Abstract
Adrenocortical carcinoma is an uncommon malignancy and feminizing symptoms secondary to adrenal estrogen-secretion are extremely rare. The direct secretion of estradiol by adrenocortical tumors requires, in addition to the expression of aromatase (CYP19), the expression of one or more of the reductive 17β-hydroxysteroid dehydrogenases. The expression of CYP19 transcripts and protein were markedly induced in the H295 adrenocortical carcinoma cell line after treatment with either forskolin or vasoactive intestinal peptide (VIP). Western immunoblotting demonstrated a marked induction of the CYP19 protein of characteristic size after only a short (6 h) treatment period with VIP or forskolin. The CYP19 mRNA transcripts were derived from both promoters PII (Ic) and I.3 (Id) after treatment with both agents. The reductive type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) was also constitutively expressed in the H295 cells but neither its mRNA transcript nor protein levels were altered after forskolin or VIP treatment. Western immunoblotting of an estrogen-secreting adrenal carcinoma revealed notable levels of both aromatase and AKR1C3 expression while an aldosterone-producing adrenal adenoma lacked aromatase expression and showed a reduced level of AKR1C3 expression. Immunohistochemistry of the carcinoma-bearing adrenal revealed localization of AKR1C3 not only in the tumor but also principally in the zona reticularis of the normal adrenal tissue. Adrenal aromatase and AKR1C3 expression therefore appear to be features of adrenocortical malignancies that are associated with biosynthesis of active estrogen.
Keywords: human adrenocortical H295 cells, estrogen biosynthesis, aromatase, CYP19, 17β-hydroxysteroid dehydrogenase type 5, AKR1C3, human adrenal cortex
INTRODUCTION
Adrenocortical carcinoma is an uncommon malignancy with an incidence of 1-2 per million population (Allolio and Fassnacht, 2006). Feminizing symptoms secondary to estrogen-secretion are extremely rare (Gabrilove et al., 1965; Kimura et., 1995) and those arising in cases of adrenocortical carcinomas were attributed to the peripheral aromatization of adrenal androgens (Hemsell et al., 1977) as the aromatase enzyme is not usually considered a member of the adrenocortical cytochrome P450 family. However, recent case reports have demonstrated local aromatase cytochrome P450 (CYP19) mRNA and aromatase activity in such adrenal tumors (Phornphutkul et al., 2001). The secretion of bioactive estrogen, specifically estradiol, by adrenocortical tumors requires, in addition to the expression of aromatase, the expression of one or more of the reductive 17β-hydroxysteroid dehydrogenases (17-ketosteroid reductases). Candidate 17-ketosteroid reductases to be considered are types 1, 3, 5 and 12 17β-hydroxysteroid dehydrogenases.
The NCI-H295 cell line was originally derived from a human adrenocortical carcinoma that expressed a multitude of steroidogenic cytochrome P450s including aromatase (Gazdar et al., 1989). The cell lines derived from this carcinoma have become popular and standard models for the study of human adrenocortical steroidogenesis (see Rainey et al., 1993; 1994; 2004). Both NCI-H295A and NCI-H295R cells have been shown to express aromatase mRNA (Staels et al., 1993) as well as enzyme activity (Watanabe and Nakajin, 2004; Watanabe et al., 2006). The principal limitation to the utility of H295 cells as a primary model cell line for the study of the underlying mechanisms involved in the diverse regulation of adrenocortical steroidogenesis has been the apparent absence of functional ACTH receptors. This has required many investigators to provoke the activation of the important PKA-cAMP signaling pathway for steroidogenesis by the use of pharmacological interventions, e.g., addition of forskolin or cAMP in its various forms. We were however able to demonstrate that vasoactive intestinal peptide (VIP) acting via the VPAC1 receptors of H295 cells promoted increased cAMP and subsequent a highly stimulated secretion of cortisol (Nicol et al., 2004). VIP was used as a physiological peptide agent to evaluate aromatase expression in H295 cells because cAMP signaling pathways have been shown to be important in aromatase expression in classical steroidogenic tissues.
The expression of 17-ketosteroid reductases in the adrenal has also not been investigated in depth, largely due to neither 17-hydroxy-C19-steroid nor estradiol secretion is commonly associated with the human adrenal cortex. Therefore in the present study we describe our initial studies to evaluate the putative pathway involving both aromatase and 17-ketosteroid reductase in the biosynthesis of the active estrogen, estradiol, in H295 cells. We have also compared the expression pattern of steroidogenic genes observed in H295 cells to the patterns observed in two distinct adrenocortical tumors. The products of the first tumor were believed to promote feminization (gynecomastia, loss of libido) in an adult male since these features were resolved after a successful adrenalectomy. The second tumor was considered to be an aldosterone-producing adrenal adenoma based on the clinical and biochemical history, and the post-operative remission of hypertension and hypokalemia.
MATERIALS AND METHODS
H295 cells
NCI-H295 cells were originally derived from a primary human adrenocortical carcinoma removed at surgery from an adult female (Gazdar et al., 1990). The multipotent nature of the cell line has been previously described and, in particular, the up-regulation of steroidogenic gene expression promoted via cAMP-PKA and PKC intracellular signaling pathways (Staels et., 1993, Rainey et al., 1994).
