Summary
Attention can selectively enhance neuronal responses and exclude external noise, but the neuronal computations underlying these effects remain unknown. At the neuronal level noise exclusion might result in altered spatial integration properties. We tested this proposal by recording neuronal activity and length tuning in macaque V1 when attention was directed towards or away from stimuli presented in the neuron’s classical receptive field. For cells with central-parafoveal receptive fields, attention indeed reduced spatial integration demonstrated by a reduction in preferred length and in the size of the spatial summation area. Conversely, in cells representing more peripheral locations, attention increased spatial integration by increasing the cell’s summation area. This previously unknown dichotomy between central and peripheral vision could support accurate analysis of attended foveal objects and target selection for impending eye-movements to peripheral objects.
Introduction
Attention exerts a critical influence over information processing in striate and extrastriate cortex1-5. This is evident psychophysically as visual perception becomes more accurate, less strongly influenced by external noise6 and has a higher resolution7 at attended locations. Neurophysiological data show that directed attention causes multiplicative enhancements in response rates when isolated stimuli are attended to8, 9. However, attentional effects are greater and more complex when attention is directed to one of two stimuli presented in the classical receptive field (CRF)2, 8, 10. Under such conditions, attention alters the interaction between these multiple stimuli, causing the influence of the non-attended stimulus to be suppressed10, 11. This effect could be mediated by changes in the profile of extrastriate CRFs, which have recently been shown to shift towards, and somewhat shrink around, attended objects 12. In primary visual cortex (V1) CRFs are too small to present multiple stimuli within their boundaries, however, attention may still influence the interaction between multiple stimuli by acting on the non-classical receptive field (nCRF). A reduction in the strength of the influence of the nCRF during attentive states could potentially mediate the exclusion of noise6 and enhancement of spatial resolution7 at attended locations. This would also be expected to reduce contextual influences in visual perception, in line with a number of psychophysical reports13, 14. The precise mechanisms by which attention mediates changes in nCRF influences are not understood. This study investigates these mechanisms in primary visual cortex of the macaque monkey. We found that attention at parafoveal sites reduced spatial pooling by reducing the size of a neuron’s summation area. Notably, at more peripheral sites, attention increased spatial pooling by increasing the size of the summation area in conjunction with changes of the excitatory and inhibitory gain.
Results
Effects of attention on neuronal length tuning
Length tuning is a classic demonstration of nCRF modulation15. It allows determination of the spatial extent and strength of a neuron’s summation area, consisting of the CRF and an excitatory inner-fringe of the nCRF15-17. Additionally, it allows the spatial extent and strength of the neuron’s inhibition area to be determined 15-17. Since length is a continuous variable, a model can be fit to the data, thereby allowing changes in summation and inhibition areas to be quantified. Thus, by measuring length tuning, the impact of the nCRF and its modulation by directed spatial attention can be assessed. We measured length tuning under conditions where the monkey was cued to attend to the stimulus presented in the RF of the neuron under study (attend-RF condition) and when the monkey was cued to attend to a stimulus presented in the opposite hemifield (attend-away condition, see Fig. 1). In the majority of cells with parafoveal RFs, the preferred length was shorter in the attend-RF condition (Fig. 2).
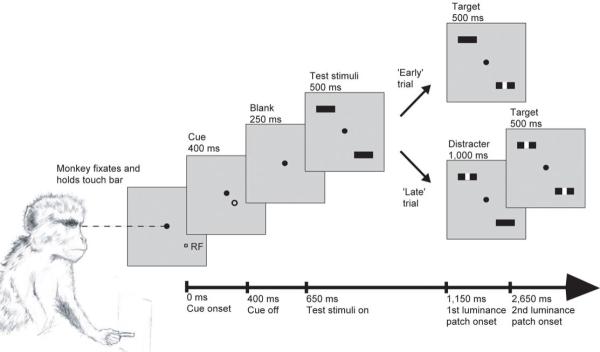
Figure 1.
Representation of the main experimental task. The timing of events relative to the start of the trial is marked along the bottom axis. Presentation time is given above each frame. The monkey initiated the trial by fixating centrally (filled circle) and holding a touch-bar. At the start of the trial a cue (open circle) indicated the location to which the monkey should attend, while the cue itself was spatially and temporally separated from the stimulus and thus the attended location. In the current example the cue directs attention towards the RF of the neuron under study. Test stimuli were two identical bars, one presented at the RF of the neuron under study, the other in the opposite hemifield. The monkey’s task was to detect the presentation of a 0.1°×0.1° luminance patch occurring centrally on the cued bar. This occurred at either 500 ms or 1,500 ms after the onset of the test stimuli. In an ‘early’ trial the first luminance patch was presented on the cued stimulus (‘target’). In a ‘late’ trial the first luminance patch was presented on the un-cued test stimulus (‘distracter’) and the second patch occurred on the cued stimulus. The monkey had 500 ms to release the touch bar following the presentation of a target, in order to receive a juice reward.
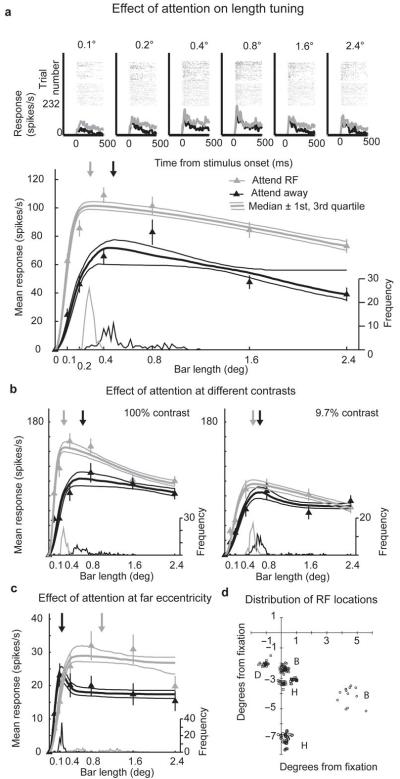
Figure 2.
Effect of attention on length tuning for individual cells. a) Upper plots show raster plots and histograms of single cell responses for each bar length. Data in grey show the attend-RF condition, data in black show the attend-away condition. Lower plot shows the effect of attention on length tuning. Triangles show mean response at each bar length, error bars s.e.m. Bold line fitted to the data shows the median DOG model fit from the bootstrap procedure, flanking upper and lower narrow lines show the 75th and 25th percentile fits. Curves at the base of each plot show the distribution of preferred lengths taken from 100 iterations of the bootstrap procedure. The frequency values of the histogram are shown on the rightward y-axis. The median preferred length is marked with the downwards-pointing arrow. Grey triangles, lines and arrows show data from the attend-RF condition; black triangles, lines and arrows show data from the attend-away condition. Error bars show s.e.m. This cell was recorded using high contrast stimuli. b) Example cell showing the effect of attention on length tuning at both high and medium contrast. Data are shown in the same format as in part a, the cell was recorded from monkey D. c) Example cell with a receptive field eccentricity of ~6° (monkey B). d) Distribution of RF locations. Each point marks the location of a RF. Capital letters next to RF clusters indicate which monkey the respective cells were recorded from (e.g. B:=monkey B).
