Abstract
Targeted radionuclide therapy (TRT) seeks molecular and functional targets within patient tumor sites. A number of agents have been constructed and labeled with beta, alpha, and Auger emitters. Radionuclide carriers spanning a broad range of sizes; e.g., antibodies, liposomes, and constructs such as nanoparticles have been used in these studies. Uptake, in percent-injected dose per gram of malignant tissue, is used to evaluate the specificity of the targeting vehicle. Lymphoma (B-cell) has been the primary clinical application. Extension to solid tumors will require raising the macroscopic absorbed dose by several-fold over values found in present technology. Methods that may effect such changes include multistep targeting, simultaneous chemotherapy, and external sequestration of the agent. Toxicity has primarily involved red marrow so that marrow replacement can also be used to enhance future TRT treatments. Correlation of toxicities and treatment efficiency has been limited by relatively poor absorbed dose estimates partly because of using standard (phantom) organ sizes. These associations will be improved in the future by obtaining patient-specific organ size and activity data with hybrid SPECT∕CT and PET∕CT scanners.
Keywords: radionuclide therapy, antibodies, absorbed dose
PRESENT CLINICAL SITUATION
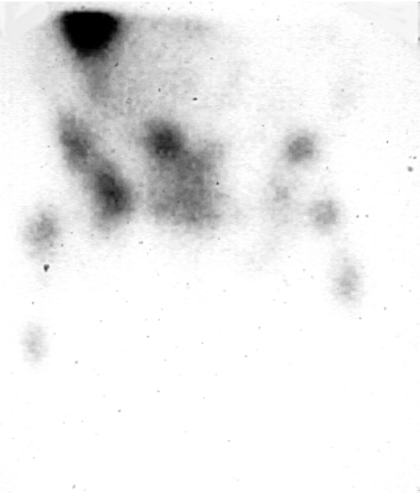
A possible imaging result with a cancer patient is shown in Fig. 1, whereby multiple lesions appear using nuclear (or other) technology. It is important to find a method to target both seen and unseen tumor locations anywhere in the body. It would be desirable that this strategy be as specific as possible to the tumor with minimal collateral toxicity. Targeting of this sort is measured in units of percent-injected dose per gram of tissue (% ID∕g). The mouse would generally be the test species for preclinical development of radiopharmaceuticals for targeted radionuclide therapy (TRT). Following IV injection into a 20 g animal, nonspecific uptake should be
| (1) |
for any of the tissues. Extrapolating the calculation to a 70-kg man, the comparable nontargeting value is 1.4% ID∕kg. Tumor uptakes (u) exceeding these respective tissue sample numbers can then be taken as evidence of specific targeting of the agent of interest. Tumor absorbed dose rate for particulate emissions is also proportional to the tumor u value so that applicability of uptake to radiotherapy is direct.
Figure 1.
Anterior gamma camera image of a medullary thyroid cancer patient. Image obtained at 48 h post-IV injection of 5 mCi (185 MBq) of 111In-cT84.66 anti-CEA antibody. Multiple bone lesions are seen in the pelvis and both femurs. Part of the right lobe of liver appears superiorly (see Ref. 37).
While intravenous injection is probably the only way to target lesions at any site, direct injections may be used to enhance tumor accumulation. Typically, this intervention occurs postsurgery. For example, intraperitoneal applications of antibodies to ovarian cancer1 and directly into brain tumor sites2 are currently being evaluated.
Many agents have been investigated as possible tumor-targeting vehicles. Some, such as antibodies, may also have intrinsic antitumor effects. While the following will describe radionuclide-effected therapy, antitumor chemical species may also be loaded into some tumor seekers such as liposomes. A partial list is given in Table 1 that includes the traditional use of the 131I ion as postsurgery treatment of choice for some types of thyroid cancer. This compilation is capable of expansion using either further engineering of the agents listed, direct invention of novel entities, or combinations of multiple technologies; e.g., liposomes with antibodies on their outer surface.
Table 1.
