Abstract
Objective
Methotrexate is an effective therapy for Rheumatoid Arthritis (RA) but is also associated with toxicity. Pharmacogenetics is the systematic evaluation of the role of genetic differences in the efficacy and toxicity of therapeutic interventions. Because the results of small pharmacogenetic studies are often misleading, we undertook a meta-analysis of published studies to determine the role of polymorphisms in the therapeutic efficacy and toxicity of methotrexate.
Methods
A search of PUBMED produced 55 publications which were then reviewed for relevance to methotrexate toxicity and efficacy in patients with RA. To ensure that no studies were missed, each polymorphism found was then entered as an independent search string and all results were again reviewed.
Results
Only 2 polymorphisms (C677T and A1298C in methylenetetrahydrofolate reductase, MTHFR, total: 8 studies) relevant to methotrexate metabolism and efficacy had sufficient data to perform a meta-analysis of their association with toxicity; there was no polymorphism with sufficient data to perform a meta-analysis of efficacy. In a fixed effects model, the C677T polymorphism was associated with increased toxicity (OR 1.71, CI 1.32 – 2.21, p<0.001). The A1298C polymorphism was not associated with increased toxicity (OR 1.12, CI 0.79 – 1.6, p=0.626).
Conclusions
As pharmacogenetics evolves, more data are needed to assess the role of various polymorphisms for drug efficacy and toxicity. These results illustrate the paucity of reliable pharmacogenetic data on a commonly used anti-rheumatic drug and the potential role of pharmacogenetics in tailoring drug therapy for an individual patient.
Key Terms: Methotrexate, Polymorphism, Toxicity
Introduction
Rheumatoid Arthritis (RA) is among the best studied chronic inflammatory diseases. One of the most effective therapies for RA, and the ‘anchor’ of many therapeutic regimens, is Methotrexate (MTX). Reports of the use of aminopterin (MTX) to treat RA date back to 1951, and the initial clinical trials showing the efficacy of MTX date back to the mid 1980s1–5. While highly effective, it is also associated with toxicity, including worsening of nodulosis, pneumonitis, neurologic toxicity, gastrointestinal complications including nausea, vomiting and diarrhea, transaminitis, hematologic abnormalities, rash, stomatitis, and alopecia6–14.
MTX is a structural analogue of folic acid15. It enters cells via solute carrier family 19 member 1 (SLC19A1), the reduced folate carrier, and then needs to be activated by a gamma-glutamyl hydrolase (GGH) to a polyglutamated form. This blocks the enzyme dihydrofolate reductase (DHFR), inhibiting purine metabolism, as well as impairing protein synthesis by blocking the conversion of other amino acids. In addition, the polyglutamated MTX can also interfere with thymidylate synthetase (TYMS) and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) transformylase (also called ATIC). Lastly, the polyglutamated MTX can interfere with methylenetetrahydrofolate reductase (MTHFR), causing elevated homocysteine levels and toxicity15.
The sequencing of the human genome, and the understanding of the potential function of single nucleotide polymorphisms (SNPs) of appropriate genes, have provided a vast amount of data to study associations between toxicity or efficacy of different medications, a burgeoning field known as pharmacogenetics. As the technology to study SNPs has advanced, reducing costs, more studies of SNPs related to specific medications have been published. However, many of these studies are small and of limited utility.
The results of pharmacogenetic studies are often both conflicting and difficult to understand. A 2002 study examining genetic associations found that of 166 putative associations that had been studied 3 or more time between a SNP and disease susceptibility, only 6 could be reproduced consistently16.
Because the results of small pharmacogenetic studies are often misleading we undertook a meta-analysis of published studies to determine the role of polymorphisms in MTHFR, an enzyme affected by methotrexate and its metabolites, in the therapeutic efficacy and toxicity of methotrexate.
Materials and Methods
We searched PUBMED using keywords Methotrexate, Arthritis, and either SNP (single nucleotide polymorphism) or polymorphism. Fifty five articles were identified, and each was individually reviewed for relevance to efficacy of treatment of rheumatoid arthritis or toxicity. Over 20 different polymorphisms were identified that impacted either efficacy or toxicity (Table 1). Because several of the efficacy trials had widely disparate definitions of efficacy, it was the opinion of the authors that an adequate meta-analysis could not be done on that literature. Only two SNPs were identified with three or more articles published with sufficient data on toxicity; MTHFR C677T and MTHFR A1298C (Table 2).
Table 1.
