Abstract
Chronic hypobaric hypoxia (CHH) increases load on the right ventricle (RV) resulting in RV hypertrophy. We hypothesized that CHH elicits distinct responses, i.e., the hypertrophied RV, unlike the left ventricle (LV), displaying enhanced mitochondrial respiratory and contractile function. Wistar rats were exposed to 4 weeks CHH (11% O2) versus normoxic controls. RV/body weight ratio increased (P < 0.001 vs. control) while RV systolic and developed pressures were higher. However, LV systolic and developed pressures were significantly reduced. Mitochondrial O2 consumption was sustained in the hypertrophied RV, ADP/O increased (P < 0.01 vs. control) and proton leak significantly decreased. Conversely, LV mitochondrial O2 consumption was attenuated (P < 0.05 vs. control) and proton leak significantly increased. In parallel, expression of mitochondrial regulators was upregulated in the hypertrophied RV but not the LV. Our data show that the hypertrophied RV induces expression of mitochondrial regulatory genes linking respiratory capacity and enhanced efficiency to sustained contractile function.
Keywords: Gene expression, Heart, Mitochondria, Right ventricular hypertrophy, Hypobaric hypoxia
Introduction
Chronic exposure to hypobaric hypoxia results in pulmonary hypertension and pressure overload on the right ventricle (RV) leading to the development of right ventricular hypertrophy. We have previously reported a robust hypertrophic response in the RV with sustained function and a lack of fibrosis in rats exposed to 2–12 weeks of hypobaric hypoxia [1, 2], suggesting a physiologic hypertrophic response [2, 3].
An emerging paradigm suggests that during the onset of physiologic cardiac hypertrophy multiple adaptive pathways are triggered by the increase in ATP breakdown and production, which enhance mitochondrial respiratory function to meet higher myocardial energy demand in response to increased load. In agreement, we recently demonstrated the coordinate induction of several genes regulating mitochondrial respiratory capacity, and increased mitochondrial DNA (mtDNA) content in the hypertrophied RV of rats exposed to 2 weeks of hypobaric hypoxia [3]. Transcript levels of nuclear respiratory factor 1 (NRF-1) and peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α), key transcriptional modulators of mitochondrial biogenesis, were increased in the hypertrophied RV but not the left ventricle (LV). Such alterations in cardiac metabolic gene remodeling were associated with enhanced mitochondrial respiratory function in the hypertrophied RV. These data are in agreement with previous work showing sustained mitochondrial respiration in the hypoxia-induced hypertrophied RV but not the LV [4]. However, others reported that chronic high altitude hypoxia decreased ATP synthesis in both ventricles, albeit a delayed response in the RV [5].
For this study, we hypothesized that exposure to chronic hypobaric hypoxia (CHH) results in distinct remodeling of the RV and LV of the heart. We propose that while the hypertrophied RV adapts by enhanced mitochondrial function and increased expression of genes encoding key mitochondrial proteins, this response is lacking in the LV thereby decreasing its respiratory and contractile function. To test our hypothesis, we exposed rats to 4 weeks of hypobaric hypoxia (11% O2) and separately determined cardiac contractile and mitochondrial respiratory function for the right and LVs. Moreover, we performed real-time quantitative RT-PCR analysis to measure transcript levels of several mitochondrial regulators. Our data demonstrate an induction of genes regulating mitochondrial respiratory function accompanied by sustained mitochondrial and contractile function in the hypertrophied RV. In contrast, the LV displayed decreased mitochondrial respiratory function without any adaptive gene remodeling.
