Abstract
Few studies have defined how the risk of hospital-acquired acute renal failure varies with the level of estimated glomerular filtration rate (GFR). It is also not clear whether common factors such as diabetes mellitus, hypertension and proteinuria increase the risk of nosocomial acute renal failure independent of GFR. To determine this we compared 1,746 hospitalized adult members of Kaiser Permanente Northern California who developed dialysis-requiring acute renal failure with 600,820 hospitalized members who did not. Patient GFR was estimated from the most recent outpatient serum creatinine measurement prior to admission. The adjusted odds ratios were significantly and progressively elevated from 1.95 to 40.07 for stage 3 through stage 5 patients (not yet on maintenance dialysis) compared to patients with estimated GFR in the stage 1 and 2 range. Similar associations were seen after controlling for inpatient risk factors. Pre-admission baseline diabetes mellitus, diagnosed hypertension and known proteinuria were also independent risk factors for acute kidney failure. Our study shows that the propensity to develop in-hospital acute kidney failure is another complication of chronic kidney disease whose risk markedly increases even in the upper half of stage 3 estimated GFR. Several common risk factors for chronic kidney disease also increase the peril of nosocomial acute kidney failure.
Keywords: acute kidney injury, epidemiology
Acute renal failure (ARF; also known as ‘acute kidney injury’) is among the most important complications observed in hospitalized patients. When severe enough to require dialysis, ARF is associated with a high rate of in-hospital mortality (exceeding 30%).1,2 Even modest declines in kidney function have been associated with a significantly heightened risk of death, prolonged length of stay, and increased costs.3,4 Several recently published studies have suggested that the incidence of ARF has increased dramatically over the past two decades.2,5 Several studies have attempted to identify determinants of ARF, but most have focused on hospital-based or intensive care unit-based risk factors6–9 and results have been inconsistent.1,10,11
Existing kidney disease (that is, chronic kidney disease) appears to be among the most potent predictors of acute declines in kidney function following exposure to radio-contrast,12 major surgery,13 and other medical conditions.6 However, a common limitation in prior studies is to use serum creatinine concentrations obtained at admission to the hospital or intensive care unit to indicate underlying kidney function. This would bias risk estimation if these serum creatinine concentrations represented early acute kidney injury rather than true baseline kidney function. Few studies have incorporated preadmission outpatient or other steady-state serum creatinine measurements in studies of hospital-acquired ARF. Moreover, the degree to which impaired kidney function renders persons at risk for ARF episodes independent of other disease states and procedures performed in hospital has not been established.
We hypothesized that severity of baseline kidney function impairment would be strongly associated with risk of hospital-acquired ARF, and this would be independent of other demographic factors and comorbid conditions. We hypothesized that the increase in risk of ARF—like other adverse consequences of chronic kidney disease—would be evident below an estimated glomerular filtration rate (GFR) of 60 ml per min per 1.73 m2 and become stronger with progressively worse renal function. We further hypothesized that common risk factors for chronic kidney disease—such as diabetes mellitus, hypertension, and proteinuria—are also risk factors for ARF and at similar estimated GFR level, patients with diabetes mellitus would be at higher risk of ARF compared with their counterparts without.
RESULTS
From 1996 to 2003, a total of 1764 Kaiser members developed hospital-acquired ARF treated with dialysis. These patients were compared with 600 820 members who were hospitalized but did not develop ARF requiring dialysis. All cases and controls had outpatient serum creatinine measurements prior to hospitalization.
A review of medical records among a random sample of 100 subjects classified as having dialysis-requiring ARF and baseline estimated GFR of ≥45 ml per min per 1.73 m2 showed that all had ARF and received acute dialysis. A review of medical records among a random sample of 100 subjects classified as having dialysis-requiring ARF and baseline GFR <45 ml per min per 1.73 m2 showed that all had ARF and 99 received acute dialysis. In one instance, acute dialysis was initially planned but not performed and the patient died. There were no observed cases of elective hospitalization for initiation of chronic maintenance dialysis.
Compared with those who did not develop dialysis-requiring ARF, those who did were older and more likely to have baseline diabetes mellitus, hypertension, and proteinuria (Table 1), and they had on average worse renal function (Table 2). Patients who developed ARF were also more likely to have had an intensive care unit stay, to require mechanical ventilation, and to suffer from sepsis and hyperbilirubinemia (Table 1).
