levels including transcription, mRNA stability, and elongation [Cuff and Shirazi-Beechey, 2004].
In the present study, we have investigated the mechanism of up-regulation of MCT1 gene transcription by butyrate at the promoter level. We have shown that butyrate markedly stimulates MCT1 promoter activity and also provided evidence for the involvement of NF-κB mediated pathway in stimulatory effect of butyrate on MCT1 promoter.
MATERIALS AND METHODS
Reagents
All of the following protein kinase inhibitors were purchased from Biomol (Plymouth Meeting, PA): Rp-cAMP [adenosine 3′,5′-cyclic monophosphorothionate, Rp-isomer, triethyl ammonium salt], Bisindolylmaleimide I [2-[1-(3-dimethylaminopropyl)-1H-indol-2-yl-3(1H-indol-3-yl)-maleimide], Genistein [4′,5,7-trihydroxyisoflavone], and Herbimycin. The NF-κB inhibitors lactacystein and CAPE were obtained from Biomol and Calbiochem (San Diego, CA), respectively. SCFAs were obtained from Sigma (St. Louis, MO) and TSA from Calbiochem. Reporter vector pGL2-basic and luciferase assay reagents were from Promega (Madison, WI).
Cell Culture and Transfection
Caco-2 cells were grown at 37°C in an atmosphere of 5% CO2. Cells were maintained in DMEM with 4.5 g/l glucose, 50 U/ml penicillin, 5 μg/ml streptomycin, 2 μg/ml gentamicin, and 20% FBS. HT-29 cells were grown in the DMEM media with 10% FBS. Cloning of the MCT1 promoter region and preparation of its progressive 5′ deletion constructs in pGL2 reporter plasmid have been described earlier [Hadjiagapiou et al., 2005]. Cells were transiently transfected with the indicated plasmid at 80–90% confluency in 24-well plates using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to manufacturer’s protocol. Twenty-four hours after transfection, fresh medium containing sodium salts of various SCFAs or sodium gluconate as control at the indicated concentrations were added. For all studies with inhibitors, cells were pre-treated for 1 h with the inhibitor, and then treated with 5 mM sodium butyrate, and/or the inhibitor for 24 h before assaying for luciferase activity.
Luciferase Assay
Cell monolayers were washed with PBS and lysed by alternate freezing and thawing in 50 μl of reporter lysis buffer (Promega). Twenty μl of the lysate was used for firefly luciferase assays with luciferase assay reagent (Promega) in a optocomp I BG I luminometer (GEM Biomedical, Inc.). Relative luciferase unit (RLU) values obtained from the assay were normalized by the amount of protein used as determined by the method of Bradford, [1976] and were expressed as RLU per milligram protein.
Nuclear Extracts and Western Blot
Nuclear extracts were prepared from cells treated for 24 h with 5 mM butyrate, 5 mM gluconate, or 1 μM TSA following the protocol as described previously [Hadjiagapiou et al., 2005]. Briefly, Caco-2 monolayers were washed twice with PBS, and transferred to Eppendorf tube. Cells were lysed in lysis buffer [in mM: 10 HEPES [pH 7.9], 10 KCl, 1.5 MgCl2, 0.1 EDTA, 1 DTT, and 1 PMSF], by keeping in ice for 10 min, and then douncing 10 times on ice in a Dounce homogenizer. Nuclear pellet obtained by centrifugation was washed with lysis buffer and resuspended in 50–80 μl of nuclear extraction buffer [in mM: 20 HEPES [pH 7.9], 400 NaCl, 1.5 MgCl2 1 EDTA, 1 DTT, 1 PMSF, with 25% glycerol] and incubated at 4°C on a rotary shaker for 3 h. Supernatant (nuclear extract) obtained by centrifugation was stored at −80°C till use. Nuclear proteins were separated by SDS–PAGE, were transferred to a nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ), and probed with the indicated antibodies procured from Santa Cruz Biotechnology (Santa Cruz, CA). Immunoreactive bands were visualized using the ECL detection kit (Amersham).
Gel Mobility Shift Assay
The oligonucleotide sequence encompassing the potential USF binding element in the MCT1 promoter (5′CAAGCCGGTCACGTGGCGGGAGGGGGCG3′) [Hadjiagapiou et al., 2005] was synthesized by Sigma. The complementary oligonucleotides were annealed, end labeled with (γ-32P)ATP, and T4 polynucleotide kinase (Promega), and purified with NucTrap probe purification columns (Stratagene, TX). The DNA–protein binding reactions were carried out in a binding buffer consisting of 10 mM Tris-HCl [pH 7.5], 1 mM MgCl2 0.5 mM EDTA, 0.5 mM DTT, 50 mM NaCl, 4% glycerol, and 0.05 mg/ml poly(dI–Dc). Nuclear proteins (4–5 μg) were added to the binding buffer and incubated for 10 min at room temperature. Then, radiolabeled oligonucleotide (50,000 cpm) was added and the incubation was further continued for 20 min. In competition assays, 100-fold molar excess of unlabeled oligonucleotides were added at the start of the reaction.
NF-κB-Luciferase Reporter Assay
To study the NF-κB activation by butyrate, NF-κB-Luciferase reporter assay was performed according to manufacturer’s instructions (Clontech, CA). Briefly, Caco-2 cells were transfected with p-NF-κB-Luc (Clontech) using Lipofectamine 2000 reagent (Invitrogen). This plasmid vector contains NF-κB binding consensus element located upstream of luciferase reporter gene. Twenty-four hours after transfection, cells were treated for 6 h and 24 h with butyrate (5 mM) or other SCFAs (acetate, propionate), or TNF-α (100 ng/ml) as positive control. Luciferase reporter assay was subsequently performed as described above and results were expressed as RLU/mg protein.
Statistical Analysis
Results are expressed as mean ± SEM. Each independent set of results represents data from at least three wells used in three to four separate experiments.
