Abstract
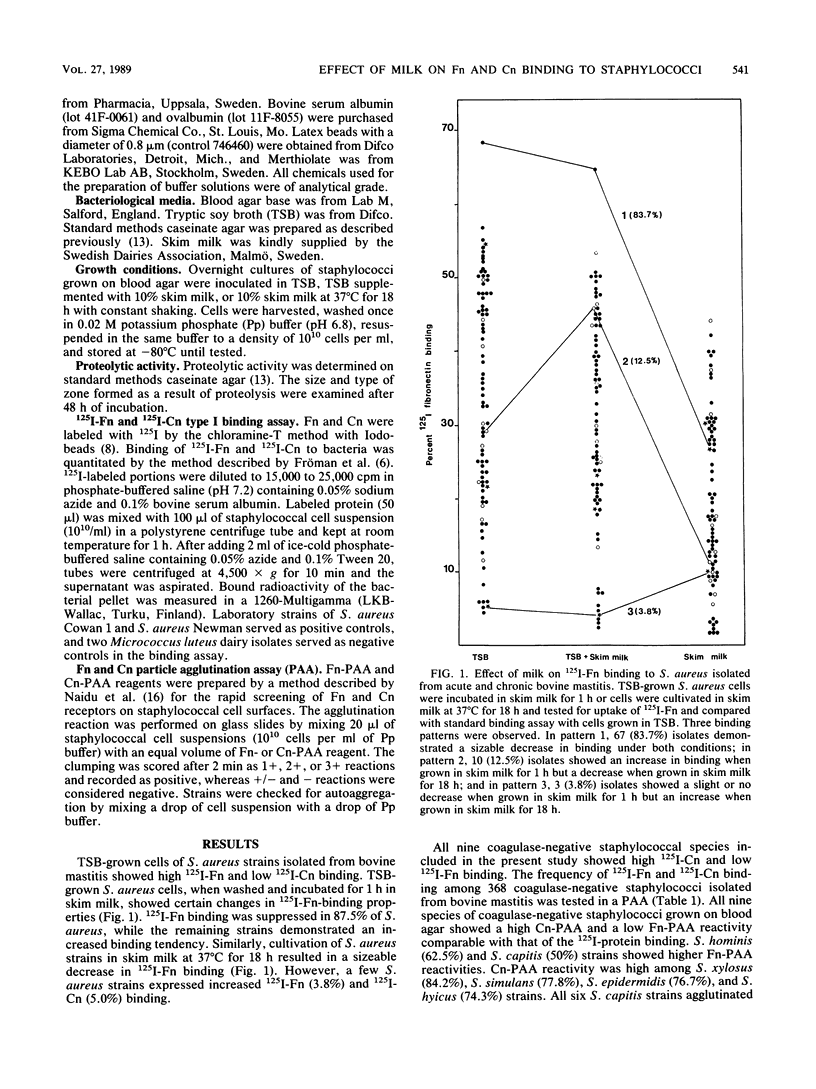
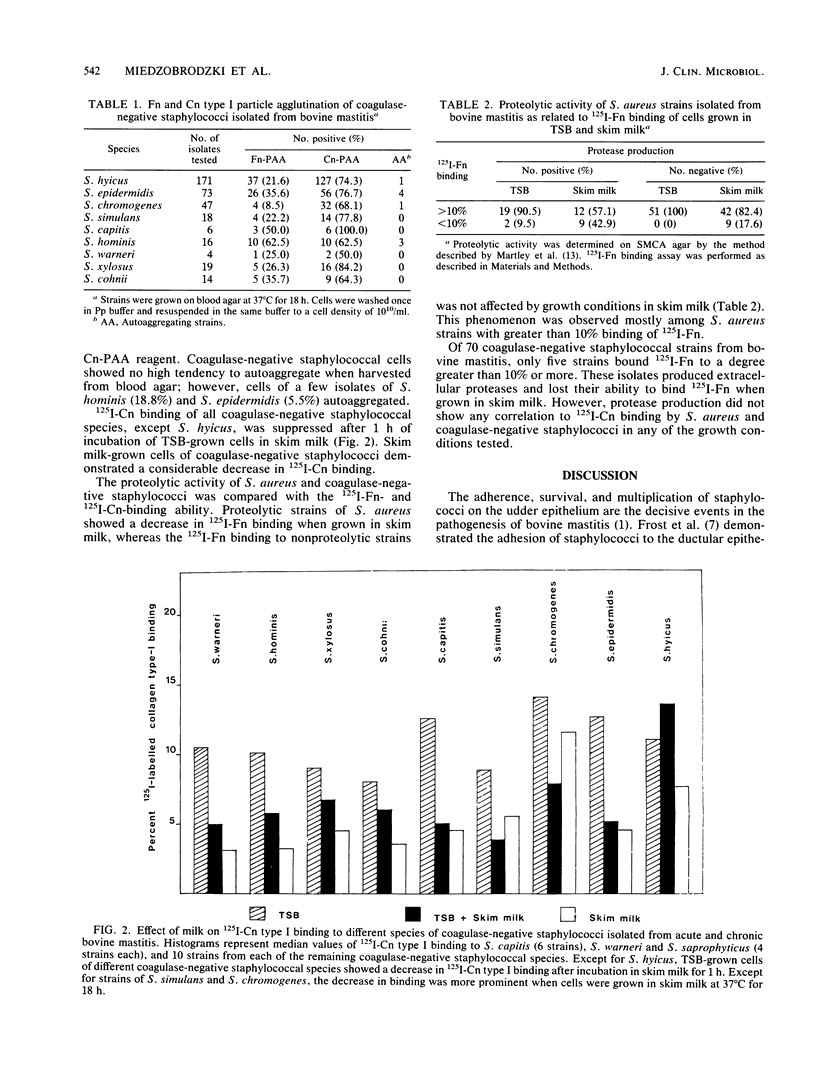
Tryptic soy broth (TSB)-grown cells of Staphylococcus aureus isolated from acute and chronic bovine mastitis bound mainly 125I-fibronectin (Fn) [corrected], whereas strains of nine species of coagulase-negative staphylococci showed a predominant interaction with 125I-collagen (Cn) [corrected] type I. A particle agglutination assay (PAA) was used to examine the interaction of coagulase-negative staphylococci with 125I-Fn and 125I-Cn immobilized on latex. All 368 coagulase-negative staphylococci demonstrated high 125I-Cn and moderate to low 125I-Fn interactions in the PAA. Cn-PAA reactivity was high among strains of Staphylococcus xylosus (84.2%), Staphylococcus simulans (77.8%), Staphylococcus epidermidis (76.7%), and Staphylococcus hyicus (74.3%), whereas all six Staphylococcus capitis strains clumped Cn-PAA reagent. Incubating TSB-grown cells in 10% skim milk for 1 h decreased the 125I-Fn- and 125I-Cn-binding affinity in most of the S. aureus and coagulase-negative staphylococci, while growth in 10% skim milk for 18 h resulted in more than 90% decrease or complete loss of interaction with these proteins. Decreased 125I-Fn binding in the presence of milk was correlated with protease production but not with 125I-Cn binding.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock J. H., Turvey A., Reiter B. Virulence of two mastitis strains of Staphylococcus aureus in bovine skin: enhancement by growth in high carbohydrate-high salt medium or in raw milk. Infect Immun. 1973 Jun;7(6):865–872. doi: 10.1128/iai.7.6.865-872.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R. Parameters affecting the association of vibrios with the intestinal surface in experimental cholera. Infect Immun. 1972 Aug;6(2):134–141. doi: 10.1128/iai.6.2.134-141.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A. J., Wanasinghe D. D., Woolcock J. B. Some factors affecting selective adherence of microorganisms in the bovine mammary gland. Infect Immun. 1977 Jan;15(1):245–253. doi: 10.1128/iai.15.1.245-253.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Faris A., Wadström T., Hök M. Binding of Escherichia coli to fibronectin. A mechanism of tissue adherence. J Biol Chem. 1984 Dec 10;259(23):14899–14905. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbitt K. G., Benians M. Some effects in vivo of the teat canal and effects in vitro of cationic proteins on Staphylococci. J Gen Microbiol. 1971 Sep;68(1):123–128. doi: 10.1099/00221287-68-1-123. [DOI] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Mamo W., Fröman G., Sundås A., Wadström T. Binding of fibronectin, fibrinogen and type II collagen to streptococci isolated from bovine mastitis. Microb Pathog. 1987 Jun;2(6):417–424. doi: 10.1016/0882-4010(87)90048-9. [DOI] [PubMed] [Google Scholar]