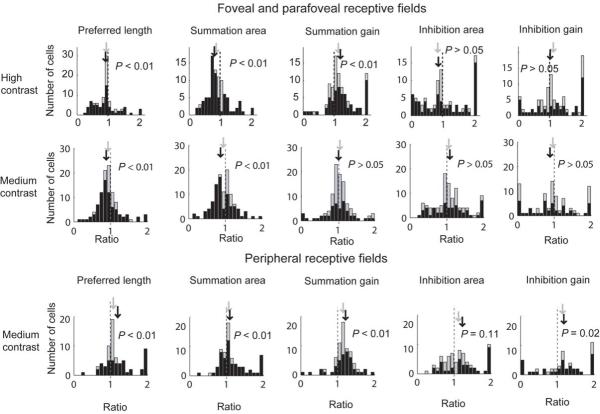
We quantified neuronal length tuning by repeatedly fitting a difference of Gaussians (DOG) model 17 to bootstrapped data. For this, we calculated 100 model fits where the mean firing rate at each bar length in both attention conditions was calculated over a new random-with-replacement selection of trials. We determined goodness of fit in two ways. (A) Goodness-of-fit for each cell was taken to be the variance accounted for 18 obtained from fitting the DOG model to the raw, non-bootstrapped data. The model gave good fits (the population median percentage variance accounted for was 95%, 25th percentile 90%, 75th percentile 98%). (B) Goodness-of-fit for each cell was taken to be the median variance accounted for 18 obtained from the bootstrap method. This approach equally resulted in good fits (the population median percentage variance accounted for was 96%, (25th percentile 93%, 75th percentile 98%). Each iteration of the bootstrap procedure returned an estimate of the preferred length and of the four fitting parameters for each attention condition. We compared the distributions of these estimates to determine whether changes in length tuning due to directed attention were statistically significant on an individual cell basis (two sample t-test). For statistical comparison across the population we calculated, for each cell, medians for the preferred length and fitting parameters under both attention conditions. We then calculated the ratio of these medians between the two conditions by dividing the parameter value for the attend-RF condition by the parameter value for the attend-away condition for each cell. This was done separately for high and medium (non-saturating) contrasts (for details see Methods). At both contrasts the preferred length was significantly reduced in the attend-RF condition compared with the attend-away condition (signed-rank test [SRT], Ho: average ratio = 1, P < 0.01; Fig. 3; see table 1 for additional details). The reduction in preferred length was mediated by a reduction in the summation area in all three monkeys (Fig. 3 and table 1). Additionally, the summation gain was significantly enhanced by attention in the high contrast condition (Fig. 3 and table 1). The inhibitory area and gain were not significantly affected by attention at either contrast level (table 1). This demonstrates that attention directed to foveal and para-foveal visual field locations reduced the influence of the nCRF mostly by reducing the size of the summation area.
Figure 3.
Effect of attention on preferred length and on DOG summation area and summation gain for high (top row) and medium contrast data (middle row) recorded at ~ 2-3° eccentricity location, as well as for medium contrast data recorded at the ~6-7° eccentricity location (bottom row). Values below 1 (vertical dashed line) indicate that the parameter of interest was reduced in the attend-RF condition. Light shaded histograms show the distribution of ratios across the population of cells. Dark shaded histograms show the distribution of ratios across cells for which the parameter of interest was significantly different between the two conditions, assessed by bootstrapping. The median ratio for the whole population and the population of significant cells is marked with downwards pointing arrows, shaded grey and black respectively. P values give the significance of changes in the parameter of interest with attention (whole population signed rank test Ho median ratio=1).
Table 1.
Each box lists the median ratio (25th and 75th percentiles in brackets) of the parameter of interest (attend-RF / attend-away). Additionally it lists the number of cells that were recorded, the number of cells for which the parameter of interest was significantly affected (bootstrap method), and the p-value under the null hypothesis that the average ratio was equal to 1 (signed rank test). Ratios < 1 indicate that the parameter of interest was reduced by attention
| Median Ratio (25th & 75th percentiles) | ||||||
|---|---|---|---|---|---|---|
| Preferred Length | Summation Area | Inhibition Area | Summation Gain | Inhibition Gain | ||
|
Fovea/parafovea
High Contrast |
All Cells | 0.94 (0.69, 1.03) n = 92, p < 0.01 |
0.87 (0.72, 1.06) n = 92, p < 0.01 |
0.93 (0.52, 1.64) n = 92, p = 0.81 |
1.14 (0.99, 1.41) n = 92, p < 0.01 |
1.09 (0.78, 1.79) n = 92, p = 0.06 |
| Significant Cells | 0.91 (0.59, 1.07) n = 66, p < 0.01 |
0.81 (0.70, 1.14) n = 75, p < 0.01 |
0.97 (0.47, 1.80) n = 66, p = 0.88 |
1.21 (1.03, 1.58) n = 62, p < 0.01 |
123 (0.66, 1.09) n = 49, p = 0.38 |
|
|
Fovea/parafovea
Medium Contrast |
All Cells | 0.92 (0.78, 1.06) n = 106, p < 0.01 |
0.94 (0.77, 1.09) n = 106, p < 0.01 |
1.04 (0.85, 1.29) n = 106, p = 0.47 |
1.02 (0.83, 1.19) n = 106, p = 0.41 |
0.96 (0.59, 1.28) n = 106, p = 0.22 |
| Significant Cells | 0.88 ( 0.73, 1.06) n = 80, p < 0.01 |
0.86 (0.72, 1.09) n = 82, p < 0.01 |
1.08 (0.69, 1.46) n = 53, p = 0.17 |
0.99 (0.83, 1.19) n = 50, p = 0.52 |
0.88 (0.33, 1.28) n = 44, p = 0.04 |
|
|
Periphery Medium
Contrast |
All Cells | 1.08 (0.97, 1.32) n = 69, p < 0.01 |
1.07 (0.92, 1.35) n = 69, p < 0.001 |
1.12 (0.83, 1.40) n = 69, p = 0.11 |
1.16 (1.04, 1.30) n = 69, p < 0.001 |
1.15 (0.78, 1.56) n = 69, p = 0.02 |
| Significant Cells | 1.19 (0.96, 1.51) n = 47, p = 0.02 |
1.09 (0.92, 1.42) n = 57, p < 0.001 |
1.22 (0.83, 1.83) n = 37, p = 0.02 |
1.21 (1.04, 1.37) n = 42, p < 0.001 |
1.17 (0.64, 1.51) n = 36, p = 0.067 |
|
Data recorded at greater retinal eccentricity
In two of the three monkeys (monkey B and monkey H) we collected data from neurons representing two different retinal eccentricities using medium non-saturating stimulus contrast (10-25% Michelson contrast). In the first ‘parafoveal’ recording location, RFs were at an eccentricity of ~2° in monkey B (n = 17, 25th percentile 2.3°, 50th percentile 2.4°, 75th percentile 2.5°) and they were at an eccentricity of ~3° in monkey H (n = 56, 25th percentile 3.1°, 50th percentile 3.2°, 75th percentile 3.3°). In the second ‘peripheral’ location RFs were at an eccentricity of ~6° in monkey B (n = 22, 25th percentile 5.6°, 50th percentile 5.8°, 75th percentile 6.5°), and they were at an eccentricity of ~ 7° in monkey H (n = 47, 25th percentile 6.7°, 50th percentile 6.9°, 75th percentile 7.2°). All cells recorded in monkey D were at a parafoveal eccentricity of around 2° (n = 37, 25th percentile 2.2°, 50th percentile 2.3°, 75th percentile 2.4°).
