Abstract
Circadian rhythms, endogenously generated by the suprachiasmatic nucleus (SCN), can be synchronized to a variety of photic and non-photic environmental stimuli. Neuropeptide Y (NPY) is produced in the intergeniculate leaflet (IGL) and known to mediate both photic and non-photic influences on the SCN. We recently found that npy-/- mice were slower to shift their locomotor activity onset to the new time of light offset when photoperiod was abruptly changed from light/dark (LD) cycle 18:6 to LD 6:18. In the present study, we measured the locomotor response of npy-/- mice to gradual changes in photoperiod (4 min a day) for 141 days (LD 16:8 changing to LD 8:16), mimicking external LD cycles in nature. When the photoperiod approached LD 8:16, npy-/- mice showed a significantly delayed onset of activity compared to wild-type mice. Activity patterns disintegrated into multiple bouts and intensity of activity decreased as the photoperiod changed and these changes were more pronounced in npy-/- mice. Our results lend further support to the idea that NPY is involved in circadian entrainment responses to seasonal photoperiod changes.
Keywords: Neuropeptide Y, Photoperiod, Circadian rhythm, Intergeniculate leaflet, Entrainment
1. INTRODUCTION
Photoperiodic responses depend on circadian entrainment. In nature, animals are exposed to daily changes in the time of dawn and dusk. These small changes in day length are translated into changes in the duration of nocturnal secretion of pineal melatonin, an important signal for transduction of the environmental daylength to adaptive physiological responses (Goldman, 2001). Duration of melatonin secretion is strongly correlated with duration of nocturnal locomotor activity in hamsters, and changes in phase of locomotor activity relative to the light:dark cycle are paralleled by changes in phase of melatonin secretion to the light:dark cycle (Elliott and Tamarkin, 1994). The phase relationship between the changing external day:night cycle and the internal circadian clock allows animals to detect seasonal changes (Elliott, 1976; Goldman, 2001).
Whereas entrainment is largely mediated via the retino-hypothalamic tract input to suprachiasmatic nucleus (SCN) of the hypothalamus, an input pathway from the intergeniculate leaflet (IGL) of the thalamus appears to play an important role in entrainment and photoperiodic responses to short days. In Siberian hamsters, IGL ablation resulted in significantly shorter total duration of activity (alpha) and more negative phase angles of entrainment when exposed to a decreasing simulated natural photoperiod (Freeman et al., 2004). These changes in circadian entrainment were associated with reduced likelihood of molting to a winter pelage (Freeman et al., 2004). IGL-ablated hamsters were reported to demonstrate reduced hibernation after transfer to cold temperatures and a short photoperiod (Menet et al., 2003). A role for the IGL in discriminating dusk and dawn and integrating photoperiodic information by the SCN has been suggested by other studies (Jacob et al., 1999; Menet et al., 2001; Shinohara et al., 1993; Smale and Morin, 1990).
The IGL is thought to mediate these influences on the SCN through neuropeptide Y (NPY) along with other neurotransmitters (Harrington, 1997). The Syrian hamster shows an increase in the number of neurons expressing NPY when hamsters are housed under short photoperiods (Jacob et al., 1998). We recently found that npy-deficient (npy-/-) mice were slower to shift their locomotor activity onset to the new light/dark cycle than WT mice when photoperiod was altered abruptly from LD 18:6 to LD 6:18 (Harrington et al., 2007). In that study the mice were not housed with running wheels during the time of the switch in photoperiod, but the motion sensor activity records indicated the npy-/- mice took approximately 10 days longer to re-entrain to the short day photoperiod, and adopted a more negative phase angle of entrainment under LD 6:18 relative to WT animals (i.e., time of activity onset was delayed relative to WT). In the present study, we examined whether npy-/- mice would show different timing of activity onset when photoperiod very gradually changed from LD 16:8 to LD 8:16, more realistically mimicking photoperiodic changes in nature. We also examined the wheel-running records for changes in intensity and fragmentation of daily activity.
