Abstract
Background
Human spatial navigation can be conceptualized as egocentric or exocentric, depending on the navigator’s perspective. While navigational impairment occurs in individuals with cognitive impairment, less is known about navigational abilities in non-demented older adults. Our objective was to develop tests of navigation and study their cognitive correlates in non-demented older adults.
Methods
We developed a Local Route Recall Test (LRRT) to examine egocentric navigation and a Floor Maze Test (FMT) to assess exocentric navigation in 127 older adults without dementia or amnestic Mild Cognitive Impairment. Factor analysis was used to reduce neuropsychological test scores to three cognitive factors representing Executive Function/Attention, Verbal Ability, and Memory. We examined relationships between navigational tests and cognitive function (using both cognitive factors and the highest loading individual test on each factor) in a series of regression analyses adjusted for demographic variables (age, sex, and education), medical illnesses, and gait velocity.
Results
The tests were well-tolerated, easy to administer, and reliable in this non-demented and non-MCI sample. Egocentric skills on the LRRT were associated with Executive Function/Attention (B -0.650, 95% C.I. -0.139, -0.535) and Memory (B -0.518, 95% C.I. -0.063, -4.893) factors. Exocentric navigation on the FMT was related to Executive Function/Attention (B -8.542, 95% C.I. -13.357, -3.727).
Conclusions
Our tests appear to assess egocentric and exocentric navigation skills in cognitively-normal older adults, and these skills are associated with specific cognitive processes such as executive function and memory.
INTRODUCTION
Successful navigation requires highly complex interactions among multiple cognitive processes, including visual perception, spatial orientation, learning, and memory (1). These cognitive processes may be differentially activated depending on the relationship of the navigator to the objects within the environment being negotiated. For this reason, navigation tasks can be divided according to the navigator’s perspective (2). In an egocentric navigation paradigm, the navigator maintains a static spatial orientation to objects in the environment. This type of ‘route-based’ navigation is essentially linear and sequential: an egocentric navigator progresses from a fixed origin to a fixed final destination, always passing intervening landmarks in the same order and with the same spatial orientation to them. By contrast, in an exocentric type of navigation the navigator’s spatial orientation to objects in the environment is fluid. This type of ‘map-like’ navigation relies on more flexible Euclidian spatial elements rather than linear sequence: the navigator’s origin, destination, spatial orientation to intervening landmarks, and path all can vary.
The two types of navigation described above have been linked to distinct aspects of the brain’s visuospatial mechanisms (1). The ventral (“what”) pathway has been associated with the processing of visual landscapes and the objects within them, while the dorsal (“where”) pathway has been associated with processing spatial relationships (3). In addition, functional neuroimaging has been used to investigate the specific neural substrates underlying different navigation paradigms. These studies have implicated posterior parietal and basal ganglia regions in egocentric navigation, while hippocampal and parahippocampal regions have been linked to exocentric navigation (4). Neuroimaging studies have often been conducted in normal young subjects or, when examining older subjects, have focused on those with brain damage (i.e. stroke) or cognitive impairment (i.e. dementia). In addition, many such investigations utilize virtual reality to test navigation, a methodology that may present unique challenges to older subjects. By comparison, the cognitive processes underlying navigational abilities in non-demented older adults have been infrequently studied, particularly in assessments oriented toward real-world conditions.
In the present study we developed two navigational tests with real-world applicability: the Local Route Recall Test (LRRT), an egocentric task, evaluated subjects’ accuracy in recalling walking routes to landmarks in their own neighborhoods; the Floor Maze Test (FMT), an exocentric task, required navigation through an unfamiliar two-dimensional floor maze.
We used these tests to explore the associations between egocentric and exocentric navigational skills and specific underlying cognitive processes in older adults without dementia or amnestic Mild Cognitive Impairment (MCI). Based on prior studies, we postulated that in our population of non-demented, non-MCI older adults exocentric navigation on the FMT would be associated with measures of executive function (5-6) and egocentric navigation on the LRRT with memory measures (7-8).
METHODS
Study Population
Eligible subjects for this pilot study included community-dwelling participants in the Einstein Aging Study (EAS: 85%) and a smaller sample of community volunteers (15%) evaluated at our research center between July 2001 and March 2002. EAS subjects were systematically recruited from population lists of Medicare recipients, age 70 and over, living in Bronx County. All subjects had been living at their current address for at least four years at the time of testing. Overall EAS study design and methods have been previously reported (9-11). EAS exclusion criteria included severe visual/auditory impairment, English language facility precluding cognitive testing, inability to ambulate, and institutionalization. Written informed consent was obtained from each subject at study entry according to protocols approved by the local institutional review board. Additional exclusion criteria for this study included use of walking aids and presence of either dementia (12) or amnestic MCI (13) (see below for diagnostic procedures). After all exclusions, 127 of 405 subjects (31.4%) seen during the 9-month study period were eligible for this study. Compared to those excluded, eligible subjects were younger (mean 77.9 vs. 80.4 years, p<0.001), better educated (mean 13.8 vs. 12.8 years, p=0.006), and had better general cognition (mean Blessed Information-Memory-Concentration test scores 1.9 vs. 3.8, p<0.001).