Methods
Animal studies
Six-week-old male Wistar rats (weighing 190–230 g) were initially housed for 7 days at room temperature for acclimatization on a 12-h reverse light/dark cycle (lights off at 4 a.m. zeitgeber time (ZT12); lights on at 4 p.m. (ZT0), with access to a conventional laboratory diet and water ad libitum. The hypoxic groups were housed inside a lexan chamber (45 kPa—11% O2) (SciTech, Cape Town, South Africa) for 4 weeks as described previously [3], and compared to age-matched normoxic controls. On the last day of the experiments, animals were anesthetized with sodium pentobarbital (100 mg kg−1 i.p.) whereupon the hearts were isolated and perfused for functional assessment (n = 6 for hypoxic and normoxic groups, respectively). In parallel experiments, additional tissues were collected for: (a) mitochondrial respiration, (b) cardiac morphometrics, and (c) gene expression determination (n = 6 for hypoxic and normoxic groups, respectively, for each of these three study groups). Here the RV was carefully dissected from the LV plus the inter-ventricular septum (LV + S) and each ventricle thereafter separately investigated. All rats were sacrificed at ZT16-ZT18 since they are most metabolically active during this period [6]. The University of Cape Town’s Animal Research Ethics Committee approved all animal experiments and the investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Cardiac mitochondrial isolation and functional characterization
Mitochondria were isolated according to the method of Sordahl et al. [7] with modifications. Briefly, RV and LV tissue were homogenized separately in 10 ml ice-cold potassium-EDTA (KE) buffer (0.18 M KCl, 10 mM EDTA, [pH 7.4]), whereafter the homogenate was centrifuged at 755 × g for 5 min. The supernatant was subsequently filtered through 41 μm nylon mesh (Spectrum, USA) and the filtrate centrifuged at 1480 × g for 5 min. The mitochondrial pellet was resuspended in 50 μl KE buffer, and was subsequently used for mitochondrial respiration measurements.
Respiratory rates were polarographically measured using a Clark-type electrode (Hansatech Instruments, London, UK) at 25°C with constant stirring as previously described [3] with modifications. We found 25°C to be the optimum temperature for our experiments as described before [8-10] and used this temperature to allow for comparisons with previous studies within the field. Briefly, freshly isolated rat ventricular mitochondria (0.5 mg/ml) were added to the electrode chamber containing incubation medium (10 mM Tris–HCl, 0.25 M sucrose, 8.5 mM KH2PO4, [pH 7.4]). We employed a mixture of 5mM malate and 40 μM palmitoyl-l-carnitine as oxidative substrates. State 3 respiration was determined by measuring mitochondrial oxygen uptake after the addition of ADP to a final concentration of 350 μM. State 4 respiration was determined by measuring mitochondrial oxygen uptake upon complete phosphorylation of ADP to ATP. Basal proton leak in the isolated mitochondria was determined by measuring the rate of mitochondrial respiration after addition of 10 μg/ml oligomycin to state 4 mitochondria.
The ADP/O ratio, a measure of mitochondrial oxidative phosphorylation efficiency, was calculated as the ratio between the ADP added and oxygen consumed during ADP phosphorylation. The rate of ADP phosphorylation was calculated as nanomoles of ADP phosphorylated per minute during state 3 respiration as described before [11]. Mitochondria were considered viable where the respiratory control index (RCI) (state 3/state 4) were ≥4. The RCI and the ADP/O ratios were calculated according to Estabrook [12] using 253 nmol O2/ml as the value for the solubility of oxygen at 25°C. Specifically, ADP/O was calculated as: ADP/O = 350 μM ADP × H ÷ 253 nmol O2/ml × 2 × h (H = height of calibration trace for oxygen detected (i.e., from equilibrated buffer to zero); h = height of decline in the oxygen concentration during state 3 respiration; 350 μM = exogenous ADP added to stimulate state 3 respiration; 2 = factor for the conversion of oxygen to atoms of oxygen; 253 nmol O2/ml—value for the solubility of oxygen at 25°C). All mitochondrial polarographic studies were normalized to total mitochondrial protein content, determined using the Lowry assay [13].
Langendorff heart perfusions
Isolated hearts were perfused in the Langendorff mode with ice-cold Krebs-Henseleit buffer (11 mM Glucose, 118 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM KH2PO4 · 1.2 mM MgSO4 · 7H2O, 1.8 mM CaCl2 · 6H2O, pH 7.4). The aorta was located and cannulated on the Langendorff perfusion rig, and a retrograde perfusion of the coronary arteries via the aorta was immediately initiated. The perfusion was performed with oxygenated (95% O2, 5% CO2) Krebs–Henseleit buffer at a constant pressure (104 cm H2O) and temperature (37°C). During perfusion, a latex balloon attached to a pressure transducer was first inserted into the left ventricular cavity for stabilization and inflated to produce a diastolic pressure of 4–12 mmHg, whereupon the balloon was then inserted into the RV to determine the right ventricular developed pressure (RVDP) as described before [3]. The RV and LV functional parameters were measured and included heart rate, systolic and diastolic pressure, developed pressure, coronary flow, and rate pressure product (heart rate × developed pressure). The RV/LV coronary flow and RV/LV heart rate measurements were obtained when the balloon was placed in the RV and LV, respectively. The duration of the perfusion experiments was limited to 15 min for each ventricle.
