Abstract
There is limited information about the true incidence of acute renal failure (ARF). Most studies could not quantify disease frequency in the general population as they are hospital-based and confounded by variations in threshold and the rate of hospitalization. Earlier studies relied on diagnostic codes to identify non-dialysis requiring ARF. These underestimated disease incidence since the codes have low sensitivity. Here we quantified the incidence of non-dialysis and dialysis-requiring ARF among members of a large integrated health care delivery system –Kaiser Permanente of Northern California. Non-dialysis requiring ARF was identified using changes in inpatient serum creatinine values. Between 1996 and 2003, the incidence of non-dialysis requiring ARF increased from 322.7 to 522.4 whereas that of dialysis-requiring ARF increased from 19.5 to 29.5 per 100 000 person-years. ARF was more common in men and among the elderly, although those aged 80 years or more were less likely to receive acute dialysis treatment. We conclude that the use of serum creatinine measurements to identify cases of non-dialysis requiring ARF resulted in much higher estimates of disease incidence compared with previous studies. Both dialysis-requiring and non-dialysis requiring ARFs are becoming more common. Our data underscore the public health importance of ARF.
Keywords: acute renal failure, dialysis, epidemiology, acute kidney injury, acute dialysis, disease incidence
Acute renal failure (ARF; also known as ‘acute kidney injury’) is among the most important complications observed in hospitalized patients. When severe enough to require dialysis, ARF is associated with a high rate of in-hospital morbidity, mortality, and prolonged length of stay.1 However, few epidemiological studies have defined the incidence of ARF in the community. A recent American Society of Nephrology Renal Research Report identified as one of the ‘critically important gaps in knowledge’ about ARF the fact that ‘incidence and prevalence of diseases are unknown.’2
We addressed this by determining the community-based incidence of non-dialysis requiring and dialysis-requiring ARF in a large, diverse cohort of patients receiving usual medical care.
RESULTS
Between 1 January 1996 and 31 December 2003, a total of 3 787 410 members contributed 15 953 549 person-years of observation. The average age of this cohort was 42.1±15.8 years and 50.6% were women.
Incidence of non-dialysis requiring ARF
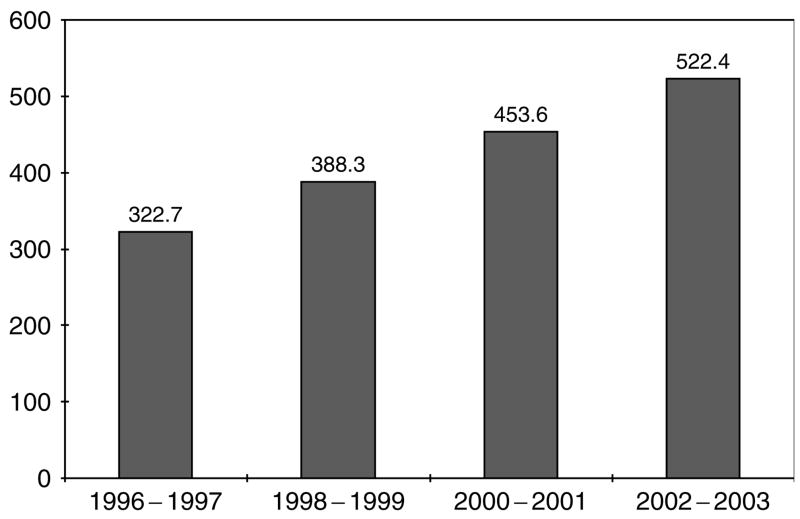
Overall, 61 280 subjects suffered non-dialysis requiring ARF, yielding a disease incidence of 384.1 per 100 000 person-years (95% confidence interval (CI) 381.1–387.2). The incidence of non-dialysis requiring ARF increased over time (Figure 1), and it was consistently higher in men than women and with advancing age (Table 1).
Figure 1.
Community-based incidence rates (per 100 000 person-years) of non-dialysis requiring ARF by calendar year.
Table 1.
