Abstract
Purpose
Docetaxel (DTX), usually administered according to maximum tolerated dosing (MTD), can inhibit endothelial cell (EC) proliferation at low nM concentrations. DTX may exert antiangiogenic effects if dosed so plasma levels are maintained at low nM concentrations over a prolonged time. We evaluated metronomic and MTD-based dosing of DTX with and without vandetanib (VAN), a VEGFR-2 and EGFR tyrosine kinase inhibitor with antiangiogenic and anti-tumor activity, in a head and neck xenograft model.
Experimental Design
A murine physiologically-based pharmacokinetic model was modified to predict DTX distribution following intraperitoneal administration to design dosing regimens that target pre-specified plasma concentrations, for anti-endothelial effects (metronomic), or exposure, to mimic 30mg/m2 (weekly/MTD) DTX in humans. Animals were treated for 28 days with 1mg/kg/day (DTX1) or 6mg/kg q4d (DTX6) DTX with or without VAN (15 mg/kg/day p.o.) in mice bearing UMSCC2 tumor xenografts.
Results
The DTX1 dosing scheme was adjusted to treatment for 10 days followed by 9 days off due to severe GI toxicity. All treatment groups significantly reduced tumor volume, tumor proliferation (Ki-67) and tumor EC proliferation (Ki-67/vonWillebrand factor) compared with control. Addition of VAN to DTX treatment significantly enhanced tumor growth inhibition over single agent therapy.
Conclusions
A positive correlation of tumor EC proliferation with tumor growth rates demonstrates VAN and DTX antiangiogenic effects. Due to the morbidity observed with DTX1 treatment it is difficult to clearly ascertain if metronomic schedules will be effective for treatment. DTX with VAN is effective in treating UMSCC2 xenografts at concentrations relevant to exposures in humans.
Keywords: pharmacokinetic modeling, docetaxel, tyrosine kinase inhibitor, head and neck cancer, xenograft
INTRODUCTION
Angiogenesis is a process largely regulated by paracrine signaling through growth factors and their binding to receptor tyrosine kinases (RTKs). As tumors grow in size, cells up-regulate the expression of pro-angiogenic factors to recruit endothelial cells to form a vascular network that will provide oxygen and nutrients to the tumor (1,2). Vascular endothelial growth factor (VEGF), a key pro-angiogenic factor, plays a critical role in endothelial cell survival, proliferation, migration and capillary tube formation (3,4). The importance of VEGF signaling, through activation of vascular endothelial growth factor receptor-2 (VEGFR2), in pathogenic angiogenesis has been recognized and has led to the recent development of compounds designed to inhibit this and other receptor mediated signal transduction pathways important in tumor progression such as epidermal growth factor receptor (EGFR) signaling. EGFR and its ligands, epidermal growth factor (EGF) and transforming growth factor-α (TGF-α), are overexpressed in several different tumor types (5) and EGFR can be found on tumor-associated endothelial cells (6,7). Activation of EGFR leads to down stream signaling of pathways that are critical regulators of tumor growth, invasion, angiogenesis and metastasis (8,9).
Vandetanib (Zactima™/ZD6474, AstraZeneca), a 4-anilinoquinazoline, is an orally available synthetic small molecule designed to bind to the intracellular kinase domain of RTKs, preventing phosphorylation and disrupting the necessary signal transduction for cell proliferation (10,11). Vandetanib has demonstrated potent, selective activity against VEGFR2 and has activity against EGFR (10,11). The dual activity of vandetanib allows a single molecularly targeted agent to inhibit two key pathways in tumor growth by targeting the tumor vasculature and the tumor cell population. Preclinical models have demonstrated vandetanib’s utility as a single agent in numerous tumor models (12) as well as its ability to enhance the activity of cytotoxic chemotherapy (13) and radiation therapy (RT) (14,15).
A recent clinical study reported that the addition of vandetanib to docetaxel therapy in patients with stage III/IV non-small cell lung cancer (NSCLC) led to a significant increase in progression-free survival versus docetaxel alone (16). Docetaxel, a semi-synthetic taxane that exerts its effects by promoting microtubule assembly and inhibiting their depolymerization, is an important chemotherapeutic agent currently approved for use for the treatment of breast, prostate and NSCLC and has demonstrated activity against ovarian, bladder and head and neck cancers (17-21). Docetaxel use in head and neck squamous cell carcinoma (HNSCC) is often combined with RT and the dose and schedule of docetaxel is modified due to excessive non-hematologic (i.e., grade III/IV mucositis) toxicity (22,23). The incorporation of anti-EGFR therapies in HNSCC has been effective with added efficacy and no increase in toxicity when combined with RT (14,15,24,25). Vandetanib’s ability to enhance docetaxel effects (13,16), have positive interactions with RT (14,15), and the lack of increased toxicity with anti-EGFR/RT combinations gives potential for this agent to be beneficial in HNSCC. An important question to be addressed is the optimization of docetaxel schedules with vandetanib and whether metronomic schedules can be considered. Docetaxel has shown antiangiogenic activity in pre-clinical studies (26-28) and the potential for enhanced activity due to VEGFR2 inhibition by vandetanib warrants investigation of schedules optimized for anti-endothelial activity.
Docetaxel exhibits a complex pharmacologic profile with high interpatient variability in pharmacokinetics (PK) (29) making the use of PK models that can predict concentrations in vivo under various dosing schema attractive. Physiologically-based pharmacokinetic (PBPK) models can aid in the development of optimized clinical protocols that incorporate chemotherapeutics, molecularly targeted agents and combinations of the two. PBPK models utilize biochemical, physiologic and chemical engineering principles to mathematically describe blood and tissue distribution of compounds. One of the greatest strengths of PBPK models, compared to classical compartmental or non-compartmental PK modeling, is the ability to predict a priori drug distribution and to extrapolate between doses, routes of administration and species (30).
One of the shortcomings of preclinical studies is the use of doses in mice that are not achievable in humans. Ideally, dose levels and schedules used in preclinical studies should simulate exposures observed in human patient populations. This may increase the potential of preclinical animal models to better predict the efficacy of treatment modalities, combinations and schedules in humans. In the study presented here, we conducted an in vivo preclinical study of vandetanib, docetaxel and combinations of the two in a mouse xenograft model of HNSCC using dose levels and schedules derived using PBPK model simulations for docetaxel (31) and empirically determined steady-state PK of vandetanib (32) to target pre-specified plasma concentrations based on human drug exposure scenarios and putative anti-endothelial drug levels. We compared anti-endothelial schedules, where drug levels targeted were based on in vitro endothelial cell response to docetaxel and vandetanib, to MTD-based schedules of docetaxel.
MATERIALS AND METHODS
Chemicals and Reagents
Docetaxel (Taxotere®, Sanofi-Aventis) for in vitro studies was purchased from LKT Labs (St. Paul, MN) and docetaxel for in vivo studies was obtained from the University of Colorado Hospital Pharmacy and as a gift from Sanofi-Aventis. Vandetanib (ZD6474, Zactima™) was a generous gift from AstraZeneca (Macclesfield, UK). Docetaxel and vandetanib stock solutions for in vitro experiments were made in DMSO (Fisher Scientific, Fairlawn, NJ). All other materials used were purchased from either Fisher Scientific (Fairlawn, NJ) or Sigma (St. Louis, MO) unless specified.