2. RESULTS
Baseline measures were taken while WT and npy-/- mice were entrained to LD 16:8 for 2 weeks and no differences were observed between the groups. Following this, we changed the day length by 4 min a day (delayed the light offset by 2 min and advanced the light onset by 2 min) until reaching LD 8:16. Locomotor activity of WT and npy-/- mice as assessed with running-wheel records in response to these gradual changes revealed differences in the change of the phase angle of entrainment, intensity of locomotor activity, and fragmentation of daily activity into multiple bouts (Fig 1).
Figure 1.
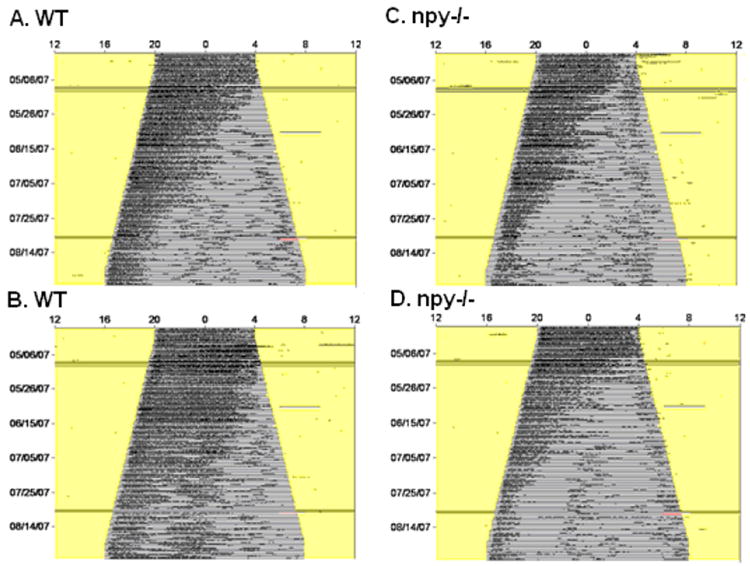
Representative actograms of WT (A and B) and npy-/- (C and D) mice. Actograms show days on the y-axis and 24 hours on the x-axis. The activity levels are marked by black bars and the number of wheel revolution is plotted in 6-min bins. The three black lines across each actogram indicate compute malfunctioning, but the light cycle was not affected by the computer problems on these days. Notice that activity of both groups started to fragment into smaller bouts as the day length gradually changed from LD 16:8 to LD 8:16.
The phase angle of entrainment gradually become more negative as the photoperiod shortened, and npy-/- mice showed a significantly delayed onset of activity compared to WT mice (the difference of phase angle of entrainment between LD 16:8 and LD 8:16 ; WT -15.4 min vs. npy-/- mice -42.9 min; t(17) = -2.11, P < 0.05) (Fig 2).
Figure 2.
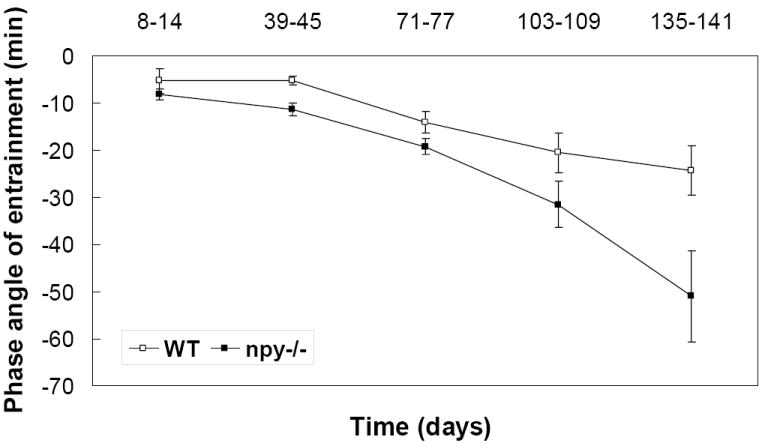
Delayed phase angle of entrainment. Phase angles of entrainment was recorded over 5 one-week periods selected at 3-5 week intervals throughout the experiment, and the mean (± S.E.M.) was used to compare between two groups. In the final week (134-141 days) of the experiment, the npy-/- mice showed a significantly delayed onset of activity compared to WT mice (the difference of phase angle of entrainment between start point and end point of the treatment WT -15.4 min vs. npy-/- mice -42.9 min; t(17) = -2.11, P < 0.05). Groups did not significantly differ before this time point.