All Methotrexate Single Nucleotide Polymorphisms Studied in Rheumatoid Arthritis Efficacy and Toxicity
| Polymorphism | Number of articles | Efficacy, Toxicity, or Both |
|---|---|---|
| MTHFR C677T | van Ede, 200123 Urano 200222 Kumagi 200320 Berkun 200418 Wessels 200624 Kim 200619 *Dervieux 200626 Aggarwal 200617 Taniguchi 200721 *Kurzawski 200729 |
Toxicity Both Both Toxicity Both Toxicity Both Both Both Efficacy |
| MTHFR A1298C | Kumagi 200320 Berkun 200418 Wessels 200624 *Dervieux 200626 Taniguchi 200721 *Kurzawski 200729 |
Both Toxicity Both Both Both Efficacy |
| TYMS 3’UTR | Kumagi 200320 Takatori 200631 |
Both Both |
| TSER*2*3 |
*Dervieux 200427 *Dervieux 200626 |
Efficacy Both |
| RFC1 G80A |
*Dervieux 200427 *Wessels 200624 *Dervieux 200626 Takatori 200631 Drozdzik 200628 |
Efficacy Both Both Both Efficacy |
| ATIC C347G |
*Dervieux 200427 *Wessels 200624 *Dervieux 200626 Takatori 200631 |
Efficacy Both Both Both |
| ITPA C94A | Wessels 200624 | Both |
| MTXPGs | *Dervieux 200427 | Efficacy |
| DHFR –G473A | Wessels 200624 | Both |
| DHFR G35289A | Wessels 200624 | Both |
| HLA-G 14b | Rizzo 200630 | Efficacy |
| HLA DRB1 | Ali 200625 | Efficacy |
| HLADQB1 | Ali 200625 | Efficacy |
| MDR1 C3435T | Drodzik 200628 | Efficacy |
| AMPD1 C34T | Wessels 200624 | Both |
| MTR A2756G | Wessels 200624 | Both |
| MS A2756G | *Dervieux 200626 | Both |
| MTRR A66G | Wessels 200624 *Dervieux 200626 |
Both Both |
| GGH C401T | *Dervieux 200626 | Both |
| GGH C452T | van der Stratten 200732 | Both |
| GGH T16C | van der Stratten 200732 | Both |
| SHMT1 C1420T | Dervieux 200626 | Both |
| ABCB1 C3435T | Takatori 200631 | Both |
| FPGS A1994G | van der Stratten 200732 | Both |
| FPGS G114A | van der Stratten 200732 | Both |
MTHFR Methylenetetrahydrofolate Reductase, TYMS thymidylate synthase, TSER thymidilate synthase enhancer region, RFC1 Reduced Folate Carrier1, ATIC 5 aminoimidazole-4-carboxamide ribonucleotide transformylase, ITPA inosine triphosphate phosphatase, MTXPGs Methotrexate Polyglutamtes, DHFR dihydrofolate reductase, HLA Human Leukocyate Antigen, MDR1 multidrug resistance 1, AMPD1 adenosine monophosphate deaminase 1, MTR methionine synthase, MTRR methionine synthase reductase, GGH gamma glutamyl hydrolase, MS methionine synthase, SHMT1 serine hydroxymethyl transferase 1, ABCB1 ATP binding cassette transporter B1, FPGS folylpoly-gamma-glutamase synthetase
Insufficient data in article to permit inclusion in meta-analysis
Table 2.