Community-based incidence rates (per 100 000 person-years) of non-dialysis requiring ARF (further stratified by gender and age)
| Overall rate (95% CI) | By gender | By age group | |
|---|---|---|---|
| 1996–2003 | 384.1 (381.1–387.2) | Male: 443.1 (438.4–447.9) Female: 330.4 (326.5–334.3) |
Age<50: 78.0 (76.3–79.7) Age 50–59: 320.0 (313.2–326.9) Age 60–69: 814.8 (801.3–828.3) Age 70–79: 1809.1 (1783.5–1834.7) Age⩾80: 3545.4 (3481.4–3609.5) |
| 1996–1997 | 322.7 (316.7–328.5) | Male: 383.8 (379.4–397.9) Female: 267.2 (259.9–274.5) |
Age<50: 64.7 (61.4–68.0) Age 50–59: 224.5 (212.6–236.3) Age 60–69: 597.8 (574.4–621.3) Age 70–79: 1362.1 (1318.3–1405.9) Age⩾80: 2867.5 (2760.9–2974.2) |
| 1998–1999 | 388.3 (382.1–394.5) | Male: 451.8 (442.2–461.4) Female: 330.5 (322.6–338.3) |
Age<50: 72.9 (69.5–76.2) Age 50–59: 303.8 (290.4–317.3) Age 60–69: 796.6 (770.0–823.3) Age 70–79: 1813.2 (1762.2–1864.2) Age⩾80: 3796.3 (3665.5–3927.1) |
| 2000–2001 | 453.6 (447.1–460.1) | Male: 520.5 (510.4–530.6) Female: 392.1 (383.8–400.5) |
Age<50: 90.7 (87.1–94.3) Age 50–59: 393.7 (378.6–408.9) Age 60–69: 985.9 (956.4–1015.4) Age 70–79: 2220.7 (2164.1–2277.4) Age⩾80: 4388.0 (4293.9–4590.4) |
| 2002–2003 | 522.4 (515.5–529.3) | Male: 588.9 (578.3–599.4) Female: 462.3 (453.3–471.2) |
Age<50: 106.4 (102.6–110.2) Age 50–59: 483.8 (467.2–500.3) Age 60–69: 1238.2 (1205.2–1271.3) Age 70–79: 2741.3 (2677.1–2805.4) Age⩾80: 4884.3 (4722.8–5045.7) |
ARF, acute renal failure; CI, confidence interval.
Bold indicates data for overall time period (1996–2003).
Incidence of dialysis-requiring ARF
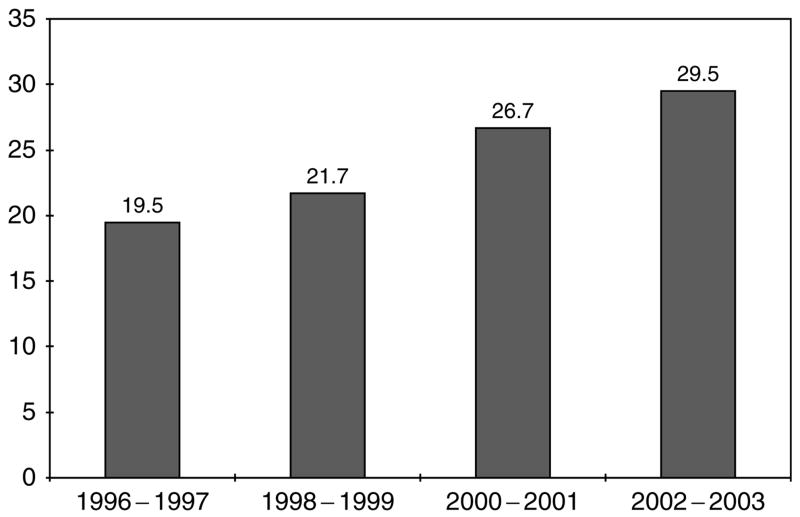
Over the same period, 3885 subjects suffered dialysis-requiring ARF, yielding a disease incidence of 24.4 per 100 000 person-years (95% CI 23.6–25.1). The incidence of dialysis-requiring ARF also increased over time (Figure 2). It was also consistently higher in men than women and with advancing age, although there was a drop off in incidence among those persons aged ⩾80 years (Table 2).
Figure 2.
Community-based incidence rates (per 100 000 person-years) of dialysis-requiring ARF by calendar year.
Table 2.