RNA isolation and real-time quantitative RT-PCR analysis
Total RNA was isolated separately from the LV and RV from all rat hearts and real-time quantitative RT-PCR of samples were performed using previously described methods [3, 14]. We determined transcript levels of: cytochrome c oxidase subunits II and IV (COXII and COXIV, respectively); NRF-1, a well-described transcriptional modulator regulating expression of several mitochondrial proteins; PGC-1α, a transcriptional coactivator controlling cellular energy metabolic pathways; and heart isoforms of uncoupling protein (UCP2 and UCP3). Standard RNA was made for all assays by the T7 polymerase method (Ambion, Austin, TX, USA), using total RNA isolated from rat hearts. The correlation between the Ct (the number of PCR cycles required for the fluorescent signal to reach a detection threshold) and the amount of standard was linear over at least a 5-log range of RNA for all assays (data not shown). Gene expression data are represented as mRNA molecules per ng total RNA.
Statistical analysis
Data are presented as the mean ± standard error of mean (SEM). Statistically significant differences between normoxic and hypoxic groups were calculated using Student t-test. Statistical significance was considered when P < 0.05.
Results
Morphometrics and cardiac function
In rats exposed to hypobaric hypoxia for 4 weeks, the RV/ LV + S ratio increased by 106 ± 2% (P < 0.001 vs. normoxic control) while RV/body weight (BW) ratio increased by 117 ± 7.4% (P < 0.001 vs. normoxic control) (Table 1). The LV + S/BW ratio was not significantly altered following exposure to hypobaric hypoxia for 4 weeks. Hypobaric hypoxia did not significantly affect the heart rate (Table 2). However, coronary flow was significantly increased in response to hypobaric hypoxia. Chronic hypoxia also enhanced RV systolic and RV developed pressures (P < 0.05 vs. normoxic control). Conversely, LV systolic and developed pressures were reduced (P < 0.05 vs. normoxic control). However, the rate pressure product was not significantly changed in both ventricles following exposure to hypobaric hypoxia.
Table 1.
Effects of 4 weeks hypobaric hypoxia (11% O2) on hemodynamic parameters
| Normoxic | Hypoxic | |
|---|---|---|
| BW (g) | 320 ± 18.5 | 273 ± 12.2 |
| HW (mg) | 860 ± 14.8 | 1,125 ± 23.2* |
| RV (mg) | 270 ± 4.5 | 486 ± 14.2* |
| LV + S (mg) | 588 ± 28 | 634 ± 34 |
| RV/LV + S ratio | 0.46 ± 0.01 | 0.96 ± 0.02** |
| RV/BW | 0.84 ± 0.1 | 1.83 ± 0.12** |
| LV + S/BW | 1.86 ± 0.1 | 2.23 ± 0.1 |
Rats were exposed to hypobaric hypoxia (11% O2) for 4 weeks and compared with age-matched normoxic groups. Data are presented as means ± SEM.
P < 0.01 and
P < 0.001 vs. normoxic controls (n = 6 animals per group)
Table 2.
Effects of hypobaric hypoxia on cardiac contractile function
| Normoxic | Hypoxic | |
|---|---|---|
| RV cardiac parameters | ||
| Coronary flow (ml/min) | 10.6 ± 0.4 | 14.3 ± 1.1** |
| Heart rate (beats min−1) | 260 ± 11.5 | 253 ± 26 |
| Systolic pressure (mmHg) | 55 ± 2.1 | 66.3 ± 5.1* |
| Developed pressure (mmHg) | 43 ± 2.1 | 54.3 ± 5.1* |
| Rate pressure product (beats min−1 mmHg) | 11,257 ± 842 | 13,993 ± 2,214 |
| LV cardiac parameters | ||
| Coronary flow (ml/min) | 12.4 ± 0.97 | 17.1 ± 1.5* |
| Heart rate (beats min−1) | 266 ± 12.9 | 306 ± 21.7 |
| Systolic pressure (mmHg) | 95.3 ± 5.6 | 81.7 ± 8.1* |
| Developed pressure (mmHg) | 83.3 ± 5.6 | 69.7 ± 8.1* |
| Rate pressure product (beats min−1 mmHg) | 22,395 ± 2,082 | 20,700 ± 1,517 |
Rats were exposed to hypobaric hypoxia (11% O2) for 4 weeks and compared with age-matched normoxic groups. Data are presented as means ± SEM.