Cells and Culture Conditions
Head and neck (H&N) tumor cells, UMSCC2 (University of Michigan), were maintained on tissue culture plates (BD Falcon, Bedford, MA) in DMEM (Cellgro, Herndon, VA) supplemented with 10% heat-inactivated FBS (Cellgro) and penicillin (100 units/ml)/streptomycin (100 μg/ml) (GIBCO, Carlsbad, CA). Primary endothelial cells were grown on 2% gelatin-coated tissue culture plates in DMEM (Biowhitaker, East Rutherford, NJ) supplemented with 2mM L-glutamine (GIBCO), 1 mM sodium pyruvate (GIBCO), 1% non-essential amino acids (GIBCO), 50 mM 2-mercaptoethanol (GIBCO), 20 mM HEPES (GIBCO), penicillin (100 units/ml)/streptomycin (100 μg/mL) (GIBCO), 12 units/ml heparin, 20% heat-inactivated FBS (GIBCO) and 100 μg/ml endothelial cell growth supplement (BD Biosciences, Bedford, MA). MCF-7 cells (ATCC, Manassas, VA) were maintained on tissue culture plates in RPMI supplemented with 10% heat-inactivated FBS with penicillin/streptomycin and 0.0015 units/ml of insulin (Humulin R, Eli Lilly, Indianapolis, IN). All cells were maintained at 37°C in a humidified incubator with 5% CO2. All in vitro drug treatments were conducted with the use of complete growth media.
Animals
Female Balb/c mice (retired breeders) were purchased from Charles River Laboratories (Wilmington, MA) and 5-10 week old female athymic nude mice were purchased from the National Cancer Institute (Bethesda, MD). Animals were housed (three to five per cage) in polycarbonate cages and kept on a 12 hour light/dark cycle. Food and water were given ad libitum. All studies were conducted in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals, and animals were housed in a facility accredited by the American Association for Accreditation of Laboratory Animal Care.
Isolation of Primary Endothelial Cells from Mouse Lung
Primary endothelial cells were isolated from the lungs of female Balb/c mice by immuno-magnetic bead separation. The method used is a slightly modified procedure from Marelli-Berg, et al (33). Briefly, mice were anesthetized with isoflurane and sacrificed by cardiac stick exsanguination. Mouse lung was excised, minced, rinsed in cold PBS, and digested by type II collagenase (Worthington Biochemical, Lakewood, NJ) and collagenase A (Roche, Indianapolis, IN) at 37°C for 1 hour. The digest was then passed through a 50 m cell strainer (BD Bioscience) to remove undigested tissue. Cells were subsequently washed with PBS with 1% FCS (GIBCO, Carlsbad, CA), counted and incubated in 10% mouse serum for 30 minutes at 4°C. Following a wash step, cells were incubated with rat anti-CD31, rat anti-CD105 (BD Pharmigen, San Diego, CA) and biotinylated isolectin B4 (Vector Laboratories, Burlingame, CA) for 30 minutes at 4°C. Cells were washed then incubated with anti-rat and streptavidin conjugated magnetic micro beads (Miltenyi Biotec Auburn, CA) for 30 minutes at 4°C. Magnetically labeled cells were then separated on a Vario Macs magnetic column (Miltenyi Biotec, Auburn, CA). Cells were seeded on 2% gelatin (Sigam, St. Louis, MO) coated tissue culture plates (BD Falcon, Bedford, MA) and cultured as described earlier. Cells were kept for up to 10 passages.
Isolation of Primary Endothelial Cells from MCF-7 Tumor Xenografts
Endothelial cells from MCF-7 tumor xenografts were isolated similar to the method described by St. Croix, et al (34) and similar to the method used to isolate lung endothelial cells as described above. Tumors were resected, minced, rinsed in cold PBS, and digested by type II collagenase, collagenase A and elastase (Roche, Indianapolis, IN) with DNAseI (Roche, Indianapolis, IN) at 37°C for 1 hour with gentle shaking. Following filtration through a 50μm cell strainer, cells were separated through a 30% Percoll gradient (Sigma, St. Louis, MO). The remainder of the isolation was performed as described above for the mouse lung endothelial cells.
Cell Proliferation Assay
Growth Inhibitory effects of vandetanib and docetaxel were determined by MTT assay. Cells were plated in 96-well plates (BD Falcon) at a density of 1,000 to 2,000 cells per well and incubated overnight prior to treatment. Drug solutions were made up in appropriate growth media and all stock solutions were diluted at least 1:100. Cells were treated with drug-containing media continuously for 120 hours followed immediately by the MTT assay. Fifty μg of MTT (thiazolyl blue tetrazolium bromide, Sigma, St. Louis, MO) per well was added to cells in serum-free DMEM. Cells were incubated at 37°C with 5% CO2 for 4 hours. Media was removed and replaced with 100 μl of DMSO. Plates were read on a plate-reader (Molecular Devices, Sunnyvale, CA) at 550 nm with Softmax version 2.32. The assay was repeated a minimum of three times for each drug.
MCF-7 Xenografts
Animals were implanted with slow-release Silastic® 17β-estradiol pellets (35) subcutaneously between the shoulders, at least 24 hours prior to MCF-7 tumor cell inoculation. MCF-7 cells were harvested and resuspended in a 1:1 mixture of serum free RPMI 1640 and matrigel (BD Bioscience, Bedford, MA). Five million cells per mouse were injected subcutaneously into the rear flank.
UMSCC2 Xenograft Studies
UMSCC2 cells were harvested and resuspended in a 1:1 mixture of serum free DMEM and matrigel. Two million cells per mouse were injected subcutaneously into the rear flank. Tumors volumes, measured by digital calipers, were calculated by V(mm3)=length × (width)2 × 0.5236. When tumors reached an average volume of 125 mm3 mice were randomized into groups (9-10 mice per group) and treatment began on day 1 and continued for 28 days. Animals received either vehicles, 15 mg/kg vandetanib (VAN) alone, 1 mg/kg docetaxel (DTX1) alone, 6 mg/kg docetaxel (DTX6) alone, or combinations of VAN and DTX1 or VAN and DTX6. Docetaxel (DTX) doses administered were determined by murine PBPK model simulations that approximated an MTD dose observed in humans (DTX6) or targeted trough plasma concentrations greater than 1nM upon daily administration (DTX1). Mice received vandetanib resuspended in 1% Tween-80 by oral gavage daily for 28 days. Docetaxel was prepared and diluted in 0.9% sodium chloride. Mice receiving 6 mg/kg docetaxel were treated every 4 days for 28 days (8 total treatments) by IP injection. Mice receiving 1 mg/kg docetaxel were treated IP for 10 consecutive days, had a 9 day recovery period, and then received treatment again for the remaining 9 days. For combination treatment, mice were administered VAN immediately followed by DTX injection. Tumor measurements were made every-other-day during treatment (days 1-28) and twice weekly post-treatment (days 29-54). Animals that experienced treatment related toxicity were placed on subcutaneous saline and NutriCal support. Animals were sacrificed when they were moribund. On day 10 of treatment, 3 animals per group were sacrificed, approximately 2 hours post treatment, and tumors excised and formalin-fixed for IHC analysis.