Table 1.
Characteristics of subjects who did and did not suffer dialysis-requiring ARF during hospitalization
| Characteristic | Subjects who developed ARF during hospitalization (N=1764) | Subjects who did not develop ARF during hospitalization (N=600 820) |
|---|---|---|
| Mean age at hospitalization±s.d., years | 65.4±14.1 | 57.3±17.2 |
| Women, n (%) | 735 (41.7%) | 340 940 (56.8 %) |
| Race/ethnicity | ||
| White/European | 1086 (61.6%) | 385 314 (64.1%) |
| Black/African | 251 (14.2%) | 48 257 (8.0%) |
| American | ||
| Hispanic | 147 (8.3%) | 41 194 (6.9%) |
| Asian/Pacific Islander | 167 (9.5%) | 55 778 (9.3%) |
| Native American/Mixed | 101 (5.7%) | 30 369 (5.1%) |
| Unknown | 12 (0.7%) | 39 908 (6.6%) |
| Selected medical history | ||
| Diabetes mellitus | 851 (48.2%) | 87 133 (14.5%) |
| Diagnosed hypertension | 1268 (71.9%) | 221 422 (36.9%) |
| Documented proteinuria | 969 (54.9%) | 65 679 (10.9%) |
| Chronic heart failure | 809 (45.9%) | 31 754 (5.3%) |
| Chronic liver disease | 111 (6.3%) | 10 903 (1.8%) |
| Index hospitalization features | ||
| Hyperbilirubinemia | 347 (19.7%) | 6419 (1.1%) |
| Intensive care unit stay | 1101 (62.4%) | 34 077 (5.7%) |
| Sepsis | 488 (27.7%) | 6879 (1.1%) |
| Mechanical ventilation | 551 (31.2%) | 6345 (1.1%) |
| Diagnostic cardiac catheterization involving intravenous contrast | 127 (7.2%) | 24 024 (4.0%) |
| Percutaneous coronary intervention | 31 (1.8%) | 4491 (0.8%) |
| Coronary artery bypass graft surgery | 52 (2.9%) | 3144 (0.5%) |
| Other cardiac surgery | 39 (2.2%) | 650 (0.1%) |
ARF, acute renal failure.
P-value for all comparisons <0.0001.
Table 2.
Baseline renal function before hospitalization among case and control subjects.
| Cases who developed ARF (N=1764) | Controls who did not develop ARF (N=600 820) | |||
|---|---|---|---|---|
| Last observed outpatient serum creatinine (mean±s.d.), mg per 100 ml | 2.42±1.70 | 1.05±0.50 | ||
| Last observed outpatient estimated GFR (mean±s.d.), ml per min per 1.73 m2 | 43.5±30.7 | 74.2±21.3 | ||
| Last observed outpatient estimated GFR, ml per min per 1.73 m2 | Median (interquartile range) duration between admission and last known outpatient estimated GFR before hospitalization (in days) | Median (interquartile range) duration between admission and last known outpatient estimated GFR before hospitalization (in days) | ||
| ≥60 | N=469 | 79 (15, 294) | N=464 720 | 98 (7, 346) |
| 45–59 | N=234 | 35 (4, 149) | N=97 356 | 67 (6, 250) |
| 30–44 | N=294 | 25 (4, 110) | N=28 434 | 25 (2, 135) |
| 15–29 | N=476 | 17 (3, 51) | N=7763 | 6 (1, 49) |
| <15 not requiring dialysis | N=291 | 13 (3, 40) | N=2547 | 2 (1, 9) |
ARF, acute renal failure; GFR, glomerular filtration rate.
Relation with baseline GFR
The median duration of time between last outpatient estimated GFR and admission was 83 days (interquartile range: 6–312). Baseline estimated GFR was a very strong risk factor for ARF. Overall, of the 1746 cases of dialysis-requiring ARF, 1295 (74%) occurred among those with baseline estimated GFR of <60 ml per min per 1.73 m2 (Table 2).
After adjusting for age, sex, race/ethnicity, and also other baseline conditions, there remained a strong graded relationship between risk of ARF and baseline estimated GFR. Even subjects with estimated GFR of 45–59 ml per min per 1.73 m2 had a twofold increase in adjusted odds ratio of ARF compared with subjects with estimated GFR of 60 ml per min per 1.73 m2 or higher. The risk then increases sharply for increasing severity of baseline kidney disease (Table 3).
