Abstract
We conducted larval surveys in habitats located in urban Malindi, Kenya, in 2005 and 2006 with the goal of determining the productivity of unused swimming pools in relation to other habitats. Of the 250 habitats sampled, 66 were unused swimming pools, 93 were wells, 60 were drainage troughs, and 31 were miscellaneous areas, such as septic tanks, swamps, concrete tanks, fish ponds, car-track depressions, and drainage ponds. Anopheles gambiae s.l. was the only anophelines species found in the habitats, whereas Culex quinquefasciatus made up >95% of all culicine immature stages found. Of the 110 habitats found to be positive for mosquitoes, unused swimming pools represented 42.7%. One hundred and forty-eight anopheline pupae were found in 8 of the 66 unused swimming pools, but none was found in the other habitats. Using a nonparametric test, Kruskal–Wallis H test, there was no significant difference in the abundance of culicine pupae found in the 4 habitat types (χ2 = 7.350, df = 3, p = 0.062). Unused swimming pools in Malindi provide ideal habitats that should be targeted for mosquito control.
Keywords: Unused swimming pools, Anopheles gambiae s.l., Malindi, Kenya
Studies of larval habitats in urban environments have identified wells (Robert et al. 1998, Afrane et al. 2004, Matthys et al. 2006), puddles in unpaved streets (Afrane et al. 2004), ditches (Afrane et al. 2004), and agricultural areas (Matthys et al. 2006) as major proliferation sites for Anopheles gambiae Giles. In Malindi, Kenya, unused swimming pools have been found to collect rainwater, which provides optimal conditions for mosquito development during off-tourist/vacation seasons (Carlson et al. 2004, Keating et al. 2004). During the off-season in 2005 and 2006, we conducted larval surveys in the urban environment of Malindi to further explore the importance of unused swimming pools for An. gambiae propagation relative to other habitat types, such as wells, drainage troughs, and others.
This study was conducted in the coastal town of Malindi, the 10th largest town in Kenya and a major tourist destination. The study area has been described previously (Macintyre et al. 2002; Keating et al. 2003, 2004). Malindi is located approximately 120 km north of Mombasa bordering the Indian Ocean. Climatic conditions are generally hot and humid all year with 2 rainy seasons: 3 months of rain occurring April through June and 2 months of rain occurring October through November.
A geographic sampling strategy, described previously (Keating et al. 2003), was used as a framework to randomly select areas to conduct larval surveys in urban Malindi. Larval surveys were conducted from May 26 to November 18, 2005, and from April 06 to August 17, 2006, using standard sampling (O’Malley 1989) and identification procedures for immature mosquitoes (Gillies and Coetzee 1987). Different areas and sets of habitats were sampled in the 2 years of the study. Within each area, all accessible habitats found by our field team were sampled for mosquito immature stages. All habitats were classified according to type. Since this study was exploratory, no polymerase chain reaction analysis was done to identify members of the An. gambiae complex, although other studies in the area have shown over 85% of the Anopheles collected are An. gambiae Giles s.s.; other species include An. arabiensis Patton and An. merus Dointz (Keating et al. 2004, Jacob et al. 2005). The percentage of emergence—defined as the ratio of pupae to the total abundance of immature stages × 100%—was calculated for Anopheles and culicine immature stages within each habitat type. For habitats positive for pupae, nonparametric tests, Kruskal–Wallis H test and Dunn’s multiple comparison test (Glantz 2002), were used to compare pupal abundance among the 4 different habitat types. For habitats that were sampled multiple times (≥2 times), the mean pupal abundance value per habitat over the entire study period was used in the analysis. Data were analyzed in SPSS 14.0® for Windows (Chicago, IL) and Microsoft Excel 2003®.
The most frequently encountered habitat types included unused swimming pools, wells, and drainage troughs. Other habitat types were grouped as miscellaneous (e.g., septic tanks, swamps, concrete tanks, fish ponds, car-track depressions, and drainage ponds) due to the low frequency upon which they were encountered.