Immunohistochemical Staining for Active Caspase-3, Ki-67 and von Willebrand Factor (vWF)
IHC staining was preformed on formalin-fixed and paraffin-embedded tumor sections collected on day 10 of the study. Four-μm thick tissue sections (on positively charged slides), were deparaffinized in xylene with subsequent rehydration. Heat-induced epitope retrieval was done by incubating the slides at 125°C for one minute in an EDTA based solution (DAKO Cytomation; Carpinteria, CA) at pH 9.0 (active-caspase 3) or pH 6.0 (for Ki-67 and vWF). Active-caspase 3 was visualized using active-caspase-3 rabbit anti-human/mouse (1:1000 dilution overnight at 4°C; R&D Systems, Minneapolis, MN) followed with Texas-Red anti-rabbit IgG (1:100 dilution; one hr at 4°C; Vector Laboratories; Burlingame, CA). For the dual stain of Ki-67 and vWF, sections were stained sequentially. Ki-67 rat anti-mouse monoclonal antibody (1:25 dilution; overnight at 4°C; Dako) was applied followed by the secondary antibody, fluorescein anti-rat IgG (1:100 dilution; one hr at 4°C; Vector Laboratories). Next, a polyclonal rabbit vWF was applied (1:100 dilution; one hr at room temperature; Dako) pursued with Texas Red anti-rabbit IgG secondary antibody (1:100 dilution; one hr at 4°C). Slides were mounted using Vecta Shield with DAPI (Vector Laboratories). For each slide, five still images (at each wavelength) were acquired using an Axioplan 2 imaging universal microscope with the necessary fluorescent wavelengths to visualize fluorescein (ex. 495nm, em. 515nm), Texas-Red (ex. 595nm, em. 615nm) and DAPI (ex 360nm, em. 460nm). Images were analyzed using AxioVision to count the positive fluorescent pixels per image section, ensuring at least a 95% capture rate. For determination of epithelial proliferation, single images visualizing fluorescein (Ki-67) and Texas-Red (vWF) were overlaid and resulting double positive cells (yellow) counted.
Docetaxel Intraperitoneal PBPK Model Development
A PBPK model for docetaxel plasma and tissue distribution in mice was previously developed in our laboratory (31). This model describes docetaxel distribution following a single IV dose. We modified this PBPK model to describe docetaxel distribution following a single IP bolus dose and repeated IP dosing. All parameters, listed in supplementary Tables S1 and S2, used for the IV docetaxel PBPK model remained the same as previously reported (31). A schematic representation of the model used here is shown in supplementary Figure S1. Additional details of the development of the IP docetaxel PBPK model are described in the supplement.
Determination of Median Lethal Dose Values for In Vitro Experiments
A general equation used to describe dose-response relationship was derived by Chou (36,37) as:
| Eq. 2.1 |
where fa is the fraction affected by the dose, fu is the fraction unaffected (fu=1-fa), D is the dose of drug, Dm is the median-effect dose, and m is an exponent signifying the sigmoidicity (shape) of the dose-effect curve. A median-effect plot, plot of x = log (D) versus y = log (fa/fu), described in equation 2.2, is the logarithmic form of equation 2.1.
| Eq. 2.2 |
Using the slope and intercept obtained from the median-effect plot, the median lethal dose can be determined by,
| Eq. 2.3 |
Tumor Growth Rate Calculations and Statistical Analysis
Tumor growth rates were calculated by liner regression of the tumor volume versus time plots for individual tumors for the time periods of 1-14 and 15-28 days. The Student’s t test was used to determine statistical significance between two groups when data was normally distributed and one-way Anova analyses with a Tukey post-test was used for the comparison of multiple groups. Analysis was performed with Prism version 4.02. Values of P < 0.05 were considered statistically significant.
RESULTS
In Vitro Antiproliferative Activity of Vandetanib and Docetaxel
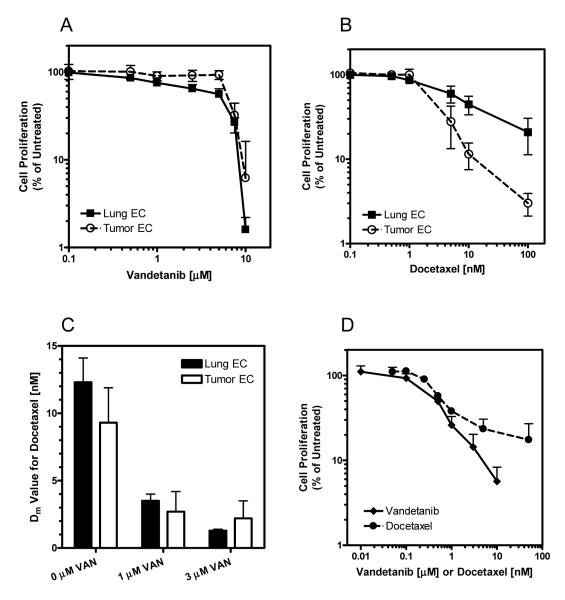
We first investigated the antiproliferative activity of vandetanib and docetaxel in murine primary endothelial cells. Endothelial cells were isolated from mouse lung and from human tumor xenografts grown in the flanks of mice. We verified the selection of endothelial cells by detection of surface receptors VEGFR2, CD105 and CD106 (data not shown). Cells were exposed to vandetanib or docetaxel in normal growth media for 120 hours. This prolonged exposure time was chosen since vandetanib is administered as a once daily dose and steady state levels should be maintained and since we were interested in investigating the use of more frequent, protracted schedules of docetaxel for anti-angiogenic therapy. Lung and tumor endothelial cells responded similarly to vandetanib with median lethal dose values (Dm) of 3.8 ± 1.1 μM and 6.1 ± 1.5 μM respectively (Figure 1 A). Although these values appear to be high, and are in fact higher than previously reported IC50 values of vandetanib in HUVEC cells (10), our treatments took place in complete media conditions (20% FBS + ECGS) and not under direct and exclusive stimulation of VEGF. At low concentrations (<10nM) tumor and lung endothelial cell lines responded similarly with Dm values of 9.3 ± 2.6 nM and 12.3 ± 1.8 nM respectively (Figure 1 B).
Figure 1.
Effect of vandetanib (A), docetaxel (B), and vandetanib with docetaxel (C) on primary lung (black symbols/bars) and primary tumor (open symbols/bars) endothelial cells. The effect of vandetanib (◆) and docetaxel (•) were also evaluated in UMSCC2 head and neck carcinoma cells (D). Cells were exposed continuously for 120 hours prior to MTT assay. Data represents the mean ± SD of a minimum of three experiments.