Table 3.
Risk factors for development of dialysis-requiring acute renal failure among hospitalized adults
| Unadjusted odds ratio (95% confidence interval) | Multivariablea odds ratio (95% confidence interval) | Multivariablea odds ratio after adjusting for in-patient risk factorsb for ARF (95% confidence interval) | |
|---|---|---|---|
| Baseline GFR (ml per min per 1.73 m2) | |||
| ≥60 | Reference | Reference | Reference |
| 45–59 | 2.38 (2.04–2.79) | 1.95 (1.66–2.30) | 1.66 (1.40–1.97) |
| 30–44 | 10.25 (8.85–11.86) | 6.54 (5.57–7.69) | 4.75 (4.01–5.63) |
| 15–29 | 60.76 (53.38–69.16) | 28.50 (24.50–33.14) | 20.42 (17.40–23.96) |
| <15 | 113.21 (97.31–131.71) | 40.07 (33.75–47.58) | 47.17 (39.22–56.74) |
| Diabetes mellitus | 5.50 (5.00–6.04) | 2.07 (1.86–2.30) | 1.99 (1.78–2.23) |
| Diagnosed hypertension | 4.38 (3.95–4.86) | 1.41 (1.25–1.58) | 1.55 (1.37–1.76) |
| Documented proteinuria | 9.93 (9.04–10.91) | 2.79 (2.49–3.11) | 2.84 (2.52–3.19) |
ARF, acute renal failure; GFR, glomerular filtration rate.
Adjusted for all variables shown and also age, sex, and race/ethnicity.
Further adjusted for hyperbilirubinemia, intensive care unit stay, sepsis, receipt of mechanical ventilation, coronary artery bypass surgery, other cardiac surgery, percutaneous coronary intervention, and cardiac catheterization including intravenous contrast.
Further adjustment for in-patient risk factors for ARF—including hyperbilirubinemia, intensive care unit stay, sepsis, mechanical ventilation, cardiac surgery, and cardiac catheterization—did not significantly alter the association between baseline estimated GFR and risk of ARF (Table 3).
Several sensitivity analyses were undertaken. Similar results were seen when we excluded patients whose last observed outpatient GFR was more that 12 months or less than 30 days before hospital admission (Table 4). Similar results were seen when we further required that subjects have a second outpatient GFR reading in the same GFR category as the latest outpatient GFR reading (Table 4) or when we controlled for baseline chronic heart failure and chronic liver disease (data not shown).
Table 4.
Sensitivity analyses for assessment of risk factors for dialysis-requiring acute renal failure
| Multivariablea odds ratio (95% confidence interval) | |||
|---|---|---|---|
| After excluding those with outpatient estimated GFR>1 year before hospitalization | After excluding those with outpatient estimated GFR>1 year or <30 days before hospitalization | Among subset of subjects with 1 outpatient estimated GFR measured 1–12 months before hospitalization and who had a prior estimated GFR in the same stage | |
| Baseline estimated GFR (ml per min per 1.73 m2) | |||
| ≥60 | Reference | Reference | Reference |
| 45–59 | 2.13 (1.79–2.54) | 1.96 (1.53–2.51) | 2.03 (1.43–2.90) |
| 30–44 | 6.80 (5.73–8.07) | 6.57 (5.10–8.47) | 8.48 (6.12–11.74) |
| 15–29 | 28.99 (24.72–34.00) | 30.44 (23.70–39.11) | 42.63 (31.46–57.76) |
| <15 | 40.03 (33.46–47.90) | 80.37 (58.47–110.46) | 107.12 (73.18–156.84) |
| Diabetes mellitus | 2.06 (1.85–2.30) | 2.08 (1.75–2.47) | 1.88 (1.52–2.33) |
| Diagnosed hypertension | 1.37 (1.21–1.55) | 1.52 (1.25–1.86) | 1.21 (0.95–1.56) |
| Documented proteinuria | 2.77 (2.46–3.11) | 2.89 (2.41–3.47) | 2.22 (1.76–2.81) |
GFR, glomerular filtration rate.
Adjusted for all variables shown and also age, sex, and race/ethnicity.