Community-based incidence rates (per 100 000 person-years) of dialysis-requiring ARF (further stratified by gender and age)
| Overall rate (95% CI) | By gender | By age group | |
|---|---|---|---|
| 1996–2003 | 24.4 (23.6–25.1) | Male: 29.2 (28.0–30.4) Female: 20.0 (19.0–20.1) |
Age<50: 8.3 (7.7–8.8) Age 50–59: 27.8 (25.8–29.9) Age 60–69: 61.1 (57.4–64.8) Age 70–79: 95.6 (90.0–101.5) Age⩾80: 75.4 (66.0–84.7) |
| 1996–1997 | 19.5 (18.1–21.0) | Male: 23.2 (21.0–25.5) Female: 16.2 (14.4–18.0) |
Age<50: 6.4 (5.4–7.4) Age 50–59: 19.6 (16.1–23.1) Age 60–69: 44.6 (38.2–52.0) Age 70–79: 73.7 (63.5–83.9) Age⩾80: 65.1 (49.0–81.1) |
| 1998–1999 | 21.7 (20.3–23.2) | Male: 26.2 (23.9–28.5) Female: 17.8 (16.0–20.0) |
Age<50: 7.1 (6.1–8.1) Age 50–59: 23.1 (19.4–26.8) Age 60–69: 51.6 (44.7–58.4) Age 70–79: 89.2 (77.9–100.5) Age⩾80: 83.2 (63.9–102.6) |
| 2000–2001 | 26.7 (25.1–28.3) | Male: 31.5 (29.0–34.0) Female: 22.3 (20.3–24.3) |
Age<50: 9.2 (8.1–10.3) Age 50–59: 32.1 (27.8–36.4) Age 60–69: 68.5 (60.8–76.3) Age 70–79: 100.5 (88.5–112.6) Age⩾80: 95.3 (73.6–117.1) |
| 2002–2003 | 29.5 (27.9–31.1) | Male: 35.6 (33.0–38.2) Female: 24.0 (22.0–26.0) |
Age<50: 10.3 (9.1–11.5) Age 50–59: 36.8 (32.3–41.3) Age 60–69: 81.5 (73.0–89.9) Age 70–79: 123.2 (109.6–136.8) Age⩾80: 62.5 (44.2–80.75) |
ARF, acute renal failure; CI, confidence interval.
Bold indicates data for overall time period (1996–2003).
Results of chart validation
A review of medical records among a random sample of 100 subjects classified as having dialysis-requiring ARF showed that 94 patients did receive dialysis for acutely worsening kidney function. The remaining six cases were either patients receiving maintenance dialysis before hospital admission or patients who did not receive dialysis.
DISCUSSION
Although ARF is one of the most common medical problems encountered among hospitalized patients, much remains unknown about its epidemiology, especially on a population level. As stated in a recent review, such a basic epidemiologic attribute as incidence of disease has remained ‘enigmatic and debated.’3
A major reason for this gap in knowledge is that almost all earlier studies of ARF have been based on hospitalized patients or on the subgroup of patients in the intensive care unit (ICU).4–8 Some studies only counted in the numerator cases of ARF in the ICU that required renal replacement therapy,6 which gives an incomplete picture of the actual incidence of ARF. Others studies used as the denominator hospitalization or ICU admission,4–8 which are suboptimal as rates of hospitalization (or ICU admission) per population are not defined – and these vary in different countries and across time. For example, Hou et al.4 found among patients from Tufts-New England Medical Center in Boston from 1978 to 1979 that the incidence of ARF was 4.9% per hospitalization. Applying the identical criteria to patients admitted to Rush Presbyterian-St Luke’s Medical Center, Chicago in 1996, the same investigative team found that the incidence of ARF was 7.2% per hospitalization.5 However, it not possible to determine how much of this change is influenced by variation in threshold for hospital admission compared with underlying changes in the incidence of ARF. In contrast, in this study, we are able to directly calculate disease incidence relative to the source population.