Due to the potential for additive and synergistic benefit of administering molecularly targeted agents in combination with cytotoxic agents, we investigated how vandetanib affected endothelial cell response to docetaxel (Figure 1 C). The addition of vandetanib to docetaxel provided an additive to synergistic benefit with CI values ranging from 0.99 for tumor endothelial cells treated with 3 μM vandetanib and docetaxel to 0.47 for lung endothelial cells treated with 1 μM vandetanib and docetaxel.
The antiproliferative effects of vandetanib and docetaxel were also evaluated in an EGFR positive HNSCC cell line, UMSCC2 (Figure 1 D). UMSCC2 cells exhibited sensitivity to both vandetanib and docetaxel with Dm values of 0.42 ± 0.07 μM and 0.74 ± 0.10 nM respectively.
PK-Directed Dosing of Vandetanib
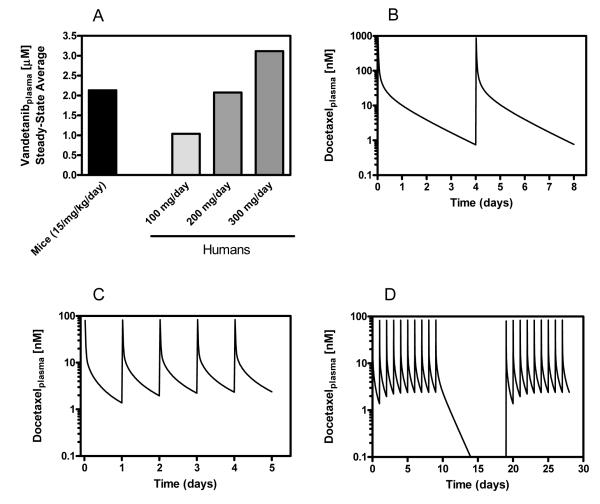
In a previous study, we used data from human clinical trials with vandetanib and data we gathered from mice and calculated daily dosing that reflected human PK parameters (32). Our calculations show that mice should receive daily dosing of 17.4, 48.6, and 17.8 mg/kg to simulate Cmax, Cmin and AUC levels achieved in humans at 300 mg/day, respectively. Based on this result we decided to use a daily p.o. dose of 15 mg/kg vandetanib, which should reasonably represent 100-300 mg/day vandetanib in humans, doses achieved in the clinic. Figure 2A shows predicted average steady state vandetanib plasma concentrations in mice at 15 mg/kd/day and in humans.
Figure 2.
(A) Average steady-state plasma levels of vandetanib in mice and humans. (B) PBPK model simulation showing docetaxel plasma concentrations following a MTD-based dosing schedule in mice. Simulations were generated based on a 6 mg/kg dose administered IP every 4 days. (C) PBPK model simulation showing docetaxel plasma concentrations following a metronomic dosing schedule in mice. Simulations were generated based on a daily IP dose of 1 mg/kg. (D) PBPK model simulation of the 1 mg/kg daily dose, modified to account for a 9 day break following the first 10 days of treatment. This break period was included due to unexpected toxicity observed in animals treated with the metronomic schedule of docetaxel.
Docetaxel PBPK Model Simulations for the Development of PK-Directed Dosing Protocols
Our previously published docetaxel PBPK model (31) was developed to design PK-directed dosing protocols to be tested in vivo, the objective of the studies described here. We were interested in investigating the effects of low-dose metronomic schedules of docetaxel on in vivo tumor growth/regression and tumor growth rates. Since repeated, frequent (> 2 × per week) intravenous injections in a nude mouse is technically very difficult, we decided to administer docetaxel by intraperitoneal (IP) injections. This required a modification to the mouse PBPK model to account for the different route of administration and for repeated dosing. The route modified model was validated (see supplementary material for further detail), and then used to determine the amount and schedule to be used that would achieve docetaxel plasma levels and exposures that were clinically relevant. We developed a schedule to be administered to mice that achieved roughly the same AUC seen in a 30 mg/m2 dose in humans administered on a weekly schedule. A single dose of 6 mg/kg delivered IP to mice achieved an AUC of 1.2 μM*h compared to an AUC of approximately 1.0 μM*h seen in humans (Table 1). The terminal half-life at 30 mg/m2 in humans was about two times longer than the terminal half-life at 6 mg/kg in mice. Therefore, to roughly match a weekly regimen of docetaxel in humans, the 6 mg/kg dose was delivered every 4 days. The resulting model simulation is shown in Figure 2 B. Model simulations were generated using a dosing interval of 96 hours for 28 days (duration of the study).
Table 1.
Comparison of pharmacokinetic parameters for single-dose and multiple-dose docetaxel in mice and humans.
| t1/2 (h) | AUC0→24 (μM*h) |
Cmax(nM) | Cmin(nM) | Total Dose Delivered (nmol) |
|
|---|---|---|---|---|---|
| Single Dose PK | |||||
| Human 30 mg/m2 * | 27.5 ± 5.5 | 1.02 ± 0.39 | 1342 ± 655 | 63.6 ± 9.6 μmol | |
| Mouse 6 mg/kg IP † | 11.5 | 1.23 | 870 | 139 | |
| Mouse 1 mg/kg IP † | 11.0 | 0.14 | 80 | 23 | |
| Multiple Dose PK | |||||
| Mouse 6 mg/kg q4d | 12.5‡ | 876 | 0.8 | 975 | |
| Mouse 1 mg/kg qd | 5.9‡ | 84 | 1.4 | 650 | |
| Mouse 1 mg/kg — modified schedule |
3.9‡ | 84 | 0 | 441 | |
Human plasma pharmacokinetic parameters were calculated using a three-compartment models with IV-infusion input of drug. Data from reference 31.
Mouse single-dose plasma pharmacokinetic parameters were calculated using non-compartmental analysis of simulated data.
AUC for multiple doses calculated in Microsoft Excel using trapezoidal rule.
To investigate the use of a metronomic or anti-angiogenic schedule with docetaxel, we determined a dose and frequency that would maintain low plasma levels of docetaxel (>1nM). A single IP dose of 1 mg/kg docetaxel results in approximately 10% of the exposure seen at the 6 mg/kg dose in mice or the 30 mg/m2 dose in humans (Table 1). The Cmax at this dose is 80 nM, which drops off to below 25 nM by 1 hour post administration. Based on simulations, to maintain a Cmin above 1nM, a 1 mg/kg dose of docetaxel needs to be administered daily. The resulting model simulation of 1 mg/kg delivered IP in mice daily is shown in Figure 2 C. Model simulations were generated using a dosing interval of 24 hours for 28 days (duration of the study). Table 1 shows comparative PK parameters for docetaxel in humans at 30mg/m2 and in mice at single doses of 6mg/kg and 1mg/kg. We also calculated the total exposure, Cmax, Cmin, and total dose delivered (based on a 25 g mouse) based on model simulations for schedules developed for in vivo testing; 6 mg/kg q4d and 1 mg/kg qd for 28 days.
