Abstract
Purpose of Review
Rotaviruses cause life-threatening gastroenteritis in children throughout the world. The burden of disease has resulted in the development of two live, attenuated vaccines that are now licensed in many countries. This review summarizes new data on these vaccines, their effectiveness, and remaining challenges including new data on the rotavirus enterotoxin, a potential antiviral target.
Recent findings
Live attenuated rotavirus vaccines are used to protect infants against severe rotavirus-induced gastroenteritis and, RotaTeq®, a pentavalent bovine based vaccine, and, Rotarix®, a monovalent human rotavirus, are now currently licensed in many countries. Initial results of the licensed RotaTeq® vaccine have been promising in the United States and results of immunogenicity and efficacy in developing countries are expected soon. However universal vaccine implementation is challenging due to age limitations on administration of these vaccines. Chronic rotavirus infections in immunocompromised children may remain a problem and require the development of new treatments including antiviral drugs. Increasing data on the mechanisms of action of the rotavirus enterotoxin highlight this pleiotropic protein as a good target as well as unique calcium agonist.
Summary
Rotavirus is now a commonly occurring vaccine-preventable disease among children in developed countries and hopefully this also will soon be true for developing countries. Future studies will determine if other methods of prevention, such as nonreplicating vaccines and antiviral drugs, will be needed to treat disease in immunocompromised children.
Keywords: Live, attenuated vaccines, enterotoxin, antivirals, rotavirus
Introduction
Rotavirus (RV) is the leading cause of life-threatening diarrheal disease among infants and young children resulting in ∼ 600,000 deaths in children less than 5 years of age worldwide [1]. This global disease burden stimulated efforts to develop vaccines, some of which are licensed and now being used. This review summarizes current information about RV vaccines as well as remaining issues about them. New information about RV pathogenesis that may be key for future therapeutics is also summarized.
Rotavirus Structure and Classification
RVs are members of the Rotavirus genus of the Reoviridae family of viruses, which contains viruses with segmented RNA genomes. RV particles are large (1000 Å) and complex with three concentric protein shells that surround the viral genome of 11 segments of double-stranded RNA (Figure 1 ) [2]. Each RV genome segment codes for at least one viral protein that functions either as a structural component of the virus particle (viral protein or VP) or as a nonstructural protein made in infected cells that is involved in various aspects of the viral replication cycle. Two RV proteins, VP7 that makes up the outer capsid protein shell and VP4 that makes up spikes that emanate through the outer capsid shell, induce neutralizing antibody and these two proteins are the basis of a binary classification system for viral serotypes. Thus, VP7 (a glycoprotein or G-type antigen) and VP4 (a protease sensitive protein or P-type antigens) are used to classify RVs. VP7 types are classified as serotypes by neutralization assays or as genotypes by sequencing and these two assays yield concordant results so viruses are referred to by their G serotype alone (e.g., G1, G2, G3, etc). VP4 serotypes are also classified by both neutralization and sequencing assays but such results do not always agree so P typing has a dual system. P serotypes are referred to by their serotype numbers (e.g., P1, P2) and P genotypes are denoted in brackets (e.g., P[8], P[4]). P genotyping is the most widely used method for classification. Currently, 19G and 28[P] types are known.
Figure 1.
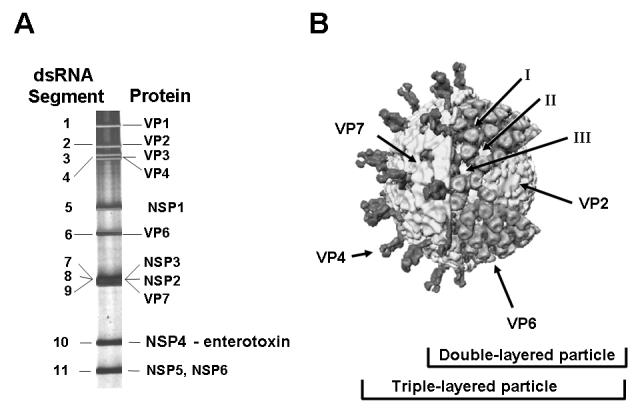
Structure and Proteins of Rotavirus. Panel A. The eleven segments of genomic double-stranded RNA are shown separated after electrophoresis on a polyacrylamide gel. Each numbered gene segment codes for at least one protein that is either a virion protein (VP) or a nonstructural (NSP) protein. The gene coding assignments are shown for the simian agent SA11. Panel B. This image shows the structure of rotavirus particles as determined by image reconstruction after cryo electron microscopy. The three concentric protein shells are seen in the cutaway and the proteins are color-coded. Different types of particles can be observed in stool samples named triple-layered particles, double-layered particles and core particles and the proteins in each type of particle are shown. Modified from [2]
To prepare for the introduction of RV vaccines, many studies characterized RV strains worldwide and found previously unappreciated strain diversity. Diversity is theoretically high because the segmented nature of the RV genome allows for gene reassortment when a single cell is co-infected with two different virus strains. From the 19G and 28[P] types known, 211 different combinations of G and P proteins can be generated to create high serotype diversity. In practice, most combinations are not fit and do not survive subsequent rounds of replication in humans so the actual number of G and P combinations is less the than possible number. Viruses circulating in humans now are characterized as being common human genotypes (G1P[8], G2P[4], G3P[8], G4P[8]), reassortants among human genotypes (G1P[4], G2P[8], G4P[4]), reassortants between animal and human genotypes (G1P[9], G4P[6], G9P[8], G12P[8]), and likely zoonotic introductions (G9[P6], G9P[11], G10P[11], G12P[6]) [3]. The common human genotypes represent the most prevalent viruses worldwide although their relative prevalence and distribution change with regard to location and time. The recently developed RV vaccines targeted the common human genotypes of RV.
