Abstract
This study investigated whether Anopheles gambiae s.l. and Anopheles funestus Giles mosquito populations were distributed randomly among houses on the coast of Kenya. Sample means and variances of mosquitoes were estimated from bimonthly pyrethrum spray collections at 30 villages from July 1997 through May 1998. In total, 5,476 An. gambiae s.l. and 3,461 An. funestus were collected. The number of An. gambiae s.l. collected was highest in November/December and lowest in May. The number of An. funestus collected was highest during September/October and lowest during May. As the density of mosquitoes decreased, there was a tendency toward randomness in the distribution. The proportion of An. gambiae s.l. and An. funestus mosquitoes collected per house for each sampling period also showed patterns of clustering, with 80% of An. gambiae s.l. collected from <30% of the houses and 80% of An. funestus collected from <20% of the total houses. The total number of mosquitoes collected from any one house ranged from 0 to 121 for An. gambiae s.l. and from 0 to 152 for An. funestus. This coupled with the results of the variance to mean ratio plots suggests extensive clustering in the distribution of An. gambiae s.l. and An. funestus mosquito populations throughout the year along the coast of Kenya.
Keywords: Anopheles, mosquito distribution, Kenya, malaria
Stable endemic malaria is a serious public health threat for much of sub-Saharan Africa (SSA), with ≈70% of the population living in areas infested with malaria vectors, mainly Anopheles gambiae s.l. and Anopheles funestus Giles. Between 200 and 300 million cases of malaria are reported each year in SSA (WHO 2000). This underscores the importance of understanding mosquito population dynamics and corresponding malaria parasite transmission to obtain significant reductions in transmission intensity.
Malaria transmission intensity can be expressed as the entomological inoculation rate (EIR), which is the product of the mosquito biting rate and the proportion of infective mosquitoes (MacDonald 1957). This indicator measures the exposure of humans to Plasmodium falciparum parasites and serves as a proxy for malaria parasite transmission intensity. EIRs can range from <1 to >1000 infective bites per year in many parts of Africa (Beier et al. 1999, Robert et al. 2003). Community-based estimates of EIR may show tremendous variability within small geographic regions (Mbogo et al. 1993, 1995) as a result of different human and physical environmental characteristics, as well as disparate measurement methodologies, thus further challenging community-based malaria control efforts.
Because the relationship between the sample means and sample variances generally obeys a power law, which provides a quantitative measure of insect distributions at specific locations, and insect populations are rarely distributed randomly in nature (Taylor 1961), detecting where and when disease vectors aggregate within and among communities is possible. Service (1971) investigated the biological distribution of larvae of the An. gambiae complex in Kisumu, Kenya, and Kaduna, Nigeria, and detected highly clustered larval distributions within habitats. Interestingly, Service (1971) also found that the density of predators in the larval habitats influenced adult mosquito distributions, and predator density varied as a function of habitat size and the level of habitat permanence. Other studies also have identified nonrandom distributions of adult Anopheles mosquitoes (Omer and Cloudsley-Thompson 1970, Ribeiro et al. 1996).
Determining how Anopheles mosquitoes cluster allows us to identify which areas have the highest transmission intensity potential. That information can be used to design targeted vector control programs. Moreover, identifying houses, or communities, with a high propensity to harbor Anopheles mosquitoes could assist with the development of novel methods for standardizing surveillance or control tools in areas with low mosquito densities or highly aggregated mosquito populations. This article examines the distribution of An. gambiae s.l. and An. funestus mosquitoes in relation to households at 30 villages in three districts along the coast of Kenya and builds upon previous research by Mbogo et al. (2003).
Materials and Methods
Study Sites
Malaria transmission along the coast of Kenya has been the subject of several entomological and epidemiological studies over the past decade (Mbogo et al. 1993, 1995, 1999; Snow et al. 1993, 1998). This analysis uses a subset of data collected at 30 villages in three districts of Coast Province (Malindi, Kilifi, and Kwale) in Kenya, as described by Mbogo et al. (2003). The coast of Kenya has two distinct rainy seasons, from April to June and from October to November. Precipitation levels vary from 750 to 1,200 mm/yr. Mean daily minimum and maximum temperatures are 22 and 30°C, respectively, as reported by the Mtwapa Meteorological Research Station, 20 km north of Mombasa.