Approximately 7 days into the in vivo therapeutic study intestinal toxicity was observed in the animals being treated with 1 mg/kg docetaxel. Due to this unexpected toxicity, the 1 mg/kg treatment did not continue for the duration of the experiment. Instead, mice to be treated with 1 mg/kg docetaxel were given a 9 day break-period. On day 20 of the study mice resumed treatment with 1 mg/kg docetaxel IP. Figure 2 D shows the PBPK model simulation reflecting the change in the treatment schedule. Table 1 also reflects the changes to the PK parameters. By day 14, plasma docetaxel levels were negligible. This break period resulted in a reduction of about 34% in the total exposure.
UMSCC2 Tumor Xenograft Response to Vandetanib, Docetaxel and Combination Therapy
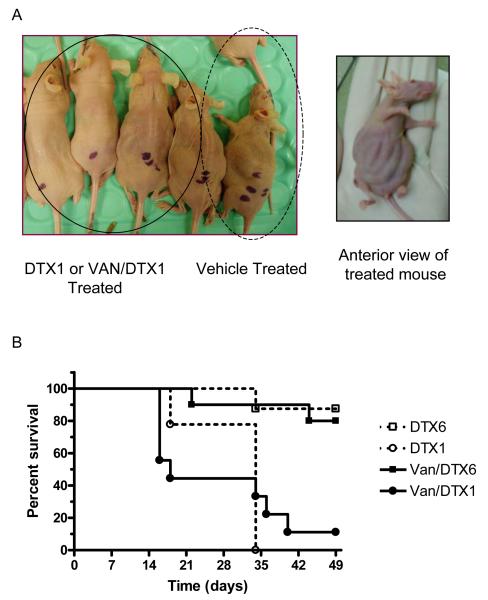
Docetaxel was delivered by IP injection at either 6 mg/kg every 4 days (DTX6) for 28 days or 1 mg/kg daily for 10 days, followed by a 9 day break period, then daily for 9 days (DTX1). Vandetanib was administered by oral gavage at a dose of 15 mg/kg daily for 28 days. The study was initially designed to have 1 mg/kg docetaxel delivered daily for the entire study, however, severe intestinal toxicity was observed by 10 days (Figure 3 A). In the sick mice the intestines were visually distended and upon necropsy the entire length of the intestine was found to be full, indicating a problem with absorption and motility. Some intestinal toxicity was also observed in the 6 mg/kg docetaxel groups, however, it was not as pronounced as the 1 mg/kg groups so treatment remained as scheduled. Figure 3 B shows the survival curves of the different docetaxel treatment groups. No animals from the control or vandetanib treated groups died during the study. Seventeen of 18 animals treated with DTX1 died whereas only 3 of 18 animals treated with DTX6 died.
Figure 3.
Intraperitoneal metronomic dosing of docetaxel resulted in severe gastrointestinal toxicity in nude mice. (A) Pictures of mice on day nine of DTX1 or VAN/DTX1 treatment compared to vehicle control treated mice. (B) Survival curves of all docetaxel treated mice. Animals treated with DTX1 alone or with VAN experienced significantly more mortality than DTX6 or VAN/DTX6 treated mice.
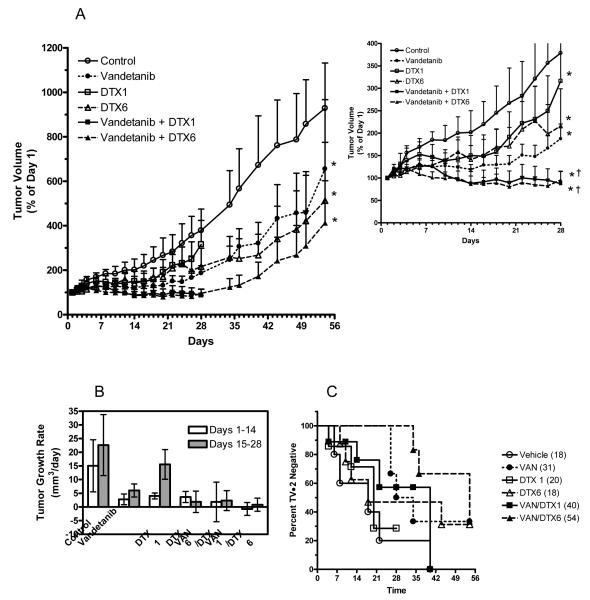
The efficacy of vandetanib, docetaxel, and combinations of the two was determined in UMSCC2 tumor xenografts. Figure 4A shows the tumor growth curves for the 28 days of treatment and for 28 days following the conclusion of treatment. Tumors were monitored following treatment to assess tumor regrowth patterns. The inset graph in Figure 4 A shows tumor growth for only the 28 day treatment period. All treatment groups significantly reduced tumor volume compared to control, p<0.05. For the 28-day treatment period, the addition of vandetanib to DTX1 and DTX6 treatment resulted in significantly reduced tumor growth compared to DTX1 or DTX6 single agent treatment.
Figure 4.
(A) Tumor growth profiles of mice bearing UMSCC2 tumor xenografts treated with vandetanib, docetaxel and combinations of the two. Treatment began on day 1 and continued for 28 days (inset graph). Following treatment, tumor volumes were measured in surviving mice to determine re-growth characteristics. Data represents the mean ± SE. *P<0.05 versus control, †P<0.05 versus DTX1 or DTX6 treatment alone. (B) Tumor growth rates were calculated for days 1-14 and days 15-28 of treatment for all mice that survived through day 28. Rates were calculated by linear regression of tumor size data for individual tumors. Data represents the mean ± SD. (C) Percentage of UMSCC2 tumors that have not doubled from their initial starting volume for each treatment group.
Tumor growth rates for individual tumors were calculated for various treatment intervals, days 1-14 and days 15-28 (Figure 4 B). Although tumor measurements were made for 4 weeks post treatment, growth rates were not calculated for this time frame due to the high mortality rates and resulting lack of sufficient data for some groups. Although none of the groups has significantly different growth rates, the trend indicates a reduction in tumor growth rates for all treatment groups for days 1-14. For days 15-28, tumor growth rates appeared to be slower for all treatment groups except for DTX1. It is also worth noting that the vandetanib + DTX6 group had a negative growth rate for the first 14 days of the study indicating tumor regression.
Tumor doubling time was also calculated and is shown in figure 4 C. The vandetanib, DTX6, and vandetanib + DTX6 groups all had animals with tumors that did not double in volume up to 28 days post treatment. Additionally, complete responses, no palpable tumor remaining and no re-growth up to 28 days post treatment, were observed in the vandetanib, DTX6, and vandetanib + DTX6 groups (n=1, 2, 1, respectively).