RESULTS
Butyrate Activates MCT1 Promoter in Human Intestinal Cells
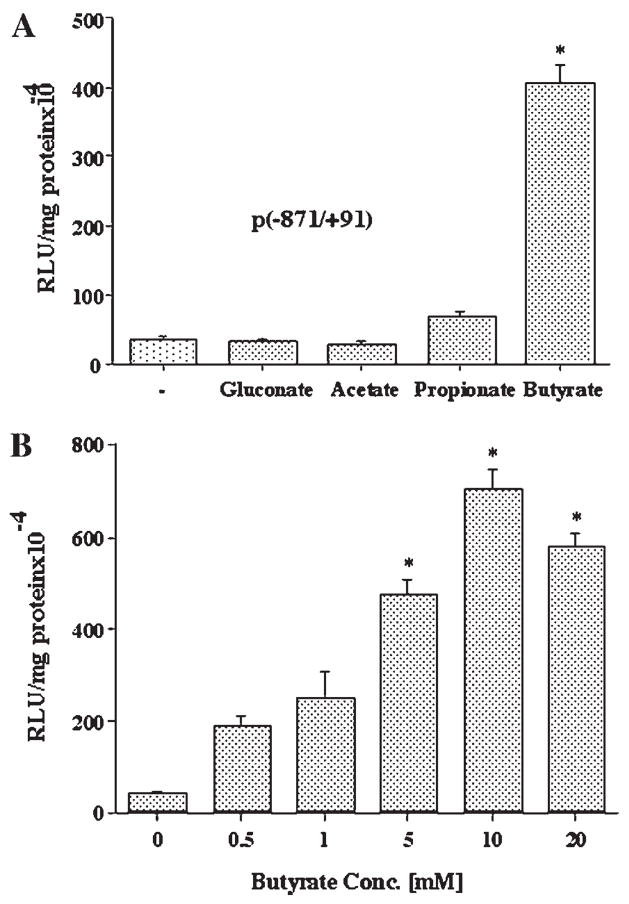
Sodium butyrate has been shown to up-regulate MCT1 mRNA and protein expression in human colonic AA/C1 cells [Cuff et al., 2002]. Therefore, we examined the effect of sodium salts of acetate, propionate, and butyrate, major SCFAs in the human colonic lumen, on the MCT1 promoter activity in Caco-2 cells. As shown in Figure 1A, 5 mM concentration of butyrate caused ~10 fold increase in the activity of the promoter construct comprising the region −871/+91 of the MCT1 gene, compared to untreated cells or cells treated with 5 mM sodium gluconate. At the same concentrations, acetate was ineffective, while activation by propionate was not significant. The stimulatory effect of butyrate on MCT1 promoter was dose dependent, with maximum stimulation observed at a concentration of 10 mM (Fig. 1B).
Fig. 1.
The effects of short chain fatty acids on the MCT1 gene promoter activity in Caco-2 cells. A: Caco-2 cells were transiently transfected with the promoter construct comprising −871/+91 region of MCT1 gene in pGL2 and 24 h later treated with 5 mM of the indicated SCFA or gluconate as control, for additional 24 h. Cells were then harvested, lysed, and assayed for firefly luciferase activity. Data were normalized by protein concentrations and expressed as relative luciferase units (RLU)/mg protein. B: Caco-2 cells, 24 h post-transfected with the promoter construct −871/+91 were treated for 24 h with butyrate at the indicated concentrations. Luciferase activity was measured and expressed as described above. Results are mean ± SEM of three independent experiments. Statistical significance was determined by Student’s paired two-tailed t-test. Asterisks indicate differences between groups, (A) control versus SCFAs or (B) control vs different conc. of butyrate are statistically significant at P < 0.001.
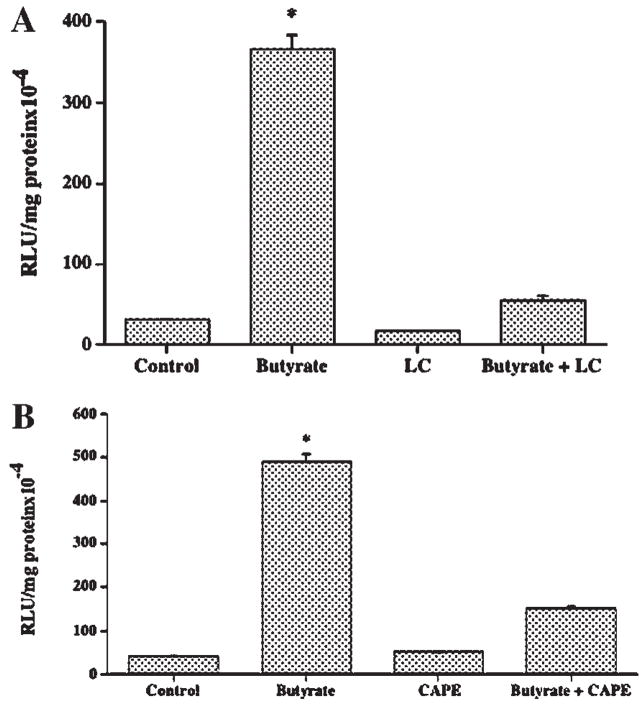
Fig. 5.
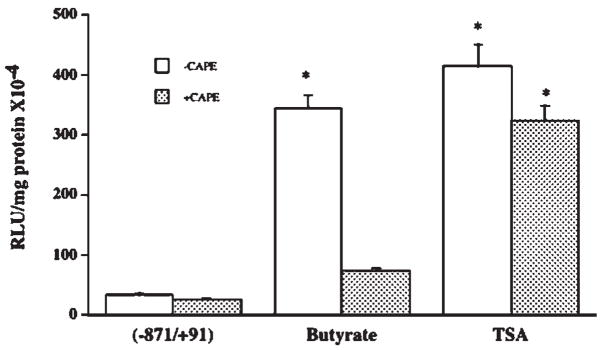
NF-κB pathway is involved in butyrate-mediated stimulation of MCT1 promoter activity. Caco-2 cells, transiently transfected with the promoter construct comprising −871/+91 region of MCT1 gene in pGL2, were treated with 5 mM butyrate for 24 h in the presence or absence of (A) 5 μM lactacystein (LC) or (B) 25 μM CAPE. Luciferase activity was subsequently determined and expressed as described above. Results are mean ± SEM of three independent experiments. Statistical significance was determined by Student’s paired two-tailed t-test. Asterisks indicate differences between groups, control versus butyrate, are statistically significant at P < 0.001.