The intensity of locomotor activity, measured as the number of running-wheel revolutions per min (rev/min), decreased as photoperiod decreased, with npy-/- mice showing a more dramatic reduction in activity (npy-/- mice decreased by 9.25 ± 14.56 rev/min; WT mice decreased by 4.54 ± 19.35 rev/min; t(16) = -2.41, P < 0.05) (Fig 3A). Groups were significantly different on Days 71-77, 103-109, and 135-141. The reduction in intensity of activity was accompanied by fragmentation of daily wheel-running activity. The number of daily activity bouts increased as the dark period increased, and the number of bouts in records from npy-/- mice was significantly greater than that seen in records from WT mice on Days 39-45 and Days 135-141 (see Fig 3). The final number of bouts for WT mice was 4.38 ± 0.42 bouts/day vs. npy-/- 5.58 ± 0.34 bouts/day; t(18) = 2.26, P < 0.05). Total duration of activity (alpha) increased as photoperiod decreased for both groups similarly (P > 0.5) (Fig 3B). Thus, our results suggest that npy-/-mice show reduced level of activity and increased rate at which activity fragments into multiple bouts within the prolonged dark phase as photoperiod decreases.
Figure 3.
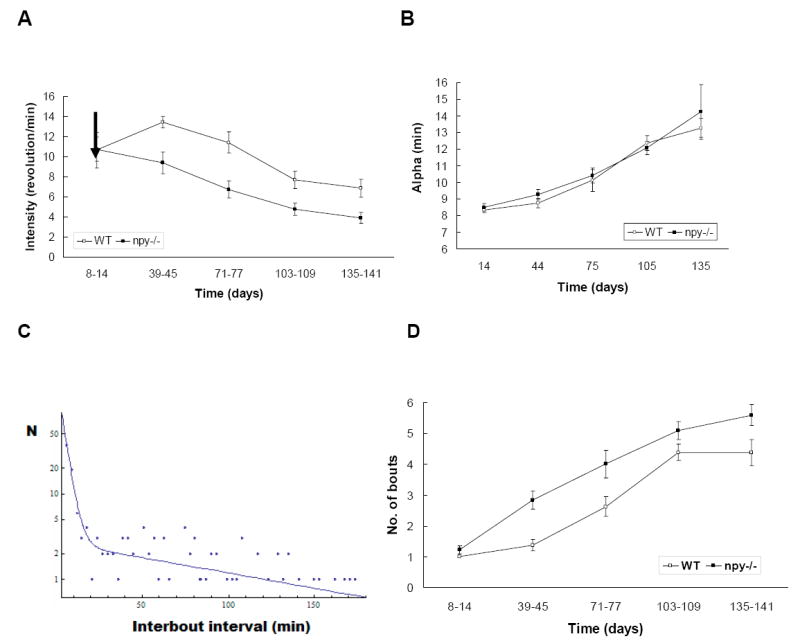
(A) Intensity of activity was measured as average wheel running counts per min. Mean (± S.E.M.) intensity data recorded for one week, and five data points were selected with 3-5 week intervals throughout the experiment. (B) Mean (± S.E.M.) of total duration of activity (alpha) of WT and npy-/- mice at 5 time points was selected from 141 days of experiment. (C) First 3h segment of the log-survivorship curve based on the analysis of intervals between discrete wheel-running events recorded over a period of 14 days from 8 young male WT mice under LD 16:8. On the X axis we plot results for varied interbout intervals and the Y axis shows the number of instances showing that interval, using a block size of 3 min and a threshold of 4 counts/min. The position where the slope changes dramatically defines the minimum time between bouts of activity, giving our 27-min bout criterion interval (see Penev et al., 1997). (D) The numbers of daily activity bouts (mean ± S.E.M.) recorded over 5 weeks selected throughout the photoperiod treatment. The number of bouts showed an increase throughout the experiment, but npy-/- mice exhibited more activity fragments compared to WT (the final number of bouts WT 4.38 ± 0.42 vs. npy-/- 5.58 ± 0.34; t(18) = 2.26, P < 0.05).