Methotrexate Studies Included in Analysis
| C677T: | ||||||
|---|---|---|---|---|---|---|
| Authors | Year | CT or TT (#) | CC (#) | OR | CI | P value |
| Van Ede23 | 2001 | 114 | 122 | 2.383 | 1.063 – 5.341 | 0.035 |
| Urano22 | 2002 | 71 | 35 | 3.623 | 0.989 – 13.274 | 0.052 |
| Kumagai20 | 2003 | 69 | 46 | 0.626 | 0.295 – 1.328 | 0.222 |
| Berkun18 | 2004 | 48 | 45 | 1.200 | 0.512 – 2.813 | 0.675 |
| Kim19 | 2006 | 252 | 133 | 3.989 | 2.445 – 6.507 | 0.000 |
| Aggarwal17 | 2006 | 63 | 87 | 0.757 | 0.332 – 1.729 | 0.509 |
| Wessels24 | 2006 | 111 | 89 | 0.802 | 0.437 – 1.471 | 0.475 |
| Taniguchi21 | 2007 | 90 | 66 | 3.242 | 1.460 – 7.200 | 0.004 |
| FIXED | 1.708 | 1.321 – 2.207 | 0.000 | |||
| RANDOM | 1.603 | 0.897 – 2.864 | 0.111 | |||
| A1298C: | ||||||
|---|---|---|---|---|---|---|
| Authors | Year | AC or CC (#) | AA (#) | OR | CI | P value |
| Urano22 | 2002 | 32 | 74 | 0.908 | 0.317 – 2.602 | 0.857 |
| Kumagi20 | 2003 | 35 | 80 | 1.029 | 0.464 – 2.285 | 0.944 |
| Berkun18 | 2004 | 43 | 50 | 0.438 | 0.181 – 1.059 | 0.067 |
| Wessels24 | 2006 | 115 | 83 | 2.319 | 1.206 – 4.456 | 0.012 |
| Taniguchi21 | 2007 | 32 | 74 | 1.016 | 0.486 – 2.125 | 0.965 |
| FIXED | 0.826 | .0541 – 1.260 | 0.375 | |||
| RANDOM | 0.826 | .0541 – 1.260 | 0.375 | |||
Meta-analysis performed on those studies examining toxicity, using both random effects model and fixed effects models. There was insufficient data on populations to know if appropriate haplotype stratification had been done in each study to know which model was appropriate
All analyses were done using Comprehensive Meta Analysis Version 2.2.046.
Results
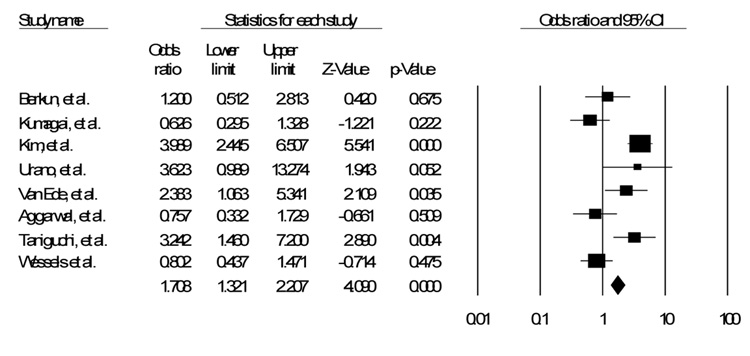
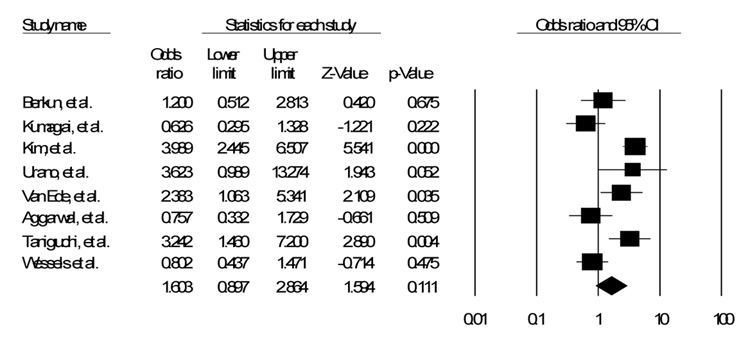
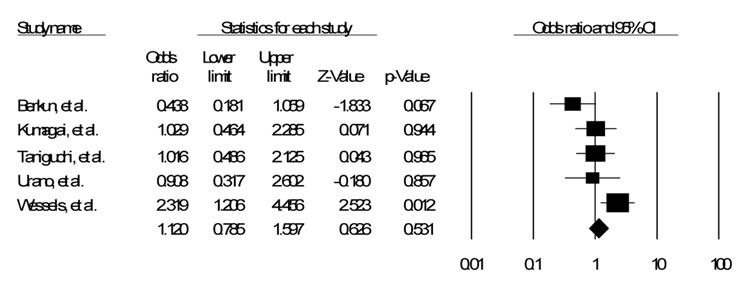
Of the fifty five studies identified in the literature, eight were identified that discussed the C677T polymorphism17–24. Of those eight, five also discussed the A1298C polymorphism18, 20–22, 24. Table 1 shows a list of all polymorphisms identified with studies documenting impact on efficacy, toxicity, or both17–33. Table 2 shows the details of the studies in this analysis. Figure 1, Figure 2 and Figure 3 show the funnel plot of the effects of the studies.