Other clinical risk factors for ARF
Baseline diabetes mellitus, diagnosed hypertension, and documented proteinuria were independent risk factors for ARF in multivariable models adjusted for age, sex, race/ethnicity, and estimated GFR (Table 3).
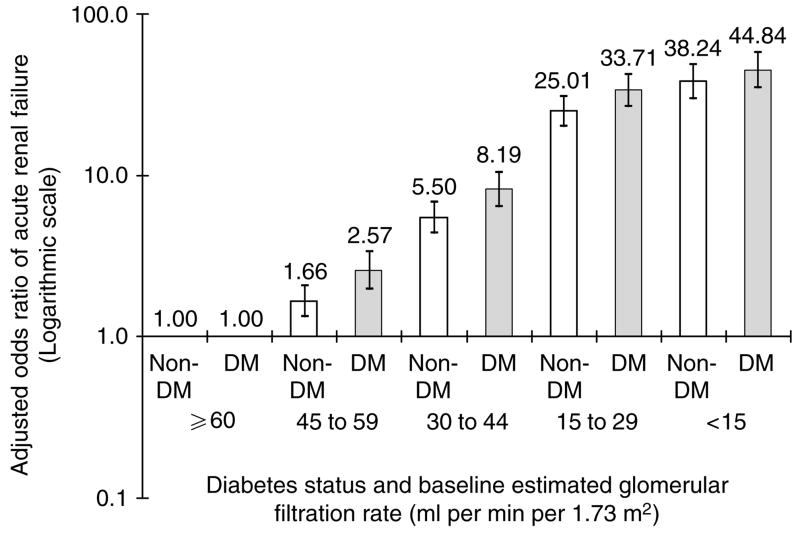
For the GFR categories of 45–59 ml per min per 1.73 m2 and 30–44 ml per min per 1.73 m2, there were statistically significant interactions between diabetes mellitus and severity of chronic kidney disease with regard to risk of ARF (P = 0.01 and 0.04, respectively). Patients with diabetes mellitus were at higher risk for ARF compared with their counterparts without diabetes mellitus in the same GFR category. Similar trends were seen among patients with GFR 15–29 ml per min per 1.73 m2 and <15 ml per min per 1.73 m2, although the interactions were not statistically significant. Because of this, we repeated our logistic regression model stratified by diabetes status, and Figure 1 shows the separate adjusted odds ratios for subjects with and without diabetes.
Figure 1. Multivariable association of baseline estimated GFR and dialysis-requiring ARF stratified by the presence or absence of diabetes mellitus (DM).
Each model adjusted for age, sex, race/ethnicity, diagnosed hypertension, and documented proteinuria.
DISCUSSION
Although ARF is one of the most common renal problems encountered among hospitalized patients, there is a paucity of research about its epidemiology and risk factors. For example, only recently has the incidence of ARF been determined using representative populations.2,5 A recent American Society of Nephrology Renal Research report identified better understanding of risk factors for ARF as a top research priority.14
Although baseline renal insufficiency is known to be a risk factor for ARF, few studies actually quantified the association between the level of preexisting kidney function and subsequent risk of ARF. Many prior studies used arbitrary dichotomous cutoffs to define ‘normal’ or ‘abnormal’ baseline renal function (for example, above or below serum creatinine of 1.2 mg per 100 ml8 or above or below 150 mmol/l−1 (Parfrey et al.).12 None to our knowledge has used the consensus National Kidney Foundation Chronic Kidney Disease staging system. In addition, previous studies often did not explicitly delineate how ‘baseline’ kidney function was determined. In many cases, serum creatinine concentrations observed only during the index hospitalization were used.7,8 Serum creatinine values in the hospital may be higher than actual baseline (usual) values because of incipient ARF or they may be lower because of acute events such as blood loss, volume resuscitation, and hemodilution.4 Past studies have also been limited because of inclusion of only one type of ARF15,16 or underrepresentation of women and racial/ethnic minorities.17
The current study contributes to the literature in several important ways. We studied a large, diverse population and did not limit the type of ARF. We focused on ARF requiring dialysis, which is known to be associated with very high risk of mortality and morbidity. Careful attention was given in defining ‘baseline’ kidney function using calibrated pre-admission outpatient measurements of serum creatinine and the Modification of Diet in Renal Disease (MDRD) equation. The comprehensive clinical information in the Kaiser databases also allowed us to capture important comorbid conditions preceding and coincident with hospitalization. Our large study sample was diverse in terms of race/ethnicity and included both men and women across a wide age range.