3. DISCUSSION
Our results support that NPY is involved in photoperiodic time measurement and responsiveness. We replicated our prior finding that npy-/- mice entrain to LD 8:16 with a delayed phase of entrainment (Harrington et al., 2007). This finding is apparently robust in that the prior study used an abrupt change of photoperiod and no access to running wheels, but produced the same difference in phase of entrainment. We interpret these changes in phase angle of entrainment as arising from altered circadian period and photic and non-photic responsiveness in npy-/- mice (Harrington et al., 2007). IGL ablation produced a similar effect in Siberian hamsters (Freeman et al., 2004). The hamsters in that study also showed shorter total duration of activity following IGL ablation, whereas our npy-/- mice did not differ in this measure, although by the end of our experiment the measures of activity duration showed high variability. The npy-/-mice showed reduced intensity of activity and greater fragmentation of wheel-running activity into multiple bouts as compared to the WT mice. Since our study continued over a 20-week period, we could not avoid the possibility that changes in locomotor activity might be age-related; our animals were more than 180 days old by the end of the experiment. Several studies indicated that considerable fragmentation in the diurnal rhythm of locomotor activity with advanced age in golden hamsters and C57BL/6 mice (Valentinuzzi et al., 1996; Penev et al., 1997). The activity fragmentation might also be related to weight gain during the photoperiod experiment. Although the amount of body weight gain as day length shortened was not statistically different between two animal groups, average body weight of npy-/- mice was always larger than that of WT (see Supplementary Figure 1). If age or body weight explain our results then it would be necessary to posit that npy-/- mice are more susceptible than WT mice to the effects of these variables on circadian parameters; at this moment we do not know of any evidence that suggests this to be the case.
Because the npy-/- model is a mouse, not a reproductively photoperiodic animal, one might question the relevance of our findings. We believe that the behavioral changes mice show in response to a change in photoperiod, such as a change in phase angle of entrainment, are fundamentally related to the reproductive responses of animals such as hamsters that show major changes in reproductive response depending on the photoperiod. On the other hand it is an open question if the role of the NPY input pathway is the same in both animals; our results suggest that there are at least some commonalities in the role of this pathway in modulating circadian responses to photoperiod.
The results of IGL ablation revealed a role of the IGL in synchronizing physiological seasonal adjustments (e.g., gonadal regression, and pelage molt) elicited by exposure to decreasing day lengths (Freeman et al., 2004) and in processing nonphotic cues that can alter the photoperiodic response (Freeman et al., 2006). Moreover, the number of NPY mRNA containing IGL neurons significantly increased in a short photoperiod compared to those kept in a long photoperiod (Jacob et al., 1989). Many prior studies of IGL function were conducted in long photoperiod but these results suggest this input pathway to the SCN may play a more important role in regulating circadian responses to short photoperiod.
4. EXPERIMENTAL PROCEDURES
Animals
The 129S6 npy-/- mice were back-crossed for 15 generations onto a C57BL/6 background at Taconic Farms (Germantown, NY; B6.129-npytm1 N15) and provided by E. Maratos-Flier (Harvard Medical School, Boston, MA) (Segal-Liberman et al., 1990). Mice were weaned at 3 wk and housed under LD 12:12. Male WT mice (n = 8) and littermate npy-/- mice (n = 12) were 6 ± 1 weeks of age at the start of the experiment. Food and water were available ad libitum. The experimental protocol was reviewed and approved by the Smith College Institutional Animal Care and Use Committee.