Figure 1.
SNP C677T Fixed Effects Model
Figure 2.
SNP C677T Random Effects Model
Of the eight studies that assessed the C677T polymorphism, either homozygous or heterozygous, only three showed a significant increase in toxicity with the use of MTX 19, 21, 23. Two others also showed an increase in toxicity, though not significant18, 22. The other three studies showed a possible decrease in toxicity, though again not approaching significance17, 20, 24. When assessed together, and weighting for the relative sizes of the different studies, assuming a fixed effects model, there is a significant, though small, increase in toxicity (OR 1.71, CI 1.32 – 2.21, p < 0.001). Assuming a random effects model, however, the confidence interval crosses the null hypothesis (OR 1.60, CI 0.90 – 2.86, p = 0.11).
Of the five studies that assessed the A1298C polymorphism, again either homozygous or heterozygous, only one showed a significant increase in toxicity24. Three of the remaining studies showed almost no impact at all20–22, and the fourth showed a possible decrease in toxicity18, approaching but not reaching significance in a fixed effects model (OR 1.12, CI 0.79 – 1.6, p = .53). A random effects model showed similar results (OR 1.04, CI 0.6 – 1.81, p = 0.88).
All studies used any toxicity as an endpoint. As such, a mild elevation in LFTs or stomatitis was treated the same as nausea or as LFT elevations greater than 3 times the upper limit of normal. In addition, almost all studies did not discriminate between whether patients had only one copy of the polymorphism or two copies of the polymorphism.
Discussion
The primary findings of this investigation are the increased OR of Methotrexate toxicity used to treat Rheumatoid Arthritis associated with the C677T polymorphism in a fixed effects model. There was no association between the A1298C polymorphism and toxicity.
This meta-analysis illustrates the paucity of data about the pharmacogenetics of one of the most commonly used DMARDs. The C677T and A1298C polymorphisms are just two of over a dozen polymorphism reported in the MTHFR gene; of those 12, only 7 have been associated with efficacy or toxicity in RA34. The C677T polymorphism was first described in the mid-1990s, leading to decreased activity of the MTHFR enzyme; the homozygous variant has about 30% of the function of the wild type35, 36. The heterozygous variant has about 60% of the function of the wild type. The A1298C polymorphism was first discovered in 1998; the homozygous variant has about 60% of the function wild type37, 38.
In attempting to draw a collective conclusion from the individual trials, it is important to comment on the strengths and weaknesses of each. The first article assessing the connection between the C677T polymorphism and toxicity, published by van Ede in 2001, focused on discontinuation due to toxicity or elevation of LFTs23. In addition, patients filled out a ‘standard toxicity questionnaire’ to assess other side effects. The primary purpose of this study was actually to assess the impact of folic acid and folinic acid supplementation on MTX efficacy and toxicity in RA patients, and was performed in a prospective manner, and this analysis only used a random subset of patients from that original study. This study is confounded somewhat by the variable use of folic acid supplementation among the RA patients – 1/3 of patients received placebo, 1/3 received daily folic acid, and 1/3 received folinic acid weekly. While this study’s strengths include a thorough statistical analysis, including defined patient numbers needed for adequate power, toxicity in this study was defined as discontinuation. Many patients suffer from side effects insufficient to warrant discontinuation, and most of the other studies did not discriminate between more minor and more significant toxicities in their analyses.
Urano and colleagues assessed the role of both the C677T and A1298C polymorphisms22. There is no discussion of numbers needed for adequate power of this cross-sectional analysis, and there is no description of how these patients were chosen from the outpatient clinics population in Tokyo. In addition, patients in this study did not receive doses of MTX higher than 12.5mg, markedly different from conventional therapy elsewhere. The authors also do not discriminate between transaminitis and less severe side effects, such as stomatitis or alopecia. The authors do note that no patients in their study had both the 677T and 1298C haplotype. Urano’s group published a second paper on MTX polymorphisms several years later, this time with Taniguchi as the lead auther21. The purpose of this study was to validate their previous work. The design was retrospective, with patients chosen randomly from their outpatient clinic population at the Institute of Rheumatology, Tokyo Women’s Medical University. This study also examined both polymorphisms. Again, there is no discussion of power. In addition, less than 1/3 of the patients in this study received folic acid supplementation, and greater than half of patients received 6mg or less of MTX. Toxicities and adverse events are not clearly defined in this study beyond a definition of transaminitis.