Cell culture
NCI-H295R cells (CRL-2128, ATCC, Rockville, MD, USA) were seeded into 12-well tissue culture plates (5 × 105 cells per well) and maintained in Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12, Invitrogen, Paisley, Renfrew, UK) containing 2% Ultroser SF (Biosepra, France), 5 μg/ml insulin, 5 μg/ml transferrin and 5 ng/ml sodium selenite (as 1% ITS, Invitrogen) at 37°C with 5% CO2–95% air. For experiments, the cells were treated in the above medium with the appropriate addition of vasointestinal peptide VIP (100 nM) or forskolin (10 μM) for 6 or 12 h (mRNA analysis) or 6-48 h (protein immunoblotting). After treatment, the cells were washed with Hank’s balanced salt solution (HBSS, Invitrogen) and cell monolayers harvested for either mRNA or protein analysis. Unless otherwise specified, all reagents were obtained from Sigma, Poole, UK.
Subjects
Estrogen-producing adrenocortical carcinoma tissue was freshly obtained at adrenalectomy to remove a right adrenal mass from a 54-year-old man who had presented with a short (2 month) history of gynecomastia and loss of libido. Aldosterone-producing adrenal adenoma tissue was obtained after laparoscopic surgery to remove a 2.6 cm left adrenal mass from a 53 year-old woman who presented with a 10 year history of hypertension and hypokalemia. Endocrine testing had conformed pre-operatively by 131I-norcholesterol scintigraphy. Written informed consent was obtained from both patients pre-operatively to permit research studies to be performed on the tissue removed at surgery.
Western immunoblotting
Tissue and cell extracts were prepared by sonication in 50mM Tris-HCl pH 7.6, 0.1% SDS, 1% deoxycholate (all from Sigma) containing a cocktail of proteinase inhibitors (Roche Diagnostics Gmbh, Mannheim, Germany). After determination of protein concentration (Bradford, 1976) tissue/cell protein was electrophoretically separated in a 10% SDS/PAGE gel and transferred to a PVDF membrane (Millipore, Bedford, MA) followed by blocking in 5% dried semi-skimmed milk diluted in PBST for 2h. This was followed by an overnight incubation at 4°C with the mouse monoclonal antibody (Novartis #677, Sasano et al., 2005) against human aromatase at 1:3000 dilution in 5% dried semi-skimmed milk/PBST, or a mouse monoclonal antibody (1:1000 dilution) against human AKR1C3 (17β-HSD5) (Lin et al., 2004), before incubation (1h) with a donkey anti-mouse IgG conjugated to horseradish peroxidase (Sigma) at 1:20000 dilution. Proteins were detected by an ECL detection kit (Millipore). To verify the specificity of the aromatase monoclonal antibody, we used samples of CHO-K1 cells that had been transiently transfected with human aromatase in a pCMV expression vector (Corbin et al., 1988) using the GeneJuice® transfection reagent (Novagen Merck Biosciences, Nottingham, UK) as we have described previously (Kowalewski et al., 2006).
RNA extraction and quantity measurement
Following in vitro treatments, cells were harvested and lyzed in lysis buffer (RLT buffer, Qiagen, West Sussex, UK) before RNA extraction with the RNeasy mini-kit (Qiagen) per manufacturer’s guidance. Exclusion of genomic DNA was achieved with DNase treatment of samples, on-column, with the RNase free DNase set (Qiagen) according to supplier’s protocol. Purification and quantification were assessed using a Nanodrop spectrophotometer (ND-1000, Nanodrop Technologies Inc, Wilmington, DE, USA).
Quantitative measurement of CYP19 and AKR1C3 (17β-HSD5) mRNA
Quantitative Taqman Real-Time PCR was performed to measure relative expression levels of CYP19 and AKR1C3 mRNA in response to treatments. Briefly, pure RNA (200ng) was reversed transcribed to cDNA using the RT-Reagent kit (Applied Biosystems, Applera, Warrington, UK) per supplier’s instructions in a final reaction of 10μl. Then, Real-Time PCR was performed using commercial Applied Biosystems reagents. Briefly, 2μl cDNA was used as a template mixed with 1X universal Taqman master cocktail (Applied Biosystems) and the specific set of 1X primer/probe mixture. Primer/probe sets for all the reported enzyme mRNA transcripts were purchased and pre-validated (Assay-on-Demand Systems, Applied Biosystems). A ribosomal 18S primer/probe set was also included and served as an internal reference control. The CT value obtained for the 18S species was also used to confirm the quality of the cDNA samples. Mean values reflecting the PCR cycle when the target transcript started to accumulate relative to 18S (mean dCT in a 40 cycle PCR reaction) are illustrated in Table 1. Values more than 36 out of 40 PCR cycles were assessed as beyond the limit of robust detection; however they were included in analysis for comparison reasons. Each reaction was carried out in duplicate. Samples were evaluated in 96-well plates using an ABI Prism 7900 Sequence Detector (Applied Biosystems).
Table 1.