Locomotor Activity Measurement
Mice were housed in cages fitted with running wheels (15 cm in diameter). Wheel revolutions were constantly monitored using Clocklab computer software, with 1-min sampling epochs (Actimetrics, Evanston, IL). Mice were initially placed in LD 16:8 for 14 days until they showed synchronized circadian rhythms (lights on 0500-2100). An automated light timer (Thermo Fisher Scientific Inc., Waltham, MA) was programmed to simulate natural photoperiod changes experienced from July 16 to November 16 at 55°N latitude. The time of light offset was advanced by 2 min a day, and the time of light onset was delayed by 2 min a day until the light/dark cycle reached LD 8:16 (lights on 0900-1700). Total duration of the study was 141 days, including the initial 14 days of baseline. Standard overhead room lighting (50 lux) used for entrainment and maintenance, and dim red light (<.1 lux) was used for routine checks of the animals.
Behavioral Analysis and Statistics
The patterns of wheel-running activity, recorded over a 16-week period of npy-/- mice were compared with that of WT animals. Each activity pattern was characterized by several parameters, including time of onset and offset, total duration of the activity phase, intensity, and total number of wheel revolutions. All parameters were determined with Clocklab software. The phase angle of entrainment was defined as the difference in hours between the time of light offset and activity onset. A positive value indicates that activity onset preceded light offset, whereas negative value indicates that activity began subsequent to light offset. We applied the procedure proposed by Penev et al. (1997) to define a minimal bout criterion interval (between-bout time criterion) using the activity data of WT mice (n = 8) over 7-day period before the photoperiod treatment. A block size of 3 min and threshold of 4 counts/min were optimal to determine the bout criterion interval for the experiment. The resulting function confirmed the presence of discrete clusters of wheel-running behavior in mice activity records and was used to select a 27-min bout criterion interval (Fig 3C).
All results throughout the text are reported as means ± standard error (SE). For comparison of the two groups over time, data were analyzed by analysis of variance (ANOVA) followed by post-hoc tests of Dunnet or Bonferroni multiple comparison. All statistical tests were carried out using MatLab. A value of p < 0.05 was regarded as statistically significant.
Supplementary Material
Body weight in WT and npy-/- mice. Average body weight (mean ± S.E.M.) was recorded during the photoperiod treatment. Body weight increased in both groups as day length shortened. Although the amount of weight gain was not significantly different between the two animal groups (weight gain during the experiment WT 9.36g vs. npy-/- 7.10g; t(18) = 1.80, P = 0.089), average body weight of npy-/- mice was always larger than that of WT.
Acknowledgments
This work was funded by a Schultz Undergraduate Research Fellowship awarded to Hyun Jung Kim and by NIH R15MH074591 (MEH). We would like to thank Penny Molyneux, Dr. Joanne Huyler, Victoria Flood, and Donna Ewell (Smith College Animal Quarters). We would also like to thank Professor Tanya Leise at Amherst College for help with log-survivorship analysis.