In the ~6-7° eccentricity sample (n = 69), the effect of attention on length tuning was opposite to the effect at ~2-3° eccentricity, i.e. preferred bar length was increased in the attend-RF condition (Fig. 2c and Fig. 3). The increase in preferred length was mediated by a significant increase of the summation area in the attend-RF condition, as well as by an increase in summation gain and an increase in inhibition gain (P = 0.02, SRT; Fig. 3 and table 1). The inhibition area was not significantly affected by attention (table 1 for details).
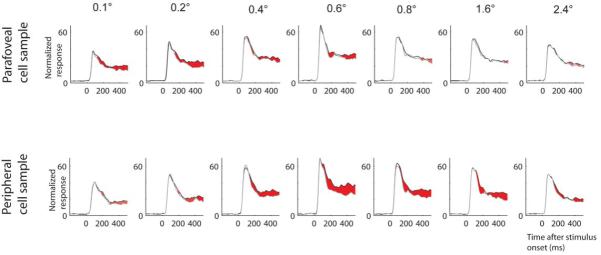
Given that attention affects length tuning differently at parafoveal and peripheral recording sites, it could be predicted that attentional modulation is stronger for shorter bar-length at parafoveal recording sites, while it is stronger for medium to larger bars at more peripheral recording sites. We indeed found this difference (Fig. 4). For parafoveal recording sites attentional modulation was stronger when shorter bars were presented (0.1-0.2° bar-length), while for peripheral sites attentional modulation was stronger for medium bar lengths (0.6-0.8° bar-length). We quantified this finding by calculating the receiver operating characteristic (ROC19) as a function of bar-length (Fig. 5). The time period to calculate the ROC value was from 200-500 ms after stimulus onset. For both eccentricities we found a significant effect of bar-length on the attentional modulation (expressed in terms of the ROC value, P < 0.001, one way RM-ANOVA). Importantly, the maximum ROC values for parafoveal sites occurred at a bar-length of 0.2°, while they were largest for a bar-length of 0.8° at more peripheral recording sites (Fig. 5).
Figure 4.
Population response as a function of bar length. Upper row of histograms: Response from cells recorded in monkey B (n = 17) and H (n = 56) at medium contrast from parafoveal sites. Lower row of histograms: Response from cells recorded in monkey B (n = 22) and H (n = 47) at medium contrast from peripheral sites. Black curves: response for the attend RF condition, grey curves: response for the attend away condition. Red shaded areas: time periods during which response differences were significant for the population (P < 0.01, rank sum test). Response normalization was performed for each cell across all conditions initially. Population responses were calculated from these individually normalized responses. Population histogram for bar-length 0.6 deg is from monkey H only, as recordings in monkey B were restricted to 6 different bar-lengths. Data from monkey B and H are included as both contributed to our parafoveal and peripheral cell sample.
Figure 5.
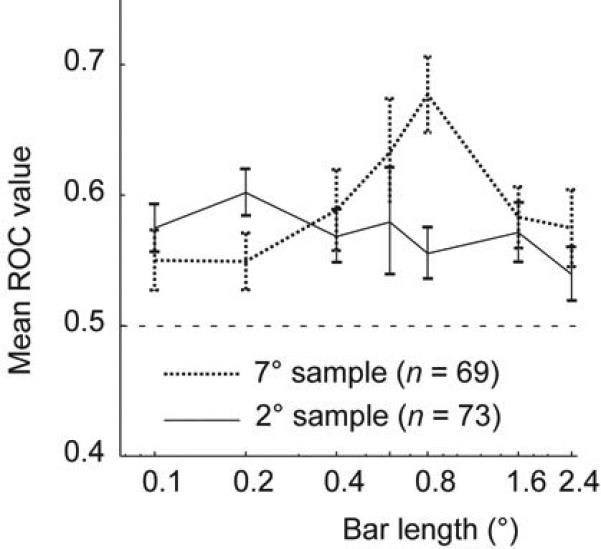
Quantitative comparison of attentional modulation as a function of bar-length and recording location. Attentional modulation was assessed by calculating a receiver operating characteristic (ROC) as a function of bar-length for each cell from monkey B and monkey H (recordings from medium contrast experiments) for parafoveal sites (black solid curve) and for peripheral sites (black dotted curve). ROC values differed significantly as a function of bar-length for both recording sites (P < 0.001, ANOVA on ranks). Error bars show s.e.m. Data for bar-length 0.6 deg are from monkey H only, as recordings in monkey B were restricted to 6 different bar-lengths. Data from monkey D are not included, as he did not contribute to data from peripheral sites.