Butyrate Activation of MCT1 Promoter Was Not Specific to Caco-2 Cells
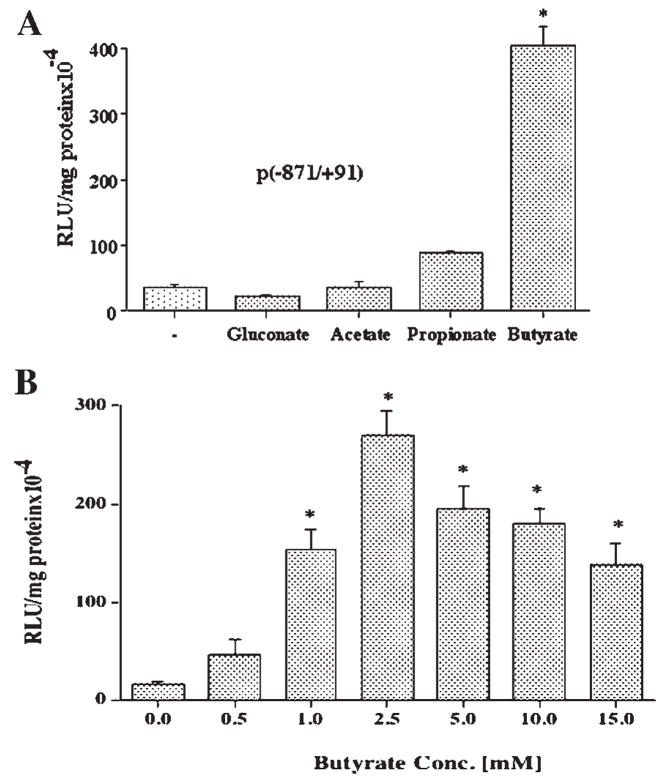
In order to investigate whether the effects of butyrate on MCT1 promoter activity were relevant to other colonic epithelial cells, the entire set of experiments described above were repeated in HT-29 colonic cells. As depicted in Figure 2, similar to our observations in Caco-2 cells, butyrate at 5 mM concentration also caused a significant increase (~10-fold) in MCT1 promoter activity in HT-29 cells, while the effect of propionate was not significant, and acetate was ineffective (Fig. 2A). The dose response to butyrate in HT-29 cells (Fig. 2B) showed that maximal stimulation of promoter activity was achieved at 2.5 mM concentration, indicating that these cells were more responsive to butyrate stimulation than Caco-2 cells.
Fig. 2.
The effects of short chain fatty acids on the MCT1 gene promoter activity in HT-29 cells. A: HT-29 cells were transiently transfected with the promoter construct comprising −871/+91 region of MCT1 gene in pGL2 and 24 h later treated with 5 mM of the indicated SCFA or gluconate as control, for additional 24 h. Cells were then harvested, lysed, and assayed for firefly luciferase activity. Data were normalized by protein concentrations and expressed as relative luciferase units (RLU)/mg protein. B: HT-29 cells, 24 h post-transfected with the promoter construct −871/+91 were treated for 24 h with butyrate at the indicated concentrations. Luciferase activity was measured and expressed as described above. Results are mean ± SEM of three independent experiments. Statistical significance was determined by Student’s paired two-tailed t-test. Asterisks indicate differences between groups, (A) control versus SCFAs or (B) control vs different conc. of butyrate are statistically significant at P < 0.001.
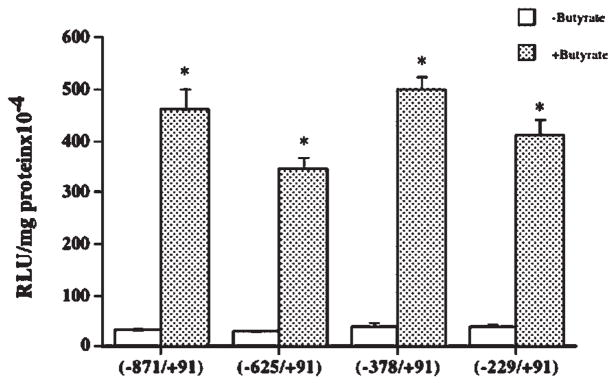
Putative Butyrate Responsive Elements Are Located in the −229/+91 Region of the Promoter
The 5′ deletion constructs of the MCT1 promoter were made by PCR method as described previously [Hadjiagapiou et al., 2005]. The effects of 5 mM butyrate on the activity of each deletion construct were determined. As shown in Figure 3, stimulation of promoter activity by butyrate was quantitatively similar for all MCT1 promoter constructs. These results indicate that the putative butyrate responsive element(s) are located in the −229/+91 region of the MCT1 promoter. Our previous results [Hadjiagapiou et al., 2005] have shown that any further deletion beyond −229 caused substantial decrease in promoter activity in Caco-2 cells, and were, therefore, not tested for the effect of butyrate.
Fig. 3.
Butyrate responsive elements are located in the −229/+91 region of the MCT1 promoter. Caco-2 cells were transfected with the promoter construct comprising −871/+91 region of MCT1 gene in pGL2 or with the 5′-truncated constructs as indicated. After 24 h, cells were treated with 5 mM butyrate for 24 h and luciferase activity was measured. Results are mean ± SEM of three independent experiments. Statistical significance was determined by Student’s paired two-tailed t-test. Asterisks indicate differences between groups control versus butyrate are statistically significant at P < 0.001.
Butyrate Response of the MCT1 Promoter Is Not Mediated by Protein Kinases
Involvement of protein tyrosine kinases and protein serine/threonine kinases has been previously implicated in activation of gene transcription by butyrate [Basson and Hong, 1998; Kiela et al., 2001]. In an effort to elucidate the signaling mechanisms in the activation of MCT1 gene promoter by butyrate, specific inhibitors for PKA, PKC, and protein tyrosine kinases were utilized. Caco-2 cells, 24 h post-transfected with the MCT1 promoter construct −871/+91, were treated with 5 mM butyrate in presence or absence of the inhibitors. Results presented in Table I. show that 5 μM bisindolylmaleimide (BIM) had no effect on stimulation of MCT1 promoter activity by butyrate, suggesting that PKC is not involved. Role of PKA also appeared unlikely as 25 μM RpcAMP could not alter the stimulation of MCT1 promoter by butyrate. Similarly, inability of 20 μM genistein or 5 μM herbimycin to alter the stimulation of MCT1 promoter activity by butyrate ruled out the involvement of protein tyrosine kinases.
TABLE I.