A partial list of tumor-targeting agents.
| Agent | Molecular weight (Da) or physical size | Application | Representative radiolabels |
|---|---|---|---|
| NaI | 154 Da | Thyroid cancer (follicular and papillary) | 131I |
| MIBGa | 130 Da | Neuroendocrine tumors | 131I |
| Octreotideb | 100 Da | As above | 90Y, 111In |
| SHALsc | <2 kDa | Lymphoma | 131I |
| Nucleotidesd | 10 kDa | Solid tumors | 99mTc, 111In |
| Antibodiese | 25–150 kDa | Lymphomas, solid tumors | 90Y and 131I |
| Liposomesf | 100 nm | Solid tumors | 111In, 99mTc |
| Nanoparticlesg | 10 nm | Solid tumors | |
| Morpholinosh | 2 kDa | Solid tumors | 188Re |
| Spheresi | 30 μm | Hepatic lesions | 90Y |
Optimization of tumor targeting is complicated due to the number of possible agents. Using an antibody as an example, a single amino acid exchange can cause order-of-magnitude variation in blood circulation times of the mutated protein.13 Since there are several thousand amino acids in the original antibody sequence and essentially 20 possible amino acid inserts at each location, the number of variations is astronomical. Thus, only a very limited subset of all possible agents will ever be investigated experimentally.
Only four agents have progressed to FDA-approved clinical therapies as shown in Table 2. Note that two of these therapies involve direct catheter injection into tumor sites in the liver. Intravenous injections have been limited to treatment of lymphoma using anti-B cell (anti-CD20) monoclonal antibodies.
Table 2.
FDA-Approved TRT protocols.
| Agent | Label | Disease | Method |
|---|---|---|---|
| SIR spheresa (plastic) | 90Y (99mTc MAA for images) | Metastatic liver cancer | Catheter via hepatic artery |
| Theraspheresb (glass) | 90Y (99mTc MAA for images) | Primary liver cancer | Catheter via hepatic artery |
| Zevalinc (Ibritumomab) | 90Y (111In for images) | B-cell lymphoma | IV injection |
| Bexxard (Tositumomab) | 131I | B-cell lymphoma | IV injection |
CURRENT LIMITATIONS
Because of the small number of FDA-approved agents, it is clear that significant constraints have hindered development of TRT. Primarily, the limitation has been relatively poor tumor uptake. This has led to correspondingly low absorbed doses and lack of tumor response. Table 3 contains estimated absorbed doses for a standard phase III lymphoma trial14 and an experimental colon cancer therapy.16 These protocols were at previously determined maximal values of activity per body mass14 or per body surface area.16 Notice that both lymphoma and colon cancer regimens have achieved similar numerical tumor absorbed dose values. Clinical application of TRT to the former disease state has followed from the relatively high sensitivity of B-cells to ionizing radiation.
Table 3.
Comparison of two TRT clinical antibody protocols involving 90Y. Absorbed doses were computed using the MIRD formalism (Refs. 20, 21) and refer to whole organ mean values. Ranges are shown in parentheses. The clinical protocols were at maximum activity per body mass (Ref. 14) or body surface area (Ref. 16) determined from previously established hematological toxicity levels.
Increasing injected total activity (A0) cannot be used to arbitrarily raise tumor absorbed doses because of limits set by normal organ toxicities.17 Bone marrow is the most probable absorbed dose-limiting tissue in TRT when the activity is injected IV. Presently, red marrow (RM) absorbed dose is difficult to estimate due to uncertainty in patient-specific information. Recall that internal emitter absorbed dose (D) is usually estimated by the simple vector equation
| (2) |
where S is a rectangular matrix relating source organs to targets and is a vector containing the integrals of source organ activities (A) over time. S contains information on the radionuclide’s emission energies and probabilities as well as the geometry of the source and target organs. For particulate emitters that stop in the source, we concern ourselves with diagonal elements of S where source and target are the same tissue. In this case, S matrix elements are inversely proportional to the total organ mass. For red marrow, this parameter is unknown since the individual patient has genetic, age-related, and therapy-associated variability. One would expect that the RM mass would be significantly different from—and probably lower than—values found in phantom-based absorbed dose estimation algorithms. There is no present-day routinely used method for estimating this unknown tissue mass value. Thus, phase I TRT trial escalation has generally not been based on absorbed dose toxicity but usually on the injected activity per meter squared or activity per total body mass. Neither of these parameters, which are based on traditional chemotherapy treatment planning, has correlated well with patient marrow toxicity.18
Given the above constraints, an approximate formula to estimate the circulating blood contribution to marrow absorbed dose has been developed by the AAPM task group19
| (3) |
where f is a fraction on the order of 0.3 and the ratio represents the correction for marrow mass (1500 g in a particular standard phantom20) in a patient with an assumed 5000 g total blood mass. In the ideal case of no direct marrow targeting of the radioactive agent or its label, the result of Eq. 3 is used as an input into Eq. 2 to estimate total RM absorbed dose.