PCR primer sets for the determination of aromatase promoter usage
| Sense | Antisense | |
|---|---|---|
| Exon II-III coding region | GACTCTAAATTGCCCCCTCTG | CAGAGATCCAGACTCGCAATG |
| Exon I.1-specific | CTGGAGGGCTGAACACGTGG | CAGAGATCCAGACTCGCAATG |
| Exon I.3-specific | CCTTGTTTTGACTTGTAACCA | CAGAGATCCAGACTCGCAATG |
| Exon I.4-specific | GACCAACTGGAGCCTGACAG | CAGAGATCCAGACTCGCAATG |
| Promoter II-specific | AACAGGAGCTATAGATGAAC | CAGAGATCCAGACTCGCAATG |
PCR determination of aromatase promoter usage in H295 cells
Reverse transcription of 2μg of total RNA from 6 h VIP-treated H295 cells was performed with oligo-dT primers using the Invitrogen SuperscriptTM III reverse transcriptase kit according to the manufacturer’s instructions. Primer pairs specific for the various alternate-spliced variants of human aromatase mRNA (Larionov et al., 2003) were used in RT-PCR reactions to identify which aromatase promoter was being utilized to express aromatase in the H295 cells. Expression of the coding part of aromatase mRNA was verified examining the region across the coding aspects of exons II and III as well as the three most common promoter variants that use the first exon (I.1, I.3, I.4) and the promoter II variant based on our previously described methodology (Larionov et al. 2003). Primer sets for the PCR are shown in Table 1. PCR was carried out using Promega PCR master mix with the following cycling conditions; initial denaturation: 94°C for 2 mins, 35 cycles of 94°C for 30 sec, 58°C for 30 sec, 72°C for 60 sec, final elongation: 72°C for 5 min. PCR products were detected following electrophoresis in a 2% agarose gel and staining with ethidium bromide.
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue (3 μm sections) with blocks selected to show tumor and tumor with adjacent normal adrenal tissue. Sections were deparaffinised in xylene, followed by serial dilutions of ethanol, phosphate-buffered saline (PBS) and distilled H2O. Microwave permeabilisation was achieved in 0.01 M sodium citrate buffer pH 6.0 (Sigma, Poole, UK) for 15min followed by cooling to room temperature for 20 min. After blocking of endogenous peroxidase (3% H2O2 in methanol), slides were incubated with avidin and biotin (Vector, Peterborough, UK). Sections were then treated with non-immunized normal goat serum (NGS; Sigma) diluted in PBS (1:5 v/v) containing 5% (w/v) BSA (Sigma) for 20min at room temperature followed by overnight incubation at 4°C with a mouse monoclonal antibody against human AKR1C3 (17β-HSD5) at a 1:1000 dilution (Lin et al., 2004, Vani et al., 2007). Negative controls incubated with unconjugated mouse IgG1 antibody (Vector) were run routinely at matched concentrations. Sections were washed with PBS supplemented with 0.05% Tween 20 (v/v) (PBST; Sigma) and then incubated with anti-mouse biotinylated IgG1 (1:200 in NGS/PBS/BSA; Vector) before use of a RTU-ABC elite kit (avidin-biotin complex; HRP conjugated; Vector) for 1h each. Finally, slides were treated with HRP-conjugated diaminobenzidine (DAB; Vector) chromagen for 5min, lightly counterstained with hematoxylin and dehydrated in serial ethanol dilutions (50-100%) and xylene. A similar protocol was employed with the mouse monoclonal antibody (Novartis #677) against human aromatase diluted 1:1200 in NGS/PBS/BSA at 4°C (Sasano et al., 2005).
Statistical analysis
Basic statistical analysis was run using the GraphPad Prism 4.00 software (GraphPad Software Inc., San Diego, USA). Multiple comparisons were performed with one-way ANOVA and Neuman-Keuls post-hoc tests, whilst single comparisons were achieved with paired Student t-tests. Statistical significance was considered at p-values ≤ .05 on a minimum of 3 independent observations.
RESULTS
Expression of aromatase (CYP19) protein in H295 cells treated with either VIP or forskolin
Western immunoblot analysis of H295 cells treated with either VIP (100 nM) or forskolin (10 μM) indicated a marked induction of aromatase protein within 6 h after commencement of treatment. A representative blot is shown in Figure 1. The identification of a single immunoreactive species of appropriate molecular size (~55 kDa) in aromatase-transfected CHO-K1 cells but absence of immunoreactivity in both non-transfected CHO-K1 cells and untreated H295 cells, confirmed the specificity and sensitivity of the anti-aromatase monoclonal antibody.
Figure 1.
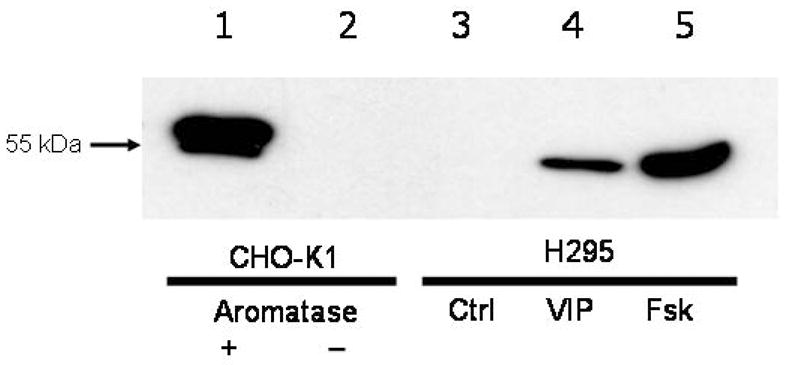
Representative western immunoblot of aromatase (CYP19) protein in H295 cells after treatment (6 h) with VIP (100 nM) or forskolin (10 μM). Lane 1: CHO-K1 cells transfected with human CYP19 cDNA (25 μg protein); lane 2: non-transfected CHO-K1 cells (25 μg protein); lane 3: untreated (Ctrl) H295 cells (25 μg protein); lane 4: VIP-treated H95 cells (25 μg protein); lane 5: Forskolin (Fsk)-treated H295 cells (25 μg protein).