Effects of Protein Kinase Inhibitors on the Stimulation of MCT1 Promoter Activity by Butyrate
| Inhibitor | Control | Butyrate | Inhibitor | Inhibitor + butyrate |
|---|---|---|---|---|
| Promoter activity (% of Control) | ||||
| RpcAMP (25 μM) | 100 | 706 ± 108 | 98 ± 13 | 718 ± 65 |
| BIM (5 μM) | 100 | 672 ± 99 | 95 ± 21 | 646 ± 60 |
| Genistein (20 μM) | 100 | 668 ± 84 | 107 ± 15 | 690 ± 65 |
| Herbimycin (5 μM) | 100 | 680 ± 94 | 96 ± 10 | 655 ± 73 |
Values represent mean ± SEM of three independent experiments performed in triplicate.
Butyrate Does Not Alter Expression of USF2, the Repressor of MCT1 Promoter
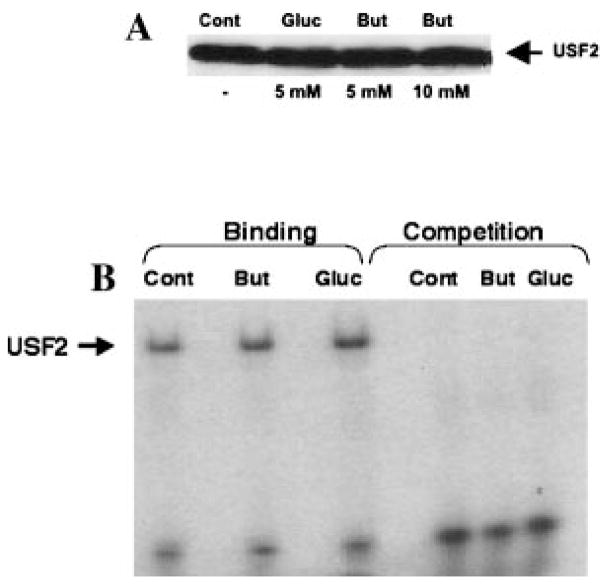
We have recently reported USF2 as a potent repressor of MCT1 promoter activity [Hadjiagapiou et al., 2005]. Therefore, it was of interest to examine if the stimulatory action of butyrate on the MCT1 promoter was secondary to its potential effect on USF2 expression. Results shown in Figure 4A demonstrate that treatment of Caco-2 cells with 5 and 10 mM butyrate did not alter the nuclear level of USF2. Similarly, electrophoretic mobility shift assay showed that butyrate had no effect on the binding of USF2 to the MCT1 promoter (Fig. 4B). These studies suggest that butyrate stimulation of MCT1 promoter activity was not mediated via alteration in expression of USF2 or its DNA binding activity.
Fig. 4.
Butyrate does not alter USF2 expression and DNA binding activity. A: Nuclear extracts were prepared from control, butyrate, and gluconate treated Caco-2 cells as described in Materials and Methods Section. Nuclear proteins were separated by SDS–PAGE and probed with anti-USF2 antibody in Western blot analysis. B: The double-stranded oligonucleotide corresponding to potential USF2 binding site in MCT1 promoter was radiolabeled with γ32P-ATP. Gel mobility shift assay was carried out with various nuclear extracts as described in Materials and Methods section.
NF-κB Pathway Is Involved in the Activation of MCT1 Promoter by Butyrate
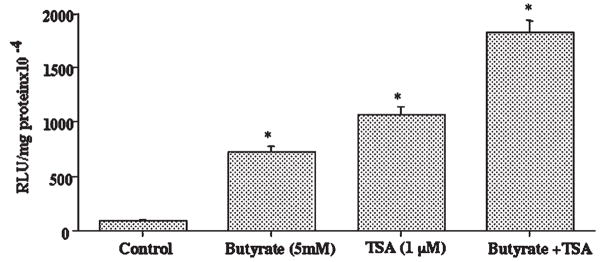
Previous reports have shown a link between NF-κB activity and butyrate’s known actions in the colon [Inan et al., 2000; Place et al., 2005]. Therefore, it was of interest to examine whether the stimulatory effect of butyrate on MCT1 promoter activity is related to NF-κB pathway. We used two selective inhibitors of NF-κB pathway, lactacysteine (LC) and caffeic acid phenyl ester (CAPE) and studied their effects on butyrate stimulation of MCT1 promoter activity. As is evident from the results shown in Figure 5, stimulatory effect of butyrate on the MCT1 promoter was significantly decreased in the presence of 5 μM LC (A) or 25 μM CAPE (B), indicating the involvement of NF-κB pathway. Since butyrate is known to modulate gene transcription primarily through its histone deacetylase (HDAC) inhibitory activity, the effect of trichostatin A (TSA), a specific HDAC inhibitor, on the MCT1 promoter activity was also investigated. As shown in Figure 6, MCT1 promoter activity was strongly enhanced by 1 μM TSA. However, unlike butyrate, this stimulatory effect of TSA was not reduced by CAPE, the NF-κB inhibitor. Figure 7 shows that 5 mM butyrate and 1 μM TSA together showed an additive, rather than a synergistic effect on the promoter activity. These results suggest that mechanism of stimulation of the MCT1 promoter activity by butyrate in Caco-2 cells is different from that of TSA.
Fig. 6.
Butyrate stimulation of MCT1 promoter is independent of its HDAC inhibitory activity. Caco-2 cells, transiently transfected with the promoter construct comprising −871/+91 region of MCT1 gene, were treated with 5 mM butyrate or 1 μM TSA for 24 h in presence or absence of 25 μM CAPE. Luciferase activity was subsequently determined and expressed as described before. Results are mean ± SEM of three independent experiments. Statistical significance was determined by Student’s paired two-tailed t-test. Asterisks indicate differences between groups control versus butyrate, control versus TSA, and control versus TSA + CAPE, which are statistically significant at P < 0.001.
Fig. 7.
Butyrate stimulates MCT1 promoter activity by a mechanism distinct from that of TSA. Caco-2 cells, transiently transfected with the promoter construct comprising −871/+91 region of MCT1 gene, were treated with 5 mM butyrate or 1 μM TSA or both for 24 h. Luciferase activity was subsequently determined and expressed as described before. Results are mean ± SEM of three independent experiments. Statistical significance was determined by Student’s paired two-tailed t-test. Asterisks indicate differences between groups, control versus butyrate, control versus TSA, and control versus butyrate + TSA are statistically significant at P < 0.001.