Issues of accurate absorbed dose estimation have been at the heart of TRT since its inception in the 1950s; e.g., there is little knowledge of the absorbed dose to remnant thyroid tissue during 131I treatment. While MIRD-type calculations are often used to justify clinical trials to the FDA, use of the associated phantom mass values contained in programs like MIRDOSE3 (Ref. 20) and OLINDA (Ref. 21) cannot be directly applied to a given patient. It has been shown, for example, that hepatic and splenic masses may differ by factors of two- and threefold in individuals undergoing TRT of colon cancer.22 Patient-specific treatment planning23 requires actual knowledge of patient anatomy (organ separations) and organ sizes (masses). This problem is thus one of image fusion whereby the activity found using quantitative nuclear imaging can be assigned correctly to a particular anatomic structure.
FUTURE DEVELOPMENTS
Medical physicists can be expected to contribute directly to the growth of TRT in the next 7–10 years. Advances would be of two distinctly different but complementary types. Initially there will have to be a strategy for developing, testing, and generally enhancing the tumor-targeting capability of radiopharmaceuticals. Computer modeling of biodistributions and comparisons of figures of merit are aspects of this work relevant to the physicist. This is particularly important for solid tumors. Beyond the engineering and inventive phase will be the necessity of establishing improved methods of patient-specific absorbed dose calculations. These estimates will become more useful when they relate to voxels rather than whole organ values as indicated above. Among target tissue calculations, red marrow absorbed dose estimates will continue to be the most important.
Pharmaceutical development and testing
Engineering is the operative word for the future. TRT agents of the present time are usually constructed by crude analogy to naturally occurring entities in the bloodstream of common mammals. Protein configurations appear to be one of the most likely for future manipulation. After in vitro testing, the putative tumor seekers would be evaluated via sequential animal three-dimensional imaging rather than by biodistribution studies as are done today. One particularly fruitful area of this research would be the use of mouse-sized PET or SPECT scanners to obviate the need for large numbers of animals being sacrificed at each time point in the biodistribution evaluation. Statistically, this method is also preferred since the same animals are being followed serially. Flexibility of usage may favor SPECT over PET in that more radionuclide labels are available for the former technique.
Use of PET scanners requires availability of suitable positron emitters as labels. Two types of labeling may be anticipated: Radioiodine and radiometal. In the case of proteins, there is a developmental advantage in using radioiodines. Iodine can be directly attached to tyrosine moieties in the amino acid sequence with protein amounts as small as 100 μg. This is in contrast to the radiometal case where milligram amounts are required since a chelator must be first attached to the protein. For iodines, the physical half-life of 124I is 100 h and appears to be a good theoretical choice for PET. Limitations of availability and accurate quantitation have stymied extensive use. Recent work24 has shown that measurement of the amount of 124I is possible with a commercial PET imager and human-scale phantoms.
Iodination of tumor-tracking agents has one disadvantage. Because of thyroid needs, multiple enzymes have evolved to extract iodine atoms from sites on proteins and other agents. Using an iodinated species for extended periods in vivo may lead to unwanted targeting of freed activity to stomach, gut, and thyroid. Because of this limitation, use of radiometal labels of proteins and other entities is expected to increase. This will require production facilities for 64Cu and 86Y, for example. With their relatively simple gamma spectra, these labels will probably become the marker of choice in many preclinical and clinical trials that study entities with relatively rapid biological targeting (i.e., biological half-life <12 h). Species that remain in the blood for periods exceeding one day, however, will remain difficult to label with these radiometals and may require use of 124I.
Several ideas have shown promise in the enhancement of tumor therapy using IV-injected agents. One of these is the use of a sequence of steps to optimize the placement of activity on or within the tumor.25 In this strategy, a large unlabeled molecule, which is at least partially an antibody to the tumor-associated antigen, is injected initially. After some time, and perhaps a clearance step, a low-mass radiolabeled entity is injected. The labeled agent would be an antibody fragment or other small ligand that reacts to the complex of original antigen and the first large molecule. Since the labeled molecule would be of relatively low molecular weight, it would diffuse more rapidly to the lesion sites. Such quick movement would reduce red marrow absorbed dose induced by the circulating activity in the blood.26 Limitations to the method involve the possible increase in the renal absorbed dose due to the anticipated urinary excretion of the radiolabeled entity.