P < 0.05 and
P < 0.01 vs. normoxic controls (n = 6 animals per group)
Mitochondrial respiratory function
Following 4 weeks of hypobaric hypoxia, the ADP/O ratio was increased by 36 ± 1% (P < 0.01 vs. normoxic control) in the RV (Table 3). Also, the basal leak was decreased by 78 ± 18% (P < 0.01 vs. normoxic control) in the RV. RV state 3 and 4 respiration, rate of ADP phosphorylation and RCI were not significantly different in response to hypobaric hypoxia. In the LV, however, oxygen consumption (state 3) was decreased by 36 ± 5% (P < 0.05 vs. normoxic control) and RCI by 67 ± 14% (P < 0.05 vs. normoxic control). Furthermore, this was associated with increased proton leak (P < 0.05 vs. normoxic control).
Table 3.
Effects of hypobaric hypoxia on mitochondrial respiration
| Normoxic | Hypoxic | |
|---|---|---|
| RV mitochondrial function | ||
| State 3/mg protein (nmol O2/min/mg protein) | 57.4 ± 8.3 | 65.3 ± 20.3 |
| ADP/O ratio | 2.02 ± 0.2 | 2.7 ± 0.1** |
| Rate of ADP phosphorylation (nmol ADP/min/mg protein) | 122 ± 20.2 | 125 ± 32.1 |
| State 4 respiration (nmol O2/min/mg protein) | 3.0 ± 0.7 | 4.3 ± 0.9 |
| Basal leak (nmol O2/min/mg protein) | 12.1 ± 1.5 | 2.6 ± 0.6** |
| RCI (State 3/State 4) | 14.7 ± 4.3 | 15.3 ± 6.5 |
| LV mitochondrial function | ||
| State 3/mg protein (nmol O2/min/mg protein) | 118 ± 11.6 | 75.8 ± 10.5* |
| ADP/O ratio | 2.6 ± 0.2 | 2.5 ± 0.2 |
| Rate of ADP phosphorylation (nmol ADP/min/mg protein) | 231 ± 17.8 | 186 ± 22.9 |
| State 4 respiration (nmol O2/min/mg protein) | 6.6 ± 1.6 | 3.9 ± 0.8 |
| Basal leak (nmol O2/min/mg protein) | 8.1 ± 1.9 | 17.8 ± 4.8* |
| RCI (State 3/State 4) | 39.1 ± 10 | 12.7 ± 2.6* |
Rats were exposed to hypobaric hypoxia (11% O2) for 4 weeks and compared with age-matched normoxic groups. Data are represented as means ± SEM.