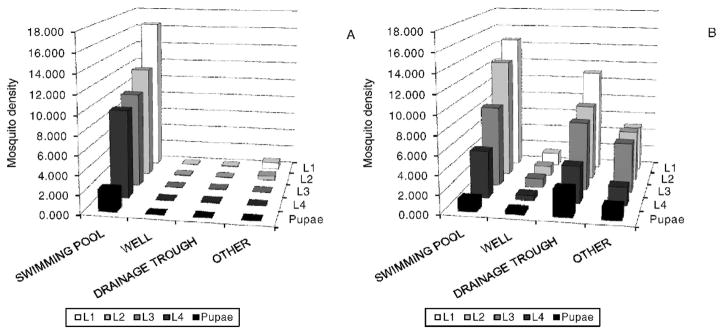
Figure 1 shows two of the typical unused swimming pools that were sampled. In Malindi, 250 individual habitats were identified: 66 unused swimming pools, 93 wells, 60 drainage troughs, and 31 miscellaneous others. Overall, there was a higher abundance of culicine immature stages (n = 17,750) than Anopheles immature stages (n = 3,362). All Anopheles mosquitoes were identified as An. gambiae s.l., whereas >95% of culicine mosquitoes were identified as Culex quinquefasciatus Say. Unused swimming pools had the highest abundance of Anopheles (n = 3,295) and culicine (n = 11,269) immature stages (Fig. 2). Of the 110 habitats found positive for immature mosquitoes, 42.7% were swimming pools, 20.0% were wells, 25.5% were drainage troughs, and 11.8% were miscellaneous others. The percentages of habitats positive for Anopheles immature stages were 43.9% of 66 unused swimming pools, 2.2% of 93 wells, 1.7% of 60 drainage troughs, and 6.5% of 31 miscellaneous. The percentages of habitats positive for culicine immature stages were 71.2% of 66 unused swimming pools, 22.6% of 93 wells, 45.0% of 60 drainage troughs, and 41.9% of 31 miscellaneous. Figure 2 illustrates the decreasing abundance of each subsequent immature stage to the next, as expected. All of the 148 Anopheles pupae collected were found in 8 of the unused swimming pools (no pupae were found in the other habitat types). The percentage of emergence of adult Anopheles from unused swimming pools was 4.5% (148/3,295), assuming 100% survival of pupae. No statistical tests were done to compare abundance among the 4 habitat types because only unused swimming pools contained Anopheles pupae. The percent adult emergence of culicine pupae was 3.1% (347/11,269) from unused swimming pools, 9.2% (131/1,429) from wells, 10.5% (402/3,837) from drainage troughs, and 10.0% (121/1,215) from miscellaneous others, assuming 100% survival of pupae. Among the 4 habitats positive for culicine pupae (n = 44 of 250 habitats), there was no significant difference in pupal abundance of (χ2 = 7.350, df = 3, p = 0.062). The Dunn’s test also revealed no significant differences between pairwise combinations of habitat types (P > 0.05).
Fig. 1.
Pictures illustrating unused swimming pool in up-market areas of Malindi (A) on the grounds of a private residential home and (B) on the grounds of a tourist hotel.
Fig. 2.
Mosquito density (number of mosquitoes per habitat) of (A) Anopheles immature stages and (B) culicine immature stages sampled from swimming pools (n = 66), wells (n = 93), drainage troughs (n = 60), and other miscellaneous habitat types (n = 31; i.e. septic tanks, swamps, concrete tanks, fish ponds, car-track depressions, and drainage ponds) located in different urban areas of Malindi, Kenya.
In this study, we explored the productivity of different habitats for Anopheles and culicine immature stages. Unused swimming pools, accounting for 42.7% of all 110 positive habitats, harbored the highest numbers of immature mosquitoes. These unused swimming pools are located in up-market residential and tourist areas. These results suggest that unused swimming pools are productive habitats for mosquitoes in urban Malindi; this may be particularly true for Anopheles, since their pupae were recovered from this habitat type only. It is not clear why Anopheles pupae were not recovered from the other habitats. For that reason, it would be useful to investigate the developmental rate of immature stages of Anopheles in the urban habitats of Malindi. Nonetheless, at the beginning of the high-peak tourist/vacation periods, after the rainy season, Malindi’s residents, hotel and domestic workers, and tourists may be at risk of being bitten by mosquitoes emerging from unused swimming pools and, consequently, exposed to mosquito-borne pathogens. Thus, unused swimming pools in Malindi should be targeted for mosquito control. For example, city ordinances can be passed requiring swimming-pool owners to regularly drain, clean, cover, or treat swimming pools to prevent rainwater collection during off-peak periods.