Immunohistochemical Analysis of UMSCC2 Tumors
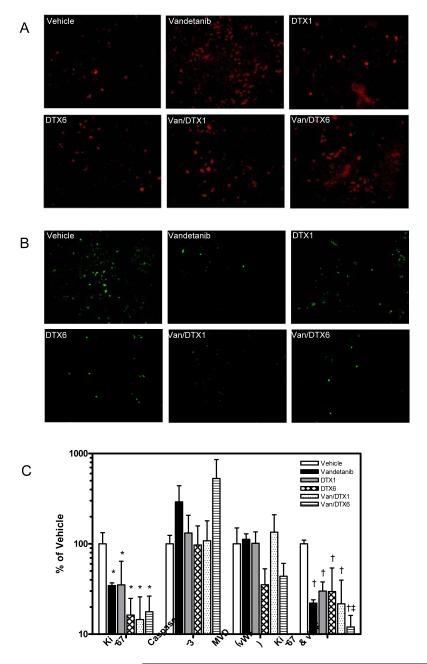
To investigate the anti-proliferative effects of treatment on tumors, on day 10, 3 animals per group were sacrificed approximately 2 hours post treatment and tumor sections collected for immunohistochemical analysis of apoptosis (active caspase 3) and proliferation (Ki-67) by caspase-3 and Ki-67. The largest increase in caspase-3, compared to control, was found in the vandetanib and vandetanib + DTX6 groups (Figure 5 A & C) and all treatment groups showed a significant (p<0.05) decrease in Ki-67 staining (Figure 5 B & C).
Figure 5.
Immunohistochemical analysis of apoptosis (A) and proliferation (B) in UMSCC2 tumor sections from mice treated with vandetanib, docetaxel and combinations of the two. Three animals per group were sacrificed on day 10 of treatment, approximately 2 hours post-treatment, and tumors removed and formalin fixed. Activated caspase-3, used to determine apoptosis, was visualized by an active-caspase-3 rabbit anti-mouse/human antibody followed by Texas-Red anti-rabbit IgG. Ki-67, used to determine proliferation, was visualized by a rat anti-mouse monoclonal antibody followed by a FITC-conjugated anti-rat IgG. (C) Quantitative analysis of activated caspase-3, Ki-67, vWF and dual vWF/Ki-67 on UMSCC2 tumor sections. Data represents the mean ± SD of data obtained from sections from three different tumors from each treatment group.
Since we were investigating the use of antiangiogenic schedules of docetaxel and since vandetanib also inhibits VEGFR2 in addition to EGFR, we wanted to investigate the effects of treatment on tumor endothelium. Tumor sections were stained with von Willebrand Factor (vWF) and vessels counted to assess the microvessel density (MVD). Only the DTX6 and vandtanib + DTX6 treatment groups appeared to affect MVD (Figure 5 C). However, Ki-67 staining co-localized with vWF, showed a significant (p<0.001) decrease in endothelial cell proliferation in all treatment groups (Figure 5 C). Additionally, analysis of tumor growth rate (Fig. 4B) with IHC indices showed that only proliferation within endothelial cells (dual vWF and Ki-67) showed a positive correlation (p<0.05) whereas neither active caspase 3 or Ki-67 alone correlated. Although these results clearly indicate endothelial cell effects of vandetanib and docetaxel treatments, the docetaxel dose and frequency did not appear to have different effects on tumor endothelium, but did appear to affect overall tumor growth characteristics.
DISCUSSION
Preclinical pharmacokinetic models capable of predicting concentrations/exposure in vivo under different dosing schema can provide an invaluable tool for the rational design of clinical dosing protocols. The overall goals of the studies described herein were to design and evaluate the use of rational dosing protocols for vandetanib, docetaxel, and combinations of the two using pharmacokinetically-directed dosing protocols that were reflective of exposures attainable in humans, and that target anti-tumor and antiangiogenic responses. We utilized a PBPK model for docetaxel to develop dosing protocols that would allow us to examine the effects of standard docetaxel treatment versus a metronomic or antiangiogenic schedule of docetaxel. PK data on vandetanib was used to determine a steady state dose level that was reflective of human vandetanib exposure (32).
Preclinical testing is an integral part of the development of new therapeutics and new therapeutic strategies. With increasing interest in angiogenesis as a cancer therapy target, either through metronomic scheduling of chemotherapeutics, molecularly targeted agents, or a combination of the two, the need for appropriate in vitro and in vivo models is essential. We first established anit-proliferative activity of docetaxel, vandetanib and combinations of the two in murine primary endothelial cells of lung and tumor origin. Murine endothelial cells were selected since the vasculature that supports the growth and maintenance of human tumor cell line xenografts is of murine origin, regardless of the tumor type or species of origin (38). Due to the difficulty in isolating pure populations of murine endothelial cells from tumors, and the short duration in which these cell can remain in culture, we wanted to determine if endothelial cells isolated from normal tissue would respond similarly to treatment and therefore serve as a reasonable alternative in vitro cell model for future experiments. We also determined the anti-proliferative effects of vandetanib and docetaxel in a HNSCC cell line, UMSCC2. This data was used to establish desired in vivo plasma concentrations to target to achieve an anti-endothelial effect.
Our results showed that primary endothelial cells derived from mouse lung and from tumor xenografts respond similarly to vandetanib, docetaxel and combinations of the two in vitro and that vandetanib additively to synergistically enhanced docetaxel growth inhibition. Both cell lines were relatively sensitive to docetaxel treatment with median lethal dose values in the low nanomolar range. Based on this data, and data from previously published studies examining endothelial cell treatment with docetaxel (26), we decided to develop an antiangiogenic dose schedule with the use of our route-modified PBPK model that maintained a minimum plasma concentration of 1nM for the course of the study.
Unexpectedly, the low-dose metronomic schedule of 1 mg/kg docetaxel per day resulted in severe toxicity and significant death (17 of 18 animals). Ten days into the daily treatment schedule we modified the schedule to include a nine day break period. Since the daily dose of docetaxel administered was significantly lower than MTD doses (∼20mg/kg) (39) and even our own 6 mg/kg dose, we speculate that the frequency of treatment may have resulted in a sort of continuous reservoir of docetaxel in the peritoneal cavity, which resulted in the dramatic and severe intestinal toxicity observed. Only mild toxicity was observed in animals treated with 6 mg/kg docetaxel with some, but significantly less, death than the 1 mg/kg groups (3 of 17 animals).
Our PK-directed dose schedules for docetaxel and vandetanib were tested in a mouse xenograft model of head and neck squamous cell carcinoma to determine anti-tumor and anti-endothelial responses. Tumor growth inhibition data showed that all treatment groups had some effect on reducing tumor growth with the greatest effect in groups given vandetanib with docetaxel. Despite the significant effect of the various treatment regimens it is important to note that tumor regression was not observed in the majority of animals, merely a reduction in tumor growth versus control was observed. Additionally, once treatment was discontinued the tumors that remained began to re-grow in all treatment groups in surviving animals. The immunohistochemical analysis of tumors on day 10 of treatment revealed significant effects of treatment only on total (tumor and endothelium) proliferation and endothelial cell proliferation as measured by Ki-67 and Ki-67/vWF dual staining of tumor sections. While trends in caspase-3 staining and microvessel density were observed, these results were not significant. The IHC results are relatively consistent with tumor growth data indicating that in general vandetanib added to docetaxel therapy resulted in the most dramatic anti-tumor and anti-endothelial effects. Although it is difficult to clearly ascertain, due to the severe toxicity and death observed, it appears that the metronomic dosing protocol developed for docetaxel was not as effective as higher dose docetaxel or even single agent vandetanib treatment in this tumor model.