We found that an increase in risk of ARF becomes apparent starting below an estimated GFR of 60 ml per min per 1.73 m2. Even subjects with estimated GFR of 45–59 ml per min per 1.73 m2 have on average a twofold increased in adjusted odds ratio of ARF compared with subjects with estimated GFR of 60 ml per min per 1.73 m2 or above—with risk being higher among subjects with diabetes than those without. We believe that rigorously quantifying risk of ARF in the large number of patients with milder stage ‘3a’ chronic kidney disease is an important contribution of this study. This threshold is consistent with prior research showing that several other adverse consequences of chronic kidney disease, such increase in risk of cardiovascular disease and anemia, also become apparent below an estimated GFR of 60 ml per min per 1.73 m2.18–20 These data support the National Kidney Foundation Chronic Kidney Disease Guidelines in which persons with GFR chronically below 60 ml per min per 1.73 m2 are classified as having chronic kidney disease, regardless of other factors.
Relatively little attention has been paid to the relationship between severity of chronic kidney disease and risk of ARF. For example, National Kidney Foundation Clinical Practice Guidelines for Chronic Kidney Disease devoted specific chapters to discuss the association of level of GFR with hypertension, anemia, nutritional status, bone and mineral metabolism disease, neuropathy, and indices of functioning and well being.21 But there is no discussion on the association of level of GFR with ARF. In fact, the magnitude of the increase in odds ratio of ARF with progressively more severe chronic kidney disease—up to 20- or 30-fold higher adjusted risk – underscores the fact that ARF may in fact be the one adverse sequela of chronic kidney disease most tightly linked with estimated GFR.
Better quantification of the impact of baseline kidney function on risk of ARF should lead to improved risk stratification and clinical decision making regarding whether or not to proceed with interventions that may predispose to ARF or adopt preventive measures to reduce ARF risk. Beyond direct patient care issues, these data have implications for the design of future studies of ARF. The fact that most cases of ARF occur among those with some degree of reduced renal function means that ARF trials (some of which have excluded patients with mild or moderately elevated ‘baseline’ serum creatinine22,23) should be more inclusive of those with preexisting chronic kidney disease to be more generalizable. These data also highlight the need for more epidemiology studies to understand the potential impact of episodes of ARF on the course of chronic kidney disease in addition to focusing on the impact of ARF on those with normal renal function.24
Our study also indicated that another marker for chronic kidney disease—proteinuria—is an important independent risk factor for ARF. Patients with documented dipstick proteinuria appear to be two to three times as likely as patients without this to develop ARF, independent of estimated GFR. It is possible that patients with proteinuria—similar to those with chronically low GFR—have less physiological adaptability and are therefore less able to tolerate reduced renal blood flow and other nephrotoxic insults.
We found that two major risk factors for chronic kidney disease—hypertension and diabetes—also were risk factors for development of ARF. Few studies have investigated hypertension as a risk factor for ARF.25 Diabetes has been recognized as a risk factor for contrast nephropathy12 and other types of ARF.15,16 We noted an important interaction between presence of diabetes and risk of ARF. Within the same estimated GFR category, patients with diabetes appear to have higher risk of ARF than their counterparts without. This interaction had been well-known for one particularly subtype of ARF—contrast nephropathy12—but our study show that this interaction seems generalizable to other causes of dialysis-requiring ARF.
Limitations of this study include the fact that we did not study hospitalized patients without serum creatinine determinations before admission. We may have missed cases of dialysis-requiring ARF, as it was not feasible to review medical records of all cases. However, recent data show that administrative codes for dialysis-requiring ARF have a sensitivity of over 90%.26 Baseline renal function was not measured but rather estimated using the MDRD equation. As a result of the known limitations of the MDRD equation, we did not report estimated GFR levels above 60 ml per min per 1.73 m2. It was also not possible to have assessments of renal function on all subjects at a fixed time point prior to the development of ARF, because the occurrence of ARF is usually unpredictable. However, our sensitivity analysis suggests that this does not appear to be an important source of bias. Our definition of ARF may be considered arbitrary but there is no commonly accepted definition of ARF and the ≥50% increase in serum creatinine requirement fits the recently proposed Acute Kidney Injury Network’s diagnostic criteria for acute kidney injury.27 Among patients with very advanced chronic kidney disease, it may be difficult to distinguish between the final stages of progression to end-stage renal disease from potentially reversible acute-on-chronic renal failure so the very high odds ratio observed among those with estimated GFR of <15 ml per min per 1.73 m2 must be interpreted with caution. We did not have direct measures of blood pressure and relied on physician-assigned codes to ascertain hypertension. Proteinuria was analyzed as a dichotomous variable and assessed only by dipstick urinalysis among those who had this test done as part of routine clinical care. However, misclassification would generally tend to bias toward the null and so the underlying associations between ARF and hypertension or proteinuria may be stronger than what we observed. As our study was conducted among insured members of a northern California integrated healthcare delivery system, our results may not be completely generalizable to other populations.