Clinical and Cognitive Evaluations
Using structured questionnaires, research assistants obtained information on demographic variables (age, sex, and education), medical history, and presence of depressive symptoms (15-item Geriatric Depression Scale, GDS) (14). Cumulative medical burden was calculated using a summary score (range 0-19) based on self-report of 19 possible medical conditions: stroke, Parkinson’s disease (PD), depression, other psychiatric disorder, rheumatoid or osteoarthritis, hip/neck/skull fracture, cardiovascular disease (heart failure, myocardial infarction, angina, arrhythmia, heart murmur, claudication), hypertension, diabetes, chronic lung disease, or cancer. A board-certified neurologist examined each subject and obtained additional detail on medical history. Finally, all subjects completed a series of basic gait and mobility assessments, including gait velocity and falls history.
All subjects underwent an extensive battery of neuropsychological tests validated in our population (15-17) and other aging cohorts. Testing assessed global cognition (Blessed-Information-Memory-Concentration test; BIMC) (18), attention (Trail-Making Test A) (19) estimated Verbal IQ (Vocabulary, Similarities, Information, and Digit Span subtests from the Wechsler Adult Intelligence Scale (WAIS-R) (20), language (Category fluency for animals, vegetables, and fruit) (21), verbal memory (free recall on the Free and Cued Selective Reminding Test-FCSRT) (22), and executive function (Block Design and Digit Symbol Substitution subtests from the WAIS-R, Controlled Oral Word Association test-FAS, and Trail-Making Test B) (20, 23, 19). Although the battery did not include a specific test of visual memory, tests such as Block Design, Digit Symbol, and the Trail-Making Test all incorporate abilities from the visuospatial and construction domains. Additional testing of visuospatial reasoning for this pilot study included a paper-and-pencil Porteus maze test from Level VIII of the Vineland Revision (24). For data reduction purposes, we submitted neuropsychological test scores to principal components factor analysis (PCA, see below) (25).
As described previously (10, 26), using review of all available clinical and neuropsychological data, diagnoses of dementia (DSM-IV criteria) and amnestic MCI (Peterson criteria) were made by unanimous consensus at case conferences attended by the study neurologist, neuropsychologist, and social worker. Neuroimaging was not done in all subjects as a standard component of our research protocols.
Egocentric and Exocentric Navigational Skills
In order to reduce variability in testing conditions as much as possible, a single research assistant performed all navigation testing using a standard set of instructions for each test.
Floor MazeTest (FMT)
To test exocentric navigation, we constructed a 7′×10′ floor maze approximately based on the paper Porteus Maze-Extension VIII test (24) using white tape on blue carpet in a large well-lit room in our research center. The Extension series is slightly less complex than the Vineland Revision used in the paper-and-pencil Porteus test (27). The research assistant positioned subjects at the entry point and instructed them to find their way to the exit. Using a stopwatch, the research assistant individually recorded time elapsed from end of instructions to maze entry (Planning Time, PT, seconds) and total time from maze entry to successful exit (Immediate Maze Time, IMT, seconds). Subjects repeated the maze (Delayed Maze Time, DMT, seconds) after a delay of ten minutes, during which they performed gait and mobility tests in a separate room. For the IMT, subjects were permitted to correct any wrong turns while in the maze, although errors were tabulated. No planning period was allowed for DMT and errors were not tabulated for this portion of the test. Subjects received as much time as they needed for each segment of the test. Each of the three segments (PT, IMT, and DMT) were timed and analyzed separately.
Local Route Recall Test (LRRT)
From a list of common landmarks (bank, school, park, etc.), subjects were asked to choose three that they encountered at increasing levels of annual frequency while walking in their local neighborhoods. For the least-frequently visited landmark, we established a minimum visit rate of three times per year to ensure a minimum level of familiarity with the route and to minimize recall bias. Subjects were asked to estimate the number of times they passed each landmark in an average year and specifically in the month immediately preceding testing. Using self-report of encounters during the month preceding testing, the most- and least-frequently visited landmarks were identified for each subject. For each landmark, subjects described their usual route from home to landmark, including street names and turns. The route for the most frequently visited landmark was designated the High-Frequency Route and that for the least frequently visited landmark the Low-Frequency Route. Street-naming error rates on the High- and Low-frequency routes were the main outcomes. Error rates for each route were calculated as the percentage of streets named incorrectly from the total number of streets named, irregardless of sequence along the route. A standard street atlas of each subject’s neighborhood was used to verify route description accuracy. The LRRT was introduced approximately four weeks after the FMT, and data is available for a slightly smaller group of subjects for this test.