Kumagai et al., another group based in Japan, studied both polymorphisms. This was a prospective analysis, with the primary purpose of assessing the impact of several polymorphisms. The authors do not state where the patients were recruited from. They also do not discuss how many patients they needed for adequate power20. The authors also had a maximum dose of 12mg of MTX in this study. While toxicities are broken down by frequency, the authors use the aggregate of all adverse events, not discriminating between minor and more significant side effects. Unlike most of the other studies, this one does discriminate between heterozygous and homozygous genotypes and rate of adverse events.
Berkun and colleagues also studied both polymorphisms18. This is a prospective study, with 93 consecutive RA patients recruited from three different rheumatology outpatient clinics in Israel. As opposed to the previous studies, the definition of toxicity is more clearly described. However, the authors use a composite ‘side effects’ result, and do not discuss severe versus mild effects. Methotrexate doses are a little higher in this population, with an average dose just under 12mg weekly. In addition, patients in this analysis received an average dose of over 5mg of folate supplementation daily.
Aggarwal et al. analyzed only the C677T polymorphism. This is a retrospective study selecting patients randomly from an outpatient clinic in Lucknow, India. All patients in this study received folic acid supplementation, and MTX doses were similar to Berkun’s study. Toxicity was better defined in this study; the authors broke down rates of toxicity for specific genotypes as any, hematologic, hepatic, gastrointestinal, and pulmonary. Only one other study in this analysis provided similar data on toxicity.
Kim and colleagues also only studied the C677T polymorphism. Of the eight studies, this prospective study in Seoul, South Korea, was by far the largest. The mean MTX dose is similar to the previous two studies, 11.6mg weekly, and all patients received daily folic acid supplementation. Toxicities were well defined by the authors, and they note which patients required temporary versus permanent withdrawal. The authors also provide data on specific toxicities related to genotype.
Lastly, Wessels et al. assessed toxicity related to both C677T and A1298C polymorphisms. These patients were a subcohort of the BeSt trial. All patients received folic acid supplementation, but average MTX dose is not noted. Toxicity is well defined, and the authors present data on specific toxicities for each genotype, and the authors also discriminate between the heterozygous and homozygous genotype.
An additional variable that may have clinical impact is the time from initiation or titration of MTX dose to onset of adverse effects. This clearly would be an important component in assessing the risk of medication and for patient counseling. However, the data presented in the articles in this analysis did not include sufficient data to assess whether the presence or absence of the above SNPs impacted time to adverse event.
Another potential issue in studying pharmacogenetics is the impact of multiple SNPs on the efficacy or toxicity of a drug. While a single SNP may not have significance alone, the combination of several SNPs for a given protein may lead to significant changes in function that either increase or decrease toxicity or efficacy or both. To date, no study has been published assessing the presence and impact of both C677T and A1298C in patients with rheumatoid arthritis. Other studies have found a correlation between the presence of both SNPs and outcome, including increased frequency of neural tube defects (NTDs), and patients heterozygous for both SNPs have significantly decreased MTHFR activity, compared to patients with only one SNP, and the expected increase in homocysteine levels as well.37
In analyzing the data presented here it is unclear whether the fixed or random effects model is the most appropriate analytic model as the frequency of the respective SNPs in various populations has not been fully explored among all RA patients. In Caucasians and Asians, 12 to 15% of individuals are homozygous TT and as many as 50% are heterozygous for the C677T polymorphism 39, 40. The C677T polymorphism has a frequency of about 35% in North America36, 41, 42. For the A1298C polymorphism, the homozygous CC polymorphism among Caucasians, was present in 7–12% of the population, and the allelic frequency was about 33%37, 43, 44. Nonetheless it is likely that, regardless of penetrance of the polymorphism, the clinical impact that it has would be the same regardless of where the study was performed or the frequency of the polymorphism within each study population, so the fixed-effects model, which demonstrated a clear and significant association between the C677T polymorphism and methotrexate toxicity, may be more applicable. It is also notable that none of the studies in this analysis discuss the racial or ethnic background of their study participants. As the rate of the different SNPs may be different in different ethnic groups, this data would be useful to help further understand the impact of a given SNP, and the utility of studying different SNPs in different patient populations.