Rotavirus Vaccines
Estimates of the burden of RV disease have been determined in individual countries in recent years in preparation for being able to evaluate the effectiveness of newly licensed vaccines. In 2006 in the United States, RV caused few deaths (20-60), but substantial morbidity among children, resulting in 55,000-70,000 hospitalizations, 200,000 to 272,000 emergency department visits and 410,000 physician office visits [4]. In 2006 in Europe, RV caused an estimated 3.5 million episodes of disease among the 236 million children younger than 5 years of age, resulting in 231 deaths, >87,000 hospitalizations and almost 700,000 outpatient visits [5]. These statistics alone document that RV disease constitutes a large public heath burden in developed countries, and with new RV vaccines available, RV is now the single most frequent vaccine-preventable gastroenterological disease among children.
Two RV vaccines are now licensed in various countries (Table 1). A live attenuated G1P[8] human RV vaccine (Rotarix® from GlaxoSmithKline Biologicals, Risensart, Belgium) has been licensed in Europe, the United States, Mexico, and several Latin American, Middle Eastern, Asian and African countries. A live pentavalent human-bovine reassortant vaccine containing G1, G2, G3, G4 and P1[8] viruses (RotaTeq® from Merck & Co., Inc., Whitehouse Station, NJ) has been licensed in Europe and the United States.
Table 1.
Licensed Rotavirus Vaccines
| RotaTeq® (Merck) | R otarix™ (GSK) | |
|---|---|---|
| Type | Pentavalent bovine-human reassortants G1, G2, G3, G4, P1[8] |
Monovalent human strain G1P[8] |
| Dosage | 3 doses, orally | 2 doses, orally |
| Age of administration | 1st dose by 6-12 weeks. Subsequent doses at 4-10 week intervals. Last dose not after 32 week |
1st dose by 6-14 weeks. 2nd dose 14-24 weeks. Interval between doses not less than 4 weeks |
| Formulation | Liquid | Lyophilized; reconstituted |
| Licensure | US FDA (2006) European Medicines Agency (2006) More than 70 countries |
European Medicines Agency (2006) US FDA (2008) More than 100 countries |
| WHO prequalification | Under review | Prequalified in countries where safety/efficacy proven (2007) |
| Efficacy against severe RV gastroenteritis |
98% | 100% |
| Efficacy versus hospitalization |
59% against hospitalization for diarrhea of any cause in 1st year of life |
42% against hospitalization for severe gastroenteritis |
| Intussusception | none | none |
The Rotateq® vaccine began to be used in the United States in the spring of 2006. A recent analysis of RV activity during the 2007-2008 season compared to the previous 15 seasons from 1991 to 2006 has provided the first indication that this vaccine is having a positive impact on RV illness [6]. CDC analyzed data from the National Respiratory and Enteric Vaccine Surveillance Network, a voluntary network of US laboratories that provide the CDC with weekly reports of the number of tests performed and positive results obtained for a variety of pathogens. For RV, fecal specimens are collected and tested using commercially available enzyme immunoassays. For this analysis, the number and proportion of acute gastroenteritis patients aged <3 years whose fecal specimens tested positive for RV during January-April in 2006, 2007, and 2008 were examined. A temporal analysis indicated that the 2007-2008 season appeared delayed in onset by 2-4 months. In addition, the number of RV tests performed and number of positive test results were substantially (>50%) lower during the current season compared to previous years. Smaller percentages of positive results were observed in all inpatient, emergency department, and outpatient clinic sites in 2008 compared with 2006 and 2007. These findings have limitations but the changes observed coincide with increased use of the RV vaccine. Continued surveillance and additional epidemiologic studies will confirm if RV vaccination is the cause of these positive effects as well as determine if vaccination has indirect benefits, such as inducing herd immunity by reducing transmission of RV in the community and thereby indirectly protecting unvaccinated children.
RV vaccines are undergoing further testing in many countries in the developing world where they are needed most. RotaTeq® and Rotarix® were found to be safe and effective in middle income countries in Latin America and Asia, in addition to the US and Europe [7-9]. Rotarix® has been found to be safe and immunogenic in South African and Bangladeshi children but its immunogenicity is lower compared with responses in children in other countries. Both RotaTeq® and Rotarix® are currently being evaluated for efficacy in Africa and in less developed countries in Asia and results are expected soon. Although it is likely that vaccine efficacy will be lower in developing countries than in Europe or the US, the public health impact of a vaccine even with a lower efficacy rate could be substantial, if mortality is substantially reduced, in developing countries.