A second targeting variation that manipulates the blood curve of the radiotracer shows significant promise for reducing bone marrow toxicities as estimated via Eq. 3. Here the clinicians would use an antigen-bearing protein column located outside the patient.27 Blood would be shunted from the patient to the column at a calculated time when the estimated marrow absorbed dose approached toxic limits. At that point, the activity level in the bloodstream would essentially be set to zero such that no further bone marrow toxicity would be anticipated. Note that this method requires that the patient be placed into an external circulatory system.
Other invasive strategies have been proposed. If the marrow is expected to be the absorbed dose-limiting tissue, the patient may have a marrow sample sequestered prior to the therapy with subsequent transplantation if the RM is sufficiently depleted. It has become conventional to replace platelets and white cells in patients undergoing TRT when these cells are below certain limits. In the future, such procedures may become economical enough such that the marrow is no longer the activity-limiting tissue. In that case, other solid organs such as the kidneys or liver may be considered limiting. This strategy may lead to greater total activities being injected and larger tumor absorbed doses being achieved.
As in the case of external beam, one anticipates that considerable effort will be expended to enhance TRT-induced absorbed dose by means of circulating chemotherapeutic and radiation-enhancing agents.28 At present, it is unclear what chemical dose levels and what timing relative to the IV injection of the radiotherapeutic agent are optimal. Again, preclinical and phase I trials will be needed to evaluate the effectiveness of this approach. Because of the multiple possible combinations of enhancing and TRT agents, extensive animal testing will become more important in the near term.
Following from the above multiple agent therapies, it is probable that future collaborations would be expanded between medical oncology and radiation oncology. Historically, chemotherapeutic drugs have been studied using only blood and excreta chemical measurements. In the next 7–10 years, it would be anticipated that present-day and future chemotherapeutic drugs would be radiolabeled and then followed, probably with PET scanning, in patients. Because of concern that the radioactive tag may affect the biodistribution, labeling will preferably be done by substituting radioactive isotopes into the chemical structure. While organic isotopes 11C, 15O, and 13N are of primary importance, there will be other possible labels such as 18F in the case of 5FU. Availability of local cyclotrons is implicit in this approach due to the short half-lives of the positron labels required.
Improved absorbed dose estimation
Improvement in patient-specific absorbed dose estimates will be one outcome achievable by medical physicists in the immediate time frame. This result may be termed a type-II absorbed dose estimate whereas the MIRD (phantom) result is a type-I calculation.29 Phantom results will still be needed for FDA approval of the trial; patient-specific calculations will evolve to be used to assess clinical outcomes for an individual. In order to achieve such specificity, knowledge of the patient’s organ masses and their spatial separations will be essential.
Instrumentation enhancements, now common in nuclear medicine, will make it possible to directly improve patient absorbed dose estimates. This follows from the two uncertainties implicit in Eq. 2: Evaluating and S for an individual. Considering the present difficulty of estimating the activity (A) at depth, it is important to realize that there are at least six different techniques30 that have been applied to this long-standing physics problem. With hybrid scanners such as SPECT∕CT and PET∕CT, gamma attenuation factors will be evaluated directly without the need for external transmission sources or complicated fusions of different modality images. Additionally, the activity being imaged will be assignable to a specific anatomic structure in the body; a structure that will also have a definite geometric volume and location relative to other tissues. As a result, uncertainties using the phantom calculations in OLINDA (Ref. 21) will be reduced and the true organ (or tumor) size obtained. Koral and co-workers have demonstrated31 that quantitative SPECT permits tumor absorbed dose estimates that do correlate with lymphoma outcomes. With hybrid scanners, we expect that such improved correlations will occur for other tumor types.