Houses in the selected rural villages are mainly of two types: the Mijikenda style house and the Swahili style house. The Mijikenda style house uses framed wooden poles with grass attached as the primary wall material and palm leaves or grass as a roofing material. Mud is often used to help support the roof. The Swahili style house also has framed wooden poles and uses mud or coral as the primary wall material. Thatched and dried coconut leaves serve as the primary roofing material. Both styles can be either multiple- or single-room structures.
Mosquito Collections
Mosquito sampling was conducted at each of the 30 villages once every 2 mo between July 1997 and May 1998. Mosquitoes were collected from inside 10 houses, within a 2-km radius of the nearest primary school, by using the pyrethrum spray collection technique (WHO 1975). The rationale for sampling houses within a 2-km radius of the nearest primary school was to ensure that children were included as residents of selected households, because investigating malaria prevalence among children and transmission intensity was one objective of a related study (unpublished data). Mosquito collections were conducted in the afternoons (12:00–3:00 p.m.). Collected mosquitoes were placed in tubes, preserved in Carnoy's solution (3:1, acetic acid:glycerol), and brought to the laboratory for identification. Carnoy's solution was used to support chromosomal determinations of anophelines, as reported by Mbogo et al. (2003). Morphological characters were used to identify An. gambiae s.l. and An. funestus mosquitoes (Gillies and DeMeillon 1968).
Data Analysis
Because mosquitoes were collected at each village every 2 mo, we divided the study period into six sampling periods: 1) July/August, 2) September/October, 3) November/December, 4) January/February, 5) March/April, and 6) May. Household mosquito data within each village were used to calculate the mean household mosquito density and corresponding variance at that village for each sample period (total n = 165). Analysis of variance (ANOVA) was conducted with log-transformed data for each sampling period to determine whether the mean household mosquito densities per village differed among districts. The estimated sample means were plotted against the corresponding estimated sample variances per village for each bimonthly sampling interval, and the ratio of the variance to the mean number of An. gambiae s.l. and An. funestus collected per village was used as one indicator of the level of mosquito aggregation (Southwood 1978). Values >1 indicate that the distribution is nonrandom, with higher numbers indicating increased aggregation.
Clustering of An. gambiae s.l. and An. funestus mosquitoes at the household level was investigated by plotting the ranked cumulative distribution of the total number of mosquitoes collected against its respective proportion of houses in which mosquito samples were taken. Houses were ranked according to the number of mosquitoes collected. Proportions were calculated by dividing the cumulative number of mosquitoes collected per house by the total number collected. Likewise, house proportions were calculated by dividing the cumulative number of houses by the total number of houses in the sampling period in which mosquito collections took place, regardless of whether mosquitoes were present. All data were analyzed using Microsoft Excel and SPSS 10 statistical software programs (SPSS Inc., Chicago, IL).
Results
In total, 5,476 An. gambiae s.l. and 3,461 An. funestus mosquitoes were collected from the 30 villages over the course of 11 mo. The number of An. gambiae s.l. collected was highest in November/December and lowest during May. The number of An. funestus collected was highest during September/October and lowest during May. The total number of mosquitoes collected from any one house ranged from 0 to 121 for An. gambiae s.l. and from 0 to 152 for An. funestus, with the highest number per house being collected in Kagombani village (November/December) in Malindi district and Magaoni village (July/August) in Kwale district, respectively. Table 1 presents summary statistics for An. gambiae s.l. and An. funestus mosquito densities.
Table 1.