In conclusion, a heightened risk of ARF is another adverse sequela of chronic kidney disease that becomes apparent at an estimated GFR of below 60 ml per min per 1.73 m2. Even subjects with estimated GFR 45–59 ml per min per 1.73 m2 had a twofold increase in adjusted odds ratio of ARF compared with subjects with estimated GFR 60 ml per min per 1.73 m2 or above. This study is distinguished from prior investigations of ARF that have mostly focused on hospital-based or intensive care unit-based risk factors. By clearly delineating the baseline kidney function against which the risk of ARF is defined, we rigorously quantified the strength of this important clinical relation. The large odds ratios observed support the notion that underlying chronic kidney disease is the single most important risk factor for ARF. Given that ARF is associated with high mortality rates, ARF may be another mechanism contributing to the high mortality rates observed among patients with chronic kidney disease. Our results suggest that other markers of chronic kidney disease—such as proteinuria—and other risk factors for chronic kidney disease—such as hypertension and diabetes—are also risk factors for ARF.
MATERIALS AND METHODS
Study population
We studied adult members (≥20 years old) enrolled in Kaiser Permanente of Northern California (Kaiser), a large integrated healthcare delivery system currently insuring more than one-third of the San Francisco Bay Area adult population. Its population is representative of local surrounding and statewide populations with only slightly lower-than-population percentages at the extremes of the socioeconomic spectrum and age.28 We included in the source population all adults who had one or more outpatient determinations of serum creatinine between 1996 and 2003.20
Identification of dialysis-requiring acute renal failure
We focused in this study on cases of dialysis-requiring ARF, which is known to be associated with high rates of morbidity and mortality. All analyses excluded patients who received a prior kidney transplant or were on maintenance dialysis. We cross-linked our study population with the nationally comprehensive US Renal Data System (USRDS) registry. At the time of cross-linkage, USRDS data were complete through 31 December 2003. Supplementary determination of end-stage renal disease status was performed using data from an internal Kaiser end-stage renal disease registry.29
Cases of dialysis-requiring ARF were identified among hospitalized patients who had outpatient measures of serum creatinine prior to admission and who were not already on maintenance dialysis. ARF requiring dialysis was defined as peak in-patient serum creatinine greater than last observed preadmission outpatient serum creatinine by ≥50% and receipt of dialysis during hospitalization. International Classification of Disease, Ninth Edition (ICD-9) procedure codes 54.98 and 39.95 and Current Procedural Terminology (CPT) codes 90935, 90937, 90945, 90947, and 90999 were used to identify episodes of acute peritoneal dialysis hemodialysis hemofiltration from health plan hospital discharge databases. A board-certified nephrologist validated this approach by auditing the charts of 200 randomly selected patients meeting the ARF definition. Only the first hospitalization for each subject during the study period was considered. Control subjects included all patients who were hospitalized but did not develop dialysis-requiring ARF and who had outpatient serum creatinine measurements prior to admission.
Assessment of baseline serum creatinine and estimated glomerular filtration rate
Glomerular filtration rate was estimated from serum creatinine and the abbreviated MDRD equation.21,30 Measurement of serum creatinine by the Kaiser regional health plan laboratory was calibrated against the MDRD core laboratory,20 which allowed for a more reliable estimate of GFR.31,32 Baseline GFR was estimated from serum creatinine determinations not associated with hospitalizations to better reflect baseline kidney function. Baseline kidney function was defined as the last outpatient GFR before admission.