Variation of tumor mass during therapy is a feature of TRT that has been observed in clinical lymphoma trials.32 This result cannot be anticipated from prior imaging sessions since the effect arises out of the relatively high magnitude of the therapeutic absorbed dose. In the future, simple relationships such as Eq. 2 will not generally be valid for tumors. Instead, dosimetrists will use the differential form of the equality whereby absorbed dose rate is evaluated over a set of time intervals during the therapy regimen. A mathematical model of the target mass variation over time can be used in the determination of S in this case.
Equation 2 has other important limitations. As defined above, it refers to average total organ or tumor absorbed doses. While of gross interest, such single value representations of the ionizing radiation would not be as useful as absorbed dose-volume histograms that have been popularized in external beam work. In this case, a voxel-based set of sources would be introduced and a voxel-by-voxel result calculated by convolution. This result, when available, will lead to greater absorbed dose heterogeneity and make treatment planning a more complicated task. A technical problem in this context would be the tracking of individual regions from one imaging session to the next during the imaging or subsequent therapy. For beta emitters, brake radiation effects can be included in this formulation.
It has been shown in both murine and some human studies that tumor uptake is an inverse function of tumor mass—at least for radiometal-labeled entities.33, 34 We would anticipate that this result would become more important in future clinical trials implying adjuvant treatment of patients postsurgery. Applications of this idea to occult lesions would also be of interest since these sites would probably have relatively small sizes. One colon cancer trial involving unlabeled antibodies has been reported.35
Like politics, absorbed dose is essentially a local phenomenon. Given surgical specimens and information on cellular localization, an optimal radionuclide label for a given tumor type can also become possible. Proof of localization within tumor cell nuclei or at least at their surfaces would imply use of alpha emitters such as 213Bi or Auger emitters as 111In. Heterogeneous distributions of activity throughout the tumor, on the other hand, would indicate longer-range beta producers such as 90Y to enhance cross-fire effects. Similar arguments can be applied to normal tissue toxicity. Thus, future improvement in therapy will depend upon greater cooperation between surgeons (and pathologists), radiation oncologists, and therapy physicists.
CONCLUSIONS
Continued growth in lymphoma treatment using TRT is expected with more patients being entered into RIT trials as part of a first-line therapy. Helping to expedite this expansion is the unexplained, growing incidence of B-cell lymphomas. We would anticipate that T-cell antibodies would become available within the next 5 years. While 90Y facilitates outpatient treatments, the utilization of 131I as a label is expected to continue due to lower radionuclide cost and the possibility of directly imaging therapeutic administrations to verify treatment planning.
With better dosimetry, repeat TRT procedures would become possible. In the next 5–7 years, repeated lymphoma therapies would become a topic of importance to patients who have recurrence or were originally undertreated. Patient-specific normal organ absorbed doses would be tabulated to make sure that the relevant total is within the levels determined in external beam therapy.17 It is likely that the toxicity levels due to TRT-derived absorbed doses will be found to be different from those seen in external beam therapies.
We can also expect extensive use of engineering and invention to contribute a number of new targeting pharmaceuticals.36 Improvement in chelates will enable greater control of radiometals so that direct targeting of the freed label to the marrow becomes improbable.
Improved patient-specific absorbed dose estimates will lead to better control of toxicities and improved clinical results for lymphomas and, eventually, solid tumors. The present tenuous connection between estimated marrow absorbed dose and its clinical toxicity will become more statistically significant as the marrow mass becomes available via marrow tracers used as adjuncts prior to the TRT protocols. As a result, escalation in phase I and other FDA-approved trials would occur with normal organ absorbed doses as the control variables and not simplistic parameters such as total activity or total activity per body surface area or body mass. Labeling and tracking of chemotherapeutics will evolve out of this work and lead to greater understanding and safety of their administration to individual patients. Combinations of radiation-enhancing compounds with TRT will expand within these two converging threads of knowledge. Patient-specific treatment is one logical outcome of this work.
ACKNOWLEDGMENTS
This work was partially supported by NIH Grant Nos. PO1-43904 and CA 33572 (L.W.).