While the current data on RV vaccines are encouraging, other challenges exist that will need to be addressed as vaccination programs continue to develop (Table 2). Partial answers to some of these issues are available. Implementation of RV immunization is more complicated than administering other childhood vaccines because of age restrictions for initiation and completion of vaccination. The preclusion of infants >12 or 14 weeks of age from initiation of a vaccine series is unprecedented and is a potential challenge for pediatricians and health care workers in both developed and developing countries. These age limitations were set due to unexpected reports of intussusception occurring in close temporal proximity to the administration of the first RV vaccine (RotaShield ®) that was licensed in the US in 1998 [10]. Most cases of intussuception were in infants to whom the first dose of vaccine had been given between 3 and 6 months of age in a so-called “catch-up program” [11;12]. This adverse event resulted in the voluntary withdrawal of RotaShield® from the market and necessitated large phase III clinical trials of the subsequent licensed vaccines (>60,000 children) to assess safety with respect to intussusception. It also led to the age restrictions for administering new vaccines to avoid vaccinating infants older than 3 months of age, the time-frame when intussusception occurred in RotaShield® vaccinees and when natural intussusception peaks. Meeting the age limits for immunization is difficult to achieve because routine vaccinations are not always made on schedule. Delayed immunization has been reported in the US, and children are being excluded from receiving any RV vaccine or completing the series [13]. These issues indicate that vaccine safety requires continued monitoring. Another safety issue that has not yet been addressed is what will occur when these live, attenuated vaccines are administered to children who have congenital or acquired immunodeficiency. RV infections in genetically immunocompromised animals or immunosuppressed children can result in chronic infections and long-term shedding, and the abnormalities leading to immunodeficiency in infants may not be detected until after the first vaccine dose is administered [14-16]. This may lead to an increase in vaccine strain-related illness in immunocompromised children; however, the occurance or consequences of such infections with RV vaccine remain to be determined.
Table 2.
Challenges Facing Rotavirus Vaccines
| Issue | Challenge |
|---|---|
| Age limits for delivery of first and last doses for safety |
Can age limitations be achieved in developed and developing countries? |
| Response of immunocompromised children |
Will live attenuated vaccines be immunogenic and cleared or cause prolonged infection? Will nonreplicating vaccines be needed? |
| Unusual and evolving RV strains are present in different geographic locations |
Circulating virus strains must be monitored. Vaccine formulations may need to be tailored for specific locations |
| Immunogenicity and protective efficacy not known yet for developing countries |
Can the often low immunogenicity of oral vaccines in developed countries be overcome? |
| No correlate of protection identified | Reduces ease of testing efficacy of future, new vaccines or measures to improve immunogenicity of any candidate vaccine. |
Chronic infections of immunocompromised children, either by community-acquired or vaccine RV strains, may lead to a need for antiviral drugs as well as nonreplicating vaccines based on either inactivated viruses, virus-like particles, or expressed proteins. Administration of subunit vaccines parenterally may offer other advantages over live-attenuated vaccines administered orally since live, oral RV vaccines may be associated with reduced take rates due to maternal antibodies transferred to infants transplacentally and by breast milk. In addition, new reassortants between vaccine strains and wild-type strains may appear. A challenge for developing alternative vaccines is the lack of a correlate of protection for existing RV vaccines. Neutralizing antibody does not correlate with protection and the mechanism(s) by which the single Rotarix vaccine strain or the pentavalent RotaTeq vaccines induce protection against human RV strains with G and P types not represented in these vaccines remains unknown. Alternative vaccines are efficacious in animal models [17] and they are ready to be tested in humans. Targets for antivirals are known and research in this area remains to be pursued.
Ongoing studies will need to continue to monitor the ability of current vaccines to induce protection against unusual and evolving RV strains. While the current vaccines appear to induce protection against strains other than those in the vaccines, several unusual strains are prevalent in specific developing countries and efficacy against challenge with these strains, or the evolution of these or other new strains need to be carefully monitored. As these vaccines are introduced into different populations globally, the appearance of new emerging RV strains that can evolve under immune selection, from immunocompromised children chronically shedding virus, or from reassortants that arise from co-circulating animal and human viruses will need to be monitored. A new classification system that characterizes RVs based on the origin of each of the 11 genome segments should be useful to quickly detect new emerging strains and possibly identify the molecular basis for new strain emergence [18;19].
NSP4: The rotavirus enterotoxin
One of the most intriguing aspects of RV pathogenesis is the involvement of a viral enterotoxin, nonstructural protein 4 (NSP4). NSP4 is initially synthesized as an endoplasmic reticulum (ER) transmembrane glycoprotein, 175 amino acids long with 2 glycosylation sites at its N-terminus. It is subsequently processed into different forms with distinct intracellular and extracellular functions. Early studies using reassortants from virulent and avirulent RV strains identified NSP4 as one of the RV virulence factors [20]. NSP4 was discovered to be an enterotoxin when it was found to induce diarrhea in neonatal mice in the absence of histological changes in the intestine [21]. Subsequent studies showed the functions of intracellular NSP4 (iNSP4) are mechanistically distinct from extracellular NSP4 (eNSP4) [22;23] and ongoing research is dissecting the molecular mechanisms responsible for each activity (Figure 2). Below is an update of the recent data for both iNSP4 and eNSP4, including which of the functions, critical for RV pathogenesis, are possible targets for vaccine or anti-viral drug design. This information updates previous summaries of the enterotoxin activity of NSP4 and its distinct activities compared to known bacterial enterotoxins [24-27].
Figure 2. Classification of iNSP4 and eNSP4 Functions.