The difference in the effect of attention on peak length and summation area between the ~2-3° and ~6-7° eccentricity samples was highly significant (P < 0.001 two sample t-test, compare ratios in table 1). Such a striking difference could be mediated by a number of factors besides the simple eccentricity difference, since many neuronal selectivities co-vary with eccentricity. These include contrast sensitivity (higher near the fovea), spatial frequency preference (also higher in central vision), and receptive field size (larger in the periphery). Under this hypothesis any cell with a large RF (or low contrast sensitivity or spatial frequency preference) would have enhanced preferred length under conditions of attention, regardless of the eccentricity of the RF. Attentional modulation would thus be determined by some low level property of the individual cell, rather than, for example, functional or network differences between central and peripheral vision. We tested for this possibility by subdividing the two eccentricity cell groups according the selectivity of the individual cells (where it had been measured). We then compared whether these preferences could account for the effect of attention on length tuning. None of the tested parameters predicted the effect of attention on length tuning in a manner similar to eccentricity (for additional details see Supplementary information1). It could still be the case that ceiling effects, or some other non-uniformity in the data, contribute to our finding of changed summation area (or other parameters of the DOG model). I.e. one might wonder whether cells belonging to the subgroup of ‘small receptive fields’ for a given eccentricity are just as likely to show attention induced changes in the summation area as cells that belong to the ‘large receptive fields’. We do not find any obvious evidence for such differences (for additional detail see Supplementary figure 1). While we cannot exclude the possibility that another co-variant of eccentricity (which was not measured in this study) was responsible for our finding, we currently suggest that attentional mechanisms may be implemented differently between parafoveal and peripheral sites, resulting in reduced spatial pooling at parafoveal sites and increased pooling at peripheral sites,
Model comparisons
Our bootstrap procedure suggested that the change in preferred length was mediated by a change in the summation area, as this was the only parameter that was significantly affected by attention in all conditions; the summation gain was not affected by attention at medium contrast at parafoveal sites, and inhibition gain was only influenced among peripheral recording sites (see table 1). An alternative account would propose that changes in gain are sufficient to explain the effect of attention on length tuning, rather than changes in the summation or inhibition area. Moreover, a divisive model, as proposed by Cavanaugh et al. 20, might explain a larger amount of variance of the data. To assess these possibilities we repeatedly fitted a DOG and a ratio of Gaussian (ROG) model to our bootstrapped data (see methods). For both models we used 3 different subtypes, namely a ‘full’ model in which all parameters were free to vary between the two attentional conditions, a ‘gain’ model where the summation and inhibition areas were forced to have the same value in both attention conditions, while the gains were allowed to vary, and an ‘area’ model where the gains were fixed but the summation and inhibition areas were allowed to vary between attention conditions. We compared the normalized χ2 values (χ2N, normalized by the degree of freedom 20) between these 6 different models for each monkey and recording condition (eccentricity, contrast level) separately as well as combined across monkeys and conditions. We found that for all conditions and all monkeys all DOG model subtypes were significantly better than the corresponding ROG model (P < 0.0001, RM-ANOVA on ranks). This demonstrates that our data are better described by a DOG model than a ROG model (for additional detail and a discussion see Supplementary information 2). Comparing the different DOG models we found that normalized χ2N was significantly smaller for the DOG area model and the DOG gain model than the full DOG model (P < 0.05, RM-ANOVA on ranks). Moreover, we found that the DOG area model resulted in significantly better fits than the DOG gain model (P < 0.05, RM-ANOVA on ranks). Although this was only significant when all conditions (high and medium contrast; parafoveal and peripheral sites) were combined, there was a trend present in each individual data set from each monkey for all conditions. This indicates that changes in gain alone are not sufficient to explain our data; a substantial (even larger) amount of variance is explained by changes of the size kernels in the DOG model (summation and inhibition areas). To further analyse this, and determine the contribution of the individual components of the DOG model (summation area, inhibition area, summation gain, inhibition gain), rather than gains combined or areas combined, we refitted our data with a series of DOG model subtypes in which just one of the four parameters was constrained to have the same value in both attention conditions, while the remaining 3 parameters could vary between the attend-away and attend-RF conditions. Fit quality was assessed by calculating the normalized chi-squared error20 (χ2N). We found that fit quality (χ2N) was significantly different for the four different models (P < 0.001, RM-ANOVA on ranks) for both eccentricities. Post-hoc testing (Tukey’s test) revealed that for the parafoveal sample constraining the summation area resulted in the highest χ2N (i.e. the worse fits) of all models. The difference was significant when compared with the model in which the excitation gain was constrained but not for the remaining two models (inhibition area and gain). Surprisingly, the model where the excitation gain was constrained gave the best fits. For the cell sample from peripheral sites we found similar results. Constraining the summation area resulted in significantly worse fits than constraining any of the other three parameters (P < 0.05, RM-ANOVA on ranks, Tukey’s test). This corroborates the previous finding that the change in length tuning in our data cannot be described by changes in the gain parameters alone, and supports the notion that changes in the summation area significantly contribute to changes in preferred length. Despite these findings, it is worthwhile to bear in mind that the extent of the summation area generally fell within the region that was directly measured in our experiment, while the inhibition area often extended beyond that region. Thus, we may have greater confidence in our estimates of the excitatory components of the DOG model than the estimates of the inhibitory components. If for example the size of the inhibition area changed from 5° to 10° our measurements might not reveal these changes, since such a change would occur beyond the maximum extent of our experimental stimuli. Changes in the parameters of the summation area could therefore have a more direct impact on our length tuning measurements. Thus, even if the inhibitory area changed in conjunction with a change of the summation area, the former may be more difficult to detect given our stimulus protocol.
Influence of eye-position
Small differences in eye position or eye movement between the attend-RF and attend-away conditions could potentially contribute to, or contaminate, the effects of attention. To control for this, we used post-hoc filtering to further restrict the eye position window (which was very small to start with: ±0.30 to 0.35°). For this analysis we first calculated the mean eye position during the analysis period (30-500 ms after stimulus onset) from all recorded trials. A threshold was set to exclude trials in which the eye position deviated from the mean position by more than the threshold (0.25°, 0.2° or 0.15°) allowed. We then used receiver operating characteristic (ROC) analysis19, 21, 22 to quantify the magnitude of attentional modulation of the neuronal response. We compared the ROC values for individual data points (one cell’s responses to one bar length) before and after filtered with increasingly restrictive thresholds. Data points where fewer than 8 trials remained after filtering were excluded. Filtering the data in this way did not reduce the attentional effect, as would be expected if the difference in response rate between attention conditions was due to differences in eye position (where the largest differences in response would occur in trials with the most deviant eye position).
Since we found no significant difference in mean ROC values between post-hoc filtered and unfiltered data at any eye window threshold, we conclude that the attentional enhancement was not due to small difference in eye movements or position between the two attentional conditions (median difference in ROC after filtering with 0.25° filter 0.00, 25th percentile −0.03, 75th percentile 0.03, n = 1078 data points, P = 0.94 paired t-test; after filtering at 0.2° median difference 0.01, 25th percentile −0.05, 75th 0.05, n = 688, P = 0.95; after filtering at 0.15° median difference 0.01, 25th percentile −0.05, 75th percentile 0.08, n = 118, P = 0.2).
Discussion
We have shown that attention affects spatial integration in primate visual cortex in a manner dependent on eccentricity, by either decreasing (central vision) or increasing (peripheral vision) the summation area. This finding demonstrates that the influence of the nCRF is dynamic and is dependent on attention. We investigated this by measuring the length tuning of V1 cells under two attentional conditions and at two eccentricities. Attention caused a reduction in preferred length for the majority of cells with RF eccentricities of ~2-3°. This change was mediated by a reduction of the summation area. The inhibitory nCRF subfield was not significantly affected by attention. For cells with eccentricities of ~6-7°, attention caused an increase in preferred length, mediated largely by an increase in the summation area, but also by a change in summation gain, and inhibition gain.
Before trying to interpret these finding, we should first consider which types of connections should be identified with the different parameters of the DOG model. We would tentatively identify the efficacy of feed-forward input (including the efficacy of recurrent excitation within a cortical column) with the summation gain. The efficacy of excitatory lateral connections and feedback connections (feedback connections that represent visual field locations outside the neuron’s CRF) would be identified with the size of the summation area. The latter two types of connections would also be responsible for the size of the inhibitory area when they target local inhibitory interneurons. The inhibition gain is likely to be influenced by a combination of feed-forward connections, local recurrent connections, lateral and feedback connections. Although we think that this could provide a reasonable framework for the current discussion, it is almost certainly an oversimplification and precise identification of the different cortical and sub-cortical connections with the different parameters of the model will require additional research (see Supplementary information 3 for further discussion).