Expression of aromatase (CYP19) mRNA in H295 cells treated with either VIP or forskolin
To determine whether this rapid induction of aromatase protein by the cAMP-PKA pathway agonists, VIP and forskolin, was transcriptionally or translationally regulated, levels of aromatase cytochrome P450 mRNA transcripts were measured in the treated H295 cells using quantitative real-time PCR. As illustrated in Figure 2 both VIP and forskolin treatments increased the levels of aromatase mRNA 4- and 10-fold respectively within 6 h after commencement of treatment, indicative that increased aromatase P450 transcription had occurred, suggesting a transcriptionally-regulated process. On the other hand, inspection of the raw qRT-PCR data for CYP19 mRNA levels (see Table 2) revealed notable levels of transcripts even in control H295 cells (mean dCT value of 16). By comparison the dCT value for CYP19 mRNA transcripts in the human NTera2/D1 neuronal cell line that is not recognized as expressing steroidogenic genes, was 26. The dCT value for aromatase transcripts in the RNA from the feminizing adrenocortical carcinoma was 16 while they were undetectable in the aldosterone-producing adrenal adenoma.
Figure 2.
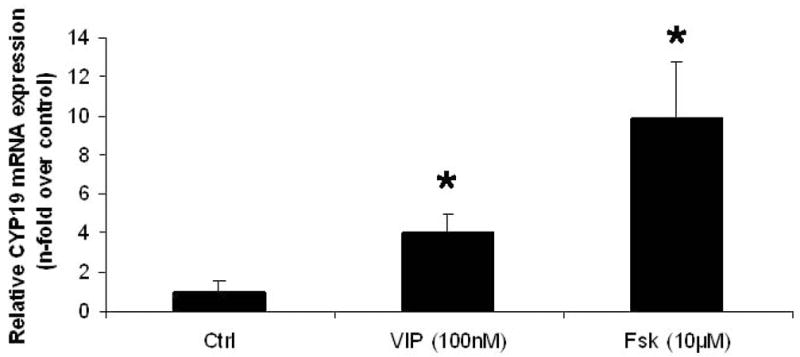
The effects of VIP and forskolin (Fsk) on CYP19 (aromatase) mRNA levels in H295 cells. H295 cells were treated with either VIP (100 nM) or forskolin (10 μM) for 6 h. RNA was isolated and mRNA levels analyzed by quantitative real-time PCR as described in Materials and Methods (*p<0.05 versus control).
Table 2.
Mean dCT values of target genes in quantitative Real-time PCR
| Gene of interest | H295 Control | H295 Forskolin | E2-adrenal Carcinoma | Aldo-adrenal Adenoma | Ntera2/D1 cells |
|---|---|---|---|---|---|
| CYP 19 | 16.1 ± 1.0 | 12.8 ± 0.7* | 15.7 | >29 | 26 |
| AKR1C3 | 13.5 ± 0.8 | 14.2 ± 0.2 | 19.6 | 17.7 | >29 |
| CYP11B1 | 20.7 ± 0.9 | 14.6 ± 1.1* | 9.5 | 8.8 | >29 |
| CYP11B2 | 21.9 ± 0.8 | 17.0 ± 0.7* | 24.8 | 3.7 | >29 |
| CYP17 | 9.9 ± 0.5 | 9.5 ± 0.4 | 3.7 | 4.3 | 26 |
| HSD3B1 | 19.8 ± 0.5 | 19.4 ± 1.3 | 18.2 | 17.5 | 28 |
| HSD3B2 | 16.4 ± 0.8 | 13.2 ± 0.6* | 13.7 | 7.1 | >29 |
Catalog numbers of Applied Biosystems Assay-on-Demand primer/probe sets used: CYP19, hs00240671_m1; AKR1C3, Hs00366267_m1; CYP11B1, Hs01596404_m1; CYP11B2, Hs01597732_m1; HSD3B1, Hs00426435_m1; HSD3B2, Hs00605123_m1.
Mean dCT reflects CT of target gene relative to CT of 18S as the internal reference;
P<0.05 versus control treatment groups
Identification of promoter-specific transcripts for CYP19 in H295 cells
As demonstrated in Figure 3, aromatase transcripts associated with utilization of the gonadal-associated aromatase promoter PII were prominently represented in H295 mRNA prepared from H295 cells treated with VIP (100 nM) for 6 hours. However, significant amounts of transcript associated with promoter I.3 were also observed, while there was no evidence for promoter I.4-associated expression.
Figure 3.
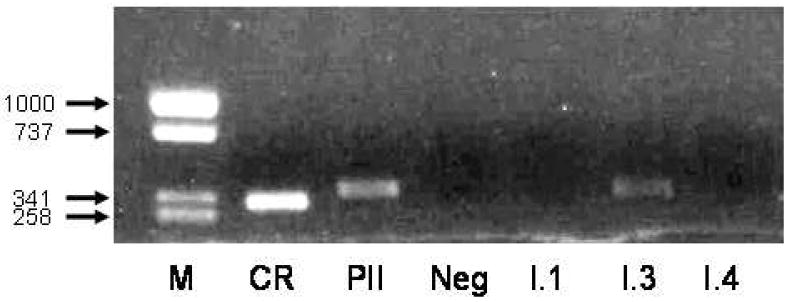
PCR amplification of aromatase (CYP19) transcripts in human H295 adrenocortical cells. After reverse transcription cDNA samples were amplified using primers specific for the various CYP19 gene transcripts. M=size markers, size of fragments is shown in bp; CR=coding region (exons II-III); PII=promoter II-specific region; I.1=promoter I.I; I.3=promoter I.3 and I.4=promoter I.4. The negative (Neg) lane is a PCR control.