P < 0.05 and
P < 0.01 vs. normoxic controls (n = 6 animals per group)
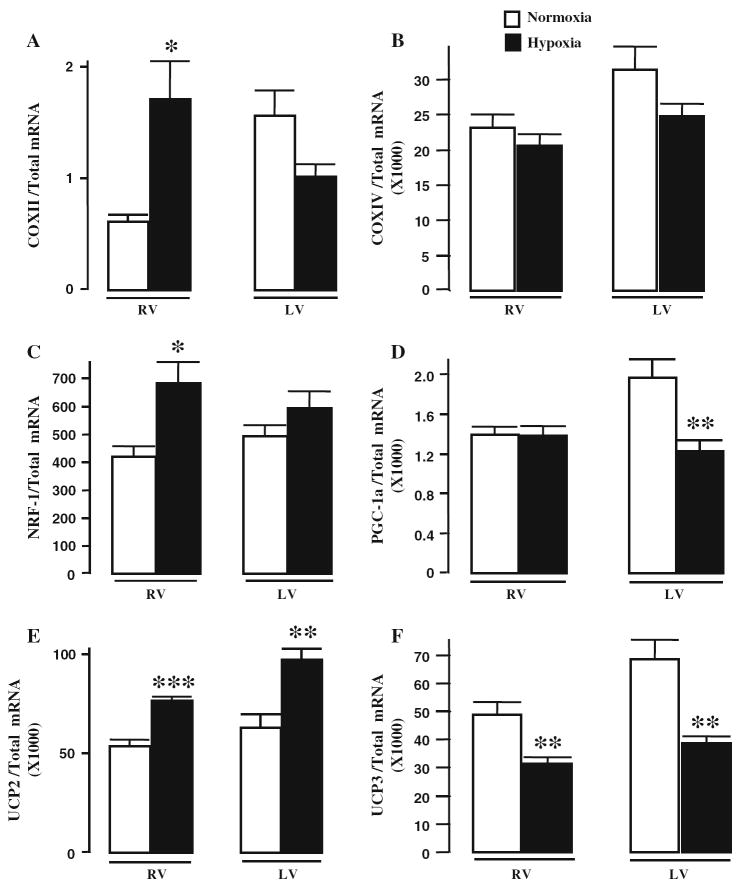
At the gene level we found that NRF-1, COXII, and UCP2 transcript levels were significantly increased in the hypertrophied RV, although PGC-1α was not different compared to controls (Fig. 1). For the LV, PGC-1α transcript levels were reduced by 38 ± 4% (P < 0.001 vs. normoxic control) while UCP2 expression was increased by 54 ± 3% (P < 0.01 vs. normoxic control). Transcript levels of COXIV were not significantly altered in either ventricle following 4 weeks of hypobaric hypoxia. UCP3 levels were reduced in RV by 36 ± 3% (P < 0.01 vs. normoxic control), and in the LV by 44 ± 3% (P < 0.01 vs. normoxic control).
Fig. 1.
Effects of hypobaric hypoxia on transcript levels of genes regulating mitochondrial respiratory function. (a) COXII, (b) COXIV, (c) NRF-1, (d) PGC-1α, (e) UCP2, and (f) UCP3. Bars represent means ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. normoxic controls (n = 6 per group)
Discussion
We hypothesized that exposure to CHH results in distinct remodeling of the right and LV, with the hypertrophied RV displaying enhanced mitochondrial respiratory capacity and contractile function. The main finding of this study is an induction of genes regulating mitochondrial function in the hypertrophied RV associated with improved efficiency of oxidative phosphorylation, enhanced respiratory function, and increased contractile function. Conversely, the LV displayed attenuated mitochondrial respiratory capacity without matching adaptive gene remodeling.
This study shows discrete responses of the right and LVs in response to CHH. Here we found a robust hypertrophic response in the RV after 4 weeks of hypobaric hypoxia accompanied by sustained contractile function. Since no visible fibrosis was previously found at this experimental time point [1], these data support a model of physiologic RV hypertrophy. At the mitochondrial level, polarographic oxygen consumption was sustained in the hypertrophied RV. Interestingly, the hypertrophied RV also displayed greater efficiency of mitochondrial oxidative phosphorylation (ADP/O) associated with reduced proton leak. Our data are in agreement with previous studies reporting sustained respiratory capacity in the hypertrophied RV in response to chronic hypobaric hypoxia [4, 5].
Since the LV is not challenged by increased load in our hypoxic model it is likely that elevated hematocrit levels [1] and/or neuroendocrine regulation are important factors regulating its response to chronic hypobaric hypoxia. Here we found decreased LV mitochondrial respiratory capacity and increased proton leak after 4 weeks exposure to chronic hypobaric hypoxia. However, our earlier work showed sustained LV mitochondrial respiratory function and unchanged proton leak after 2 weeks of hypobaric hypoxia [3]. Together these data indicate a temporal decline in LV mitochondrial function and efficiency in response to increasing hypobaric hypoxia exposure times. In agreement, others reported similar findings for the LV in response to chronic hypobaric hypoxia, albeit occurring at an earlier stage when compared to data from the present study. For example, Nouette-Gaulain et al. [5] demonstrated decreased mitochondrial electron transport chain enzyme activities in the LV following 14 days of hypobaric hypoxia. Furthermore, Rumsey et al. [4] found that oxidation of pyruvate and glutamate was diminished in the LV after 7 days of hypoxic exposure (10% O2). Adaptation of enzymes involved in cardiac energetics, total creatine kinase activity and mitochondrial creatine kinase were also lower in the LVs of rats exposed to 4 weeks of hypobaric hypoxia [15]. The precise mechanisms and rationale for attenuated mitochondrial respiratory capacity in the LV are unclear at present. It is possible that it may be due to lower ATP demand and supply pathways thereby allowing ATP levels to remain relatively constant even when ATP turnover rates decline [16, 17].