References
- Jacobs A. J., Fer M., Su F. M., Breitz H., Thompson J., Goodgold H., Cani J., Heap J., and Weiden P., “A phase I trial of rhenium-186-labeled monoclonal antibody administered intraperitoneally in ovarian carcinoma: Toxicity and clinical response,” Obstet. Gynecol. (N.Y., NY, U. S.) 82, 586–593 (1993). [PubMed] [Google Scholar]
- Riva P., Arista A., Tison V., Sturiale C., Francheschi G., Spinelli A., Riva N., Casi M., Moscatelli G., and Frattarelli M., “Intralesional radioimmunotherapy of malignant gliomas: An effective treatment in recurrent tumors,” Cancer 73, 1076–1082 (1994). [DOI] [PubMed] [Google Scholar]
- Shapiro B., Sisson J. C., Wieland D. M., Mangner T. J., Zempel S. M., Mudgett E., Gross M. D., Carey J. E., Zasadny K. R., and Beierwaltes W. H., “Radiopharmaceutical therapy of malignant pheochromocytoma with 131I metaiodobenzylguanidine: Results from ten years of experience,” J. Nucl. Biol. Med. 35, 269–276 (1991). [PubMed] [Google Scholar]
- Frilling A., Weber F., Cicinnati V., and Broelsch C., “Role of radiolabeled octreotide therapy in patients with metastatic neuroendocrine neoplasms,” Exp. Rev. Endocrinology Metabolism 2, 517–527 (2007). [DOI] [PubMed] [Google Scholar]
- DeNardo G. L., Natarajan A., Hok S., Perkins J., Cosman M., DeNardo S. J., Lightstone F. C., Mirick G. L., Miers L. A., and Balhorn R. L., “Pharmacokinetic characterization in xenografted mice of a series of first-generation mimics for HLA-DR antibody, Lym-1, as carrier molecules to image and treat lymphoma,” J. Nucl. Med. 48, 1338–1347 (2007). [DOI] [PubMed] [Google Scholar]
- Hicke B. J., Stephens A. W., Gould T., Chang Y. -F., Lynott C. K., Heil J., Borkowski S., Hilger C. -S., Cook G., Warren S., and Schmidt P. G., “Tumor targeting by an aptamer,” J. Nucl. Med. 47, 668–678 (2006). [PubMed] [Google Scholar]
- Waldmann T. A., “Monoclonal antibodies in diagnosis and therapy,” Science 252, 1657–1662 (1991). [DOI] [PubMed] [Google Scholar]
- Park J. W., “Liposome-based drug delivery in breast cancer treatment,” Breast Cancer Res. 4, 95–99 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukowska-Latallo J. F., Candido K. A., Cao Z., Nigavekar S. S., Majoros I. J., Thomas T. P., Balogh L. P., Khan M. K., and J. R.Baker, Jr., “Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer,” Cancer Res. 10.1158/0008-5472.CAN-04-3921 65, 5317–5324 (2005). [DOI] [PubMed] [Google Scholar]
- Liu G., Dou S., Mardirossian G., He J., Zhang S., Liu X., Rusckowski M., and Hnatowich D. J., “Successful radiotherapy of tumor in pretargeted mice by 188Re-radiolabeled phosphorodiamidate morpholino oligomer, as synthetic DNA analogue,” Clin. Cancer Res. 12, 4958–4964 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski R. J., Thurston K. G., Goin J. E., Wong C. Y., Gates V. L., Van Buskirk M., Geschwind J. F., and Salem R., “90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: Response to treatment at targeted absorbed doses of 135−150 Gy as measured by 18F fluorodeoxyglucose positron emission tomography and computer tomographic imaging,” J. Vasc. Interv. Radiol. 16, 1641–1651 (2005). [DOI] [PubMed] [Google Scholar]
- Kennedy A. S., Coldwell D., Nutting C., Murthy R., D. E.Wertman, Jr., Loehr S. P., Overton C., Meranze S., Niedzwecki J., and Sailer S., “Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: Modern USA experience,” Int. J. Radiat. Oncol., Biol., Phys. 10.1016/j.ijrobp.2005.12.051 65, 412–425 (2006). [DOI] [PubMed] [Google Scholar]