Comparative western immunoblot of aromatase expression in an aldosterone-producing adrenal adenoma, an estrogen-secreting adrenal carcinoma and H295 cells
Western immunoblot analysis of an aldosterone-producing adrenal adenoma, a feminizing adrenal carcinoma and H295 cells treated with either VIP (100 nM) or forskolin (10 μM) as positive controls indicated the presence of CYP19 protein of appropriate molecular size within the feminizing adrenal carcinoma sample but absence of any immunoreactivity within the aldosterone-producing adrenal adenoma. The representative blot is shown in Figure 4.
Figure 4.
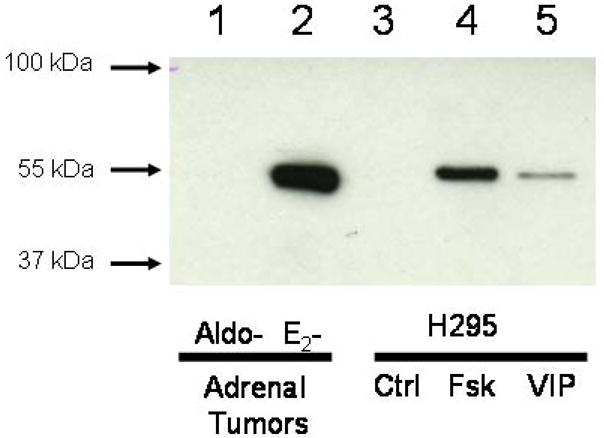
Western immunoblot of aromatase (CYP19) protein in an aldosterone-producing adrenal adenoma, a feminizing estrogen-secreting adrenal carcinoma and H295 cells after treatment (12 h) with VIP (100 nM) or forskolin (10 μM). Lane 1: Aldosterone-producing adrenal adenoma (15 μg protein); lane 2: Estrogen-secreting adrenal carcinoma (15 μg protein); lane 3: Untreated control H295 cells (15 μg protein); lane 4: Forskolin (Fsk)-treated H95 cells (15 μg protein); lane 5: VIP-treated H295 cells (15 μg protein).
Effect of VIP/forskolin treatment of H295 cells on AKR1C3 (17β-HSD5) protein expression
Western immunoblot analysis of H295 cells treated with either VIP (100 nM) or forskolin (10 μM) revealed the presence in the untreated (Ctrl) cells of a single protein of the expected molecular size of 37 kDa when probed with mouse monoclonal antibody specific for human AKR1C3. Furthermore, little if any change in level of the enzyme was found after treatments with either VIP or forskolin (Fsk) for 6, 12, or 24 hours. A representative blot for a 12 h treatment period is shown in figure 5.
Figure 5.
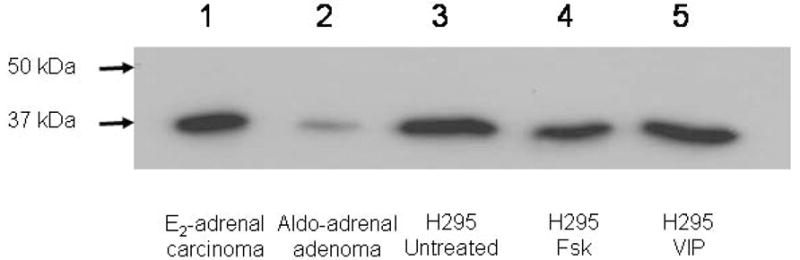
Western immunoblot of AKR1C3 (17β-HSD5) protein in a feminizing estrogen-secreting adrenal carcinoma, an aldosterone-producing adrenal adenoma, and H295 cells after treatment (24 hours) with either VIP (100 nM) or forskolin (10 μM). Lane 1: Aldosterone-producing adrenal adenoma (25 μg protein); lane 2: Estrogen-secreting adrenal carcinoma (25 μg protein); lane 3: Untreated control H295 cells (25 μg protein); lane 4: Forskolin (Fsk)-treated H295 cells (25 μg protein); lane 5: VIP-treated H295 cells (25 μg protein).
When AKR1C3 mRNA levels were assessed in H295 cells following treatment with VIP (100 nM) or forskolin (10 μM), no significant differences in mRNA levels were seen between untreated control (dCT 13.5 ± 0.8, n=3) VIP-treated (dCT 14.2 ± 0.3) or forskolin–treated (dCT 14.2 ± 0.2) cells (see also Table 2).
A single immunoreactive species of appropriate molecular size (37 kDa) was also identified in the feminizing adrenal carcinoma and the aldosterone-producing adrenal adenoma (see figure 5).
Measurement of mRNA transcript levels of CYP11B1, CYP11B2, CYP17, HSD3B1, HSD3B2 in H295 cells, a feminizing adrenal carcinoma and an aldosterone-producing adrenal adenoma
To provide a comparative analysis of the levels of mRNA transcripts of various relevant adrenocortical enzymes besides AKR1C3 and CYP19, we used quantitative real-time PCR with validated primer/probe sets for transcripts of the genes listed in Table 2. The data are provided in Table 2 as dCT values for each transcript, the cycle number CT to achieve the threshold fluorescence level for the gene of interest minus the CT value for the 18S housekeeping transcript.