Another strength of this study is that non-dialysis requiring ARF was defined based on changes in serum creatinine values. This is an advance over other epidemiologic studies which defined non-dialysis requiring ARF using only claims data.7,9 Administrative data lack sensitivity in identifying cases of non-dialysis requiring ARF, as it requires both the recognition and coding of ARF. For example, using the Hou et al. criteria for ARF as the ‘gold standard,’ Waikar et al.10 determined that International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for ARF had a sensitivity of only 28.3%. In an independent sample, Liangos et al.11 concluded that sensitivity was only 19.2%. Hence, estimates of the incidence of non-dialysis requiring ARF from studies that rely on claims data7,9,11 will substantially underestimate the true incidence of ARF. This is possibly an important reason that the observed incidence in our study is higher than previously reported: 522.4 per 100 000 person-years observed here in the calendar year 2002–2003 vs 288 per 100 000 person-years reported by Waikar et al.9 during the same time period.
In addition to problems of underascertainment of disease, claims data may be subject to bias from ‘code creep.’ Some commentators have raised the concern that observed secular trends in disease incidence are confounded by higher likelihood over time of receiving a code for the same condition or up-coding by using a more severe diagnostic code to enhance financial reimbursement.12 In fact, ‘code creep’ has been documented for ARF diagnostic codes.9 Defining disease using a change in serum creatinine should lead to greater confidence that the observed increase in the community-based incidence of non-dialysis requiring ARF (Figure 1 and Table 1) is real and not an artifact of coding bias.
Reliable quantification of the community-based incidence of non-dialysis requiring ARF is needed to provide an accurate estimate of the population attributable risk of non-dialysis requiring ARF on the development of adverse clinical outcomes, including mortality. Recently published studies suggest that even relatively small changes in serum creatinine during a hospital admission are associated with a dramatic impact of short-term mortality risk.13,14 For example, Chertow et al.13 showed that an increase in serum creatinine of ⩾0.5 mg/dl was independently associated with a 6.5-fold increase in the odds of death, a 3.5-day increase in length of stay, and nearly $7500 in excess hospital costs even after adjusting for age, sex, and measures of comorbidity. Although historically more attention has been focused on dialysis-requiring ARF, our data indicate that the incidence of non-dialysis requiring ARF is likely to be 10 to 20-fold higher, which highlights its overall importance to individuals and to the public health.
It should also be noted in this context that studying non-dialysis requiring ARF rather than focusing solely on dialysis-requiring ARF paints a more accurate picture of disease distribution. The diagnosis of dialysis-requiring ARF can only be made in cases when physicians ordered and patients accepted dialysis. The juxtaposition of the incidence of non-dialysis requiring ARF by age – which increases monotonically with older age (Table 1) – and the incidence of dialysis-requiring ARF – which drops off above 80 years (Table 2) – suggest strongly that elderly persons are not protected from ARF but rather, are far less likely to be prescribed, or to accept, acute dialysis.
We observed that men have a higher incidence of non-dialysis requiring ARF than women. One may argue that the Hou et al. criteria are sex-biased in so far as men, who generate more creatinine on average than women, are more likely to reach a certain serum creatinine threshold given an equivalent decrement in glomerular filtration rate (GFR). However, differences in creatinine generation appear unlikely to explain the observation that men also have a higher incidence of dialysis-requiring ARF. Although numerous studies have investigated sex differences in progression of chronic kidney disease,15,16 few have investigated sex differences in risk of ARF. Further studies are needed to confirm this finding and to determine the underlying explanations.
Our observed community-based incidence of dialysis-requiring ARF – 29.5 per 100 000 person-years by calendar year 2002–2003 – is considerably higher than what has been noted in the older literature,17 but similar to more recent reports.9,18,19 For example, Liano and Pascual17 found in a 9-month study conducted in 1991–1992 in Madrid, Spain that the community incidence of dialysis requiring ARF was 7.5 per 100 000 person-years. In contrast, an 11-week Scottish study conducted in 2000 by Metcalfe et al.18 determined that the incidence of dialysis-requiring ARF was 20 per 100 000 person-years. US-based studies have also shown a steady rise in the incidence of ARF over time. For example, data from the Nationwide Inpatient Sample showed that the incidence of dialysis-requiring ARF had increased from 4 to 27 per 100 000 person-years from 1988 to 2002.9 It is possible that there are more cases of dialysis-requiring ARF because the threshold for dialysis has become lower over time. This would inflate the number of observed cases of dialysis-requiring ARF but should not affect the incidence of non-dialysis requiring ARF. Since the increase in incidence of dialysis-requiring ARF does not appear greater than the increase in incidence of non-dialysis requiring ARF (Figures 1 and 2), we believe that the increase in ARF incidence is not artifactual. The increase in ARF incidence may be a result of increase in disease severity among hospitalized patients, improvements in intensive care or expanded use of procedures, such as percutaneous coronary interventions which predispose to ARF.7,9,19 Again, both the incidence of non-dialysis requiring ARF and the incidence of dialysis-requiring ARF are increasing. This is another finding that has clinical, public health, and policy implications.