Mean number of An. gambiae s.l. and An. funestus per house per village for each sampling period
| Kwale | Sampling period | Mean An. gambiae s.l. | SD | Mean An. funestus | SD | Villages sampled |
|---|---|---|---|---|---|---|
| 1 | July/Aug. | 2.85 | 3.29 | 5.04 | 12.12 | 10 |
| 2 | Sept./Oct. | 0.22 | 0.43 | 2.00 | 3.89 | 10 |
| 3 | Nov./Dec. | 5.86 | 4.88 | 10.01 | 7.88 | 7 |
| 4 | Jan./Feb. | 0.78 | 1.46 | 3.49 | 6.48 | 10 |
| 5 | Mar./April | 0.38 | 0.64 | 0.90 | 1.25 | 9 |
| 6 | May | 0.08 | 0.22 | 2.67 | 2.59 | 5 |
| Malindi | ||||||
| 1 | July/Aug. | 6.11 | 6.56 | 0.03 | 0.01 | 10 |
| 2 | Sept./Oct. | 2.11 | 3.85 | 0.36 | 0.70 | 10 |
| 3 | Nov./Dec. | 21.81 | 11.75 | 1.09 | 1.32 | 10 |
| 4 | Jan./Feb. | 1.73 | 1.87 | 0.47 | 0.50 | 10 |
| 5 | Mar./April | 0.17 | 1.16 | 0.23 | 0.66 | 10 |
| 6 | May | 0.54 | 0.42 | 0.20 | 0.16 | 5 |
| Kilifi | ||||||
| 1 | July/Aug. | 1.59 | 1.72 | 1.52 | 2.32 | 10 |
| 2 | Sept./Oct. | 0.72 | 1.00 | 1.10 | 2.07 | 10 |
| 3 | Nov./Dec. | 6.10 | 5.90 | 4.30 | 4.75 | 9 |
| 4 | Jan./Feb. | 0.46 | 0.79 | 1.10 | 1.99 | 10 |
| 5 | Mar./April | 0.41 | 1.16 | 0.25 | 0.41 | 10 |
| 6 | May | 2.72 | 3.31 | 0.50 | 0.64 | 10 |
Analysis of a subset of An. gambiae complex mosquitoes by polymerase chain reaction showed that An. gambiae s.s. was the predominant vector species at the 30 sites, accounting for >80% of those tested. However, An. funestus, Anopheles merus Dönitz, and Anopheles arabiensis Patton also were found in all three districts. Mbogo et al. (2003) report additional information about the composition and abundance of species within the An. gambiae complex.
Results from the ANOVA indicate that differences in the mean An. gambiae s.l. mosquito density differed between districts in November/December (F = 7.414; df = 2, 23; P < 0.05). Results from a posthoc comparison suggest that Malindi district has higher mean densities in period three as compared with both Kwale and Kilfi districts, although the means did not differ between Kwale and Kilifi. An. funestus mosquito densities also differed between districts during November/December (F = 4.42; df = 2, 23; P < 0.05). During May, An. gambiae s.l. densities differed between districts (F = 4.14; df = 2.9; P < 0.05), in which Kwale district showed higher mean densities than both Malindi and Kilfi districts.
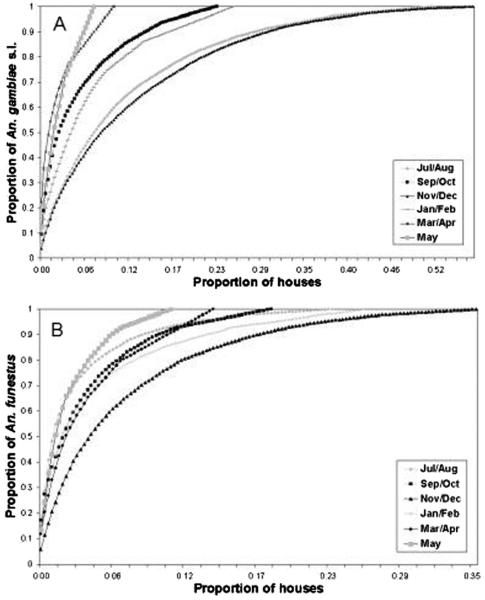
Throughout the six sampling periods, 70% of An. gambiae s.l. mosquitoes and 80% of An. funestus mosquitoes were collected from <20% of the total number of houses sampled (Fig. 1). During November/December, 80% of both An. gambiae s.l. and An. funestus were collected from <30% of the total number of houses sampled. Zero mosquito counts were obtained in >50% of the houses sampled in most sampling periods. Clustering within houses was not specific to any one house throughout the study period. Houses harboring 85% of the mosquitoes in November/December had <20% of the total number of mosquitoes collected during all other periods. This pattern further illustrates the complexity of within-village variation in the distribution of anopheline mosquitoes.
Fig. 1.
Seasonal cumulative distribution of An. gambiae s.l. (A) and An. funestus (B) mosquitoes collected from houses within the 30 coastal villages.