Abbreviations
- NPY
Neuropeptide Y
- LD
light-dark
- SCN
Suprachiasmatic nucleus
- RHT
retinohypothalamic tract
- GHT
geniculohypothalamic tract
- IGL
intergeniculate leaflet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Elliott JA. Circadian rhythms and photoperiodic time measurement in mammals. Fed Proc. 1976;35:2339–46. [PubMed] [Google Scholar]
- Elliott JA, Tamarkin L. Complex circadian regulation of pineal melatonin and wheel-running in Syrian hamsters. J Comp Physiol [A] 1994;174:469–484. doi: 10.1007/BF00191713. [DOI] [PubMed] [Google Scholar]
- Freeman DA, Dhandapani KM, Goldman BD. The thalamic intergeniculate leaflet modulates photoperiod responsiveness in siberian hamsters. Brain Res. 2004;1028:31–38. doi: 10.1016/j.brainres.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Freeman DA, Teubner BJ, Goldman BD. The thalamic intergeniculate leaflet mediates locomotor activity-induced reversal of phenotype in photoperiod nonresponsive siberian hamsters. J Biol Rhythms. 2006;21:206–213. doi: 10.1177/0748730406287996. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: Formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Harrington ME. The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci Biobehav Rev. 1997;21:705–727. doi: 10.1016/s0149-7634(96)00019-x. [DOI] [PubMed] [Google Scholar]
- Harrington M, Molyneux P, Soscia S, Prabakar C, McKinley-Brewer J, Lall G. Behavioral and neurochemical sources of variability of circadian period and phase: Studies of circadian rhythms of npy-/- mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:1306–14. doi: 10.1152/ajpregu.00383.2006. [DOI] [PubMed] [Google Scholar]
- Jacob N, Vuillez P, Moller M, Pevet P. Photoperiodic dependent changes in the number of neurons containing mRNA encoding neuropeptide Y in the intergeniculate leaflet of the syrian hamster. Brain Res. 1998;813:160–166. doi: 10.1016/s0006-8993(98)01032-4. [DOI] [PubMed] [Google Scholar]
- Jacob N, Vuillez P, Lakdhar-Ghazal N, Pevet P. Does the intergeniculate leaflet play a role in the integration of the photoperiod by the suprachiasmatic nucleus? Brain Res. 1999;828:83–90. doi: 10.1016/s0006-8993(99)01324-4. [DOI] [PubMed] [Google Scholar]
- Menet J, Vuillez P, Jacob N, Pévet P. Intergeniculate leaflets lesion delays but does not prevent the integration of photoperiodic change by the suprachiasmatic nuclei. Brain Res. 2001;906:176–179. doi: 10.1016/s0006-8993(01)02518-5. [DOI] [PubMed] [Google Scholar]
- Menet JS, Vuillez P, Saboureau M, Pévet P. Inhibition of hibernation by exercise is not affected by intergeniculate leaflets lesion in hamsters. Am J Physiol Regul Integr Comp Physiol. 2003;285:R690–700. doi: 10.1152/ajpregu.00068.2003. [DOI] [PubMed] [Google Scholar]
- Penev PD, Zee PC, Turek FW. Quantitative analysis of the age-related fragmentation of hamster 24-h activity rhythms. Am J Physiol. 1997;273:2132–7. doi: 10.1152/ajpregu.1997.273.6.R2132. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Tominaga K, Fukuhara C, Otori Y, Inouye SI. Processing of photic information within the intergeniculate leaflet of the lateral geniculate body: assessed by neuropeptide Y immunoreactivity in the suprachiasmatic nucleus of rats. Neuroscience. 1993;56:813–22. doi: 10.1016/0306-4522(93)90129-4. [DOI] [PubMed] [Google Scholar]
- Segal-Lieberman G, Trombly DJ, Juthani V, Wang X, Maratos-Flier E. NPY ablation in C57BL/6 mice leads to mild obesity and to an impaired refeeding response to fasting. Am J Physiol Endocrinol Metab. 1990;284:1131–1139. doi: 10.1152/ajpendo.00491.2002. [DOI] [PubMed] [Google Scholar]
- Smale L, Morin LP. Photoperiodic responsiveness of hamsters with lesions of the lateral geniculate nucleus is related to hippocampal damage. Brain Res Bull. 1990;24:185–90. doi: 10.1016/0361-9230(90)90204-d. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol. 1997;273:R1957–64. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Body weight in WT and npy-/- mice. Average body weight (mean ± S.E.M.) was recorded during the photoperiod treatment. Body weight increased in both groups as day length shortened. Although the amount of weight gain was not significantly different between the two animal groups (weight gain during the experiment WT 9.36g vs. npy-/- 7.10g; t(18) = 1.80, P = 0.089), average body weight of npy-/- mice was always larger than that of WT.