It has been argued that attention does not alter neuronal tuning functions8, 9. However, we found a strong and significant change of length tuning with attention. Length tuning may be a special case of cortical neuronal tuning which is particularly sensitive to changes in the balance between feed-forward and lateral or feedback inputs. With increasing stimulus length, an increasing pool of cortical neurons contribute to a cell’s response through lateral interactions23-25, or feedback connections 16, 20. Thus, as stimulus length increases, the balance of inputs shifts from being mostly feed-forward contributions at short lengths towards substantial influence from lateral or feedback contributions at longer lengths. Attention mediated changes in the synaptic efficacy of the lateral or feedback input will thus alter the response to long stimuli (heavily dependent on lateral or feedback inputs) but not the response to short stimuli (largely independent of the lateral or feedback input), thereby shifting the length tuning profile. This would not necessarily be the case for other feature domains (e.g. orientation, or direction of motion), where the relative contribution of feed-forward and lateral or feedback connections is less influenced by changing the feature variable.
Length tuning has been shown to be altered by stimulus contrast, whereby increased contrast leads to reduced spatial summation 17, 20, 26, although contrast induced changes in preferred lengths may be due to changes in excitation gain alone20. This contrast effect is strikingly similar to the effect of attention we found at parafoveal locations; an observation which is in line with suggestions that attention is equivalent to increasing the contrast of a stimulus 11, 27. However, our results from more peripheral locations are incompatible with this idea. Hence our data contribute to a growing body of recent work 28, 29 which demonstrates that the link between attention and contrast cannot always be explained by changes in contrast gain 11, 27.
We have shown that the precise nature of the effect of attention on spatial integration depends on retinal eccentricity, this has important implications for the results of a previous study 30. The authors 30 reported reduced nCRF facilitation under conditions of focused attention for one of their monkeys (SA), matching their previous psychophysical results14 and our finding of reduced spatial summation with attention in the parafovea. For their second monkey (UM) they reported increased nCRF facilitation under conditions of focused attention, supporting our data from peripheral vision. The authors suggested that these opposite effects could be explained by differences in training and the strategy of the monkey, however, there were also differences in the RF eccentricity between the two monkeys. Monkey SA, whose data were comparable with our data from ~2-3° eccentricity, had RFs with eccentricities in the range of 1.85° to 3.22°. Conversely, monkey UM, whose data were more comparable with our data from ~6-7° eccentricity, had RFs with eccentricities in the range of 3.68° to 5.25° (personal communication with M. Ito). While the difference in eccentricity between the two monkeys in Ito and Gilbert’s study was not as large as the difference between our samples, the pattern of results is consistent with our finding: attention caused reduced summation near the fovea but increased summation further towards the periphery. Therefore, the inconsistency in Ito and Gilbert’s data30 may be explained by differences in eccentricity rather than, or in addition to, differences in training and strategy.
The differential effect of attention on spatial summation as a function of eccentricity could reflect differences in the cortical network between peripheral and parafoveal vision. Evidence for such a difference has come from human psychophysical studies showing stronger suppressive contextual interactions for the periphery than for foveal-central vision 31. This was paralleled by our finding of a significant contribution of inhibitory gain to changes in length tuning in the peripheral sample but not in the parafoveal sample. For central vision it may be beneficial to exclude contextual information when objects are attended to, allowing unbiased analysis (noise and distracters excluded) of the attended location. In such circumstances attention should reduce spatial summation. Detailed analysis of visual scenes is not possible in peripheral vision due to reduced visual resolution for these locations. Here, enhancement of facilitatory interactions by attention could promote a more integrative scene analysis and highlight attended peripheral objects as targets for impending eye movements, thereby bringing the attended object into foveal vision for detailed analysis. Alternatively, differences in sensitivity between parafoveal vision and peripheral vision (in terms of contrast or spatial sensitivity) might require different amounts of neuronal pooling to adequately solve the task. The change in luminance contrast the animals had to detect was subtle. If contrast sensitivity is higher near the fovea, the animals might thus need to recruit fewer neurons. In conjunction with better spatial resolution of parafoveal neurons this could possibly account for the differences we found with respect to spatial integration at parafoveal and peripheral recording sites.
In a recent study Womelsdorf et al12 reported that spatial attention shifts the location of receptive fields of medial temporal (MT) neurons, while having only a very small effect on the size of the receptive field. At first glance this appears contrary to our findings of a significantly reduced summation area for parafoveal RFs. However, our length tuning paradigm specifically probed the effect of attention on spatial pooling over an extended area (irrespective of whether these include the CRF or the nCRF), while the study by Womelsdorf et al. exclusively measured responses to small stimuli, which were not intended to give rise to different amounts of spatial pooling and thus revealed exclusively the minimum response field. In our data the average summation area under both attention conditions was larger than the CRF and the minimum response field (see table 2 for details). Thus, since our experiment probed the effects of attention on the efficacy of spatial pooling, rather than the minimum response field size, it does not contradict, but rather complements Womelsdorf et al’s 12 finding.
Table 2.
Each table lists the median and 25th and 75th percentiles (in brackets) of the parameter of interest under the condition of attend-RF and attend-away from the receptive field. The unit for preferred length, summation area, and inhibition area is degrees of visual angle (diameter). Summation and inhibition gains are in arbitrary units. For comparison the receptive field diameter (median, 25th and 75th percentiles) is also listed (in degrees of visual angle), as determined from the initial mapping with 0.1×0.1o stimuli under ‘neutral’ attention conditions
|
N (cells) |
Receptive Field Diameter |
Attention Condition |
Preferred Length |
Summation Area |
Inhibition Area |
Summation Gain |
Inhibition Gain |
|
|---|---|---|---|---|---|---|---|---|
| High Contrast | 92 | 0.25 (0.2, 0.3) |
away | 0.38 (0.22, 0.52) |
0.34 (0.17, 0.44) |
1.40 (0.74, 2.42) |
69.2 (37.3, 125.4) |
41.2 (26.5, 90.9) |
| RF | 0.31 (0.21, 0.48) |
0.28 (0.17, 0.43) |
1.23 (0.60, 2.55) |
83.3 (47.6, 150.6) |
54.5 (31.2, 99.3) |
|||
| Medium Contrast | 106 | Not mapped with medium contrast | away | 0.47 (0.30, 0.86) |
0.42 (0.24, 0.54) |
1.50 (0.87, 2.07) |
32.93 (20.06, 68.67) |
14.5 (0.07, 29.92) |
| RF | 0.42 (0.28, 0.71) |
0.36 (0.21, 0.51) |
1.57 (0.88, 2.21) |
32.29 (19.97, 65.37) |
14.7 (0.03, 35.67) |
|||
| ~6 degrees eccentricity | 69 | 0.33 (0.25, 0.37) |
away | 0.29 (0.21, 1.12) |
0.23 (0.17, 0.47) |
1.46 (0.70, 2.46) |
30.96 (16.02, 47.87) |
7.19 (0, 25.38) |
| RF | 0.40 (0.24,v1.24) |
0.31 (0.20, 0.51) |
1.61 (0.75, 3.46) |
37.78 (20.08, 53.23) |
11.7 (0, 29.35) |
In our task, monkeys had to detect the brightening at a very small location in the visual field. This requires a specific form of attention, where spatial integration is likely to be detrimental. Under these circumstances we find a reduction in spatial integration at parafoveal sites, and an increase at more peripheral sites. Attention may not be a unitary mechanism. It is a mechanism to ensure that task-relevant information (accurately or inaccurately) has an impact on ongoing processing. Therefore, the influence of attention on the underlying computational architecture (e.g. CRF-nCRF interactions) may depend sensitively on the specific task. E.g. tasks where attention to large parts of the visual field is required 3, 32 might increase spatial integration also at parafoveal locations. Perceptual learning may further alter the network effects of attention 33. Additional studies are necessary to precisely delineate how different attentional task demands alter specific computations within the neuronal architecture.