What are the mechanisms responsible for the differences in RV and LV mitochondrial function in response to 4 weeks of chronic hypobaric hypoxia? We propose that higher mitochondrial content in the RV is an important factor that sustains its respiratory capacity, and as a consequence RV contractile function. In agreement, we reported induction of several genes encoding mitochondrial regulatory proteins and also increased mtDNA in the hypertrophied RV in response to 2 weeks of hypobaric hypoxia [3]. Likewise, at the 4-week time point transcript levels for NRF-1, COX II, and UCP2 were coordinately upregulated in the hypertrophied RV while COX IV and PGC-1α levels were maintained. Although we did not measure mitochondrial number in the current study, we propose that the hypoxia-induced hypertrophied RV continues to adapt to the greater load by increasing the expression of mitochondrial proteins/number leading to enhanced respiratory capacity, more efficient mitochondrial energy production and sustained contractile function. We are of the opinion that the latter concept also applies more broadly, such as to adaptive LV hypertrophy. In support, higher mitochondrial content was found in hypertrophic hearts [18] while reduced activation of PGC-1α and/or transcriptional co-regulators such as estrogen-related receptor α is implicated in the development of pathophysiologic hypertrophy [19].
For the LV, PGC-1α transcript levels were significantly diminished and COXIV expression unchanged compared to controls after 4 weeks of hypobaric hypoxia. However, we previously reported that COXIV expression was significantly increased and PGC-1α transcript levels maintained versus controls after 2 weeks of hypobaric hypoxia [3]. Together these data suggest that LV mitochondrial numbers may indeed decline after 4 weeks of hypobaric hypoxia, thereby leading to attenuated mitochondrial respiratory capacity. Further studies are required to confirm this suggestion. At the functional level the LV displayed lowered developed pressures suggesting that decreased mitochondrial capacity may begin to have an impact on its contractile function. However, at this stage the LV is able to respond by modifying its heart rate to maintain overall contractile function.
We are unclear regarding the mechanisms responsible for increased proton leak in the LV in response to 4 weeks of hypobaric hypoxia. Here we suggest that chronic exposure to hypobaric hypoxia may result in greater, pathological levels of reactive oxygen species (ROS) that may in turn increase mitochondrial proton leak by prolonged opening of the mitochondrial permeability transition pore (mPTP) [20]. Conversely, we propose that the hypertrophied ventricle triggers adaptive signaling pathways that decrease mPTP opening and/or lower mitochondrial uncoupling thereby leading to greater efficiency of mitochondrial oxidative phosphorylation and counteracting the effects of pathological ROS. A potential explanation may be that diminished UCP3 expression in the hypertrophied RV is implicated in this process. However, this appears less likely since decreased UCP3 expression was also found in the hypoxic LV without a corresponding increase in mitochondrial efficiency. We also found increased UCP2 transcript levels in both right and LVs. UCPs are inner mitochondrial membrane proteins that play a role in uncoupling by transporting protons into the mitochondrial matrix [21]. It also been shown that UCPs also transport nonesterified fatty acid anions out of the mitochondrial matrix [22]. If UCP2 acts as a defensive mechanism against ROS as proposed by some [23, 24], this may reflect an adaptive mechanism to deal with greater oxidant stress in response to chronic hypobaric hypoxia. Further studies are needed to delineate the regulatory mechanisms directing varying mitochondrial efficiencies observed in the hypertrophied right and hypoxic LVs.
Limitations
We report somewhat low RPP values for the LV at baseline; however, they are not outside the range found in the literature [25-28]. The LVEDP was on the high end of the normal range as also reported by others [26], but was not different among groups studied. The perfused hearts in this study were not paced and thus there is an unexplained difference between the LV and RV, but importantly, there were no differences between heart rates for normoxic and hypoxic groups.