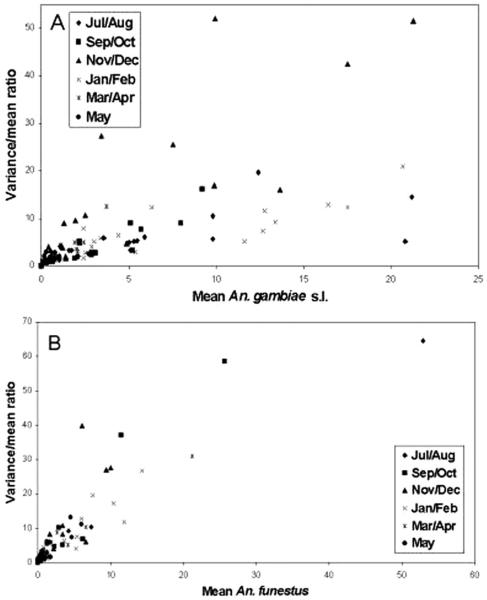
The relationship between the variance/mean ratio and the mean mosquito density per village for each sampling period is illustrated in Fig. 2 for An. gambiae s.l. and An. funestus. Strong positive correlations were detected in all sampling periods. The linear relationship observed indicates that variability in the mosquito distribution increases as the mean mosquito density per village increases.
Fig. 2.
Mean density of An. gambiae s.l. (A) and An. funestus (B) collected per house per village plotted against the variance/mean ratio for each village.
Discussion
This study reveals clustering in the distribution of An. gambiae s.l. and An. funestus adult mosquito populations. These results extends the findings of a previous study in an Ethiopian village by Ribeiro et al. (1996), which also concluded that clustering of An. gambiae was present, and changed as a function of time and mean mosquito density.
The presence of clustering in all sampling periods, irrespective of season or weather patterns, suggests extensive heterogeneity in the distribution of both An. gambiae s.l. and An. funestus. Throughout the study, 100% of An. funestus mosquitoes and 90% of An. gambiae s.l. mosquitoes were collected from less than one-half of the total number of houses. Furthermore, it was not necessarily the same houses that consistently harbored Anopheles species. In three villages, no An. funestus were collected from any of the households, and very low levels of An. gambiae s.l. were collected within the same villages. In other instances, some houses remained free of both species throughout the study, whereas nearby households had high densities of both An. gambiae s.l. and An. funestus. The results presented in this study have clear implications for sampling design. Our analysis suggests detecting mosquito distribution patterns may be harder in sites having high densities throughout the year. As a result, nonrandom selection and sampling techniques, as well as information about house characteristics, may be needed to further quantify the distribution of Anopheles mosquitoes at high densities. This information also may be useful for standardizing EIR measurements or for developing stratified sampling strategies for the surveillance or control of mosquito populations.
The variance to mean ratio changes with mean mosquito density per site, and aggregation levels are reduced at the lowest mosquito densities (Fig. 2). In essence, as precipitation levels increase and/or favorable ecological conditions are created, mosquito densities increase and exhibit patterns of aggregation. Conversely as conditions become less favorable, mosquito densities decline and the population surviving has an older age structure and tends toward randomness. This is potentially of epidemiological importance because it is the older mosquitoes that are the most dangerous in terms of sporozoite rates. Furthermore, when older mosquitoes are present in low density, the risk of encountering highly infectious mosquitoes within villages becomes essentially random. However, it should be noted that low mosquito densities and small sample sizes might reduce the power to detect overdispersion. Interpretation should therefore be done with this in mind to avoid over extrapolation.
To understand the epidemiological consequences of anopheline distribution variability along the coast of Kenya and subsequently develop control programs that target areas harboring the most mosquitoes, relationships between house types and locations, human behavior, and environmental modification must be better understood. Additional investigations into the mechanisms underlying aggregated human–vector contact will require the development of ecological tools and methods specific to heterogeneous environments. Understanding human ecological processes, and the mosquito life cycle and dispersion patterns, will assist communities in obtaining significant reductions in stable, endemic malaria morbidity, and mortality. If efficient vector control programs are to be developed and implemented at reasonable expense to health programs, future designs to enhance public health should consider how mosquitoes distribute their bites to affect individual hosts among a set of houses within communities. core funds from ICIPE (International Centre of Insect Physiology and Ecology).