We would like to thank the research staff at International Centre of Insect Physiology and Ecology (ICIPE) and Kenya Medical Research Institute (KEMRI), Kenya, and two anonymous reviewers of this manuscript. We acknowledge support from National Institutes of Health, grants U19 AI45511, FO6TWO5588, and P20RR020770, and the Abess Center for Ecosystem Science and Policy (CESP), University of Miami.
REFERENCES CITED
- Afrane YA, Klinkenberg E, Drechsel P, Owusu-Daaku K, Garms R, Kruppa T. Does irrigated urban agriculture influence the transmission of malaria in the city of Kumasi, Ghana? Acta Trop. 2004;89:125–134. doi: 10.1016/j.actatropica.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Carlson J, Keating J, Mbogo CM, Kahindi S, Beier JC. Ecological limitations on aquatic mosquito predator colonization in the urban environment. J Vector Ecol. 2004;29:331–339. [PMC free article] [PubMed] [Google Scholar]
- Gillies MT, Coetzee M. A supplement to anophelinae of Africa south of Sahara (Afro-tropical region) Publ South Africa Inst Med Res. 1987;55:1–143. [Google Scholar]
- Glantz SA. Primer of biostatistics. New York: McGraw Hill; 2002. [Google Scholar]
- Jacob BG, Arheart KL, Griffith DA, Mbogo CM, Githeko AK, Regens JL, Githure JI, Novak R, Beier JC. Evaluation of environmental data for identification of Anopheles (Diptera: Culicidae) aquatic larval habitats in Kisumu and Malindi, Kenya. J Med Entomol. 2005;42:751–755. doi: 10.1093/jmedent/42.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating J, MacIntyre K, Mbogo C, Githeko A, Regens JL, Swalm C, Ndenga B, Steinberg LJ, Kibe L, Githure JI, Beier JC. A geographic sampling strategy for studying relationships between human activity and malaria vectors in urban Africa. Am J Trop Med Hyg. 2003;68:357–365. [PubMed] [Google Scholar]
- Keating J, Macintyre K, Mbogo CM, Githure JI, Beier JC. Characterization of potential larval habitats for Anopheles mosquitoes in relation to urban land-use in Malindi, Kenya. Int J Health Geogr. 2004;3(1):9. doi: 10.1186/1476-072X-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre K, Keating J, Sosler S, Kibe L, Mbogo CM, Githeko AK, Beier JC. Examining the determinants of mosquito-avoidance practices in two Kenyan cities. Malar J. 2002;1:14. doi: 10.1186/1475-2875-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys B, N’Goran EK, Kone M, Koudou BG, Vounatsou P, Cisse G, Tschannen AB, Tanner M, Utzinger J. Urban agricultural land use and characterization of mosquito larval habitats in a medium-sized town of Cote d’Ivoire. J Vector Ecol. 2006;31:319–333. doi: 10.3376/1081-1710(2006)31[319:ualuac]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- O’Malley CM. Guidelines for larval surveillance. Proceedings of the Seventy-Sixth Annual Meeting of the New Jersey Mosquito Control Association [Internet] 1989:45–55. Available from Rutgers University [accessed March 26, 2008], http://www.rci.rutgers.edu/~insects/larvsurv.htm.
- Robert V, Awono-Ambene HP, Thioulouse J. Ecology of larval mosquitoes, with special reference to Anopheles arabiensis (Diptera: Culcidae) in market-garden wells in urban Dakar, Senegal. J Med Entomol. 1998;35:948–955. doi: 10.1093/jmedent/35.6.948. [DOI] [PubMed] [Google Scholar]