- Kenanova V., Olafsen T., Williams L. E., Ruel N. H., Longmate J., Yazaki P. J., Shively J. E., Colcher D., Raubitschek A. A., and Wu A. M., “Radioiodinated versus radiometal-labeled anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments: Optimal pharmacokinetics for therapy,” Cancer Res. 67, 718–726 (2007). [DOI] [PubMed] [Google Scholar]
- Wiseman G. A., White C. A., Sparks R. B., Erwin W. D., Podoloff D. A., Lamonica D., Bartlett N. L., Parker J. A., Dunn W. L., Spies S. M., Belanger R., Witzig T. L., and Leigh B. R., “Biodistribution and dosimetry results from a phase III prospectively randomized controlled trial of Zevalin radioimmunotherapy for low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma,” Crit. Rev. Oncol. Hematol. 39, 181–194 (2001). [DOI] [PubMed] [Google Scholar]
- Vose J. M., Wahl R. L., Saleh M., Rohatiner A. Z., Knox S. J., Radford J. A., Zelenetz A. D., Tidmarsh G. F., Stagg R. J., and Kaminski M. S., “Multicenter phase II study of iodine I-131 tositumomab for chemotherapy-relapsed/refractory low-grade and transformed low-grade B-cell non-Hodgkin’s lymphoma,” J. Clin. Oncol. 18, 1316–1323 (2000). [DOI] [PubMed] [Google Scholar]
- Wong J. Y. C., Shibata S., Williams L. E., Kwok C. S., Liu A., Chu D. Z., Yamauchi D. M., Wilcznski S., Ikle D. N., Wu A. M., Yazaki P. J., Shively J. E., Doroshow J. H., and Raubitschek A. A., “A phase I trial of 90Y-anti-carcinoembryonic antigen chimeric T84.66 radioimmunotherapy with 5-fluorouracil in patients with metastatic colorectal cancer,” Clin. Cancer Res. 9, 5842–5852 (2003). [PubMed] [Google Scholar]
- Emami B., Lyman J., Brown A., Coia L., Goitein M., Munzenrider J. E., Shank B., Solin L. J., and Wesson M., “Tolerance of normal tissue to therapeutic irradiation,” Int. J. Radiat. Oncol., Biol., Phys. 21, 109–122 (1991). [DOI] [PubMed] [Google Scholar]
- DeNardo G. L., DeNardo S. J., Shen S., DeNardo D. A., Mirick G. R., Macey D. J., and Lamborn K. R., “Factors affecting 131-I-Lym-1 pharmacokinetics and radiation dosimetry in patients with non-Hodgkin’s lymphoma and chronic lymphocytic leukemia,” J. Nucl. Med. 40, 1317–1326 (1999). [PubMed] [Google Scholar]
- Siegel J. A., Wessels B. W., Watson E. E., Stabin M. G., Vriesendorp H. M., Bradley E. W., Badger C. C., Brill A. B., Kwok C. S., Stickney D. R., Eckermann K. F., Fisher D. R., Buchsbaum D. J., and Order S. E., “Bone marrow dosimetry and toxicity in radioimmunotherapy,” Antibody, Immunoconjugates, Radiopharm. 3, 213–233 (1990). [Google Scholar]
- Stabin M. G., “MIRDOSE: Personal computer software for internal absorbed dose assessment in nuclear medicine,” J. Nucl. Med. 37, 538–546 (1996). [PubMed] [Google Scholar]
- Stabin M. G., Sparks R. B., and Crowe E., “OLINDA/EXM: The second-generation personal computer software for internal absorbed dose assessment in nuclear medicine,” J. Nucl. Med. 46, 1023–1027 (2005). [PubMed] [Google Scholar]
- Williams L. E., Liu A., Raubitschek A. A., and Wong J. Y. C., “A method for patient-specific absorbed dose estimation for internal beta emitters,” Clin. Cancer Res. 5, 3015s–3019s (1999). [PubMed] [Google Scholar]
- Akabani G., Hawkins W. G., Eckblade M. B., and Leichner P. K., “Patient-specific dosimetry using quantitative SPECT imaging and three-dimensional discrete Fourier transform convolution,” J. Nucl. Med. 38, 308–314 (1997). [PubMed] [Google Scholar]
- Holdsworth C. H., Dahlbom M., Liu A., Williams L., Levin C. S., Janecek M., and Hoffman E. J., “Expanding the versatility of a more accurate accelerated Monte Carlo simulation for 3D PET: Data correction of PET emission scans using 124-I,” Nuclear Science Symposium Conference Record 2001 IEEE (2002), Vol. 4, pp. 2105–2109.