In summary, our study demonstrates distinct responses by the RV and LV to 4 weeks of exposure to hypobaric hypoxia. The LV exhibited decreased respiratory function associated with reduced PGC-1α transcript levels. However, we found that the hypertrophied RV induces expression of mitochondrial regulatory genes that are accompanied by increased respiratory function, mitochondrial efficiency and sustained contractility. We propose that the unique remodeling observed in the RV forms part of an adaptive, physiologic hypertrophic response after 4 weeks exposure to hypobaric hypoxia.
Acknowledgments
The authors wish to thank Mr. Noel Markgraaff for technical assistance. This work was supported by an NIH-Fogarty R03 TW07344 (to M. F. Essop and W. C. Stanley), the South African Medical Research Council and the South African National Research Foundation (to M.F. Essop).
References
- 1.Adrogue JV, Sharma S, Ngumbela K, Essop MF, Taegtmeyer H. Acclimatization to chronic hypobaric hypoxia is associated with a differential transcriptional profile between the right and left ventricle. Mol Cell Biochem. 2004;278:71–78. doi: 10.1007/s11010-005-6629-5. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S, Taegtmeyer H, Adrogue J, Razeghi P, Sen S, Ngumbela K, et al. Dynamic changes of gene expression in hypoxia-induced right ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2004;286:H1185–H1192. doi: 10.1152/ajpheart.00916.2003. [DOI] [PubMed] [Google Scholar]
- 3.Zungu M, Alcolea MP, Garcia-Palmer FJ, Young ME, Essop MF. Genomic modulation of mitochondrial respiratory genes in the hypertrophied heart reflects adaptive changes in mitochondrial and contractile function. Am J Physiol Heart Circ Physiol. 2007;293:H2819–H2825. doi: 10.1152/ajpheart.00806.2006. [DOI] [PubMed] [Google Scholar]
- 4.Rumsey WL, Abbott B, Bertelsen D, Mallamaci M, Hagan K, Nelson D, et al. Adaptation to hypoxia alters energy metabolism in rat heart. Am J Physiol. 1999;276:H71–H80. doi: 10.1152/ajpheart.1999.276.1.H71. [DOI] [PubMed] [Google Scholar]
- 5.Nouette-Gaulain K, Malgat M, Rocher C, Savineau JP, Marthan R, Mazat JP, et al. Time course of differential mitochondrial energy metabolism adaptation to chronic hypoxia in right and left ventricles. Cardiovasc Res. 2005;66:132–140. doi: 10.1016/j.cardiores.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Young ME. Circadian rhythms in cardiac gene expression. Curr Hypertens Rep. 2003;5:445–453. doi: 10.1007/s11906-003-0051-8. [DOI] [PubMed] [Google Scholar]
- 7.Sordahl LA, Besch HR, Allen JC, Crow C, Lindenmayer GE, Schwartz A. Enzymatic aspects of the cardiac muscle cell: mitochondria, sarcoplasmic reticulum and noncovalent cation active transport system. Methods Achiev Exp Pathol. 1971;5:287–346. [PubMed] [Google Scholar]
- 8.Essop MF, Razeghi P, McLeod C, Young ME, Taegtmeyer H, Sack MN. Hypoxia-induced decrease of UCP3 gene expression in rat heart parallels metabolic gene switching but fails to affect mitochondrial respiratory coupling. Biochem Biophys Res Commun. 2004;314:561–564. doi: 10.1016/j.bbrc.2003.12.121. [DOI] [PubMed] [Google Scholar]
- 9.Starnes JW, Barnes BD, Olsen ME. Exercise training decreases rat heart mitochondria free radical generation but does not prevent Ca2+-induced dysfunction. J Appl Physiol. 2007;102:1793–1798. doi: 10.1152/japplphysiol.00849.2006. [DOI] [PubMed] [Google Scholar]
- 10.Redout EM, Wagner MJ, Zuidwijk MJ, Boer C, Musters RJ, van Hardeveld C, et al. Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc Res. 2007;75:770–781. doi: 10.1016/j.cardiores.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Babsky A, Doliba N, Doliba N, Saychenko A, Wehrli S, Osbakken M. Na+ effects on mitochondrial respiration and oxidative phosphorylation in diabetic hearts. Exp Biol Med. 2001;226:543–551. doi: 10.1177/153537020122600606. [DOI] [PubMed] [Google Scholar]