States of attention are known to be related to the release of acetylcholine (ACh) in the cortex34. In a previous study35 we tested the effect of iontophoretic application of ACh on length tuning in primate V1. Application of ACh caused a significant reduction in preferred length across the population of cells (which we estimated had RF eccentricities in the range of 1-10°). Fitting the data with a DOG model showed that the reduction in preferred length was mostly mediated by a reduction in a cell’s summation area. Thus, the effects of ACh application on length tuning were similar to the effects of directed spatial attention in our sample from parafoveal sites. This similarity in the effects of ACh application and of directing voluntary attention could support the hypothesis that ACh is involved in the neuronal processes that mediate attention. Moreover, our finding that attention mostly affects the later part of the response matches the results of local ACh application 35, and is in line with previous findings in V1 and V4 3, 11 (but see Supplementary information 4 for a more detailed discussion). Our findings from more peripheral sites are difficult to reconcile with the idea that ACh is the sole agent which mediates mechanisms of attention. We rather propose that the effects of spatial attention are mediated by an interaction of cholinergic input and feedback connections (possibly synapto-synaptic) from higher cortical areas. Under this hypothesis, high levels of ACh allow feedback projections to exert their specific influence. This is akin to a recent model where neuromodulator and feedback interactions allow for unsupervised learning36. As attention is normally required for learning, attention and learning may share a common neuronal substrate. Future investigations will have to determine the precise underlying neuronal substrate and mechanisms which cause attention to have different effects on spatial integration at foveal-parafoveal vs. more peripheral receptive field locations.
In summary; we have demonstrated that directed spatial attention towards the RF of a V1 neuron significantly alters its length tuning. In cells with parafoveal RFs this change in length tuning was induced by reducing the efficacy of facilitatory nCRF influences in the attend-RF condition. Thus, attention caused the neuronal response to be more strongly driven by visual stimuli within the CRF and less strongly influenced by the surrounding context (nCRF). Reduced spatial integration at foveal-parafoveal locations is likely to aid high resolution analysis of attended objects, furthermore, distracters or external noise surrounding the objects of interest will have reduced impact on processing. We also found attention caused increased spatial integration at more peripheral sites in V1. This demonstrates that cortical processes, and their modulation by attention, are not uniform across visual space. Rather, processing in the periphery may rely on fundamentally different cortical mechanisms to those operating near to the fovea, reflecting different requirements of central and peripheral vision.
Methods
All experiments were carried out in accordance with the European Communities Council Directive 1986 (86/609/EEC), the US National Institutes of Health Guidelines for the Care and Use of Animals for Experimental Procedures, and the UK Animals Scientific Procedures Act.
Surgical preparation
Following initial training monkeys were implanted with a head holder, eye coil, and recording chambers above V1 under general anesthesia and sterile conditions. All details regarding surgical procedures, postoperative care and the cleaning of the implant and recording chambers are published elsewhere 37.
Electrophysiological recordings
Once monkeys were able to perform the task reliably, a craniotomy was made above V1. Extracellular responses were recorded using tungsten-in-glass microelectrodes (0.5-2 MΩ, made in-house). Stimulus presentation and behavioral control was managed by Remote Cortex 5.95 (Laboratory of Neuropsychology, National Institute for Mental Health, Bethesda). Neuronal data was collected either by Remote Cortex 5.95 (1kHz sampling rate) or by Cheetah data acquisition (30kHz sampling rate) interlinked with Remote Cortex 5.95.
Receptive field mapping
Receptive fields (RF) were mapped by presenting a 0.1° black (100% contrast) square at pseudo-random locations on a 10×10 grid (i.e. a 1×1° area; 5 repetitions at each location; 100 ms presentation time with 100 ms gaps), while monkeys fixated centrally on the CRT. To prevent the monkey from attributing a ‘special status’ to the RF location, an identical stimulus was simultaneously presented in the opposite hemifield. The mean response at each stimulus location (calculated from 30-100 ms after stimulus onset) was determined and a 2D Gaussian was fitted to the response distribution. The RF centre was taken as the location of the peak of the fitted Gaussian.
Main experimental stimuli and protocol
The monkey’s task was to detect a small change in luminance at a cued (attended) location, while ignoring a change that occurred at a non-cued location (Fig. 1). Monkeys initiated trials by holding a touch bar and fixating a red fixation point (FP, 0.1° diameter) on a grey background (21 cd/m2) presented centrally on a 20” analogue CRT monitor (75 Hz, 1,600 * 1,200 pixels, 57 cm from the animal). The fixation window was ±0.3° - 0.35° wide, and the animal’s eye position had to remain within these boundaries throughout the trial. Eye position was recorded with a scleral search coil. A cue (blue annulus, 0.24° outer diameter, 0.18° inner diameter) was presented for 400 ms on one side of the fixation spot. The location of the cue indicated the location to which the monkey had to attend. The cue was presented displaced along the axis connecting the FP and the RF location by one quarter of the eccentricity of the neuron’s RF. The cue was displaced either towards or away from the RF to indicate whether attention should be directed towards or away from the stimulus presented in the RF. After cue offset a 250 ms blank (900 ms in monkey H) period occurred with just the FP present. Spatial and temporal separation of the cue from the test stimuli ensured that it had no direct effect on the neuronal response to the test stimulus. Thereafter, two identical stimuli were presented (test stimuli), one centered on the RF, the other at the same eccentricity in the opposite hemi-field. Test stimuli were dark bars of preferred orientation and varying length (see below). After 500 ms a brighter patch (0.1° square) appeared at the centre of one of the bars. If presented in the cued location it is referred to as ‘target’, if presented in the un-cued location it is referred to as ‘distracter’. The target or distracter was brighter than the test stimuli by 7.3 cd/m2 (±2) depending on the contrast of the test stimulus and on the training of the monkey. After the presentation of a target, the monkey had to release the touch bar within 500 ms to receive a juice reward. If a distracter was presented first the monkey had to continue to hold the touch bar and maintain fixation until target appearance. This occurred 1,000 ms after the distracter appeared. If the monkey made no response, the trial was terminated 500 ms after presentation of the target or distracter, whichever appeared last. Touch bar releases (correctly or incorrectly) or failure to maintain fixation resulted in immediate trial termination.