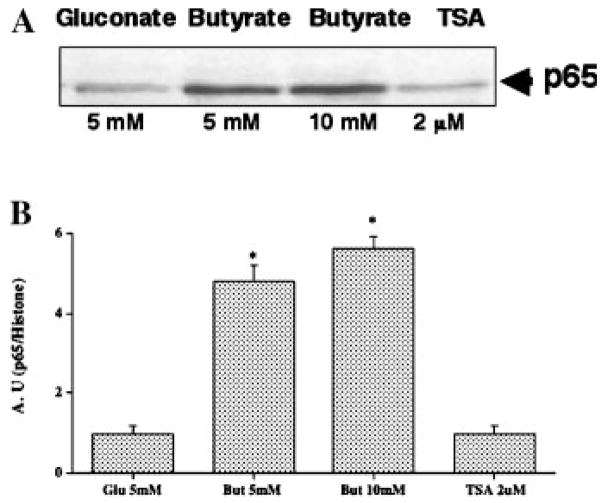
Butyrate Elevates Nuclear NF-κB Level
In order to determine whether butyrate has a direct effect on NF-κB activation, we examined the effect of butyrate, and TSA on the nuclear levels of NF-κB subunits. The most abundant forms of active NF-κB are heterodimer of p65 and p50 or p65 homodimer [Quivy and Lint, 2004]. Results shown in Figure 8 reveal that butyrate treatment of the Caco-2 cells caused a significant increase in nuclear level of p65 compared to those treated with gluconate. This elevation of nuclear p65, however, was not observed in TSA treated cells (Fig. 8), which further highlights that distinct mechanisms are involved in the actions of butyrate, and TSA in stimulating MCT1 promoter activity.
Fig. 8.
Butyrate elevates nuclear level of NF-κB (p65). Nuclear extracts were prepared from control, butyrate, and gluconate treated Caco-2 cells as described in Materials and Methods section. Nuclear proteins were separated by SDS–PAGE and probed with anti-p65 antibody in Western blot analysis. Band intensities were normalized utilizing histone as internal standard.
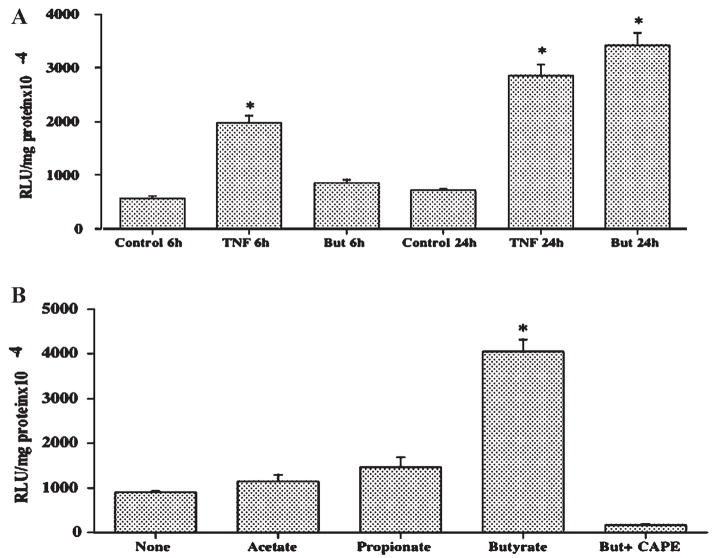
Butyrate Specifically Activates NF-κB Pathway in Caco-2 Cells
To further confirm that butyrate has a specific direct effect on the activation of NF-κB pathway in Caco-2 cells, we used the NF-κB transcription reporter vector p-NF-κB-Luc for transfection of Caco-2 cells. This vector contains NF-κB consensus sequence located upstream of the firefly luciferase reporter gene. When the plasmid is transfected into an appropriate cell line, expression of the reporter gene can be induced by adding a stimulus that activates the NF-κB pathway. We used TNF-α as the positive control as per manufacturer’s instructions. As shown in Figure 9A, after 6 h of treatment, TNF-α caused 3.5-fold activation of NF-κB-dependent reporter gene while activation by butyrate was 1.5-fold. At 24 h, however, activation by butyrate was 6-fold compared to 5-fold activation by TNF-α. Figure 9B shows that the effect of activating the NF-κB-dependent reporter was specific to butyrate, as activation by acetate, and propionate, the other two colonic SCFAs, was not significant. Interestingly, in the presence of 50 μM CAPE, the inhibitor of NF-κB pathway, butyrate had no effect on reporter gene activation.
Fig. 9.
Butyrate specifically activates NF-κB-dependent reporter gene. Caco-2 cells were transfected with the plasmid p-NF-κB-Luc using Lipofectamine 2000 as described in Materials and Methods section. A: After 24 h cells were treated with either 5 mM butyrate or 100 ng/ml TNF-α. Cells were harvested after 6 and 24 h and luciferase activity was measured. B: 24 h post-transfected cells were treated with the indicated SCFAs (5 mM) for 24 h and luciferase activity was subsequently measured. Results are mean ± SEM of two independent experiments in triplicate. Statistical significance was determined by Student’s paired two-tailed t-test. Asterisks indicate differences between groups, (A) control versus TNF 6 h and 24 h, control versus butyrate 24 h or (B) control versus butyrate 24 h, which are statistically significant at P < 0.001.