Assessment of other exposures of interest
Outpatient covariates were assessed as previously described using comprehensive laboratory, prescription medication, and diagnostic code data.20 These included age, sex, race/ethnicity, diabetes mellitus,20,29 diagnosed hypertension (by ICD-9 codes 401–405),20 and documented proteinuria. Documented proteinuria was based on laboratory database entries of urine protein dipstick results of 1 + or greater (approximately 30 mg per 100 ml or greater) in the absence of a possible urinary tract infection (that is, concomitant positive test for urinary nitrite or esterase).20
To capture important in-hospital complications associated with ARF, we determined the need for mechanical ventilation using ICD-9 code 96.7×33–35 and the presence of sepsis using ICD-9 codes 38.xx, 020.0, 790.7, 117.9, 112.5, 112.81.33 Hyperbilirubinemia was defined as observed peak in-patient serum total bilirubin level >2.0 mg per 100 ml. Intensive care unit stay was determined using hospitalization administrative records. Cases of coronary artery bypass surgery, other cardiac surgery, percutaneous coronary intervention, and diagnostic cardiac catheterization involving intravenous contrast were identified using relevant procedure codes (listed in Appendix).
APPENDIX.
| ICD-9 procedure codes | CPT codes | |
|---|---|---|
| Coronary artery bypass surgery | 36.10–36.17, 36.19 | 33510–35519, 33521–35523, 33533–35536 |
| Other cardiac surgery | 35.10–35.14, 35.20–35.28, 35.31–35.35, 35.39, 35.50–35.54, 35.60–35.63, 35.70–35.73, 35.81–35.84, 35.91–35.95, 35.98–35.99, 36.03, 37.31–37.33, 37.35 | 33030–33031, 33050, 33120, 33130, 33140,33250–33251, 33253, 33261, 33300, 33305, 33310, 33315, 33400–33401, 33403–33406, 33410–33417, 33420, 33422, 33425–33427, 33430, 33460, 33463–33465, 33468, 33470–33472, 33474–33476, 33478, 33496, 33500–33506, 33600, 33602, 33606, 33608, 33610–33612, 33615, 33617, 33619, 33641, 33645, 33647, 33660, 33665, 33670, 33681, 33684, 33688, 33690, 33692, 33694, 33697, 33702, 33710, 33720, 33722, 33732, 33735–33737, 33770–33771, 33774–33779, 33780–33781, 33786, 33788, 33935, 33945 |
| Percutaneous coronary intervention | 36.01–36.02, 36.05–36.07, 36.09, 00.66 | 92974, 92980–92982, 92984, 92995–92996 |
| Diagnostic cardiac catheterization involving intravenous contrast | 88.50–88.57 | 95908, 93539–93545 |
Statistical analysis
For this nested case–control study, we used a logistic regression model with dialysis-requiring ARF as the outcome and baseline kidney function as the main exposure of interest. The predictor variable estimated GFR was categorized using a modified National Kidney Foundation classification of chronic kidney disease: ≥60 ml per min per 1.73 m2 (reference group), 45–59 ml per min per 1.73 m2 (stage 3a), 30–44 ml per min per 1.73 m2 (stage 3b), 15–29 ml per min per 1.73 m2 (stage 4), and <15 ml per min per 1.73 m2 not requiring dialysis (stage 5). Area under the receiver operating characteristic curve for the main result multivariable analysis was 0.84, indicating good model fit. The Hosmer–Lemeshow test χ2 was 19.07, P = 0.014, indicating very mild departures from perfect model fit for a sample of this size.
Interaction terms between GFR and diabetes were tested based on our a priori consideration that the threshold for increased ARF risk by GFR might vary by diabetes status.12 Given the significant interaction, we presented models stratified by diabetes status.
In sensitivity analyses, we excluded patients whose last observed outpatient GFR was more that 12 months before the hospitalization to reduce misclassification of baseline kidney function. We also additionally excluded patients whose last observed outpatient GFR was less than 30 days before the hospitalization as the decline in GFR observed shortly before hospitalization may be part of the acute illness that led to hospitalization. Finally, we repeated our multivariate model among the subset of patients with at least one outpatient GFR estimated 1–12 months prior to hospitalization and who also had a second earlier GFR that was in the same GFR category/chronic kidney disease stage (for example, if the most recent estimated GFR prior to hospitalization was between 30–44 ml per min per 1.73 m2, then one before that would also have to be between 30–44 ml per min per 1.73 m2).