Immunolocalization of AKR1C3 (17β-HSD5) and CYP19 (aromatase) expression
Immunolocalization of AKR1C3 (17β-HSD5) and CYP19 (aromatase) in a feminizing adrenal cortical carcinoma and adjacent normal adrenocortical tissue are illustrated in Figure 6. Both localized to cytoplasm of cells. 17β-HSD5 protein was immunolocalized not only in the carcinoma cells but also principally in the lipid-poor adrenal zona reticularis with much less intense staining observed in the lipid-rich zona fasciculata. The localization of CYP19 was restricted to the carcinoma.
Figure 6.
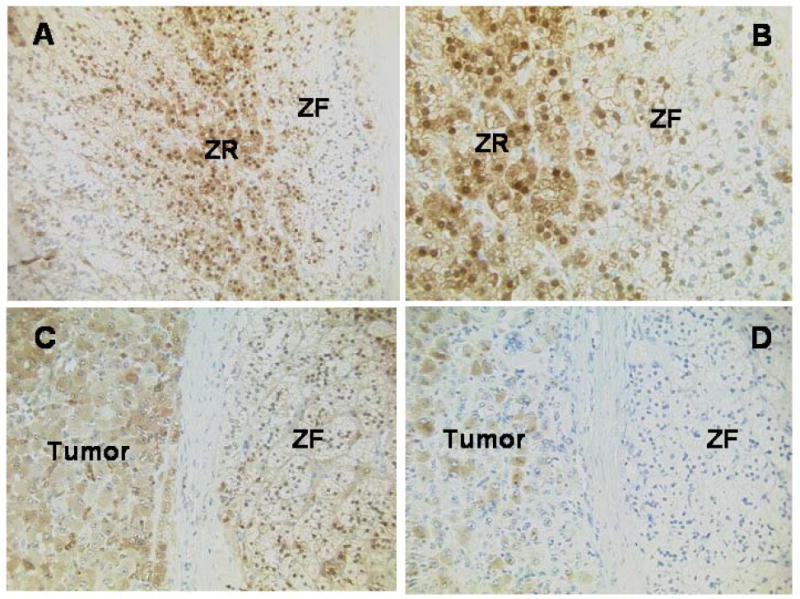
Immunolocalization of AKR1C3 and CYP19 in an adrenal containing an estrogen-secreting adrenal adenocarcinoma. A. Immunohistochemistry for AKR1C3 showing normal adrenal cortex with higher expression centrally in the lipid-poorer cells of the zona reticularis (ZR) than in the lipid-rich cells of the zona fasciculata (ZF); the adrenal capsule is located at the extreme right of the image (original magnification x20). B. Immunohistochemistry for AKR1C3 showing normal adrenal cortex with higher expression in the lipid-poorer cells of the zona reticularis (centre) than in the lipid-rich cells of the zona fasciculata (right); adrenal capsule to the far right of the image (original magnification x40). C. Immunohistochemistry for AKR1C3 showing expression in the carcinoma cells (left), tumor capsule (centrally) and some of the normal adrenocortical cells (right) (original magnification x20). D. Immunohistochemistry for aromatase, showing expression in many of the carcinoma cells (left) and the normal adrenal cortex to be negative (right) (original magnification x20).
DISCUSSION
In these current studies we have demonstrated the expression of both aromatase cytochrome P450 (CYP19) and AKR1C3 (type 5 17β-hydroxysteroid dehydrogenase) in H295 cells at the level of mRNA transcript and protein. CYP19 mRNA has been previously demonstrated in H295 cells (Staels et al., 1993; Watanabe and Nakijin, 2004; Watanabe et al., 2006) and the presence of translated protein has been assumed based on the detection of aromatase activity using the tritiated water technique. However, while AKR1C3 appeared constitutively expressed, aromatase protein was only observed after treatment with the cAMP-PKA pathway agonists, VIP and forskolin. Because AKR1C3 is a reductive NADPH-dependent 17-ketosteroid reductase capable of in vivo conversion of androstenedione to testosterone and estrone to estradiol (reviewed in Penning and Drury, 2007), our finding is indicative that H295 cells can biosynthesize the active estrogen, estradiol, directly from cholesterol.
Notwithstanding the evidence that cAMP-PKA pathway agonists, namely VIP and forskolin, increased the level of CYP19 mRNA transcripts in H295 cells suggesting an element of transcriptional control of CYP19 expression, our findings are also strongly suggestive of notable translational control of CYP19 expression. This conclusion is based on the demonstration of a quite rapid accumulation of CYP19 protein within 6 hours after commencement of treatment along with significant levels of CYP19 mRNA transcripts even in untreated H295 cells (see Table 2). One explanation from a number of plausible ones could be that a microRNA is active in the untreated cells.
The aromatase enzyme is the single product of the human CYP19 gene. Multiple signaling pathways regulate CYP19 expression in the various tissues where aromatase is found. The end response to the multiplicity of signals is under the control of multiple promoters utilizing alternative splicing of different upstream exons with exon II containing the start site of translation (reviewed in Bulun et al., 2005). In the current study using H295 cells after stimulation of the cAMP/PKA pathway with VIP we found that the principal aromatase promoters utilized were promoters PII and I.3. The proximal regions of both of these promoters contain cAMP response element (CRE)-like sequences which may be activated in a cAMP-dependent manner by VIP acting through the VPAC1 receptor. Indeed it have previously shown that forskolin most likely activates aromatase expression in H295 cells via these promoters (Watanabe and Nakajin, 2004).