Statistical analysis
Neuropsychological test results were submitted to PCA (25), using Varimax rotation to derive orthogonal factor scores accounting for 64% of test score variance. The resultant cognitive factors were used as predictors in regression analyses. In addition, we completed secondary analyses for the neuropsychological test with the highest loading score for each factor, in order not to obscure individual test contributions. For the FMT, we examined the association of neuropsychological factors with PT, IMT, and DMT using linear regression analyses adjusted for demographic variables (age, sex, and education), medical summary score, and gait velocity. Using poorest performances, we imputed IMT (100 seconds) and DMT (32 seconds) for nine subjects unable to complete all components of the FMT. Main results were not materially different after excluding these nine subjects. For the LRRT, overdispersed Poisson regression (28) was used to model street-naming error rates on the High- and Low-Frequency routes as a function of neuropsychological factors, also adjusted for demographic variables, medical summary score, and gait velocity. The models were fit by maximum quasi-likelihood using the glm () function in S-Plus (29). Those who could not perform the LRRT were omitted from the analysis; we did not impute data for this group given the relatively large number of subjects who did not complete the test (see below). Unless otherwise specified, all statistical analyses were conducted using SPSS Version 15.0 (30).
RESULTS
Baseline characteristics are shown in Table 1. The sample was predominantly Caucasian (74%), female (56.7%) and well-educated (mean 13.8 years). Neuropsychological test performance is displayed in Table 2. Mean neuropsychological test performance for all subjects was in the normal range for age and education for all tests (31).
Table 1.
Baseline demographic characteristics. Values are means ± SD unless otherwise noted
| Overall (n=127) | |
|---|---|
| Age (years) | 77.8 ± 5.3 |
| Women (%) | 56.7 |
| Caucasian (%) | 74.8 |
| Education (years) | 13.8 ± 3.1 |
| Medical Summary Score (0-19) | 3.3 ± 1.9 |
| GDS* (0-15) | 2.0 ± 1.9 |
| Falls in Past year (%) | 27.6 |
| Gait Velocity (meters/second) | 1.1 ± 0.3 |
Geriatric Depression Scale
Table 2.
Neuropsychological test performance. Values are means ± SD unless otherwise noted. Score ranges are noted in parentheses
| Domain | Test Variable (range) | Overall (n=127) |
|---|---|---|
| Global Cognition | BIMC test (0-32, higher better) | 2.2 ± 2.1 |
| Attention | Trail-Making Test-A (0-300 sec) | 54.7 ± 21.9 |
| WAIS-R Verbal IQ Subtests | Vocabulary (0-70) | 48.1 ± 14.2 |
| Similarities(0-28) | 19.3 ± 5.8 | |
| Information (0-29) | 22.5 ± 5.2 | |
| Digit Span (0-28) | 12.8 ± 3.6 | |
| Language | Category Fluency† (sum of items named in 60 seconds for each category) | 37.0 ± 9.2 |
| Memory | FCSRT, free recall (0-48) | 32.0 ± 6.4 |
| Executive Function | Block Design (0-51) | 18.8 ± 7.9 |
| Digit Symbol-Substitution (0-93) | 38.9 ± 12.2 | |
| Controlled Oral Word Association-FAS (sum of items named in 60 seconds for each phoneme) | 34.9 ± 13.2 | |
| Trail-Making Test B ( 0-300 sec) | 122.3 ± 53.2 | |
| Porteus Maze | Vineland Revision -VIII | |
| Completion Time (secs) | 21.3 ± 24.7 | |
| Committed Error (%) | 36.6 | |
| Errors (number) | 0.5 ± 0.8 | |
animals + vegetables + fruits
Our population of community-residing elderly subjects was relatively healthy. Subjects self-reported a mean of 3.3 medical problems on the 19-point medical summary score. Subjects endorsed few depressive symptoms (mean GDS score 2.0). Twenty-seven percent had fallen in the year preceding testing, consistent with patterns of fall frequency in geriatric populations (32). None of the subjects endorsed a previous history of PD. Stroke was self-reported by 17 subjects, however only four subjects had any clinically-detectable residual deficits (isolated left homonymous hemianopsia in one, mild left hemiparesis in two, and mild right hemiparesis in one). We conducted a secondary analysis after excluding all subjects who had self-reported a history of stroke and results are described below.
Neuropsychological Test Factor Analysis
PCA results are shown in Table 3. Three significant orthogonal factors associated with major cognitive domains were found. The Executive Function/Attention factor loaded highly on the Digit Symbol, Trail-Making (A and B), and Block Design tests, all of which are timed tests relying on problem-solving abilities and speed of processing; Digit Symbol was the highest loading test on this factor. The Verbal Ability factor loaded highly on WAIS-R verbal IQ subtests, with the Information subtest as the highest-loading test. The Memory Factor loaded highly on tests of working, semantic, and episodic memory, with Free-Recall (FCSRT) as the highest-loading individual test.