There are several limitations to the study. First, the selected definition of non-dialysis requiring ARF, although widely adopted, might be considered arbitrary. We chose to use the Hou et al. definition principally to allow direct comparison with earlier published studies. Second, we may have missed cases of dialysis-requiring ARF, as it was not feasible to review medical records of all cases. However, recent data show that administrative codes for dialysis-requiring ARF have a sensitivity of over 90%.10 Missing cases would, if anything, underestimate disease incidence and the public health importance of dialysis-requiring ARF. The high-positive predictive value for using administrative codes to identify dialysis-requiring ARF shown by our 100 random chart audit is consistent with earlier literature.10 Third, we did not distinguish among different etiologies of ARF. The so-called ‘pre- and post-renal’ causes are more likely to be reversible and less likely to require dialysis than cases of ‘intrinsic’ ARF such as acute tubular necrosis.1 Fourth, as we only used inpatient serum creatinine values to define ARF, we may have missed cases of ARF that occurred in the outpatient setting and did not require hospitalization. However, these are likely to be relatively uncommon and are less important from a clinical and public health point of view as they are not associated with the high morbidity and mortality seen with inpatient episodes of ARF. Finally, as our study was conducted among insured members of a Northern California integrated healthcare delivery system, our results may not be generalizable to all other populations or health care settings. In particular, patients without any form of health insurance are not represented. However, our observed incidence of dialysis-requiring ARF (29.5 per 100 000 person-years in 2002–2003) is very similar to that reported from a nationally representative survey (27 per 100 000 person-years in 2002),9 which argues for greater generalizability.
In summary, we believe our study fills an important knowledge gap by providing estimates of the incidence of non-dialysis requiring and dialysis-requiring ARF among a very large and diverse cohort of patients receiving usual medical care. Using serum creatinine measurements to identify cases of non-dialysis requiring ARF resulted in higher – and we believe much more accurate – estimates of disease incidence compared with earlier reports.
MATERIALS AND METHODS
To determine the community-based incidence of ARF, our source population included all adult members (⩾20 years old) enrolled in Kaiser Permanente of Northern California (Kaiser), a large integrated healthcare delivery system currently insuring more than one third of the San Francisco Bay Area adult population. Its population is representative of local surrounding and statewide populations with only slightly lower-than population percentages at the extremes of the socioeconomic spectrum and age.20
Kaiser delivers comprehensive inpatient and outpatient care to its members and captures many aspects of its care through the use of its comprehensive clinical and administrative databases. Fewer than 10% of hospitalizations in Kaiser members occur at a non-Kaiser facility, and the majority of those patients are repatriated to a Kaiser hospital early in their clinical course to complete their hospitalization, which allows capture of relevant diagnoses and the majority of procedures in this small subgroup of patients.
To calculate the incidence of ARF, the number of persons with one or more episodes of ARF was divided by the number of persons at risk. The number at risk was ascertained using annual health plan membership information during the period from 1 January 1996 to 31 December 2003.
We cross-linked our study population with the nationally comprehensive US Renal Data System (USRDS). At the time of cross-linkage, USRDS data were complete through 31 December 2003. Supplementary determination of ESRD status was performed using data from an internal Kaiser ESRD Registry.21 Patients with known ESRD before hospitalization were excluded from our analysis.
Cases of non-dialysis requiring ARF were identified using inpatient serum creatinine values. We used the Hou et al. criteria, which defined ARF as an increase in serum creatinine level of 0.5 mg/dl for patients with a baseline serum creatinine level of 1.9 mg/dl or less, 1.0 mg/dl for patients with a baseline level of 2.0–4.9 mg/dl, and 1.5 mg/dl for patients with a baseline level greater than or equal to 5.0 mg/dl. The lowest serum creatinine level during the hospitalization was used as the ‘baseline value,’ and renal insufficiency was defined relative to that.4,5 Patients who met these criteria but also received dialysis using that hospitalization were not counted (see below).