It was of interest to compare data obtained from the study of H295 cells with the situation existing in two different examples of human adrenocortical tumors, a feminizing adrenocortical carcinoma and an aldosterone-producing adrenal adenoma. Western immunoblotting of these tumors revealed that the feminizing adrenal carcinoma expressed notable amounts of both CYP19 and AKR1C3 consistent with clinical evidence that it was secreting bioactive estrogens. However, the aldosterone-producing adrenal adenoma did not express aromatase enzyme and the level of AKR1C3 was reduced compared to that found in the feminizing adrenal tumor. The level of CYP19 mRNA transcripts relative to 18S housekeeping gene transcripts in the feminizing adrenal tumor were similar to those observed in the H295 cells (see Table 2), suggestive that H295 cells are an appropriate model for in-depth studies of mechanisms underlying development of such tumors.
Another candidate 17-ketosteroid reductase that is effective in converting in vivo estrone to estradiol is the type 1 17β-hydroxysteroid dehydrogenase. However, we were unable to detect the expression of this enzyme on immunoblotting of H295 cells or the tumors using a rabbit polyclonal antibody raised against the human placental enzyme (data not shown).
Analysis of the mRNA transcript levels of other key steroidogenic enzymes in these two tumors demonstrated much higher levels of CYP11B2 (aldosterone synthase) transcripts in the aldosterone-producing adenoma versus the feminizing adrenal tumor (see Table 2). This could be predicted since it has recently been documented that 100% of aldosterone-producing adrenal adenomas have highly elevated CYP11B2 transcript levels compared to normal adrenals (Ye et al., 2007). The observation that CYP17 mRNA levels in the aldosterone-producing adenoma were similar to those in the estrogen-producing adrenal carcinoma is suggestive that the 17-hydroxysteroids, e.g., cortisol, were produced in the adenoma and thereby acting as a brake on the production of aldosterone, a 17-deoxysteroid. In both tumors as well as H295 cells, the predominant HSD3B gene expressed was the gonadal/adrenal-specific HSD3B2. Transcripts of the HSD3B1 gene were readily detectable, albeit at a lower level (<10%) than HSD3B2. It was observed, however, that forskolin treatment of H295 cells also increased HSD3B1 transcript levels suggestive that this isoform may be expressed at a low level in the human adrenal cortical pathophysiologies and might be responsible for the very low but nevertheless detectable plasma levels of cortisol found in individuals with 3β-hydroxysteroid dehydrogenase deficiency congenital adrenal hyperplasia due to a totally non-functional HSD3B2 gene product.
Finally we demonstrated by immunohistochemistry the presence of both AKR1C3 (17β-hydroxysteroid dehydrogenase type 5) and CYP19 in the feminizing adrenal carcinoma. While CYP19 was not present in the adjacent normal adrenocortical tissue, AKR1C3 was localized predominantly in the lipid-poor region of the human adrenal zona reticularis. This finding is supportive of the notion that the zona reticularis, the principal site of adrenal C19-steroid production, is potentially capable of synthesising the active androgen testosterone. The capacity to produce testosterone in this compartment may be favoured under conditions of stress when androstenedione production is facilitated because of increased 3β-hydroxysteroid dehydrogenase activity.
In summary, we have presented in these studies further evidence that H295 cells maintain the enzymology to produce directly the potent estrogen, estradiol, consolidating evidence that they be considered as a suitable model system to explore the mechanisms underlying feminizing adrenal cortical carcinomas.
Acknowledgments
G.P was financially supported by a Principal’s PhD studentship awarded from the University of Edinburgh. The study was also supported, in part, by R01-CA90744 awarded by the National Institutes of Health to TMP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allolio B, Fassnacht M. Clinical review: Adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab. 2006;91:2027–2037. doi: 10.1210/jc.2005-2639. [DOI] [PubMed] [Google Scholar]
- Bouraima H, Lireux B, Mittre H, Benhaim A, Herrou M, Mahoudou J, Guillon-Metz F, Reznik Y. Major hyperestrogenism in a feminizing adrenocortical adenoma despite a moderate over-expression of the aromatase enzyme. Eur J Endocrinol. 2003;148:457–461. doi: 10.1530/eje.0.1480457. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive methos for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to bedside. Pharmacol Rev. 2005;57:59–83. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- Corbin CJ, Graham-Lorence S, McPhaul M, Mason JI, Mendelson CR, Simpson ER. Isolation of a full-length cDNA insert encoding human aromatase system cytochrome P-450 and its expression in nonsteroidogenic cells. Proc Natl Acad Sci U S A. 1988;85:8948–8952. doi: 10.1073/pnas.85.23.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilove JL, Sharma DC, Wotiz HH, Dorfman RI. Feminizing adrenocortical tumors in the male. A review of 52 Cases including a case report. Medicine (Baltimore) 1965;44:37–79. doi: 10.1097/00005792-196501000-00002. [DOI] [PubMed] [Google Scholar]