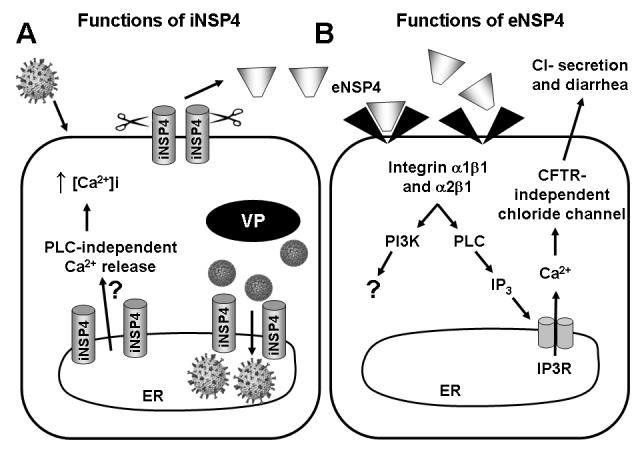
A. Upon RV infection, intracellular NSP4 (iNSP4) is synthesized in the endoplasmic reticulum (ER) as a transmembrane glycoprotein. iNSP4 induces a phospholipase C (PLC)-independent calcium leakage from the ER by an unknown mechanism and results in a sustained elevation in the intracellular calcium concentration (↑[Ca2+]i). ER-bound iNSP4 also binds VP6 on immature DLPs, facilitates (1) the budding of DLPs through the ER membrane and (2) the acquisition of the outer capsid proteins VP4 and VP7. iNSP4 that traffics to the plasma membrane is cleaved by a protease, generating the rotavirus enterotoxin, extracellular NSP4 (eNSP4). B. eNSP4 binds to integrins α1β1 or α2β1 on neighboring cells, triggering the activation of phosphoinositide-3 kinase (PI3K) and PLC. While the consequences of PI3K activation are under investigation, NSP4-induced activation of PLC leads to the production of inositol 1,4,5-trisphosphate (IP3) and IP3 receptor (IP3R)-mediated calcium release. The calcium activates chloride secretion that is independent of the CFTR channel. Chloride secretion is age-dependent, occurring in neonatal, but not adult, mice and leads to diarrhea.
eNSP4
The enterotoxin activity of NSP4 was discovered in 1996 after diarrhea was induced in neonatal mice administered either the full-length protein or a 22 amino acid synthetic peptide corresponding to a small domain (amino acids 114-135) of the cytoplasmic tail of NSP4 [21]. These initial studies predicted that an enterotoxic form of NSP4 (i) would be released from RV-infected cells, (ii) bind to receptors on uninfected cells, and (iii) activate a signaling pathway that produces diarrhea. Soon, a secreted cleavage product of NSP4 (NSP4 aa112-175) was detected [28]. The secreted NSP4 induces PLC-dependent signaling in HT-29 cells, a human intestinal cell line, and in neonatal mouse intestinal cells leading to increased levels of intracellular calcium ([Ca2+]i) and subsequent chloride secretion through activation or regulation of a CFTR-independent channel [22;29]. These early studies fulfilled the 1st and 3rd predictions but the NSP4 receptor remained elusive for nearly a decade. Recently, studies identified the I domain of integrins α1 or α2 as receptors for NSP4, and exposure of cells expressing integrin α2β1 to NSP4 activates a signal transduction pathway mediated by both phospholipase C (PLC) and phosphoinositide-3 kinase (PI3K) [30]. Integrin I domain binding is mediated by glutamic acid 120 in NSP4, corresponding to a putative calcium binding site [31;32]. Integrin signaling is initiated by NSP4 amino acids 130-140, consistent with previous work that identified specific mutations within this domain that reduce both PLC-mediated signaling and NSP4 enterotoxin activity [33]. Mutant NSP4 that cannot bind to the I domain or elicit a signal through the integrin are deficient for diarrhea induction in neonatal mice, confirming that this interaction is critical for NSP4 enterotoxin activity. These results are exciting because they identify the initial molecular interaction behind NSP4-induced diarrhea, and inhibition of this interaction might prevent disease. They also reveal a new role for integrins in enterotoxin function and further distinguish NSP4 enterotoxin activity from bacterial enterotoxins.
Extraintestinal spread of RV is now well documented and it is intriguing to postulate that RV-infected cells outside of the intestine may secrete NSP4 [34-37]. The possible release of NSP4 into multiple organs as well as into the circulatory system could be significant if NSP4-induced cell signaling and downstream effects are as detrimental to these organ systems as they are to the gut. If such events occur, NSP4 could have more far-reaching effects than currently appreciated.
iNSP4
iNSP4 has two main roles in RV infection: (i) increasing intracellular calcium, and (ii) facilitating virus assembly. These activites are critical for RV replication, since siRNA-mediated knockdown of iNSP4 increases mRNA production, reduces viral protein synthesis and genomic dsRNA replication, and causes aberrant sub-cellular localization of all of the viral proteins [38;39]. Intracellular NSP4 induces a 2- to 6-fold increase in calcium that is necessary for productive virus replication. Chelating [Ca2+]i or siRNA-mediated knockdown of NSP4 also reduce cytopathic effects and virus yield [40;41]. The mechanism for how iNSP4 alters calcium homeostasis is unknown, but understanding this process is critical to understanding the RV replication process. NSP4 increase ER leakiness that increases [Ca2+]i and entry of calcium into cells through the plasma membrane capacitative calcium entry channels. An attractive idea is that NSP4 increases plasma membrane permeability, possibly by forming a calcium channel [41-43], but this remains to be demonstrated. Since virion assembly requires high cytoplasmic and ER calcium, such an NSP4 channel would need to be regulated, possibly by another viral protein, to ensure that NSP4-induced ER-leakiness does not deplete the ER of calcium but rather calcium is elevated throughout the cell [44]. Elevated calcium may be important for RV replication beyond virus assembly. A calcium binding site is present in NSP5, a key component of intracellular structures called viroplasms where viral RNA replication occurs, and calcium binding to NSP5 appears to regulate viroplasm formation [45]. NSP4 also responds to increases in calcium by forming a vesicular compartment that associates with viroplasms. This raises the possibility that the NSP4-induced increases in [Ca2+]i, but not NSP4 itself, controls viral protein sub-cellular localization [38;46].