Cases of dialysis-requiring ARF were identified among patients who were not on maintenance dialysis on admission but who received dialysis during that hospitalization. After a review of the literature and initial pilot testing, we used the International Classification of Disease, Ninth Edition (ICD-9) procedure codes 54.98, 39.95, and Current Procedural Terminology (CPT) codes 90 935, 90 937, 90 945, 90 947, and 90 999 to identify episodes of peritoneal dialysis and hemodialysis from health plan hospital discharge databases. This approach was validated via a chart audit of 100 randomly selected episodes meeting these criteria.
Incidence of disease was presented as case per 100 000 person-years. Confidence intervals were calculated using a large sample approximation for Poisson rates.22 Given earlier literature regarding possible differences in risk of ARF by age, sex, and calendar year,7,9 data are presented stratified by these variables.
The Institutional Review Boards of collaborating institutions approved the study. Waiver of informed consent was obtained because of the nature of the study.
Acknowledgments
We thank Niela Pomernacki, RD for her expert technical assistance on the study. We thank Mr Shu-Cheng Chen of the United States Renal Data System for his assistance. Some of the data reported here were supplied by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the United States government. None of the authors declare a conflict of interest. This work is supported by a grant (R01 DK67126) from the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 2.American Society of Nephrology Renal Research Report. J Am Soc Nephrol. 2005;16:1886–1903. doi: 10.1681/ASN.2005030285. [DOI] [PubMed] [Google Scholar]
- 3.Van Biesen W, Vanholder R, Lameire N. Defining acute renal failure: RIFLE and beyond. Clin J Am Soc Nephrol. 2006;1:1314–1319. doi: 10.2215/CJN.02070606. [DOI] [PubMed] [Google Scholar]
- 4.Hou SH, Bushinsky DA, Wish JB, et al. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243–248. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- 5.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 6.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 7.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 8.Obialo CI, Okonofua EC, Tayade AS, et al. Epidemiology of de novo acute renal failure in hospitalized African Americans: comparing community-acquired vs hospital-acquired disease. Arch Intern Med. 2000;160:1309–1313. doi: 10.1001/archinte.160.9.1309. [DOI] [PubMed] [Google Scholar]
- 9.Waikar SS, Curhan GC, Wald R, et al. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 10.Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Codes for Acute Renal Failure. J Am Soc Nephrol. 2006;17:1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 11.Liangos O, Wald R, O’Bell JW, et al. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 12.Lameire N, Van Biesen W, Vanholder R. The rise of prevalence and the fall of mortality of patients with acute renal failure: what the analysis of two databases does and does not tell us. J Am Soc Nephrol. 2006;17:923–925. doi: 10.1681/ASN.2006020152. [DOI] [PubMed] [Google Scholar]
- 13.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 14.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 15.Coggins CH, Breyer Lewis J, Caggiula AW, et al. Differences between women and men with chronic renal disease. Nephrol Dial Transplant. 1998;13:1430–1437. doi: 10.1093/ndt/13.6.1430. [DOI] [PubMed] [Google Scholar]
- 16.Silbiger SR, Neugarten J. The role of gender in the progression of renal disease. Adv Ren Replace Ther. 2003;10:3–14. doi: 10.1053/jarr.2003.50001. [DOI] [PubMed] [Google Scholar]
- 17.Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Kidney Int. 1996;50:811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 18.Metcalfe W, Simpson M, Khan IH, et al. Acute renal failure requiring renal replacement therapy: incidence and outcome. QJM. 2002;95:579–583. doi: 10.1093/qjmed/95.9.579. [DOI] [PubMed] [Google Scholar]
- 19.Robertson S, Newbigging K, Isles CG, et al. High incidence of renal failure requiring short-term dialysis: a prospective observational study. QJM. 2002;95:585–590. doi: 10.1093/qjmed/95.9.585. [DOI] [PubMed] [Google Scholar]
- 20.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karter AJ, Ferrara A, Liu JY, et al. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 22.Fisher LD, van Belle G. Biostatistics: A Methodology for the Health Sciences. Wiley; New York: 1993. [Google Scholar]