Table 3.
Results of the Principal Components Factor Analysis using Varimax rotation
| Variable | Factor 1- Executive Function/Attention | Factor 2 -Verbal Ability | Factor 3 -Memory |
|---|---|---|---|
| Variance (%) for rotated | 40.22 | 13.19 | 10.21 |
| Test | |||
| Vocabulary | .096 | .845 | .219 |
| Similarities | .303 | .697 | .229 |
| Information | .084 | .867 | .144 |
| Digit Span | .177 | .132 | .541 |
| Block Design | .676 | .277 | .005 |
| Digit Symbol | .804 | .016 | .294 |
| FAS | .314 | .373 | .604 |
| Category Fluency | .259 | .326 | .693 |
| Trails A (time) | -.733 | -.250 | -.094 |
| Trails B (time) | -.744 | -.011 | -.265 |
| FCSRT (free) | .004 | .057 | .846 |
Note: bold indicates loading coefficients over .50. FAS=phonemic fluency; Category fluency= animals + fruit + vegetables, FCSRT=Free and Cued Selective Reminding Test.
Higher scores denote better performance.
Floor Maze Test
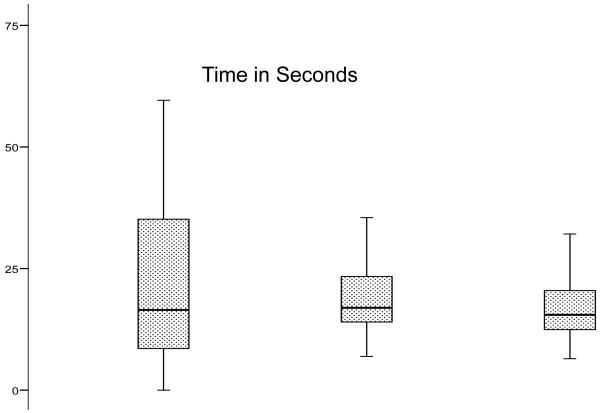
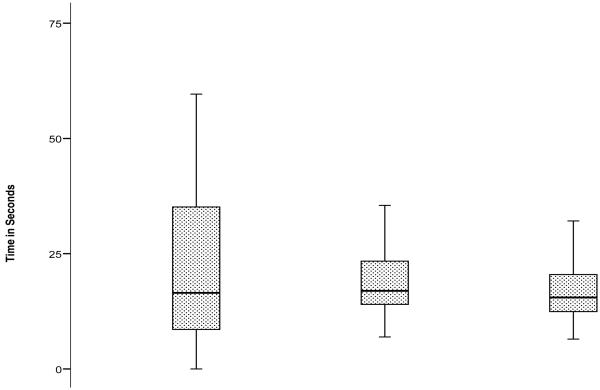
Error-free completion of the IMT was achieved by 101 of 124 subjects (the testing room was unavailable for three subjects). Of the 23 subjects (18.1%) who did not attain error-free IMT completion, 16 subjects completed the test with errors and seven subjects prematurely terminated their trials due to frustration. By comparison, 45 (36.6%) subjects committed errors on the paper-and-pencil Porteus maze. Two subjects completed the IMT but were unable to complete the DMT. There were no significant group differences on the FMT by gender, significant depressive symptoms, medical illness summary score, or history of falls. Test performance on the FMT is shown graphically in Figure 2-B. Subjects devoted more time to route planning than to IMT (mean 34.4 vs. 24.3 seconds, p = 0.002). They were faster on DMT (mean 17.0 sec) than IMT (p<0.001). Subjects with a high-school education or less were slower on both IMT (30.8 vs. 19.3 seconds, p=0.001) and DMT (19.5 vs. 15.0 seconds, p<0.001). IMT and DMT were strongly correlated (Pearson r 0.762). Paper Porteus maze times correlated weakly with IMT (r 0.299) and DMT (r 0.336); errors on the paper Porteus and the IMT were uncorrelated (r 0.019).
Figure 2b.
Performance on Planning Time, Immediate Maze Time and Delayed Maze Time on the Floor Maze Test (in seconds)
Table 5 shows that the Executive Function/Attention factor predicted performance on all components of the FMT in the fully adjusted models. In terms of individual tests, Digit Symbol similarly predicted performance on all components of the FMT, while Free Recall (FCSRT) predicted IMT.
Table 5.