The majority of published preclinical studies that have demonstrated single agent efficacy of vandetanib have used doses (25-100 mg/kg) (12, 13, 40) which would not be reflective of exposures attainable in humans. Our study utilized a dose that was reflective of exposures measured in humans at 100 - 300mg/day (32, 41). Additionally, our selected dose of 15 mg/kg/day achieved steady-state plasma minimum and maximum plasma concentrations (Cmin and Cmax of approximately 1.4 and 3.3 μM respectively) (32) in mice capable of inhibiting tumor and endothelial cell growth in vitro and in vivo.
The use of vandetanib in the clinic will probably involve combinations with other treatment modalities. Our in vitro and in vivo study findings are in agreement with several preclinical and clinical studies that have indicated that molecularly target agents, such as vandetanib, provide added benefit to chemotherapy (13, 16, 42, 43). Our growth inhibition studies in primary endothelial cells showed that sub-toxic levels of vandetanib (1 μM) significantly enhanced the effects of docetaxel treatment in endothelial cells. Numerous studies have shown that cytotoxic chemotherapy on endothelial cells can be diminished by the presence of growth factors such as bFGF or VEGF (26, 44, 45). As a result, the addition of vandetanib may enhance the growth inhibitory effects of docetaxel by blocking the pro-survival pathways activated by growth factors.
The use of PK and PBPK models to design dosing protocols to be tested preclinically is the most rational approach to developing cancer therapies that will be clinically effective. Often, doses of drugs used in preclinical models far exceed what is attainable in humans. The use of dose levels that are reflective of exposures measured or estimated in human populations may serve to improve the ability of preclinical models to better predict therapies and schedules that will be effective in obtaining tumor response clinically. Although we were unable to clearly test metronomic versus MTD schedules of docetaxel alone and in combination with vandetanib in these studies, due to toxicity observed with metronomic schedules through ip administration, we were able to utilize a PBPK model and PK data to develop dosing regimens to effectively treat head and neck squamous cell carcinoma cell xenografts at concentrations relevant to exposures in humans. Since repeated, frequent ip administration of docetaxel is not a reasonable approach for the evaluation of MTD versus metronomic schedules, we feel it is worth investigating other routes of administration.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank AstraZeneca for their generous gift of vandetanib and Sanofi-Aventis for their generous gift of docetaxel. We would also like to thank Dr. Barbara Frederick for providing cells and for helpful discussions, Ms. Andrea Merz for her assistance with the animal treatments, and Dr. E.J. Ehrhart for his assistance with the immunohistochemistry.
Financial Support: This work was supported by an NIH pre-doctoral fellowship (CA99942) from the NCI to Erica Bradshaw-Pierce, and by CA101988 from the NCI to Dr. Daniel L. Gustafson.
REFERENCES
- (1).Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- (2).Ferrara N. Vascular endothelial growth factor. Eur J Cancer. 1996;32A:2413–2422. doi: 10.1016/s0959-8049(96)00387-5. [DOI] [PubMed] [Google Scholar]
- (3).Jain R. Molecular recognition of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- (4).Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9:2–10. doi: 10.1634/theoncologist.9-suppl_1-2. [DOI] [PubMed] [Google Scholar]
- (5).Nicholson RI, Gee JMW, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37:S9–S15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- (6).Baker CH, Kedar D, McCarty MF, et al. Blockade of epidermal growth factor receptor signaling on tumor cells and tumor-associated endothelial cells for therapy of human carcinomas. Am J Pathol. 2002;161:929–938. doi: 10.1016/S0002-9440(10)64253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Sasaki T, Kitadai Y, Nakamura T, et al. Inhibition of epidermal growth factor receptor and vascular endothelial growth factor receptor phosphorylation on tumor-associated endothelial cells leads to treatment of orthotopic human colon cancer in nude mice. Neoplasia. 2007;9:1066–1077. doi: 10.1593/neo.07667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hynes N, Lane H. ErbB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:580. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- (9).Rogers S, Harrington K, Rhys-Evans P, O-Charoenrat P, Eccles S. Biological significance of c-ErbB family oncogenes in head and neck cancer. Cancer Metastasis Rev. 2005;24:47–69. doi: 10.1007/s10555-005-5047-1. [DOI] [PubMed] [Google Scholar]
- (10).Hennequin LF, Stokes ESE, Thomas AP, et al. Novel 4-anilinoquinazolines with C-7 basic side chains: Design and structure activity relationship of a series of potent, orally active, VEGF receptor tyrosine kinase inhibitors. J Med Chem. 2002;45:1300–1312. doi: 10.1021/jm011022e. [DOI] [PubMed] [Google Scholar]
- (11).Hennequin LF, Thomas AP, Johnstone C, et al. Design and structure-activity relationship of a new class of potent VEGF receptor tyrosine kinase inhibitors. J Med Chem. 1999;42:5369–5389. doi: 10.1021/jm990345w. [DOI] [PubMed] [Google Scholar]
- (12).Wedge SR, Ogilvie DJ, Dukes M, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62:4646–4655. [PubMed] [Google Scholar]
- (13).Ciardiello F, Caputo R, Damianco V, et al. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res. 2003;9:1546–1556. [PubMed] [Google Scholar]
- (14).Williams K, Telfer B, Brave S, et al. ZD6474, a potent inhibitor of vascular endothelial growth factor signaling, combined with radiotherapy: Schedule-dependent enhancement of antitumor activity. Clin Cancer Res. 2004;10:8587–8593. doi: 10.1158/1078-0432.CCR-04-1147. [DOI] [PubMed] [Google Scholar]
- (15).Damianco V, Melisi D, Bianco C, et al. Cooperative antitumor effect of multitargeted kinase inhibitor ZD6474 and ionizing radiation in glioblastoma. Clin Cancer Res. 2005;11:5639–5644. doi: 10.1158/1078-0432.CCR-05-0174. [DOI] [PubMed] [Google Scholar]
- (16).Heymach JV, Johnson BE, Prager D, et al. Randomized, placebo-controlled phase II study of vandetanib plus docetaxel in previously treated non-small cell lung cancer. J Clin Oncol. 2007;25:4270–4277. doi: 10.1200/JCO.2006.10.5122. [DOI] [PubMed] [Google Scholar]