We considered two-tailed P-values <0.05 to be statistically significant. Analyses were conducted using SAS (Cary, NC, USA).
The institutional review boards of collaborating institutions approved the study. Waiver of informed consent was obtained because of the nature of the study.
Acknowledgments
We thank Niela Pomernacki, RD for her expert technical assistance on the study. Some of the data reported here were supplied by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the United States government. None of the authors declare a conflict of interest. This work is supported by a grant (R01 DK67126) from the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 2.Waikar SS, Curhan GC, Wald R, et al. Declining mortality in patients with acute renal failure, 1988–2002. J Am Soc Nephrol. 2006;17:1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 3.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 4.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, McCulloch CE, Fan D, et al. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou SH, Bushinsky DA, Wish JB, et al. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243–248. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- 7.Chertow GM, Christiansen CL, Cleary PD, et al. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995;155:1505–1511. [PubMed] [Google Scholar]
- 8.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 9.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 10.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 11.Leblanc M, Kellum JA, Gibney RT, et al. Risk factors for acute renal failure: inherent and modifiable risks. Curr Opin Crit Care. 2005;11:533–536. doi: 10.1097/01.ccx.0000183666.54717.3d. [DOI] [PubMed] [Google Scholar]
- 12.Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989;320:143–149. doi: 10.1056/NEJM198901193200303. [DOI] [PubMed] [Google Scholar]
- 13.Browner WS, Li J, Mangano DT. In-hospital and long-term mortality in male veterans following noncardiac surgery. JAMA. 1992;268:228–232. [PubMed] [Google Scholar]
- 14.American Society of Nephrology Renal Research Report. J Am Soc Nephrol. 2005;16:1886–1903. doi: 10.1681/ASN.2005030285. [DOI] [PubMed] [Google Scholar]
- 15.Wijeysundera DN, Karkouti K, Dupuis JY, et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007;297:1801–1809. doi: 10.1001/jama.297.16.1801. [DOI] [PubMed] [Google Scholar]
- 16.Thakar CV, Arrigain S, Worley S, et al. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 17.Chertow GM, Lazarus JM, Christiansen CL, et al. Preoperative renal risk stratification. Circulation. 1997;95:878–884. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 18.Astor BC, Muntner P, Levin A, et al. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2002;162:1401–1408. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the Unites States: results from III NHANES. J Am Soc Nephrol. 2002;13:504–510. doi: 10.1681/ASN.V132504. [DOI] [PubMed] [Google Scholar]
- 20.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 22.Palevsky PM, O’Connor T, Zhang JH, et al. Design of the VA/NIH Acute Renal Failure Trial Network (ATN) Study: intensive versus conventional renal support in acute renal failure. Clin Trials. 2005;2:423–435. doi: 10.1191/1740774505cn116oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinsonneau C, Camus C, Combes A, et al. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006;368:379–385. doi: 10.1016/S0140-6736(06)69111-3. [DOI] [PubMed] [Google Scholar]
- 24.Schiffl H. Renal recovery from acute tubular necrosis requiring renal replacement therapy: a prospective study in critically ill patients. Nephrol Dial Transplant. 2006;21:1248–1252. doi: 10.1093/ndt/gfk069. [DOI] [PubMed] [Google Scholar]
- 25.Conen D, Buerkle G, Perruchoud AP, et al. Hypertension is an independent risk factor for contrast nephropathy after percutaneous coronary intervention. Int J Cardiol. 2006;110:237–241. doi: 10.1016/j.ijcard.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Codes for acute renal failure. J Am Soc Nephrol. 2006;17:1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 27.Molitoris BA, Levin A, Warnock DG, et al. Improving outcomes from acute kidney injury. J Am Soc Nephrol. 2007;18:1992–1994. doi: 10.1681/ASN.2007050567. [DOI] [PubMed] [Google Scholar]
- 28.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karter AJ, Ferrara A, Liu JY, et al. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 31.Hsu CY, Chertow GM, Curhan GC. Methodological issues in studying the epidemiology of mild to moderate chronic renal insufficiency. Kidney Int. 2002;61:1567–1576. doi: 10.1046/j.1523-1755.2002.00299.x. [DOI] [PubMed] [Google Scholar]
- 32.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 33.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 34.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992–2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]