Regression Coefficients for Neuropsychological Factors on Planning Time (PT), Immediate Maze Time (IMT) and Delayed Maze Time (DMT). Coefficients are adjusted for demographic variables (age, sex, education), medical disease burden, and gait velocity
| Variables | Planning Time (PT) | Immediate Maze Time (IMT) | Delayed Maze Time (DMT) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | B (95% C.I.) |
p-value | Beta | B (95% C.I.) |
p-value | Beta | B (95% C.I.) |
p-value | |
| Proces sing Speed Factor | 0.398 | -20.091 (-30.353, -9.829) |
0.000 | -0.364 | -8.542 (-13.357, -3.727) |
0.001 | -0.499 | -3.137 (-4.277, -1.998) |
0.000 |
| Verbal Ability Factor | -0.031 | -1.536 (-12.274, 9.203) |
0.777 | 0.020 | 0.462 (-4.515, 5.439) |
0.854 | -0.103 | -0.634 (-1.898, 0.6300 |
0.322 |
| Memory Factor | -0.060 | -2.911 (-13.433, 7.611) |
0.584 | -0.169 | -3.791 (-8.612, 1.030) |
0.122 | -0.096 | -0.578 (-1.820, 0.663) |
0.357 |
| Digit-Symbol Substitution | -0.254 | -1.008 (-1.876, -0.139) |
0.023 | -0.253 | -0.464 (-0.859, -0.068) |
0.022 | -0.370 | -0.183 (-0.278, -0.088) |
0.000 |
| WAIS-Information | -0.069 | -0.663 (-2.775, 1.449) |
0.535 | -0.101 | -0.450 (-1.410, 0.510) |
0.355 | -0.127 | -0.154 (-0.400, 0.091) |
0.215 |
| Free Recall-FCSRT | -0.096 | -.798 (-2.498, 0.902) |
0.354 | -0.199 | -0.769 (-1.533, -0.006) |
0.048 | -0.125 | -0.133 (-0.331, 0.065) |
0.187 |
Of covariates tested, age was significantly associated only with PT, while walking velocity was significantly associated with DMT for both the factors and the individual tests. None of the other adjustments were significant. Main results were unchanged after exclusion of the 17 subjects with a self-reported history of stroke (data not shown).
Local Route Recall Test
Of the 111 subjects who attempted the test, 91 (82.0%) successfully completed it. Of the 20 subjects who did not complete the test, 11 did not walk often in their neighborhoods, five were able to recall the route to only one landmark, and four self-discontinued testing. While three annual visits was the minimum required on both routes, most subjects visited the landmarks more frequently (mean 460.4 visits annually to the High-Frequency landmark and 67.9 visits to the Low-Frequency landmark). During the month immediately preceding testing subjects had encountered High-Frequency landmarks a mean of 37.8 times (range 4-120) and Low-Frequency landmarks 5.6 times (range 0-50). Distance (70%) and ease of terrain (27%) primarily influenced walking route selection to the neighborhood landmarks.
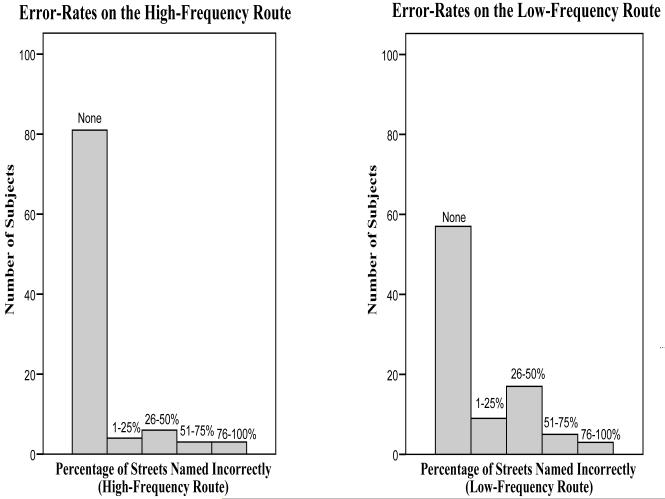
Performance on the LRRT is shown in Figure 2-A. Error-free completion was achieved by 83.5% of subjects on the High-Frequency route and by 62.6% of subjects on the Low-Frequency route. Subjects named more streets on the Low-Frequency than the High- Frequency route (mean 5.8 vs. 3.6, p ≤ 0.001). Error rates were higher on Low-Frequency street naming (15.6 vs. 7.8% streets incorrectly named, p=0.009) than on High-Frequency street-naming. Error rates on describing turns did not contribute significantly to the analysis for either route (data not shown). There were no significant group differences by gender, significant depressive symptoms (GDS> 6), medical illnesses, or educational level.
Figure 2a.
Street-Naming Errors on the High- and Low-Frequency Routes on the Local Route Recall Test.
Poisson regression (Table 4) revealed that the Executive Function/Attention and Memory factors predicted High-Frequency route error rates while for the Low-Frequency route, none of the factors were significant. Of the highest loading individual tests for each factor, Digit Symbol predicted performance on the High-Frequency route and Free Recall (FCSRT) predicted performance on the Low-Frequency route.
Table 4.