The knockdown of NSP4 during RV infection causes an increase in mRNA levels and indicates that the intracellular receptor function of NSP4 may serve as a negative regulator of transcription though its interaction with VP6 on immature double-layered particles [39]. One can speculate that NSP4 binds VP6 at the 5-fold axis of the particle and blocks the type III channel, where transcripts emerge. Alternatively, studies to recoat double-layered particles with the outer capsid proteins show that VP4 (the spike protein) needs to be assembled onto particles before VP7 (outer shell glycoprotein), suggesting that VP4 interacts with the surface of VP6 [47]. NSP4 has binding sites for both VP4 and VP6 that are closely juxtaposed, suggesting that NSP4 may act as a scaffolding protein to properly orient VP6 and VP4 during outer capsid assembly [48]. Thus, while the basic intracellular functions of NSP4 have been known for many years, recent work indicates the mechanisms of iNSP4 functions are more intricate than originally appreciated. Understanding these mechanisms is of broad interest since RV is one of an increasing number of viruses for which calcium-signaling is a key cellular target for viral infection, and further studies exploring new calcium-related therapeutic strategies could have broad applications [49].
NSP4: a future therapeutic target
The persistent infection and chronic shedding of RV from immunocompromised children points to the need for antiviral drugs that target RV replication. The establishment of high [Ca2+]i is critical for RV replication and NSP4 is the sole protein responsible for this phenotype, making NSP4 a compelling target for antiviral drug development [41;50]. While the mechanism of NSP4-induced high [Ca2+]i is unknown, if NSP4 does form a calcium channel, then compounds that block this channel should block RV replication.
For many bacterial infections, an antibody response directed solely against the secreted enterotoxin protects the host against severe disease. A similar finding seems to be true for NSP4 and RV-induced disease. Immunization of mice with either the enterotoxic peptide (aa114-135) or the entire NSP4 C-terminus (aa85-175) linked to either the cholera toxin or shiga toxin B subunit, which serves as an adjuvant, stimulates both systemic (IgG) and mucosal (IgA) antibody responses [51;52]. Pups born to NSP4-vaccinated dams exhibit significantly reduced severity and duration of diarrhea compared to pups born to unvaccinated dams [51;52]. Although these studies did not determine which epitope(s) on NSP4 stimulated the protective antibody, earlier studies found immunization with the aa114-135 peptide generates an antibody response that protects against diarrhea [21] and this protective antibody is specific to a single NSP4 epitope that is 100% conserved in all NSP4 sequences [48;53]. Interestingly, this epitope contains the integrin I domain binding site and this antibody blocks the NSP4-I domain interaction [30]. Thus, antibody to NSP4 aa114-135 may be protective against severe RV disease by blocking the interaction between eNSP4 and its receptor and eliminating the enterotoxin component of disease. The strict conservation of this epitope in all NSP4 sequences suggests that active or passive immunization strategies that include NSP4 may provide broad protection by neutralizing the enterotoxin irrespective of the G or P type of an infecting virus strain. Several studies investigating the immune response to NSP4 after natural RV infection have shown that seroconversion rates are high (54-70%, for both IgG and IgA) and heterotypic responses can be detected [54-56]. Thus, it is intriguing to speculate that antibody to NSP4 might be a correlate of heterotypic protection seen by vaccination of children or animals with a single virus who are protected against diarrhea induced by other virus serotypes. Although initial attempts to measure protective antibodies to NSP4 were not successful, the assays used may not have measured the relevant function-blocking antibodies.
Conclusion
Rotavirus infections of the gastrointestinal tract continue to cause significant disease but new vaccines are showing promise of reducing the high morbidity observed in developing countries. Hopefully, ongoing vaccines trials in developing countries will lead to the global reduction of RV-associated mortality of children. However, global implementation of rotavirus vaccination programs faces continued challenges due to age restrictions for vaccination, complex strain diversity in different locations, and some areas with high numbers of immunocompromised children. Thus, vaccine safety and virus evolution needs to continue to be monitored, and additional RV strains may need to be added to vaccines in the future to protect against unusual circulating and evolving rotavirus strains. Future needs in the armentarium of approaches to overcome RV disease may include antivirals and the viral enterotoxin is one promising target based on a new understanding of the mechanisms of enterotoxin function.
Acknowledgements
Research in the authors laboratory is supported by NIH grants R56DK30144-, T32AI007471, and P30 DK56338 that funds the Texas Medical Center Digestive Diseases Center. MKE is an inventor on candidate rotavirus VLP and NSP4 vaccines.