Attentional cueing was done in a blocked design, blocks were counterbalanced in random order. Conditions of cueing towards the location of the RF are labeled ‘attend-RF’, conditions of cueing towards the opposite hemifield are labeled ‘attend-away’. Within each block, bar length was varied in either six steps (monkey B and D 0.1°, 0.2°, 0.4° 0.8° 1.6°, 2.4°; bar width: 0.1°) or 7 steps (monkey H additional 0.6° stimulus). For each bar length the target occurred once at 500 ms after bar onset (early target condition) and once at 1,500 ms after test bar onset (late target condition). Conditions (bar length, early or late target) were presented in pseudorandom order within each block. If the monkey made an error the condition would be repeated later in the block. A four-block design was used in sessions where two test stimulus contrasts were presented. Only cells for which there were at least 8 trials per length and attention condition were included in the analysis. The median number of trials per condition for neurons reported here was 20 (25th percentile 16, 75th percentile 28).
Orientation tuning and contrast response function
For monkeys B and D the preferred orientation was measured by varying test stimuli orientations in 8 steps of 22.5° between 0° and 157.5° (stimulus size: 0.4°×0.1°, 100% contrast) while the monkey performed the task described above. Each stimulus was presented 8 times for both attention conditions. The preferred orientation was taken as the orientation with the highest mean response in either attention condition.
The contrast response function was measured either under passive viewing or by using the attentional task (described above). In both conditions we presented bars, of the preferred orientation (size: 0.4° * 0.1°), at 8 different contrasts (range: 2-100% contrast, individually adjusted to sample the steep part of the contrast response function). Each stimulus was presented at least 8 times. A Naka-Rushton function was fit to the data38 and used to select two contrasts that would give significantly different responses in the length tuning experiment.
Spatial frequency mapping
Optimal spatial frequency (and orientation) in monkey H was determined by employing a reverse correlation technique 15, 39. Stimuli were 336 circular patches of static sinusoidal gratings (1.0 ° diameter) varying in orientation (12 orientations 0-165 °), spatial frequency (1, 3, 5, 7, 8, 9, 10 cyc/°) and phase (0, 0.5π, π, 1.5π). Gratings were presented for ~60 ms in a pseudo-randomized order centred over the minimum response field. Responses were averaged over a 60 ms time window following stimulus onset at + 30 ms, and at + 60 ms. 5-10 repetitions of each stimulus were averaged. The stimulus that yielded the peak response was take to represent the preferred orientation and preferred spatial frequency in monkey H.
Recording protocol
For each cell we initially mapped the RF, then measured the orientation tuning followed by the contrast response function and then the length tuning (in monkey H we also measured the spatial frequency preference, while contrast tuning was measured at the end of the session, provided recording stability still allowed for it). To test that our results concerning the effect of attention on length tuning were not simply due to a saturation effect, we measured length tuning at two contrast categories in monkeys B and D: high and medium contrast. In recordings defined as high contrast, the contrast of the test bar was always 100% relative to the background, therefore it is possible that the cell’s response may have been saturated. However, in recordings defined as medium contrast, a lower contrast was used and the cell’s response was demonstrably not saturated i.e. the highest response to a medium contrast stimulus was significantly lower than the highest response to a high contrast stimulus (P < 0.05, one-tailed two-sample t-test). The range of contrasts used in medium contrast recordings differed slightly between the two monkeys. For monkey B contrasts were between 9% and 48% (median contrast of 18%), while for monkey D the range was between 7% and 22% (median contrast of 10%). Data obtained using stimuli from either contrast category are referred to as ‘high contrast data’ and ‘medium contrast data’ respectively throughout the text. Depending on the training and motivational status of the monkey we measured the effect of attention on length tuning at either one (either high or medium contrast) or both contrast levels. For monkey H we used a fixed contrast of 25% for our medium contrast recordings.
Calculating stimulus-driven responses
We measured the neuronal response to each bar length in the attend-RF and attend-away conditions. We calculated this response from 30 ms to 500 ms after stimulus onset (i.e. up to the time of the appearance of an early target). Spontaneous firing rates were calculated separately for each attention condition from responses during the 250 ms preceding the presentation of the test stimuli. All stimulus-driven activity presented herein was corrected for spontaneous activity (i.e. spontaneous activity subtracted). This was done to fulfill the assumption of the fitting model that the response is 0 with 0° bar length (i.e. no stimulus).
Length tuning analysis and fitting
Length tuning data, given by the mean response at each bar length, was initially fit with a difference of Gaussians (DOG) model 17. In this model the narrower Gaussian represents the RF’s excitatory centre and an excitatory fringe of the nCRF while the broader Gaussian represents the inhibitory surround (but see Supplementary information 3 and 5 for more in depth description). Each Gaussian is described by a gain and an area constant, determining its height and width respectively. The response to any bar length is modeled by the difference between the integrals of the area of each mechanism up to the length of the stimulus. This function captures the shape of measured length tuning curves and allows the relative strength and size of excitation (summation) and inhibition areas to be determined (for details of the DOG function see 40). Fits of the summation area and inhibitory area were constrained such that the inhibitory area was larger than the summation area. We optimized fits to minimize the summed squared error (SSE) between the model’s prediction and the mean firing rate. To take the variance in the data into account and to assess the significance of changes in peak length and fitting parameters, we used a bootstrap method as described previously35. The quality of the model fit was assessed by calculating the percentage of variance accounted for by the fitted model18. Additional fitting was performed by using a ratio of Gaussian model (ROG)20. This model assumes divisive inhibitory mechanisms rather than subtractive ones. Fitting was performed in an identical manner as described for the DOG otherwise.
Supplementary Material
Acknowledgements
We would like to thank P. Dayan for valuable discussions and comments on the paper. The staff of the Comparative Biology Centre (University of Newcastle upon Tyne) provided excellent technical support. The work was supported by the BBSRC (BBS/B/09325), the Wellcome Trust (070380/Z/03/Z), and the MRC (G0100407; G78/7853).
Footnotes
Competing financial interests: The authors declare that they have no competing financial interests.