Acknowledgments
We thank Thom Eisele, David Smith, and two anonymous reviewers for helpful comments. We also thank the scientific and technical staff at the Center for Geographic Medicine Research-Coast, particularly Festus Yaah, Shida David, Gabriel Nzai, and the driver Harrison Ngala who endured the rough roads during the El Nino rains. This study was supported by funds from DANIDA (Danish International Development Agency); National Institutes of Health grants U19 AI45511, D43 TW01142, and D43 TW00920; and additional
References Cited
- Beier JC, Killeen GF, Githure JI. Short report: entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am. J. Trop. Med. Hyg. 1999;61:109–113. doi: 10.4269/ajtmh.1999.61.109. [DOI] [PubMed] [Google Scholar]
- Gillies MT, DeMeillon B. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region) South African Institute of Medical Research; Johannesburg: 1968. [Google Scholar]
- MacDonald G. The epidemiology and control of malaria. Oxford University Press; London, United Kingdom: 1957. [Google Scholar]
- Mbogo CM, Mwangangi JM, Nzovu J, Gu W, Yan G, Gunter JT, Swalm C, Keating J, Regens JL, Shililu JI, et al. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am. J. Trop. Med. Hyg. 2003;68:734–742. [PubMed] [Google Scholar]
- Mbogo CM, Kabiru EW, Glass GE, Forster D, Snow RW, Khamala CPM, Ouma JH, Githure JI, Marsh K, Beier JC. Vector-related case-control study of severe malaria in Kilifi district, Kenya. Am. J. Trop. Med. Hyg. 1999;60:781–785. doi: 10.4269/ajtmh.1999.60.781. [DOI] [PubMed] [Google Scholar]
- Mbogo CM, Snow RW, Khamala CP, Kabiru EW, Ouma JH, Githure JI, Marsh K, Beier JC. Relationships between Plasmodium falciparum transmission by vector populations and the incidence of severe disease at nine sites on the Kenyan coast. Am. J. Trop. Med. Hyg. 1995;52:201–206. doi: 10.4269/ajtmh.1995.52.201. [DOI] [PubMed] [Google Scholar]
- Mbogo CM, Snow RW, Kabiru EW, Ouma JH, Githure JI, Marsh K, Beier JC. Low-level Plasmodium falciparum transmission and the incidence of severe malaria infections on the Kenyan coast. Am. J. Trop. Med. Hyg. 1993;49:245–253. doi: 10.4269/ajtmh.1993.49.245. [DOI] [PubMed] [Google Scholar]
- Omer SM, Cloudsley-Thomson JL. Survival of female Anopheles gambiae Giles through a 9-month dry season in Sudan. Bull. World Health Organ. 1970;42:319–330. [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JMC, Seulu F, Abose T, Kidane G, Teklehaimanot A. Temporal and spatial distribution of anopheline mosquitoes in an Ethiopian village: implications for malaria control strategies. Bull. World Health Organ. 1996;74:299–305. [PMC free article] [PubMed] [Google Scholar]
- Robert V, Macintyre K, Keating J, McWilson W, Trappe JP, Duchemin JB, Beier JC. Malaria transmission in urban sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2003;68:169–176. [PubMed] [Google Scholar]
- Service MW. Studies on sampling larval populations of the Anopheles gambiae complex. Bull. World Health Organ. 1971;45:169–180. [PMC free article] [PubMed] [Google Scholar]
- Snow RW, Gouws E, Omumbo J, Rapuoda B, Craig MH, Tanser FC, le Seur D, Ouma J. Models to predict the intensity of Plasmodium falciparum transmission: applications to the burden of disease in Kenya. Tran. R. Soc. Trop. Med. Hyg. 1998;92:601–606. doi: 10.1016/s0035-9203(98)90781-7. [DOI] [PubMed] [Google Scholar]
- Snow RW, Armstrong JRM, Forster D, Winstanley PA, Mwangi I, Waruiru C, Warn P, Newbold C, Marsh K. Periodicity and time spacing clustering of severe childhood malaria on the Kenyan coast. Tran. R. Soc. Trop. Med. Hyg. 1993;87:386–390. doi: 10.1016/0035-9203(93)90007-d. [DOI] [PubMed] [Google Scholar]
- Southwood TRE. Ecological methods with particular reference to the study of insect populations. Chapman & Hall; London, United Kingdom: 1978. [Google Scholar]
- Taylor LR. Aggregation, variance and the mean. Nature (Lond.) 1961;189:732–735. [Google Scholar]
- [WHO] World Health Organization . Manual on practical entomology in malaria. World Health Organization; Geneva, Switzerland: 1975. (Offset publication no. 13). [Google Scholar]
- [WHO] World Health Organization . WHO expert committee on malaria: technical report series 892. World Health Organization; Geneva, Switzerland: 2000. pp. 1–71. [PubMed] [Google Scholar]