- 12.Estabrook R. Mitochondrial respiratory control and the polarographic measurement of ADP/Oratios. Methods Enzymol. 1967;10:41–47. doi: 10.1016/0076-6879(67)10010-4. [DOI] [Google Scholar]
- 13.Lowry OH, Rosenbrough N, Farr AL, Radall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Stavinoha MA, Rayspellicy JW, Hart-Sailors ML, Mersmann HJ, Bray MS, Young ME. Diurnal variations in the responsiveness of cardiac and skeletal muscle to fatty acids. Am J Physiol Endocrinol Metab. 2004;287:E878–E887. doi: 10.1152/ajpendo.00189.2004. [DOI] [PubMed] [Google Scholar]
- 15.Pissarek M, Bigard X, Mateo P, Guezennec CY, Hoerter JA. Adaptation of cardiac myosin and creatine kinase to chronic hypoxia: role of anorexia and hypertension. Am J Physiol. 1997;272:H1690–H1695. doi: 10.1152/ajpheart.1997.272.4.H1690. [DOI] [PubMed] [Google Scholar]
- 16.Hochachka PW, Lutz PL. Mechanism, origin, and evolution of anoxia tolerance in animals. Comp Biochem Physiol B Biochem Mol Biol. 2001;130:435–459. doi: 10.1016/S1096-4959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- 17.Essop MF. Cardiac metabolic adaptations in response to chronic hypoxia. J Physiol. 2007;584:715–726. doi: 10.1113/jphysiol.2007.143511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goffart S, von Kleist-Retzow JC, Wiesner RJ. Regulation of mitochondrial proliferation in the heart: power plant failure contributes to cardiac failure in hypertrophy. Cardiovasc Res. 2004;64:198–207. doi: 10.1016/j.cardiores.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 19.Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, et al. The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Essop MF, Opie LH. Metabolic therapy for heart failure. Eur Heart J. 2004;25:1765–1768. doi: 10.1016/j.ehj.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Jezek P. Fatty acid interaction with mitochondrial uncoupling proteins. J Bioenerg Biomembr. 1999;31:457–466. doi: 10.1023/A:1005496306893. [DOI] [PubMed] [Google Scholar]
- 22.Jezek P, Engstová H, Zácková M, Vercesi AE, Costa ADT, Arruda P, et al. Fatty acid cycling mechanism and mitochondrial uncoupling proteins. Biochim Biophys Acta. 1998;1365:319–327. doi: 10.1016/S0005-2728(98)00084-X. [DOI] [PubMed] [Google Scholar]
- 23.Teshima Y, Akao M, Jones SP, Marban E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ Res. 2003;93:192–200. doi: 10.1161/01.RES.0000085581.60197.4D. [DOI] [PubMed] [Google Scholar]
- 24.Horimoto M, Fulop P, Derdak Z, Wands JR, Baffy G. Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology. 2004;39:386–392. doi: 10.1002/hep. 20047. [DOI] [PubMed] [Google Scholar]
- 25.Chatham JC, Seymour AM. Cardiac carbohydrate metabolism in Zucker diabetic fatty rats. Cardiovasc Res. 2002;55:104–112. doi: 10.1016/S0008-6363(02)00399-1. [DOI] [PubMed] [Google Scholar]
- 26.Milano G, Corno AF, Lippa S, von Segesser LK, Samaja M. Chronic and intermittent hypoxia induce different degrees of myocardial tolerance to hypoxia-induced dysfunction. Exp Biol Med. 2002;227:389–397. doi: 10.1177/153537020222700604. [DOI] [PubMed] [Google Scholar]
- 27.Kurzelewski M, Duda M, Stanley WC, Boemke W, Beresewicz A. Nitric oxide synthase inhibition and elevated endothelin increase oxygen consumption but do not affect glucose and palmitate oxidation in the isolated rat heart. J Physiol Pharmacol. 2004;55:27–38. [PubMed] [Google Scholar]
- 28.Mozaffari MS, Patel C, Ballas C, Schaffer SW. Effects of excess salt and fat intake on myocardial function and infarct size in rat. Life Sci. 2006;78:1808–1813. doi: 10.1016/j.lfs.2005.08.014. [DOI] [PubMed] [Google Scholar]