Results of Overdispersed Poisson Regression Models for Street-Naming Error Rates on the Route Recall Test for the High-Frequency and Low-Frequency routes. Regression coefficients are shown for each of the three factors and for the highest loading individual test on each factor. For each model shown, coefficients are adjusted for demographic variables (age, sex, years of education), medical disease burden, and gait velocity
| Variables | High-Frequency route Error Rate | Low-Frequency route Error Rate | ||||||
|---|---|---|---|---|---|---|---|---|
| Relative Risk | B | (95% C.I.) | p-value | Relative Risk | B | (95% C.I.) | p-value | |
| Processing Speed Factor | 0.522 | -0.650 | (-0.139, -.535) | 0.013 | 1.172 | 0.159 | (0.477, 2.081) | 0.327 |
| Verbal Ability Factor | 1.453 | 0.374 | (0.930, 2.956) | 0.188 | 1.100 | 0.094 | (0.412, 1.239) | 0.559 |
| Memory Factor | 0.596 | -0.518 | (-0.063, -4.893) | 0.026 | 0.815 | -0.204 | (0.049, -3.308) | 0.113 |
| Digit-Symbol Substitution Test | 0.941 | -0.061 | (-0.020, -5.739) | 0.004 | 1.008 | 0.008 | (0.030, 1.292) | 0.512 |
| WAIS-R Information Subtest | 1.030 | 0.030 | (0.132, 1.157) | 0.565 | 1.017 | 0.017 | (0.075, 1.151) | 0.563 |
| FCSRT-Free Recall | 0.968 | -0.032 | (0.034, -1.889) | 0.344 | 0.952 | -0.049 | (-0.007, -4.520) | 0.023 |
Several potential confounders were significantly associated with test outcomes. On the High-Frequency route, age was associated with performance for all three factors and on the Digit Symbol test. The medical summary score affected the Memory and (non-significant) Verbal Ability factors. Gait velocity, sex, and education had no significant association with outcomes on either the factors or the individual tests. On the Low-Frequency route, except for the Memory factor, age influenced performance for all predictors, including individual tests, but none of the other covariates were significant. In the secondary analysis excluding the 17 subjects with a self-reported history of prior stroke, main results were unchanged for the High-Frequency route but Free Recall no longer predicted performance on the Low-Frequency route (data not shown). No significant correlations were found between either the High-Frequency or Low-Frequency route outcome measures and any of the components of the FMT.
Discussion
In this study we developed and examined clinical tests of egocentric and exocentric navigation in a group of non-demented community-residing elderly subjects. The tests were well tolerated, quick, and easy to administer. The majority of subjects were able to complete both tests. Our results demonstrated strong associations between the two navigation tests and specific cognitive processes in older adults without dementia or amnestic MCI, supporting the construct validity of these two new tests. While both the FMT and the LRRT tests are associated with cognitive processes related to executive function and memory, the lack of correlation between the two suggests that they may be tapping into complementary but distinct human navigational abilities.
On the exocentric FMT, the Executive Function/Attention factor and its highest loading individual test, Digit Symbol, were related to performance on all three components of the test in models adjusted for potentially significant confounders such as demographic variables (age, sex, and education), gait velocity, and medical summary score. The strong association indicates that this test of exocentric navigation required subjects to tap into cognitive abilities associated with mental flexibility and psychomotor processing speed. Memory scores predicted performance on the IMT but not on the DMT, suggesting that episodic memory was not a strong contributor to delayed navigation performance. With the exception of PT, results on the FMT were independent of age. Walking velocity was related to performance only on the DMT. Because this was a novel testing paradigm we wanted to reduce variability in testing conditions as much as possible. For this reason, a single research assistant performed all testing, using a standard set of instructions. Alternate versions of the FMT were not tested, due to space and time constraints. We are addressing these issues in ongoing studies. Nonetheless, there was high correlation between IMT and DMT on the FMT indicating very good test-retest reliability despite possible practice effects due to repeat administration and planning.
On the egocentric LRRT, both the Executive Function/Attention and Memory factors were associated with High-Frequency route performance, while none of the factors were significant on the Low-Frequency route. Digit Symbol and not FCSRT predicted High-Frequency route performance, raising the possibility that, for frequently-traveled routes, route recall relies on cognitive abilities beyond memory. On the Low-Frequency route the FCSRT demonstrated the anticipated association and the Memory Factor did not, possibly indicating a specific role for episodic memory in recall of routes less-often traveled. The associations between aspects of the egocentric LRRT and memory may represent co-localization of these functions in common neuroanatomical sites, such as the hippocampus or frontal lobes. Maguire and colleagues (33) reported relative hypertrophy of the posterior hippocampus in London taxi drivers with extensive navigation experience compared to controls. Another study reported that AD and MCI patients recognized landmarks as well as controls on a route learning task, but could not find their locations on maps or recall the order in which they were encountered (6). Location identification correlated with right posterior hippocampal and parietal volumes, whereas recall order correlated with bilateral inferior frontal volumes.