- Gazdar AF, Oie HK, Shackleton CH, Chen TR, Triche TJ, Myers CE, Chrousos GP, Brennan MF, Stein CA, La Rocca RV. Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res. 1990;50:5488–5496. [PubMed] [Google Scholar]
- Hemsell DL, Edman CD, Marks JF, Siiteri PK, MacDonald PC. Massive extranglandular aromatization of plasma androstenedione resulting in feminization of a prepubertal boy. J Clin Invest. 1977;60:455–464. doi: 10.1172/JCI108796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Itoh N, Tsukamoto T, Kumamoto Y, Takagi Y, Mori Y. Aromatase activity in an estrogen-producing adrenocortical carcinoma in a young man. J Urol. 1995;153:1039–1040. [PubMed] [Google Scholar]
- Kowalewski MP, Mason JI, Howie AF, Morley SD, Schuler G, Hoffmann B. Characterization of the canine 3beta-hydroxysteroid dehydrogenase and its expression in the corpus luteum during diestrus. J Steroid Biochem Mol Biol. 2006;101:254–262. doi: 10.1016/j.jsbmb.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Kruk PA, Maines-Bandiera SL, Auersperg N. A simplified method to culture human ovarian surface epithelium. Lab Invest. 1990;63:132–136. [PubMed] [Google Scholar]
- Larionov AA, Vasyliev DA, Mason JI, Howie AF, Berstein LM, Miller WR. Aromatase in skeletal muscle. J Steroid Biochem Mol Biol. 2003;84:485–492. doi: 10.1016/s0960-0760(03)00059-1. [DOI] [PubMed] [Google Scholar]
- Lin HK, Steckelbroeck S, Fung KM, Jones AN, Penning TM. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3α-hydroxysteroid dehydrogenase/type 5 17β-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids. 2004;69:795–801. doi: 10.1016/j.steroids.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Nicol MR, Cobb VJ, Williams BW, Morley SD, Walker SW, Mason JI. Vasoactive intestinal peptide (VIP) stimulates cortisol secretion from H295 human adrenocortical tumour cell line via VPAC1 receptors. J Mol Endocrinol. 2004;32:869–877. doi: 10.1677/jme.0.0320869. [DOI] [PubMed] [Google Scholar]
- Penning TM, Drury JE. Human aldo-ketoreductases: Function, gene regulation, and single nucleotide polymorphisms. Arch Biochem Biophys. 2007;464:241–250. doi: 10.1016/j.abb.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phornphutkul C, Okubo T, Wu K, Harel Z, Tracy TF, Jr, Pinar H, Chen S, Gruppuso PA, Goodwin G. Aromatase P450 expression in a feminizing adrenal adenoma presenting as isosexual precocious puberty. J Clin Endocrinol Metab. 2001;86:649–652. doi: 10.1210/jcem.86.2.7201. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Bird IM, Sawetawan C, Hanley NA, McCarthy JL, McGee EA, Wester R, Mason JI. Regulation of human adrenal carcinoma cell (NCI-H295) production of C19 steroids. J Clin Endocrinol Metabol. 1993;77:731–737. doi: 10.1210/jcem.77.3.8396576. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Bird IM, Mason JI. The NCI-H295 cell line: a pluripotent model for human adrenocortical studies. Mol Cell Endocrinol. 1994;100:45–50. doi: 10.1016/0303-7207(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Saner K, Schimmer BP. Adrenocortical cell lines. Mol Cell Endocrinol. 2004;22:23–38. doi: 10.1016/j.mce.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Sasano H, Anderson TJ, Silverberg SG, Santen RJ, Conway M, Edwards DP, Krause A, Bhatnagar AS, Evans DB, Miller WR. The validation of new aromatase monoclonal antibodies for immunohistochemistry--a correlation with biochemical activities in 46 cases of breast cancer. J Steroid Biochem Mol Biol. 2005;95:35–39. doi: 10.1016/j.jsbmb.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Staels B, Hum DW, Miller WL. Regulation of steroidogenesis in NCI-H295 cells: a cellular model of the human fetal adrenal. Mol Endocrinol. 1993;7:423–433. doi: 10.1210/mend.7.3.8387159. [DOI] [PubMed] [Google Scholar]
- Vani S, McDonald SE, Williams AR, Mason JI, Thong KJ, Critchley HO. Mid-luteal endometrial intracrinology following controlled ovarian hyperstimulation involving use of a gonadotrophin releasing hormone antagonist. Hum Reprod. 2007;22:2981–2991. doi: 10.1093/humrep/dem269. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Nakajin S. Forskolin up-regulates aromatase (CYP19) activity and gene transcripts in the human adrenocortical carcinoma cell line H295R. J Endocrinol. 2004;180:125–133. doi: 10.1677/joe.0.1800125. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Noda M, Nakajin S. Effect of epidernal growth factor and prostaglandin on the expression of aromatase (CYP19) in the human adrenocortical cell line NCI-H295R. J Endocrinol. 2006;188:59–68. doi: 10.1677/joe.1.06214. [DOI] [PubMed] [Google Scholar]
- Ye P, Marinello B, Mantero F, Shibata H, Rainey WE. G-protein-coupled receptors in aldosterone-producing adenomas: a potential cause of hyperaldosteronism. J Endocrinol. 2007;195:39–48. doi: 10.1677/JOE-07-0037. [DOI] [PubMed] [Google Scholar]
- Young J, Bulun SE, Agarwal V, Couzinet B, Mendelson CR, Simpson ER, Schaison G. Aromatase expression in a feminizing adrenocortical tumor. J Clin Endocrinol Metab. 1996;81:3173–3176. doi: 10.1210/jcem.81.9.8784064. [DOI] [PubMed] [Google Scholar]