- Goodwin D. A., “Tumor pretargeting: Almost the bottom line,” J. Nucl. Med. 36, 876–879 (1995). [PubMed] [Google Scholar]
- Goldenberg D. M. and Sharkey R. M., “Novel radiolabeled antibody conjugates,” Oncogene 26, 3734–3744 (2007). [DOI] [PubMed] [Google Scholar]
- Linden O., Kurkus J., Garkavij M., Cavallin-Stahl E., Ljungberg M., Nilsson R., Ohlsson T., Sandberg B., Strand S. -E., and Tennvall J., “A novel platform for radioimmunotherapy: Extracorporeal depletion of biotinylated and 90Y-labeled rituximab in patients with refractory B-cell lymphoma,” Cancer Biother. Radiopharm. 20, 457–466 (2005). [DOI] [PubMed] [Google Scholar]
- Welt S., Ritter G., C.Williams, Jr., Cohen L. S., Jungbluth A., Richards E. A., Old L. J., and Kemeny N. E., “Preliminary report of a phase I study of combination chemotherapy and humanized A33 antibody immunotherapy in patients with advanced colorectal cancer,” Clin. Cancer Res. 9, 1347–1353 (2003). [PubMed] [Google Scholar]
- Williams L. E., Liu A., Yamauchi D. M., Lopatin G., Raubitschek A. A., and Wong J. Y. C., “The two types of correction of absorbed dose estimates for internal emitters,” Cancer 94, 1231–1234 (2002). [DOI] [PubMed] [Google Scholar]
- Macey D. J., Williams L. E., Breitz H. B., Liu A., Johnson T. K., and Zanzonico P. B., A Primer for Radioimmunotherapy and Radionuclide Therapy AAPM Report No. 71 (Medical Physics, Madison, WI, 2001). [Google Scholar]
- Koral K. F., Dewaraja Y., Clarke L. A., Li J., Zasadny R., Rommelfanger S. G., Francis I. R., Kaminski M. S., and Wahl R. L., “Tumor-absorbed dose estimates versus response in tositumomab therapy of previously untreated patients with follicular non-Hodgkin’s Lymphoma: Preliminary report,” Cancer Biother. Radiopharm. 15, 347–355 (2000). [DOI] [PubMed] [Google Scholar]
- Hindorf C., Linden O., Stenberg L., Tennvall J., and Strand S. -E., “Change in tumor-absorbed dose due to decrease in mass during fractionated radioimmunotherapy in lymphoma patients,” Clin. Cancer Res. 1, 4003S–4006S (2003). [PubMed] [Google Scholar]
- Williams L. E., Duda R. B., Proffitt R. T., Beatty B. G., Beatty J. D., Wong J. Y. C., Shively J. E., and Paxton R. J., “Tumor uptake as a function of tumor mass: A mathematic model,” J. Nucl. Med. 29, 103–109 (1988). [PubMed] [Google Scholar]
- Macey D. J., DeNardo S. J., DeNardo G., Goodnight J. L., and Unger M. W., “Uptake of In-111 labeled monoclonal antibody ZME018 as a function of tumor size in a patient with melanoma,” Am. J. Physiol. Imaging 3, 1–6 (1988). [PubMed] [Google Scholar]
- Riethmuller G., Schneider-Gadicke E., Schlimok G., Schmiegel W., Raab R., Hoffken K., Gruber R., Pichlmaier H., Hirche H., Pichlmayr R., Buggisch P., and Witte J., “Randomised trial of monoclonal antibody for adjuvant therapy of resected Dukes’C colorectal carcinoma,” Lancet 343, 1177–1183 (1994). [DOI] [PubMed] [Google Scholar]
- Carlsson J., Aronsson Forsell E., Hietala S. O., Stigbrand T., and Tennvall T., “Tumor therapy with radionuclides: Assessment of progress and problems,” Radiother. Oncol. 10.1016/S0167-8140(02)00374-2 66, 107–117 (2003). [DOI] [PubMed] [Google Scholar]
- Reprinted by permission of the Society of Nuclear Medicine from Wong J. Y. C., Thomas G. E., Yamauchi D., Williams L. E., Odom-Maryon T. L., Liu A., Esteban J. M., Neumaier M., Dresse S., Wu A. M., Primus F. J., Shively J. E., and Raubitschek A. A., “Clinical evaluation of indium-111-labeled chimeric anti-CEA monoclonal antibody,” J. Nucl. Med. 38, 1951–1959 (1997). [PubMed] [Google Scholar]