The strengths of this study include the size of the analysis, with over 1400 patients for the C677T analysis, and over 660 for the A1298C analysis. In addition, the relative merits of each study are discussed, with a focus on the differences in both the treatments and toxicity analyses of the different studies. This analysis has limitations as well. First, there is an inherent heterogeneity to meta-analysis, and there were differences in definition of toxicity, methotrexate dose, and folic acid supplementation among the different studies examined. Second, not all studies discriminated between the heterozygous and homozygous genotype. Because of this, the meta-analysis was performed combining all patients who deviated from the wild type, allowing all studies to be compared in the meta-analysis.
In conclusion, as pharmacogenetics evolves more and larger studies are needed to assess the role of various polymorphisms for drug efficacy and toxicity. However, until larger studies are carried out meta-analysis of pooled data is the best available tool to validate genetic associations with efficacy and toxicity. The results presented here illustrate both the paucity of reliable pharmacogenetic data on a very commonly used anti-rheumatic drug as well as the potential role that pharmacogenetics can play in tailoring drug therapy for an individual patient.
Figure 3.
SNP A1298C Fixed Effects Model
Acknowledgments
Grant Support - Mark C. Fisher: None
Bruce N. Cronstein: NIH (AA13336, AR41911 and GM56268), King Pharmaceuticals, the General Clinical Research Center (M01RR00096) and by the Kaplan Cancer Center of New York University School of Medicine
References
- 1.Gubner R, August S, Ginsberg V. Therapeutic suppression of tissue reactivity. II. Effect of aminopterin in rheumatoid arthritis and psoriasis. The American journal of the medical sciences. 1951;221:176–182. [PubMed] [Google Scholar]
- 2.Andersen PA, West SG, O'Dell JR, Via CS, Claypool RG, Kotzin BL. Weekly pulse methotrexate in rheumatoid arthritis. Clinical and immunologic effects in a randomized, double-blind study. Annals of internal medicine. 1985;103:489–496. doi: 10.7326/0003-4819-103-4-489. [DOI] [PubMed] [Google Scholar]
- 3.Thompson RN, Watts C, Edelman J, Esdaile J, Russell AS. A controlled two-centre trial of parenteral methotrexate therapy for refractory rheumatoid arthritis. The Journal of rheumatology. 1984;11:760–763. [PubMed] [Google Scholar]
- 4.Weinblatt ME, Coblyn JS, Fox DA, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. The New England journal of medicine. 1985;312:818–822. doi: 10.1056/NEJM198503283121303. [DOI] [PubMed] [Google Scholar]
- 5.Williams HJ, Willkens RF, Samuelson CO, Jr, et al. Comparison of low-dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis. A controlled clinical trial. Arthritis and rheumatism. 1985;28:721–730. doi: 10.1002/art.1780280702. [DOI] [PubMed] [Google Scholar]
- 6.Strand V, Cohen S, Schiff M, et al. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Archives of internal medicine. 1999;159:2542–2550. doi: 10.1001/archinte.159.21.2542. [DOI] [PubMed] [Google Scholar]
- 7.Weinblatt ME. Toxicity of low dose methotrexate in rheumatoid arthritis. J Rheumatol Suppl. 1985;12 Suppl 12:35–39. [PubMed] [Google Scholar]
- 8.West SG. Methotrexate hepatotoxicity. Rheumatic diseases clinics of North America. 1997;23:883–915. doi: 10.1016/s0889-857x(05)70365-3. [DOI] [PubMed] [Google Scholar]
- 9.Wernick R, Smith DL. Central nervous system toxicity associated with weekly low-dose methotrexate treatment. Arthritis and rheumatism. 1989;32:770–775. doi: 10.1002/anr.1780320616. [DOI] [PubMed] [Google Scholar]
- 10.McKendry RJ, Dale P. Adverse effects of low dose methotrexate therapy in rheumatoid arthritis. The Journal of rheumatology. 1993;20:1850–1856. [PubMed] [Google Scholar]
- 11.Kerstens PJ, Boerbooms AM, Jeurissen ME, Fast JH, Assmann KJ, van de Putte LB. Accelerated nodulosis during low dose methotrexate therapy for rheumatoid arthritis. An analysis of ten cases. The Journal of rheumatology. 1992;19:867–871. [PubMed] [Google Scholar]
- 12.Halla JT, Hardin JG. Underrecognized postdosing reactions to methotrexate in patients with rheumatoid arthritis. The Journal of rheumatology. 1994;21:1224–1226. [PubMed] [Google Scholar]