- Martley F. G., Jarvis A. W., Bacon D. F., Lawrence R. C. Typing of coagulase-positive staphylococci by proteolytic activity on buffered caseinate-agar, with special reference to bacteriophage nontypable strains. Infect Immun. 1970 Oct;2(4):439–442. doi: 10.1128/iai.2.4.439-442.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila T., Syväjärvi J., Jensen N. E., Sandholm M. Determinants of bacterial replication rates in mastitic whey. J Dairy Res. 1986 May;53(2):197–202. doi: 10.1017/s0022029900024791. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Physiology of fibronectin. Annu Rev Med. 1984;35:561–575. doi: 10.1146/annurev.me.35.020184.003021. [DOI] [PubMed] [Google Scholar]
- Naidu A. S., Paulsson M., Wadström T. Particle agglutination assays for rapid detection of fibronectin, fibrinogen, and collagen receptors on Staphylococcus aureus. J Clin Microbiol. 1988 Aug;26(8):1549–1554. doi: 10.1128/jcm.26.8.1549-1554.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Textor J. A., Vann J. M., Mosher D. F. Role of fibronectin in human monocyte and macrophage bactericidal activity. Infect Immun. 1985 Mar;47(3):629–637. doi: 10.1128/iai.47.3.629-637.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainard P. Experimental mastitis with Escherichia coli: kinetics of bacteriostatic and bactericidal activities. Ann Rech Vet. 1983;14(1):1–11. [PubMed] [Google Scholar]
- Reite B., Oram J. D. Bacterial inhibitors in milk and other biological fluids. Nature. 1967 Oct 28;216(5113):328–330. doi: 10.1038/216328a0. [DOI] [PubMed] [Google Scholar]
- Rydén C., Rubin K., Speziale P., Hök M., Lindberg M., Wadström T. Fibronectin receptors from Staphylococcus aureus. J Biol Chem. 1983 Mar 10;258(5):3396–3401. [PubMed] [Google Scholar]
- Thomas C. L., Neave F. K., Dodd F. H., Higgs T. M. The susceptibility of milked and unmilked udder quarters to intra-mammary infection. J Dairy Res. 1972 Feb;39(1):113–131. doi: 10.1017/s0022029900013911. [DOI] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadström T. Molecular aspects on pathogenesis of wound and foreign body infections due to staphylococci. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Aug;266(1-2):191–211. doi: 10.1016/s0176-6724(87)80032-9. [DOI] [PubMed] [Google Scholar]
- Watts J. L., Nickerson S. C. A comparison of the STAPH-Ident and STAPH-Trac systems to conventional methods in the identification of staphylococci isolated from bovine udders. Vet Microbiol. 1986 Jul;12(2):179–187. doi: 10.1016/0378-1135(86)90079-9. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]