DISCUSSION
We and others have previously cloned and characterized the promoter region of the gene for MCT1 [Cuff and Shirazi-Beechey, 2002; Hadjiagapiou et al., 2002, 2005], a transporter implicated in luminal transport of butyrate into colonic epithelial cells [Ritzhaupt et al., 1998; Hadjiagapiou et al., 2000]. Butyrate has multiplicity of functions in maintaining the integrity and health of the colonocytes, which emphasizes the importance of the studies on the regulation of MCT1. However, there is very little information available regarding transcriptional regulation of this transporter, and the physiological significance of its regulation in the intestine. We have recently reported the roles of USF1 and USF2 as repressor proteins of the MCT1 promoter [Hadjiagapiou et al., 2005]. A previous report [Cuff et al., 2002] showed that butyrate itself caused an up-regulation of both MCT1 mRNA and protein expression along with an increase in butyrate transport activity in human colonic epithelial AA/C1 cells. We have also previously shown butyrate-induced increase in the uptake of 14C butyrate in Caco-2 cells [Alrefai et al., 2004]. However, detailed studies on the transcriptional regulation of MCT1 gene by butyrate have not been carried out. In this report, we have demonstrated that butyrate has a strong stimulatory effect on the MCT1 promoter activity. Of the three most abundant colonic SCFAs, butyrate was the most effective in stimulating MCT1 promoter activity. We also found a dose response of butyrate effect, with 10 mM butyrate showing the highest stimulatory effect. This stimulatory effect of butyrate was also found in another colonic epithelial cell line HT-29. Preliminary mapping of the cis elements by deletion analysis of the promoter revealed that the butyrate responsive elements are located in the −229/+91 region of the promoter. We have earlier reported this region as the core promoter which has cis elements for binding key transcription factors like Sp1, AP2, USF, and HIF1-alpha [Hadjiagapiou et al., 2005]. Since USF proteins, more particularly USF2, have been shown by us as repressors of this promoter [Hadjiagapiou et al., 2005], we speculated that butyrate activation of the promoter might be secondary to down-regulation of the expression and/or DNA binding activity of USF by butyrate. However, contrary to the expectations, our results showed that butyrate did not have any effect on the expression or DNA binding activity of USF.
In an attempt to elucidate the signaling mechanisms of butyrate action on MCT1 promoter, we used specific inhibitors to block the activities of various protein kinases that have been previously shown to be important mediators of butyrate’s effects on gene transcription. For example, tyrosine kinase activity has been postulated to be an important signal-transduction event in the mechanism of butyrate action in Caco-2 cells [Basson and Hong, 1998]. Similarly, Ser/Thr kinases have also been implicated in signal transduction pathways leading to activation of gene transcription by butyrate [Kiela et al., 2001]. In the present study, however, blocking the activities of protein kinase A (PKA), protein kinase C (PKC), and tyrosine kinases with specific inhibitors did not alter the stimulatory effect of butyrate on the MCT1 promoter activity, ruling out their involvement in mediating the effect of butyrate. A regulatory link between NF-κB and butyrate has also been shown in various studies involving modulation of target gene expression in the intestine [Inan et al., 2000; Blais et al., 2005; Place et al., 2005] and other tissues [Quivy et al., 2002; Adam et al., 2003]. Therefore, in an attempt to elucidate the mechanisms associated with butyrate mediated induction of MCT1 promoter activity, specific inhibitors of NF-κB pathway were utilized. Interestingly, we observed that in presence of LC or CAPE, specific inhibitors of NF-κB pathway, stimulation of MCT1 promoter activity by butyrate was substantially decreased suggesting the importance of NF-κB pathway in butyrate’s effect on the MCT1 promoter activity.
Butyrate is known to modulate expression of an array of genes. Its action has traditionally been attributed to reversible inhibition of histone deacetylases (HDACs) [Riggs et al., 1977; Sealy and Chalkley, 1978]. However, butyrate also induces a variety of other changes both in the nucleus, and cytoplasm. These include hyperacetylation of non-histone proteins, including transcription factors, DNA methylation and modulation of intracellular kinase signaling [Cuff and Shirazi-Beechey, 2004]. This suggests that butyrate responsiveness of various genes involves multiple and distinct mechanisms/pathways. Our results showing marked reduction of stimulatory effect of butyrate on MCT1 promoter activity by NF-κB inhibitors raised the question if NF-κB involvement is related to the hyperacetylation activity of butyrate. We therefore carried out parallel experiments with TSA, another specific, and potent HDAC inhibitor. We observed that TSA also substantially activated the MCT1 promoter, but unlike butyrate, the stimulatory activity of TSA was unaffected by NF-κB inhibition. This suggests that mechanisms of action of butyrate on the MCT1 promoter activity are distinct from that of TSA and are not secondary to its HDAC inhibitory activity. This was further confirmed by the fact that combined effect of butyrate and TSA on MCT1 promoter activity is additive rather than synergistic. This mechanistic difference in the action of butyrate and TSA has also been shown in the stimulation of NHE3 gene promoter [Kiela et al., 2001].
Other studies have reported that potentiation of NF-κB mediated gene induction by TSA or sodium butyrate involves a complex acetylation dependent regulation of NF-κB activity [Quivy et al., 2002; Quivy and Lint, 2004]. The most studied NF-κB heterodimer is composed of two subunits p50, and p65, which are both acetylated at multiple lysine residues in order to regulate different functions of NF-κB including transcriptional activation, and DNA binding [Quivy and Lint, 2004]. However, the effects of butyrate on the MCT1 promoter activity via NF-κB pathway do not seem to be related to hyperacetylation of NF-κB, since our results showed the effects of NF-κB, and TSA to be independent. Although hyperacetylation of NF-κB increases its duration in the nucleus [Chen et al., 2001], our studies showing increased nuclear p65 in butyrate treated cells is unlikely to result from its hyperacetylation, because TSA treatment did not increase nuclear level of p65. Another recent study showed that potentiation of TNF-α induced NF-κB activation by butyrate might be associated with a delay in the reappearance of IκB [Adam et al., 2003]. Thus, in different systems, the mechanisms of regulation of various genes requiring an interplay between butyrate and NF-kB is complex, each involving distinct mechanisms/pathways. Our studies using 5′ deletions of the MCT1 promoter demonstrated that butyrate responsive elements are located in the core promoter [−229/+91] region which does not seem to have potential binding sites for NF-κB. Therefore, it is possible that potentiation of the stimulatory effect of butyrate on MCT1 promoter by NF-κB involves an indirect mechanism by modulating the activity of other transcription factors and/or by activating target genes for other signaling components of this mechanism. Gene activation by NF-κB has been shown to be modulated by synergistic or antagonistic interactions with other promoter-bound transcription factors [Hirano et al., 1999; Krehan et al., 2000; Liu et al., 2004]. It will be of interest to investigate in future the existence of any synergistic interaction between NF-κB and any other transcription factor that might bind to this region of the promoter and activate MCT1 gene transcription.