- 13.Carson CW, Cannon GW, Egger MJ, Ward JR, Clegg DO. Pulmonary disease during the treatment of rheumatoid arthritis with low dose pulse methotrexate. Seminars in arthritis and rheumatism. 1987;16:186–195. doi: 10.1016/0049-0172(87)90021-7. [DOI] [PubMed] [Google Scholar]
- 14.Buchbinder R, Hall S, Sambrook PN, et al. Methotrexate therapy in rheumatoid arthritis: a life table review of 587 patients treated in community practice. The Journal of rheumatology. 1993;20:639–644. [PubMed] [Google Scholar]
- 15.Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bulletin of the NYU hospital for joint diseases. 2007;65:168–173. [PubMed] [Google Scholar]
- 16.Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med. 2002;4:45–61. doi: 10.1097/00125817-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal P, Naik S, Mishra KP, Aggarwal A, Misra R. Correlation between methotrexate efficacy & toxicity with C677T polymorphism of the methylenetetrahydrofolate gene in rheumatoid arthritis patients on folate supplementation. The Indian journal of medical research. 2006;124:521–526. [PubMed] [Google Scholar]
- 18.Berkun Y, Levartovsky D, Rubinow A, et al. Methotrexate related adverse effects in patients with rheumatoid arthritis are associated with the A1298C polymorphism of the MTHFR gene. Annals of the rheumatic diseases. 2004;63:1227–1231. doi: 10.1136/ard.2003.016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SK, Jun JB, El-Sohemy A, Bae SC. Cost-effectiveness analysis of MTHFR polymorphism screening by polymerase chain reaction in Korean patients with rheumatoid arthritis receiving methotrexate. The Journal of rheumatology. 2006;33:1266–1274. [PubMed] [Google Scholar]
- 20.Kumagai K, Hiyama K, Oyama T, Maeda H, Kohno N. Polymorphisms in the thymidylate synthase and methylenetetrahydrofolate reductase genes and sensitivity to the low-dose methotrexate therapy in patients with rheumatoid arthritis. International journal of molecular medicine. 2003;11:593–600. [PubMed] [Google Scholar]
- 21.Taniguchi A, Urano W, Tanaka E, et al. Validation of the associations between single nucleotide polymorphisms or haplotypes and responses to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a proposal for prospective pharmacogenomic study in clinical practice. Pharmacogenetics and genomics. 2007;17:383–390. doi: 10.1097/01.fpc.0000236326.80809.b1. [DOI] [PubMed] [Google Scholar]
- 22.Urano W, Taniguchi A, Yamanaka H, et al. Polymorphisms in the methylenetetrahydrofolate reductase gene were associated with both the efficacy and the toxicity of methotrexate used for the treatment of rheumatoid arthritis, as evidenced by single locus and haplotype analyses. Pharmacogenetics. 2002;12:183–190. doi: 10.1097/00008571-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 23.van Ede AE, Laan RF, Blom HJ, et al. The C677T mutation in the methylenetetrahydrofolate reductase gene: a genetic risk factor for methotrexate-related elevation of liver enzymes in rheumatoid arthritis patients. Arthritis and rheumatism. 2001;44:2525–2530. doi: 10.1002/1529-0131(200111)44:11<2525::aid-art432>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Wessels JA, de Vries-Bouwstra JK, Heijmans BT, et al. Efficacy and toxicity of methotrexate in early rheumatoid arthritis are associated with single-nucleotide polymorphisms in genes coding for folate pathway enzymes. Arthritis and rheumatism. 2006;54:1087–1095. doi: 10.1002/art.21726. [DOI] [PubMed] [Google Scholar]
- 25.Ali AA, Moatter T, Baig JA, Iqbal A, Hussain A, Iqbal MP. Polymorphism of HLA-DR and HLA-DQ in rheumatoid arthritis patients and clinical response to methotrexate--a hospital-based study. J Pak Med Assoc. 2006;56:452–456. [PubMed] [Google Scholar]
- 26.Dervieux T, Greenstein N, Kremer J. Pharmacogenomic and metabolic biomarkers in the folate pathway and their association with methotrexate effects during dosage escalation in rheumatoid arthritis. Arthritis and rheumatism. 2006;54:3095–3103. doi: 10.1002/art.22129. [DOI] [PubMed] [Google Scholar]