References
- 1.Haenny PE, Schiller PH. State dependent activity in monkey visual cortex. I Single cell activity in V1 and V4 on visual tasks. Exp.Brain Res. 1988;69:225–244. doi: 10.1007/BF00247569. [DOI] [PubMed] [Google Scholar]
- 2.Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 3.Roelfsema PR, Lamme VA, Spekreijse H. Object-based attention in the primary visual cortex of the macaque monkey. Nature. 1998;395:376–81. doi: 10.1038/26475. [DOI] [PubMed] [Google Scholar]
- 4.Spitzer H, Desimone R, Moran J. Increased Attention Enhances Both Behavioral and Neuronal Performance. Science. 1988;240:338–340. doi: 10.1126/science.3353728. [DOI] [PubMed] [Google Scholar]
- 5.Treue S, Maunsell JHR. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- 6.Lu ZL, Lesmes LA, Dosher BA. Spatial attention excludes external noise at the target location. J Vis. 2002;2:312–23. doi: 10.1167/2.4.4. [DOI] [PubMed] [Google Scholar]
- 7.Yeshurun Y, Carrasco M. Attention improves or impairs visual performance by enhancing spatial resolution. Nature. 1998;396:72–5. doi: 10.1038/23936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treue S, Martinez-Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- 9.McAdams CJ, Maunsell JHR. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. Journal of Neuroscience. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci. 1999;19:1736–53. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26:703–14. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- 12.Womelsdorf T, Anton-Erxleben K, Pieper F, Treue S. Dynamic shifts of visual receptive fields in cortical area MT by spatial attention. Nat Neurosci. 2006 doi: 10.1038/nn1748. [DOI] [PubMed] [Google Scholar]
- 13.Zenger B, Braun J, Koch C. Attentional effects on contrast detection in the presence of surround masks. Vision Res. 2000;40:3717–24. doi: 10.1016/s0042-6989(00)00218-2. [DOI] [PubMed] [Google Scholar]
- 14.Ito M, Westheimer G, Gilbert CD. Attention and perceptual learning modulate contextual influences on visual perception. Neuron. 1998;20:1191–1197. doi: 10.1016/s0896-6273(00)80499-7. [DOI] [PubMed] [Google Scholar]
- 15.DeAngelis GC, Freeman RD, Ohzawa I. Length and width tuning of neurons in the cat’s primary visual cortex. J Neurophysiol. 1994;71:347–74. doi: 10.1152/jn.1994.71.1.347. [DOI] [PubMed] [Google Scholar]
- 16.Angelucci A, Levitt JB, Lund JS. Anatomical origins of the classical receptive field and modulatory surround field of single neurons in macaque visual cortical area V1. Prog Brain Res. 2002;136:373–88. doi: 10.1016/s0079-6123(02)36031-x. [DOI] [PubMed] [Google Scholar]
- 17.Sceniak MP, Ringach DL, Hawken MJ, Shapley R. Contrast’s effect on spatial summation by macaque V1 neurons. Nat Neurosci. 1999;2:733–9. doi: 10.1038/11197. [DOI] [PubMed] [Google Scholar]
- 18.Carandini M, Heeger DJ, Movshon JA. Linearity and normalization in simple cells of the macaque primary visual cortex. J Neurosci. 1997;17:8621–44. doi: 10.1523/JNEUROSCI.17-21-08621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thiele A, Distler C, Hoffmann KP. Decision-related activity in the macaque dorsal visual pathway. Eur J Neurosci. 1999;11:2044–58. doi: 10.1046/j.1460-9568.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- 20.Cavanaugh JR, Bair W, Movshon JA. Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. J Neurophysiol. 2002;88:2530–46. doi: 10.1152/jn.00692.2001. [DOI] [PubMed] [Google Scholar]
- 21.Celebrini S, Newsome WT. Neuronal and psychophysical sensitivity to motion signals in extrastriate area MST of the macaque monkey. Journal of Neuroscience. 1994;14:4109–4124. doi: 10.1523/JNEUROSCI.14-07-04109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Visual Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- 23.Shapley R, Hawken M, Ringach DL. Dynamics of orientation selectivity in the primary visual cortex and the importance of cortical inhibition. Neuron. 2003;38:689–99. doi: 10.1016/s0896-6273(03)00332-5. [DOI] [PubMed] [Google Scholar]
- 24.Angelucci A, et al. Circuits for local and global signal integration in primary visual cortex. J Neurosci. 2002;22:8633–46. doi: 10.1523/JNEUROSCI.22-19-08633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferster D, Chung S, Wheat H. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature. 1996;380:249–252. doi: 10.1038/380249a0. [DOI] [PubMed] [Google Scholar]
- 26.Kapadia MK, Westheimer G, Gilbert CD. Dynamics of spatial summation in primary visual cortex of alert monkeys. Proc Natl Acad Sci U S A. 1999;96:12073–8. doi: 10.1073/pnas.96.21.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrasco M, Ling S, Read S. Attention alters appearance. Nat Neurosci. 2004;7:308–13. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williford T, Maunsell JH. Effects of spatial attention on contrast response functions in macaque area V4. J Neurophysiol. 2006;96:40–54. doi: 10.1152/jn.01207.2005. [DOI] [PubMed] [Google Scholar]
- 29.Buracas GT, Boynton GM. The effect of spatial attention on contrast response functions in human visual cortex. J Neurosci. 2007;27:93–7. doi: 10.1523/JNEUROSCI.3162-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito M, Gilbert CD. Attention modulates contextual influences in the primary visual cortex of alert monkeys. Neuron. 1999;22:593–604. doi: 10.1016/s0896-6273(00)80713-8. [DOI] [PubMed] [Google Scholar]
- 31.Xing J, Heeger DJ. Center-surround interactions in foveal and peripheral vision. Vision Res. 2000;40:3065–72. doi: 10.1016/s0042-6989(00)00152-8. [DOI] [PubMed] [Google Scholar]
- 32.Freeman E, Sagi D, Driver J. Lateral interactions between targets and flankers in low-level vision depend on attention to the flankers. Nat Neurosci. 2001;4:1032–6. doi: 10.1038/nn728. [DOI] [PubMed] [Google Scholar]
- 33.Li W, Piech V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nat Neurosci. 2004;7:651–7. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annual Review of Psychology. 1997;48:649–84. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 35.Roberts M, et al. Acetylcholine dynamically controls spatial integration in marmoset primary visual cortex. J Neurophysiol. 2005;93:2062–72. doi: 10.1152/jn.00911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roelfsema PR, van Ooyen A. Attention-gated reinforcement learning of internal representations for classification. Neural Comput. 2005;17:2176–214. doi: 10.1162/0899766054615699. [DOI] [PubMed] [Google Scholar]
- 37.Thiele A, Delicato LS, Roberts MJ, Gieselmann MA. A novel electrode-pipette design for simultaneous recording of extracellular spikes and iontophoretic drug application in awake behaving monkeys. J Neurosci Methods. 2006;158:207–11. doi: 10.1016/j.jneumeth.2006.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiele A, Distler C, Korbmacher H, Hoffmann K-P. Contribution of inhibitory mechanisms to direction selectivity and response normalization in macaque middle temporal area. PNAS. 2004;101:9810–9815. doi: 10.1073/pnas.0307754101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ringach D, Shapley R. Reverse correlation in neurophysiology. Cognitive Science. 2004;28:147–166. [Google Scholar]
- 40.Sceniak MP, Hawken MJ, Shapley R. Visual spatial characterization of macaque V1 neurons. J Neurophysiol. 2001;85:1873–87. doi: 10.1152/jn.2001.85.5.1873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.