- (17).Gueritte-Voegelein F, Guenard D, Lavelle F, et al. Relationships between the structure of Taxol analogues and their antimitotic activity. J Med Chem. 1991;34:992–998. doi: 10.1021/jm00107a017. [DOI] [PubMed] [Google Scholar]
- (18).Engels F, Verweij J. Docetaxel administration schedule: From fever to tears? A review of randomised studies. Eur J Cancer. 2005;41:1117–1126. doi: 10.1016/j.ejca.2005.02.016. [DOI] [PubMed] [Google Scholar]
- (19).Lyseng-Williamson K, Fenton C. Docetaxel: A review of its uses in metastatic breast cancer. Drugs. 2005;65:2513–2531. doi: 10.2165/00003495-200565170-00007. [DOI] [PubMed] [Google Scholar]
- (20).Belani C. Optimizing chemotherapy for advanced non-small cell lung cancer: focus on docetaxel. Lung Cancer. 2005;50:S3–S8. [PubMed] [Google Scholar]
- (21).Engels F, Sparreboom A, Mathot R, Verweij J. Potential for improvement of docetaxel-based chemotherapy: a pharmacological review. Br J Cancer. 2005;93:173–177. doi: 10.1038/sj.bjc.6602698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Suzuki M, Nishimura Y, Nakamatsu K, et al. Phase I study of weekly docetaxel infusion and concurrent radiation therapy for head and neck cancer. Jpn J Clin Oncol. 2003;33:297–301. doi: 10.1093/jjco/hyg054. [DOI] [PubMed] [Google Scholar]
- (23).Allal AS, Zwahlen D, Becker M, Dulguerov P, Mach N. Phase I trial of concomitant hyperfractionated radiotherapy with docetaxel and cisplatin for locally advanced head and neck cancer. Cancer J. 2006;12:63–68. doi: 10.1097/00130404-200601000-00011. [DOI] [PubMed] [Google Scholar]
- (24).Kalyankrishna S, Grandis J. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- (25).Bonner B, Harari P, Giralt J, et al. Radiotherapy plus cetuximab for squamous cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- (26).Sweeny CJ, Miller KD, Sissons SE, et al. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369–3372. [PubMed] [Google Scholar]
- (27).Grant D, Williams T, Zahaczewsky M, Dicker A. Comparison of antiangiogenic activities using paclitaxel (Taxol) and docetaxel (Taxotere) Int J Cancer. 2003;104:121–129. doi: 10.1002/ijc.10907. [DOI] [PubMed] [Google Scholar]
- (28).Hotchkiss K, Ashton A, Mahmood R, et al. Inhibition of endothelial cell function in vitro and angiogenesis in vivo by docetaxel (Taxotere): Associtaion with impaired repositioning of the microtubule organizing center. Mol Cancer Ther. 2002;1:1191–1200. [PubMed] [Google Scholar]
- (29).Baker S, Zhao M, Lee C, et al. Comparative pharmacokinetics of weekly and every-three-weeks docetaxel. Clin Cancer Res. 2006;10:1976–1983. doi: 10.1158/1078-0432.ccr-0842-03. [DOI] [PubMed] [Google Scholar]
- (30).Chen H-SG, Gross J. Physiologically based pharmacokinetic models for anticancer drugs. Cancer Chemother Pharmacol. 1979;2:85–94. doi: 10.1007/BF00254079. [DOI] [PubMed] [Google Scholar]
- (31).Bradshaw-Pierce EL, Eckhardt SG, Gustafson DL. A physiologically based pharmacokinetic model of docetaxel disposition: from mouse to man. Clin Cancer Res. 2007;13:2768–2776. doi: 10.1158/1078-0432.CCR-06-2362. [DOI] [PubMed] [Google Scholar]
- (32).Gustafson DL, Bradshaw-Pierce EL, Merz AL, Zirrolli JA. Tissue distribution and metabolism of the tyrosine kinase inhibitor ZD6474 (Zactima) in tumor-bearing nude mice following oral dosing. J Pharmacol Exp Ther. 2006;318:872–880. doi: 10.1124/jpet.106.102376. [DOI] [PubMed] [Google Scholar]
- (33).Marelli-Berg FM, Peek E, Lidington EA, Stauss HJ, Lechler RI. Isolation of endothelial cells from murine tissue. J Immunol Methods. 2000;244:205–215. doi: 10.1016/s0022-1759(00)00258-1. [DOI] [PubMed] [Google Scholar]
- (34).Croix B, Rago C, Velculescu V, et al. Genes expressed in human and tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- (35).Sartorius C, Shen T, Horwitz K. Progesterone receptors A and B differentially affect the growth of estrogen-dependent human breast tumor xenografts. Breast Cancer Res Treat. 2003;79:287–299. doi: 10.1023/a:1024031731269. [DOI] [PubMed] [Google Scholar]
- (36).Chou T-C. Relationships between inhibition constants and fractional inhibitions in enzyme-catalyzed reactions with different numbers of reactants, different reaction mechanisms, and different types of mechanisms of inhibition. Mol Pharm. 1974;10:235–247. [PubMed] [Google Scholar]
- (37).Chou T-C. Derivation and properties of Michaelis-Menton type and Hill type equations for reference ligands. J Theor Biol. 1976;39:253–276. doi: 10.1016/0022-5193(76)90169-7. [DOI] [PubMed] [Google Scholar]
- (38).Walter-Yohrling J, Morgenbesser S, Rouleau C, et al. Murine endothelial cell lines as a model of tumor endothelial cells. Clin Cancer Res. 2004;10:2179–2189. doi: 10.1158/1078-0432.ccr-03-1013. [DOI] [PubMed] [Google Scholar]
- (39).Dykes D, Bissery M, Harrison SJ, Waud W. Response of human tumor xenografts in athymic nude mice to docetaxel (RP 56976, Taxotere) Invest New Drugs. 1995;13:1–11. doi: 10.1007/BF02614214. [DOI] [PubMed] [Google Scholar]
- (40).Ciardiello F, Bianco R, Caputo R, et al. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor activity. Clin Cancer Res. 2004;10:784–793. doi: 10.1158/1078-0432.ccr-1100-03. [DOI] [PubMed] [Google Scholar]
- (41).Holden S, Eckhardt S, Basser R, et al. Clinical Evaluation of ZD6474, an Orally Active Inhibitor of VEGF and EGF Receptor Signaling, in Patients with Solid, Malignant Tumors. Ann Oncol. 2005;16:1391–1397. doi: 10.1093/annonc/mdi247. [DOI] [PubMed] [Google Scholar]
- (42).Bianco C, Giovannetti E, Ciardiello F, et al. Synergistic antitumor activity of ZD6474, an inhibitor of vascular endothelial growth factor receptor and epiderman growth factor receptor signaling, with gemcitabine and ionizing radiation against pancreatic cancer. Clin Cancer Res. 2006;12:7099–7107. doi: 10.1158/1078-0432.CCR-06-0833. [DOI] [PubMed] [Google Scholar]
- (43).Troiani T, Serkova NJ, Gustafson DL, et al. Investigation of two dosing schedules of vandetanib (ZD6474), an inhibitor of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling, in combination with irinotecan in a human colon cancer xenograft model. Clin Cancer Res. 2007;13:6450–6458. doi: 10.1158/1078-0432.CCR-07-1094. [DOI] [PubMed] [Google Scholar]
- (44).Tran J, Master Z, Yu JL, Rak J, Dumont DJ, Kerbel RS. A role for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc Natl Acad Sci USA. 2002;99:4349–4354. doi: 10.1073/pnas.072586399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Gerber H-P, McMurtrey A, Kowalski J, et al. Vascular Endothelial Growth Factor Regulates Endothelial Cell Survival Through the Phosphati-dylinositol 3′-Kinase/Akt Signal Transduction Pathway. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.