Although our studies demonstrated activation of NF-κB pathway by butyrate, several earlier reports also showed suppression of NF-κB activation by butyrate, and other HDAC inhibitors. In HT-29 cells, butyrate suppressed the TNF-α-mediated activation of NF-κB by more than 10-fold, although had no effect on NF-κB activation by IL1-β [Inan et al., 2000]. TSA showed similar effects, suppressing the TNF-α-mediated activation of NF-κB, suggesting that the effects are related to HDAC inhibitory activity of butyrate, and TSA. However, our studies do not appear to contradict these results, because the effects of butyrate on MCT1 promoter activity via NF-κB pathway is not related to its HDAC inhibitory activity and are distinct from that of TSA. Although it has been reported that TNF-α up-regulates MCT1 mRNA, and protein levels in macrophages [Hahn et al., 2000], our unpublished results showed that TNF-α did not alter MCT1 promoter activity in Caco-2 cells, and had no effect on MCT1-mediated butyrate uptake in Caco-2 cells. Also, our studies using NF-κB-dependent reporter gene activation assay, clearly demonstrates that butyrate directly activates NF-κB pathway in Caco-2 cells which is independent of TNF-α activation. Therefore, NF-κB pathway mediated effects of butyrate on MCT1 promoter activity seemed to be independent of TNF-α or IL1-β.
In summary, we have demonstrated that butyrate, the key SCFA, is a specific potent stimulator of MCT1 gene promoter. We have further shown that this stimulatory effect is specific to butyrate and is dose dependent. We also provide evidence that the stimulatory effect of butyrate on the MCT1 promoter activity is not secondary to its histone deacetylase inhibitory activity, as the mechanism of action of butyrate appeared to be distinct from that of TSA. We found several experimental evidences for the involvement of NF-κB pathway in mediating the effects of butyrate on MCT1 promoter. We speculate that the involvement of NF-κB pathway in the up-regulation of MCT1 transcription, resulting in increased absorption of SCFA by colonocytes, may be an adaptive response to epithelial inflammation.
Acknowledgments
NIDDK; Grant numbers: DK 54016, DK 33349, DK 71596, DK 067887.
These studies were supported by the Department of Veterans Affairs and the NIDDK grants DK 54016 (PKD), DK 33349 (KR), and DK 71596 (WAA) and the Program Project Grant DK 067887 (PKD, KR).
References
- Adam E, Quivy V, Bex F, Chariot A, Colette T, Vanhulle C, Schoonbroodt S, Goffin V, Nguyen TLA, Gloire G, Carrard G, Frignet B, Launoit Y, Burny A, Bours V, Piette J, Lint CV. Potentiation of Tumor Necrosis Factor induced NF-κB activation by deacetylase inhibitors is associated with a delayed cytoplasmic reappearance of I-κBα. Mol Cell Biol. 2003;23:6200–6209. doi: 10.1128/MCB.23.17.6200-6209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrefai WA, Tyagi S, Gill R, Saksena S, Hadjiagapieu C, Mansour F, Ramaswamy K, Dudeja PK. Regulation of butyrate uptake in Caco-2 cells by phorbol ester. Am J Physiol Gastrointest Liver Physiol. 2004;286:G197–G203. doi: 10.1152/ajpgi.00144.2003. [DOI] [PubMed] [Google Scholar]
- Archer SY, Meng S, Shei A, Hodin RA. p21 (WAF1) is required for butyrate mediated growth inhibition of human colon cancer cells. Proc Natl Acad Sci USA. 1998;95:6791–6796. doi: 10.1073/pnas.95.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard JA, Warwick G. Butyrate rapidly induces growth inhibition and differentiation in HT-29 cells. Cell Growth Differ. 1993;4:495–501. [PubMed] [Google Scholar]
- Basson MD, Hong F. Tyrosine kinase inhibitors reverse butyrate stimulation of human Caco-2 intestinal epithelial cell alkaline phosphatase but not butyrate promotion of dipeptidyl dipeptidase. Cell Biol Int. 1998;22:339–344. doi: 10.1006/cbir.1998.0257. [DOI] [PubMed] [Google Scholar]
- Binder HJ, Mehta P. Short chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology. 1989;96:989–996. doi: 10.1016/0016-5085(89)91614-4. [DOI] [PubMed] [Google Scholar]
- Blais M, Desilets A, Asselin C. Synergy between deacetylase inhibitors and IL1-β in activation of the serum amyloid A2 gene promoter. DNA Cell Biol. 2005;24:209–217. doi: 10.1089/dna.2005.24.209. [DOI] [PubMed] [Google Scholar]
- Borthakur A, Gill RK, Hodges K, Ramaswamy K, Hecht G, Dudeja PK. Enteropathogenic Eschericia coli inhibits butyrate uptake in Caco-2 cells by altering apical membrane MCT1 level. Am J Physiol Gastrointest Liver Physiol. 2006;290:G30–G35. doi: 10.1152/ajpgi.00302.2005. [DOI] [PubMed] [Google Scholar]
- Bradford A. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen LF, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-κB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- Cook SI, Sellin JH. Review article: Short chain fatty acids in health and disease. Ailment Pharmacol Ther. 1998;12:499–507. doi: 10.1046/j.1365-2036.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- Cuff MA, Shirazi-Beechey SP. The human monocarboxylate transporter, MC T1:Genomic organization and promoter analysis. Biochem Biophys Res Commun. 2002;292:1048–1056. doi: 10.1006/bbrc.2002.6763. [DOI] [PubMed] [Google Scholar]
- Cuff MA, Shirazi-Beechey SP. The importance of butyrate transport to the regulation of gene expression in the colonic epithelium. Biochem Soc Transec. 2004;32:1100–1102. doi: 10.1042/BST0321100. [DOI] [PubMed] [Google Scholar]
- Cuff MA, Lambert DW, Shirazi-Beechey SP. Substrate-induced regulation of the human colonic monocarboxylate transporter, M CT1. J Physiol. 2002;539:361–371. doi: 10.1113/jphysiol.2001.014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki T, Yoshida Y, Lally J, Hatta H, Bonen A. Testosterone increases lactate transport, MCT1 and MCT4 in rat skeletal muscle. J Physiol. 2006;577:433–443. doi: 10.1113/jphysiol.2006.115436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill RK, Saksena S, Alrefai WA, Sarwar J, Goldstein JL, Carrol RE, Ramaswamy K, Dudeja PK. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol Cell Physiol. 2005;289:C846–C852. doi: 10.1152/ajpcell.00112.2005. [DOI] [PubMed] [Google Scholar]