While our ability to comment on the relationship between navigation and aging is limited by the fact that our sample did not include young subjects, our analysis within this age-restricted sample shows that age influenced the egocentric LRRT more than the exocentric FMT. At first glance, this might seem to suggest that street-naming errors were a function of decreased activity outside the home for older subjects. However, older subjects did not report visiting local landmarks less often, irrespective of route frequency, nor were they more likely to be unable to perform the LRRT. The ability to name streets accurately on the LRRT may be influenced by other factors, such as long habituation to the neighborhood or memory performance.
Our tests differ somewhat from previous investigations, which have included subjects’ ability to retrace routes in clinical or hospital settings (5-6). These tests, which can be cumbersome for use in a clinical rather than a research setting, often depend on the local architecture or subjects’ familiarity with the testing environment, making them prone to reduced reliability if landmarks change and limits their comparison with other settings. Virtual reality has been used to investigate navigation, usually in younger subjects offering the requisite easy familiarity with computer operations. The correlation between virtual environment navigation and real world navigation has not been well established. Hence, our tests may not be directly comparable to previous studies. On the other hand, by testing navigation in real world settings, our tests may improve ecological validity, in keeping with an elderly population’s navigational skills.
The question of ecological validity raises implications for clinical assessments of navigation in community-residing elderly individuals, including evaluation of environmental safety (i.e. giving directions, driving ability, and wandering behavior). In the relatively low correlation found between the Porteus maze and the FMT, our results seem to corroborate earlier findings that paper-and-pencil spatial tests do not necessarily correlate strongly with real-world tests of navigation ability (34). Porteus mazes were recently shown to predict driving ability in subjects with mild dementia (35) but given that ecological validity may be lower in paper tests, a test such as the FMT could be useful in clinical assessment of the cognitive and spatial skills required for safe driving in elderly individuals. The LRRT, by focusing on local neighborhoods, may be an ecologically valid method to assess navigation skills in clinical settings and possibly to aid in identification of early impairments in memory or executive function. Errors on the LRRT could be verified through an informant. These applications should be further studied in other settings.
A strength of our study is that the sample size was large enough to allow us to control for a number of key confounders. Several limitations require acknowledgment. This cognitively-normal sample from the Bronx is not necessarily representative of the general population, and our findings need to be replicated in other cohorts. The cognitive factors are unique to our sample but were similar to those obtained in our previous studies (36-37). Our standard battery of neuropsychological tests did not include a specific test of visual learning; while tests such as Block Design and TMT incorporate abilities from the visuospatial domain, it has also been suggested that two-dimensional tests of visual memory function are not equivalent to recall of three-dimensional space (4). Finally, the cross-sectional nature of the study precludes causal inferences.
Summary
We developed novel clinical tests of egocentric and exocentric navigation. Exocentric navigation in cognitively-normal elderly subjects, assessed with the Floor Maze Test, was related to executive function and to a lesser extent memory. Egocentric skills, tested by the Local Route Recall test, were associated with memory, executive function, and age. Our findings provide insight into exocentric and egocentric navigation skills in cognitively-normal older adults, which should be validated in other populations or pursued in longitudinal assessments and with techniques such as neuroimaging or transcranial magnetic stimulation to localize neuroanatomical sites or networks.
Figure 1.
The Floor Maze Test
Acknowledgments
We thank Molly Zimmerman, PhD and Mindy Katz, MPH for helpful comments on the manuscript. We thank Claudia Salazar for help with data collection.
Funding:
The Einstein Aging Study is supported by National Institutes on Aging program project grant AG03949. Dr. Sanders is supported by NRSA Institutional Training Grant T32 AG023475-04. Dr. Verghese is supported by NIA R01 AG025119.
Footnotes
Abstract Presentation:
Presented in part at the 59th annual meeting of the American Academy of Neurology, Boston, April 28-May 5, 2007.
Conflict of Interest:
No conflict of interest.