Reference List
References and recommended reading
Papers of particular interest, published within the annual period of review have beenhighlighted as:
* Of special interest
** Of outstanding interest
- 1.Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estes MK. Rotaviruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 1747–1785. [Google Scholar]
- 3.Gray J, Vesikari T, Van Damme P, Giaquinto C, Mrukowicz J, Guarino A, Dagan R, Szajewska H, Usonis V. Rotavirus. J Pediatr.Gastroenterol Nutr. 2008;46(Suppl 2):S24–S31. doi: 10.1097/MPG.0b013e31816f78ee. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Prevention of rotavirus gastroenteritis among infants and children. MMWR Morb Mortal Wkly Rep. 2006;55 (No. RR-12) [Google Scholar]
- 5.Soriano-Gabarro M, Mrukowicz J, Vesikari T, Verstraeten T. Burden of rotavirus disease in European Union countries. Pediatr.Infect Dis J. 2006;25:S7–S11. doi: 10.1097/01.inf.0000197622.98559.01. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Delayed onset and diminished magnitude of rotavirus activity - United States, November 2007 - May 2008. MMWR Morb Mortal Wkly Rep. 2008;57:1–4.First report indicating that the new rotavirus vaccines are having a positive effect in the United States.
- 7.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, Cervantes Y, Linhares AC, Lopez P, Macias-Parra M, Ortega-Barria E, Richardson V, Rivera-Medina DM, Rivera L, Salinas B, Pavia-Ruz N, Salmeron J, Ruttimann R, Tinoco JC, Rubio P, Nunez E, Guerrero ML, Yarzabal JP, Damaso S, Tornieporth N, Saez-Llorens X, Vergara RF, Vesikari T, Bouckenooghe A, Clemens R, De Vos B, O’Ryan M. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 8.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, Shinefield HR, Christie CDC, Ylitalo S, Itzler RF, Coia ML, Onorato MT, Adeyi BA, Marshall GS, Gothefors L, Campens D, Karvonen A, Watt JP, O’Brien KL, DiNubile MJ, Clark HF, Boslego JW, Offit PA, Heaton PM. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 9.Linhares AC, Velazquez FR, Perez-Schael I, Saez-Llorens X, Abate H, Espinoza F, Lopez P, Macias-Parra M, Ortega-Barria E, Rivera-Medina DM, Rivera L, Pavia-Ruz N, Nunez E, Damaso S, Ruiz-Palacios GM, De Vos B, O’Ryan M, Gillard P, Bouckenooghe A. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–1189. doi: 10.1016/S0140-6736(08)60524-3.Large trial showing safety and efficacy of the Rotarix vaccine during the first 2 years of life in Latin American infants.
- 10.Centers for Disease Control and Prevention (CDC) Intussusception among recipients of rotavirus vaccine--United States, 1998-1999. MMWR. 1999;48:577–581. [PubMed] [Google Scholar]
- 11.Simonsen L, Viboud C, Elixhauser A, Taylor RJ, Kapikian AZ. More on RotaShield and intussusception: The role of age at the time of vaccination. J Infect Dis. 2005;192:S36–S43. doi: 10.1086/431512. [DOI] [PubMed] [Google Scholar]
- 12.Rothman KJ, Young-Xu Y, Arellano F. Age dependence of the relation between reassortant rotavirus vaccine (RotaShield) and intussusception. J Infect Dis. 2006;193:898–899. doi: 10.1086/500217. [DOI] [PubMed] [Google Scholar]
- 13.Daskalaki I, Spain CV, Long SS, Watson B. Implementation of rotavirus immunization in Philadelphia, Pennsylvania: high levels of vaccine ineligibility and off-label use. Pediatrics. 2008;122:e33–e38. doi: 10.1542/peds.2007-2464.Discussion of the challenges that the age restrictions for rotavirus vaccination presents and the possible complications of vaccinating children off schedule.
- 14.Rayani A, Bode U, Habas E, Fleischhack G, Engelhart S, Exner M, Schildgen O, Bierbaum G, Eis-Hubinger A Maria, Simon A. Rotavirus infections in paediatric oncology patients: a matched-pairs analysis. Scand.J Gastroenterol. 2007;42:81–87. doi: 10.1080/00365520600842179.Report of rotavirus infection and prolonged shedding is clinically relevant in pediatric cancer patients but most cases may be preventable as they are nosocomial in origin.
- 15.Eiden J, Losonsky GA, Johnson J, Yolken RH. Rotavirus RNA variation during chronic infection of immunocompromised children. Pediatr.Infect.Dis. 1985;4:632–637. doi: 10.1097/00006454-198511000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Dharakul T, Rott L, Greenberg HB. Recovery from chronic rotavirus infection in mice with severe combined immunodeficiency: Virus clearance mediated by adoptive transfer of immune CD8+ T lymphocytes. J.Virol. 1990;64:4375–4382. doi: 10.1128/jvi.64.9.4375-4382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen K, Conner ME, Estes MK. Virus-like particles (VLP) as vaccines and vaccine delivery systems. In: Levine MM, Kaper JB, Rappuoli R, Lui MA, editors. New Generation of Vaccines. Marcel Dekker, Inc; New York: 2008. In: New Generation of Vaccines.Review of virus-like particles as vaccines.