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- Hadjiagapiou C, Schmidt C, Dudeja PK, Layden TJ, Ramaswamy K. Mechanism(s) of butyrate transport in Caco-2 cells: Role of monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol. 2000;279:G775–G780. doi: 10.1152/ajpgi.2000.279.4.G775. [DOI] [PubMed] [Google Scholar]
- Hadjiagapiou C, Dahlal K, Ramaswamy K, Dudeja PK. Molecular cloning of the human monocarboxylate transporter 1 (MCT1) promoter (Abstract) Gastroenterology. 2002;122:W945. [Google Scholar]
- Hadjiagapiou C, Borthakur A, Dahlal R, Gill RK, Malakooti J, Ramaswamy K, Dudeja PK. Role of USF1 and USF2 as potential repressor proteins for human intestinal monocarboxylate transporter 1 promoter. Am J Physiol Gastrintest Liver Physiol. 2005;288:G1118–G1126. doi: 10.1152/ajpgi.00312.2004. [DOI] [PubMed] [Google Scholar]
- Hague A, Diaz GD, Hicks DJ, Krajewski S, Reed JC, Paraskeva C. Bcl-2 and bak may play a pivotal role in sodium butyrate induced apoptosis in colonic epithelial cells; however, overexpression of bcl-2 does not protect against bak-mediated apoptosis. Int J Cancer. 1997;72:898–905. doi: 10.1002/(sici)1097-0215(19970904)72:5<898::aid-ijc30>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Hahn EL, Halestrap AP, Gamelli RL. Expression of the lactate transporter MCT1 in macrophages. Shock. 2000;13:253–260. doi: 10.1097/00024382-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Meredith D. The SLC16 gene family from monocarboxylate transporters to aromatic amino acids and beyond. Pfliigers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- Heerdt BG, Houston MA, Augenlicht LH. Short chain fatty acid-initiated cell cycle arrest and apoptosis of colonic epithelial cells is linked to mitochondrial function. Cell Growth Differ. 1997;8:523–532. [PubMed] [Google Scholar]
- Hirano F, Tanaka H, Hirano Y, Hiramoto M, Handa H, Maniko I, Scheidereit C. Functional interference of Sp1 and NF-κB through the same binding site. Mol Cell Biol. 1999;18:1266–1274. doi: 10.1128/mcb.18.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short chain fatty acid butyrate modulates NF-κB activity in human colonic epithelial cell line. Gastroenterology. 2000;118:724–734. doi: 10.1016/s0016-5085(00)70142-9. [DOI] [PubMed] [Google Scholar]
- Kiela PR, Hines ER, Collins JF, Ghishan FK. Regulation of the rat NHE3 gene promoter by sodium butyrate. Am J Physiol Gastrointest Liver Physiol. 2001;281:G947–G956. doi: 10.1152/ajpgi.2001.281.4.G947. [DOI] [PubMed] [Google Scholar]
- Kirat D, Kato S. MCT1 mediates transport of short chain fatty acids in bovine caecum. Exp Physiol. 2006;91:835–844. doi: 10.1113/expphysiol.2006.033837. [DOI] [PubMed] [Google Scholar]
- Kirat D, Masuoka D, Hayashi H, Iwano H, Yokota H, Taniyama H, Kato S. MCT1 plays a direct role in short chain fatty acid absorption in caprine rumen. J Physiol. 2006;576:635–647. doi: 10.1113/jphysiol.2006.115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krehan A, Ansuini H, Bocher O, Grein S, Wirkner U, Pyerin W. Transcription factors ets1, NF-κB, and Sp1 are major determinants of the promoter activity of human protein kinase CK2alpha gene. J Biol Chem. 2000;275:18327–18336. doi: 10.1074/jbc.M909736199. [DOI] [PubMed] [Google Scholar]
- Liu A, Hoffman PW, Lu W, Bai G. NF-κB site interacts with Sp factors and up-regulates the NR1 promoter during neuronal differentiation. J Biol Chem. 2004;279:17449–17458. doi: 10.1074/jbc.M311267200. [DOI] [PubMed] [Google Scholar]
- Mortensen FV, Nielsen H, Mulvany MJ, Hessov I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut. 1990;31:1391–1394. doi: 10.1136/gut.31.12.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place RF, Noonan EJ, Giardina C. HDAC inhibition prevents NF-κB activation by suppressing proteasome activity: Down-regulation of proteasome subunit expression stabilizes IκBα. Biochem Pharmacol. 2005;70:394–406. doi: 10.1016/j.bcp.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Quivy V, Lint V. Regulation at multiple levels of NF-κB-mediated transactivation by protein acetylation. Biochem Pharmacol. 2004;68:1221–1229. doi: 10.1016/j.bcp.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Quivy V, Adam E, Collette Y, Demonte D, Chariot A, Vanhulle C, Berkhout B, Castellano R, Launoit Y, Burny A, Piette J, Bours V, Lint CV. Synergistic activation of Human Immunodeficiency Virus Type I promoter by NF-κB and inhibitors of deacetylases: Potential perspectives for the development of therapeutic strategies. J virol. 2002;76:11091–11103. doi: 10.1128/JVI.76.21.11091-11103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs MG, Whittaker RG, Neumann JR, Ingram VM. n-butyrate causes histone modification in Hela and Friend erythroleukemia cells. Nature. 1977;268:462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi Beechey SP. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: Its potential to transport L-lactate as well as butyrate. J Physiol. 1998;513:719–732. doi: 10.1111/j.1469-7793.1998.719ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy L, Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978;14:115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- Siavoshian S, Segain JP, Kornprobst M, Bonnet C, Cherbut C, Galmiche JP, Blottiere HM. Butyrate and tricostatin A effects on proliferation/differentiation of human intestinal epithelial cells: Induction of cyclin D3 and p21 expression. Gut. 2000;46:507–514. doi: 10.1136/gut.46.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J, Zores M, Schroder O. Short chain fatty acid (SCFA) uptake into Caco-2 cells by a pH-dependent carrier-mediated transport mechanism. Eur J Nutr. 2000;39:121–125. doi: 10.1007/s003940070028. [DOI] [PubMed] [Google Scholar]