References
- 1.Van Asselen M, Kessels RPC, Kappelle LJ, Neggers SFW, Frijns CJM, Postma A. Neural correlates of human wayfinding in stroke patients. Brain Research. 2006;1067:229–238. doi: 10.1016/j.brainres.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre GK, D’Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain. 1999;122(9):1613–28. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- 3.Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. PIT Press; Cambridge, MA: 1982. pp. 549–586. [Google Scholar]
- 4.Maguire EA, Burgess N, Donnett JG, Frackowiak RSJ, Frith CD, O’Keefe J. Knowing where and getting there: a human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 5.Cherrier MM, Mendez M, Perryman K. Route learning performance in Alzheimer Disease patients. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14(3):159–168. [PubMed] [Google Scholar]
- 6.deIpolyi AR, Rankin KP, Mucke L, Miller BL, Gorno-Tempini ML. Spatial cognition and the human navigation network in AD and MCI. Neurology. 2007;69:986–997. doi: 10.1212/01.wnl.0000271376.19515.c6. [DOI] [PubMed] [Google Scholar]
- 7.Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 8.Tiernan KN, Schenk K, Swadberg D, et al. Puget Sound Route Learning Test: Examination of the validity and reliability of a novel route test in healthy older adults and Alzheimer’s disease patients. Clinical psychologist. 2004;8(1):39–42. [Google Scholar]
- 9.Verghese J, Levalley A, Hall CB, et al. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc. 2006;54:255–261. doi: 10.1111/j.1532-5415.2005.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipton RB, Katz MJ, Kuslansky G, et al. Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc. 2003;51:1382–1390. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- 11.Verghese J, Katz MJ, Derby CA, Kuslansky G, Hall C, Lipton RB. Reliability and validity of a telephone-based mobility assessment questionnaire. Age Ageing. 2004;33:628–632. doi: 10.1093/ageing/afh210. [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- 13.Petersen RC. Mild cognitive impairment: current research and clinical implications. Semin Neurol. 2007;27:22–31. doi: 10.1055/s-2006-956752. [DOI] [PubMed] [Google Scholar]
- 14.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In: Sheikh JI, Yesavage JA, editors. Clinical Gerontology: A Guide to Assessment and Intervention. The Hawarth Press; New York: 1986. pp. 165–173. [Google Scholar]
- 15.Katzman R, Aronson M, Fuld P, et al. Development of dementing illness in an 80-year-old volunteer cohort. Ann Neurol. 1989;25:317–324. doi: 10.1002/ana.410250402. [DOI] [PubMed] [Google Scholar]
- 16.Masur DM, Sliwinski M, Lipton RB, Blau AD, Crystal HA. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology. 1994;44:1427–1432. doi: 10.1212/wnl.44.8.1427. [DOI] [PubMed] [Google Scholar]
- 17.Sliwinski M, Buschke H, Stewart WF, Masur D, Lipton RB. The effect of dementia risk factors on comparative and diagnostic selective reminding norms. J Int Neuropsychol Soc. 1997;3:317–326. [PubMed] [Google Scholar]
- 18.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile changes in the cerebral gray matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 19.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. Oxford University Press; New York: 2004. pp. 371–374. [Google Scholar]
- 20.Wechsler D. WAIS-R Manual. Psychological Corporation; New York: 1981. [Google Scholar]
- 21.Monsch AU, Bondi MW, Butters N, et al. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- 22.Buschke H. Cued recall in amnesia. J Clin Neuropsychol. 1984;6:433–440. doi: 10.1080/01688638408401233. [DOI] [PubMed] [Google Scholar]
- 23.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. Oxford University Press; New York: 2004. pp. 518–520. [Google Scholar]
- 24.Porteus SD. Porteus Maze Test. Fifty Years’ Application. Psychological Corporation; New York: 1965. [Google Scholar]
- 25.Bryant FB, Yarnold PR. Principal components analysis and exploratory and confirmatory factor analysis. In: Grimm LG, Yarnold PR, editors. Reading and Understanding Multivariate Statistics. 1st ed. American Psychological Association; Washington DC: 1995. pp. 99–136. [Google Scholar]
- 26.Verghese J, Lipton RB, Hall C, Kuslansky G, Katz M, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347:1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- 27.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. Oxford University Press; New York: 2004. p. 616. [Google Scholar]
- 28.McCullagh P, Nelder J. Generalized Linear Models. 2nd ed. Chapman and Hall; London: 1989. pp. 323–352. [Google Scholar]
- 29.S-Plus. Insightful Corporation; http://www.splus.com. [Google Scholar]
- 30.SPSS 15.0 for Windows [computer software] SPSS; Chicago: 2006. [Google Scholar]
- 31.Holtzer R, Goldin Y, Zimmerman ME. Comparison of robust versus conventional norms of neuropsychological tests in aging. J Int Neuropsychol Soc. 2007;13:4. [Google Scholar]
- 32.Sattin RW. Falls among older persons: a public health perspective. Annu Rev Public Health. 1992;13:489–508. doi: 10.1146/annurev.pu.13.050192.002421. [DOI] [PubMed] [Google Scholar]
- 33.Maguire EA, Gadian DG, Johnsrude IS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA. 2000;97(8):4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadolne MJ, Stringer AY. Ecologic validity in neuropsychological assessment: prediction of wayfinding. J Int Neuropsychol Soc. 2001;7:675–682. doi: 10.1017/s1355617701766039. [DOI] [PubMed] [Google Scholar]
- 35.Ott BR, Heindel WC, Whelihan WM, Caron MD, Piatt AL, DiCarlo MA. Maze test performance and reported driving ability in early dementia. J Geriatr Psychiatry Neurol. 2003;16(3):151–5. doi: 10.1177/0891988703255688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holtzer R, Friedman R, Lipton RB, Katz M, Xue X, Verghese J. The relationship between specific cognitive functions and falls in aging. Neuropsychology. 2007;21(5):540–548. doi: 10.1037/0894-4105.21.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20(2):215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]