- 18.Matthijnssens J, Ciarlet M, Heiman E, Arijs I, Delbeke T, McDonald SM, Palombo EA, Iturriza-Gomara M, Maes P, Patton JT, Rahman M, Van Ranst M. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J Virol. 2008;82:3204–3219. doi: 10.1128/JVI.02257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthijnssens J, Ciarlet M, Rahman M, Attoui H, Banyai K, Estes MK, Gentsch JR, Iturriza-Gomara M, Kirkwood CD, Martella V, Mertens PP, Nakagomi O, Patton JT, Ruggeri FM, Saif LJ, Santos N, Steyer A, Taniguchi K, Desselberger U, Van Ranst M. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch.Virol. 2008;153:1621–1629. doi: 10.1007/s00705-008-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshino Y, Saif LJ, Kang SY, Sereno MM, Chen WK, Kapikian AZ. Identification of group A rotavirus genes associated with virulence of a porcine rotavirus and host range restriction of a human rotavirus in the gnotobiotic piglet model. Virology. 1995;209:274–280. doi: 10.1006/viro.1995.1255. [DOI] [PubMed] [Google Scholar]
- 21.Ball JM, Tian P, Zeng CQ-Y, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 22.Dong Y, Zeng CQ-Y, Ball JM, Estes MK, Morris AP. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci. 1997;94:3960–3965. doi: 10.1073/pnas.94.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian P, Estes MK, Hu Y, Ball JM, Zeng CQ-Y, Schilling WP. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J Virol. 1995;69:5763–5772. doi: 10.1128/jvi.69.9.5763-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorrot M, Vasseur M. How do the rotavirus NSP4 and bacterial enterotoxins lead differently to diarrhea? Virol J. 2007;4:31. doi: 10.1186/1743-422X-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ball JM, Mitchell DM, Gibbons TF, Parr RD. Rotavirus NSP4: A multifunctional viral enterotoxin. Viral Immunol. 2005;18:27–40. doi: 10.1089/vim.2005.18.27. [DOI] [PubMed] [Google Scholar]
- 26.Morris AP, Estes MK. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. VIII. Pathological consequences of rotavirus infection and its enterotoxin. Am J Physiol Gastrointest Liver Physiol. 2001;281:G303–G310. doi: 10.1152/ajpgi.2001.281.2.G303. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz MC, Cohen J, Michelangeli F. Role of Ca2+ in the replication and pathogenesis of rotavirus and other viral infections. Cell Calcium. 2000;28:137–149. doi: 10.1054/ceca.2000.0142. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Zeng CQ-Y, Morris AP, Estes MK. A functional NSP4 enterotoxin peptide secreted from rotavirus-infected cells. J.Virol. 2000;74:11663–11670. doi: 10.1128/jvi.74.24.11663-11670.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris AP, Scott JK, Ball JM, Zeng CQ-Y, O’Neal WK, Estes MK. NSP4 elicits age-dependent diarrhea and Ca2+-mediated I- influx into intestinal crypts of CF mice. Am J Physiol. 1999;277:G431–G444. doi: 10.1152/ajpgi.1999.277.2.G431. [DOI] [PubMed] [Google Scholar]
- 30.Seo NS, Zeng CQ, Hyser JM, Utama B, Crawford SE, Kim KJ, Hook M, Estes MK. Inaugural article: integrins alpha1beta1 and alpha2beta1 are receptors for the rotavirus enterotoxin. Proc Natl Acad Sci U S A. 2008;105:8811–8818. doi: 10.1073/pnas.0803934105.First identification of a cellular receptor for the rotavirus enterotoxin, demonstration that the secreted form of NSP4 can interact with this receptor on uninfected cells distant from rotavirus-infected cells, and elucidation of signaling pathways induced by NSP4.
- 31.Bowman G, Nodelman I, Levy O, Lin S, Tian P, Zamb T, Udem S, Venkataraghavan B, Schutt C. Crystal structure of the oligomerization domain of NSP4 from rotavirus reveals a core metal-binding site. J Mol Biol. 2000;304:861–871. doi: 10.1006/jmbi.2000.4250. [DOI] [PubMed] [Google Scholar]
- 32.Deepa R, Durga RC, Suguna K. Structure of the extended diarrhea-inducing domain of rotavirus enterotoxigenic protein NSP4. Arch Virol. 2007;152:847–859. doi: 10.1007/s00705-006-0921-x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M, Zeng CQ-Y, Dong Y, Ball JM, Saif LJ, Morris AP, Estes MK. Mutations in rotavirus nonstructural glycoprotein NSP4 are associated with altered virus virulence. J Virol. 1998;72:3666–3672. doi: 10.1128/jvi.72.5.3666-3672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blutt SE, Conner ME. Rotavirus: to the gut and beyond! Curr Opin Gastroenterol. 2007;23:39–43. doi: 10.1097/MOG.0b013e328011829d.Review of data demonstrating extraintestinal rotavirus infection in animals and children.
- 35.Crawford SE, Patel DG, Cheng E, Berkova Z, Hyser JM, Ciarlet M, Finegold MJ, Conner ME, Estes MK. Rotavirus viremia and extraintestinal viral infection in the neonatal rat model. J.Virol. 2006;80:4820–4832. doi: 10.1128/JVI.80.10.4820-4832.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feneaux M, Cuadras MA, Feng N, Jaimes MC, Greenberg HB. Extra-intestinal spread and replication of homologous (EC) and a heterologous (RRV) rotavirus in BALB/c mice. J Virol. 2006 doi: 10.1128/JVI.02664-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azevedo AS, Yuan L, Jeong KI, Gonzalez A, Nguyen TV, Pouly S, Gochnauer M, Zhang W, Azevedo A, Saif LJ. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J.Virol. 2005;79:5428–5436. doi: 10.1128/JVI.79.9.5428-5436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez T, Camacho M, Zayas M, Najera R, Sanchez R, Arias CF, Lopez S. Silencing the morphogenesis of rotavirus. J Virol. 2005;79:184–192. doi: 10.1128/JVI.79.1.184-192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silvestri LS, Tortorici A, Patton JT. The rotavirus glycoprotein NSP4 is a modulator of viral transcription in the infected cell. J Virol. 2005;79:15165–15174. doi: 10.1128/JVI.79.24.15165-15174.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez JF, Chemello ME, Liprandi F, Ruiz MC, Michelangeli F. Oncosis in MA104 cells is induced by rotavirus infection through an increase in intracellular Ca2+ concentration. Virology. 1998;252:17–27. doi: 10.1006/viro.1998.9433. [DOI] [PubMed] [Google Scholar]
- 41.Zambrano JL, Diaz Y, Pena F, Vizzi E, Ruiz MC, Michelangeli F, Liprandi F, Ludert JE. Silencing of rotavirus NSP4 or VP7 expression reduces alterations in Ca2+ homeostasis induced by infection of cultured cells. J Virol. 2008;82:5815–5824. doi: 10.1128/JVI.02719-07.Studies using siRNA-based knockdown of NSP4, VP7, and VP4 demonstrate that predominantly NSP4, and to a lesser extent VP7, are responsible for the dramatic changes in calcium homeostasis during rotavirus infection.