- 27.Dervieux T, Kremer J, Lein DO, et al. Contribution of common polymorphisms in reduced folate carrier and gamma-glutamylhydrolase to methotrexate polyglutamate levels in patients with rheumatoid arthritis. Pharmacogenetics. 2004;14:733–739. doi: 10.1097/00008571-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Drozdzik M, Rudas T, Pawlik A, et al. The effect of 3435C>T MDR1 gene polymorphism on rheumatoid arthritis treatment with disease-modifying antirheumatic drugs. Eur J Clin Pharmacol. 2006;62:933–937. doi: 10.1007/s00228-006-0192-1. [DOI] [PubMed] [Google Scholar]
- 29.Kurzawski M, Pawlik A, Safranow K, Herczynska M, Drozdzik M. 677C>T and 1298A>C MTHFR polymorphisms affect methotrexate treatment outcome in rheumatoid arthritis. Pharmacogenomics. 2007;8:1551–1559. doi: 10.2217/14622416.8.11.1551. [DOI] [PubMed] [Google Scholar]
- 30.Rizzo R, Rubini M, Govoni M, et al. HLA-G 14-bp polymorphism regulates the methotrexate response in rheumatoid arthritis. Pharmacogenetics and genomics. 2006;16:615–623. doi: 10.1097/01.fpc.0000230115.41828.3a. [DOI] [PubMed] [Google Scholar]
- 31.Takatori R, Takahashi KA, Tokunaga D, et al. ABCB1 C3435T polymorphism influences methotrexate sensitivity in rheumatoid arthritis patients. Clin Exp Rheumatol. 2006;24:546–554. [PubMed] [Google Scholar]
- 32.van der Straaten RJ, Wessels JA, de Vries-Bouwstra JK, et al. Exploratory analysis of four polymorphisms in human GGH and FPGS genes and their effect in methotrexate-treated rheumatoid arthritis patients. Pharmacogenomics. 2007;8:141–150. doi: 10.2217/14622416.8.2.141. [DOI] [PubMed] [Google Scholar]
- 33.Wessels JA, Kooloos WM, De Jonge R, et al. Relationship between genetic variants in the adenosine pathway and outcome of methotrexate treatment in patients with recent-onset rheumatoid arthritis. Arthritis and rheumatism. 2006;54:2830–2839. doi: 10.1002/art.22032. [DOI] [PubMed] [Google Scholar]
- 34.Rozen R. Molecular genetics of methylenetetrahydrofolate reductase deficiency. Journal of inherited metabolic disease. 1996;19:589–594. doi: 10.1007/BF01799831. [DOI] [PubMed] [Google Scholar]
- 35.Kang SS, Zhou J, Wong PW, Kowalisyn J, Strokosch G. Intermediate homocysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. American journal of human genetics. 1988;43:414–421. [PMC free article] [PubMed] [Google Scholar]
- 36.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nature genetics. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 37.van der Put NM, Gabreels F, Stevens EM, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? American journal of human genetics. 1998;62:1044–1051. doi: 10.1086/301825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ranganathan P, Eisen S, Yokoyama WM, McLeod HL. Will pharmacogenetics allow better prediction of methotrexate toxicity and efficacy in patients with rheumatoid arthritis? Annals of the rheumatic diseases. 2003;62:4–9. doi: 10.1136/ard.62.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey LB. Folate, methyl-related nutrients, alcohol, and the MTHFR 677C-->T polymorphism affect cancer risk: intake recommendations. The Journal of nutrition. 2003;133:3748S–3753S. doi: 10.1093/jn/133.11.3748S. [DOI] [PubMed] [Google Scholar]
- 40.Ueland PM, Hustad S, Schneede J, Refsum H, Vollset SE. Biological and clinical implications of the MTHFR C677T polymorphism. Trends in pharmacological sciences. 2001;22:195–201. doi: 10.1016/s0165-6147(00)01675-8. [DOI] [PubMed] [Google Scholar]
- 41.Jacques PF, Bostom AG, Williams RR, et al. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93:7–9. doi: 10.1161/01.cir.93.1.7. [DOI] [PubMed] [Google Scholar]
- 42.Ma J, Stampfer MJ, Christensen B, et al. A polymorphism of the methionine synthase gene: association with plasma folate, vitamin B12, homocyst(e)ine, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 1999;8:825–829. [PubMed] [Google Scholar]
- 43.Robien K, Ulrich CM. 5,10-Methylenetetrahydrofolate reductase polymorphisms and leukemia risk: a HuGE minireview. American journal of epidemiology. 2003;157:571–582. doi: 10.1093/aje/kwg024. [DOI] [PubMed] [Google Scholar]
- 44.Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Molecular genetics and metabolism. 1998;64:169–172. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]