- 42.Ruiz MC, Diaz Y, Pena F, Aristimuno OC, Chemello ME, Michelangeli F. Ca2+ permeability of the plasma membrane induced by rotavirus infection in cultured cells is inhibited by tunicamycin and brefeldin A. Virology. 2005;333:54–65. doi: 10.1016/j.virol.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 43.Newton K, Meyer JC, Bellamy AR, Taylor JA. Rotavirus nonstructural glycoprotein NSP4 alters plasma membrane permeability in mammalian cells. J.Virol. 1997;71:9458–9465. doi: 10.1128/jvi.71.12.9458-9465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz MC, Aristimuno OC, Diaz Y, Pena F, Chemello ME, Rojas H, Ludert JE, Michelangeli F. Intracellular disassembly of infectious rotavirus particles by depletion of Ca2+ sequestered in the endoplasmic reticulum at the end of virus cycle. Virus Res. 2007;130:140–150. doi: 10.1016/j.virusres.2007.06.005.Selective depletion of ER calcium pools late in infection causes disassembly of infectious virions, suggesting that high ER calcium is critical for the integrity of newly formed virions.
- 45.Sen A, Sen N, Mackow ER. The formation of viroplasm-like structures by the rotavirus NSP5 protein is calcium-regulated and directed by a C-terminal helical domain. J Virol. 2007;81:11758–11767. doi: 10.1128/JVI.01124-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berkova Z, Crawford SE, Trugnan G, Yoshimori T, Morris AP, Estes MK. Rotavirus NSP4 induces a novel vesicular compartment regulated by calcium and associated with viroplasms. J Virol. 2006;80:6061–6071. doi: 10.1128/JVI.02167-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trask SD, Dormitzer PR. Assembly of highly infectious rotavirus particles recoated with recombinant outer capsid proteins. J Virol. 2006;80:11293–11304. doi: 10.1128/JVI.01346-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hyser JM, Zeng CQ-Y, Beharry Z, Palzkill T, Estes MK. Epitope mapping of the rotavirus SA11 NSP4 cytoplasmic tail. Virology. 2007 doi: 10.1016/j.virol.2007.11.021. PMID: 18164740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chami M, Oules B, Paterlini-Brechot P. Cytobiological consequences of calcium-signaling alterations induced by human viral proteins. Biochim Biophys Acta. 2006;1763:1344–1362. doi: 10.1016/j.bbamcr.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 50.Tian P, Hu Y, Schilling WP, Lindsay DA, Eiden J, Estes MK. The nonstructural glycoprotein of rotavirus affects intracellular calcium levels. J Virol. 1994;68:251–257. doi: 10.1128/jvi.68.1.251-257.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu J, Langridge WH. A plant-based multicomponent vaccine protects mice from enteric diseases. Nat Biotechnol. 2001;19:548–552. doi: 10.1038/89297. [DOI] [PubMed] [Google Scholar]
- 52.Choi NW, Estes MK, Langridge WH. Oral immunization with a shiga toxin B subunit: rotavirus NSP4(90) fusion protein protects mice against gastroenteritis. Vaccine. 2005;23:5168–5176. doi: 10.1016/j.vaccine.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 53.Lin SL, Tian P. Detailed computational analysis of a comprehensive set of group a rotavirus NSP4 proteins. Virus Genes. 2003;26:271–282. doi: 10.1023/a:1024451314534. [DOI] [PubMed] [Google Scholar]
- 54.Vizzi E, Calvino E, Gonzalez R, Perez-Schael I, Ciarlet M, Kang G, Estes MK, Liprandi F, Ludert JE. Evaluation of serum antibody responses against the rotavirus nonstructural protein NSP4 in children after rhesus rotavirus tetravalent vaccination or natural infection. Clin Diagn Lab Immunol. 2005;12:1157–1163. doi: 10.1128/CDLI.12.10.1157-1163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johansen K, Hinkula J, Espinoza F, Levi M, Zeng CQY, Vesikari T, Estes MK, Svensson L. Humoral and cell-mediated immune responses to the NSP4 enterotoxin of rotavirus. J Med Virol. 1998;59:369–377. [PubMed] [Google Scholar]
- 56.Ray P, Malik J, Singh RK, Bhatnagar S, Bahl R, Kumar R, Bhan MK. Rotavirus nonstructural protein NSP4 induces heterotypic antibody responses during natural infection in children. J Infect Dis. 2003;187:1786–1793. doi: 10.1086/375243. [DOI] [PubMed] [Google Scholar]


